ABSTRACT
Introduction
In this study we sought to clarify the effects of early fast‐acting treatment (EFT) strategies on the time course for achieving the treatment target in generalized myasthenia gravis (MG).
Methods
This retrospective study of 923 consecutive MG patients analyzed 688 generalized MG patients who had received immunotherapy during the disease course. The time to first achieve minimal manifestations (MM) or better while receiving prednisolone at ≤5 mg/day for ≥6 months (MM‐or‐better‐5mg) up to 120 months after starting immunotherapy was compared between EFT and non‐EFT patients.
Results
Achievement of MM‐or‐better‐5mg was more frequent and earlier in the EFT group (P = 0.0004, Wilcoxon test; P = 0.0001, log‐rank test). Multivariate Cox regression analysis calculated a hazard ratio of 1.98 (P < 0.0001) for utilization of EFT. Dosing regimens of oral steroids in EFT produced no differences in the time course.
Conclusions
EFT strategies are advantageous for early achievement of MM‐or‐better‐5mg. Muscle Nerve 55: 794–801, 2017
Keywords: Kaplan–Meier method, myasthenia, oral steroids, treatment strategies, treatment target
Abbreviations
- AChR‐Ab
autoantibodies against the acetylcholine receptor
- CNI
calcineurin inhibitor
- CSR
complete stable remission
- EFT
early fast‐acting treatment
- HMP
high‐dose intravenous methylprednisolone
- HRQOL
health‐related quality of life
- IVIg
intravenous immunoglobulin
- MG
myasthenia gravis
- MGFA
Myasthenia Gravis Foundation of America
- MG‐QOL15
15‐item MG‐Specific QOL Scale
- MG‐QOL15‐J
Japanese version of the MG‐QOL15
- MM
minimal manifestations
- MuSK‐Ab
autoantibodies against muscle‐specific tyrosine kinase
- PSL
prednisolone
- QMG
MGFA quantitative MG score
Long‐term full remission without immune treatment is rare in myasthenia gravis (MG) patients, even though the frequency of patients with severe illness decreases during treatment.1, 2, 3, 4, 5 Many MG patients are still burdened by both insufficient improvement and long‐term side effects of oral corticosteroids.3, 4, 5, 6, 7 Disease severity and the dose of oral corticosteroids are major factors that negatively affect self‐perceived health‐related quality of life (HRQOL) in MG patients.5, 6 Steroid‐associated depression has also been suggested to be a significant negative factor for HRQOL of patients.5, 7 Therefore, greater effort is needed to reduce both the severity of the illness and the dose of oral corticosteroids.5, 6
It is ideal but uncommon (<10% of total patients) for MG patients to achieve Myasthenia Gravis Foundation of America (MGFA) complete stable remission (CSR)8 status.4, 5, 6 The HRQOL of MG patients who achieve minimal manifestations (MM)8 or better status on prednisolone (PSL) at ≤5 mg/day (MM‐or‐better‐5mg) is good and at almost an identical level to those who achieve CSR status.5, 6 Moreover, comparisons of the relationship between patients’ HRQOL and clinical parameters of MG with the associated treatment have led to the proposal that achieving MM‐or‐better‐5mg can be a practical treatment target.5, 6, 9 By achieving an early return to such a state, MG patients are enabled to live a normal lifestyle without worry about corticosteroid complications.6
Because the percentage of patients who achieve MM‐or‐better‐5mg is not high (<50% of subjects in cross‐sectional studies), changes in treatments are needed to further increase treatment success.5, 6 However, studies on the use of longer treatments with a higher dose of oral corticosteroids did not identify better outcomes for MG patients.10, 11 Therapeutic strategies have been reported that aggressively use non‐oral fast‐acting immunotherapies, such as plasmapheresis, high‐dose intravenous methylprednisolone (HMP), and intravenous immunoglobulin (IVIg) in the early stages of treatment.11 As these fast‐acting therapies can be repeated as needed beginning in the early stages [early fast‐acting treatment (EFT) strategies], this could possibly lead to earlier improvements with lower doses of oral corticosteroids, thereby potentially resulting in frequent achievement of MM‐or‐better‐5mg lasting at least several months.11 In fact, in Japan, some neurologists are currently utilizing the EFT strategy to treat patients.9
Recently, we conducted a survey of 923 MG patients and obtained detailed clinical information on their past immunotherapy regimen, past course of MGFA postintervention status, current immunotherapy, and present disease status. Using this database, we attempted to clarify whether utilization of EFT strategies would have an effect on the frequency and time course of achieving MM‐or‐better‐5mg lasting >6 months in generalized MG patients.
METHODS
Patients
This survey was conducted at 13 neurological centers (Japan MG Registry Study Group; refer to Supplementary Table S1, available online) in Japan. We evaluated patients with established MG between April and July 2015. To avoid potential bias, we enrolled consecutive patients over a short duration (4 months). During this period, a total of 1,088 MG patients visited our hospitals. From this group we were able to collect fully detailed clinical data from 923 patients. Data collected included past treatment regimens starting from the early stages, disease status when at its worst, current treatment and disease status, and period from start of treatment to first achieving MM‐or‐better‐5mg for ≥6 months (the treatment target). Among the 923 patients, 722 had generalized MG. From this group, 34 patients with mild disease who received no immune treatment during the study course were excluded, as they could not be classified as either EFT or non‐EFT. Finally, a total of 688 generalized patients were included in this analysis (Fig. 1). It should be noted that 263 of the 688 subjects had been included in our previous survey conducted in 2012.6 Tables 1 and 2 list the clinical data for the subjects who received immune treatment with (n = 249) and without (n = 439) EFT strategies (Fig. 1).
Figure 1.
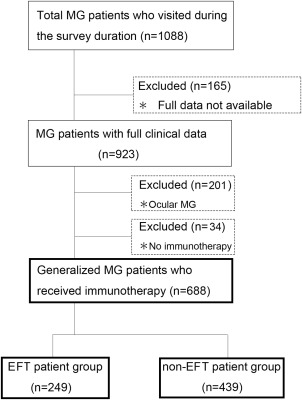
CONSORT diagram of study participants. EFT, early fast‐acting treatment; MG, myasthenia gravis.
Table 1.
Comparisons of characteristics between patients treated with early fast‐acting treatment (EFT) and non‐EFT strategies (Mann–Whitney U‐test)
|
EFT group (n = 249) |
Non‐EFT group (n = 439) |
|
|---|---|---|
| Age (years) | 56.6 ± 17.5 | 58.3 ± 15.0 |
| Women (%)a | 71.9 (179/249) | 67.9 (298/439) |
| Duration of immune treatment (years) | 6.1 ± 5.0d | 11.1 ± 8.8 |
| Age at onset (years) | 49.2 ± 18.3 | 43.9 ± 17.8 |
| Thymectomy (%)a | 56.6 (141/249) | 63.6 (279/439) |
| Thymoma (%)a | 31.3 (78/249) | 23.0 (101/439) |
| AChR‐Ab positivity (%)a | 81.9 (204/249) | 85.0 (373/439) |
| MuSK‐Ab positivity (%)a | 4.8 (12/249) | 2.5 (11/439) |
| MGFA classification (%)a (worst, II/III/IV/V) | 42.2/31.3/9.2/17.3d | 62.2/23.0/4.3/10.5 |
| Bulbar symptoms (%)a (worst) | 75.5 (188/249)d | 58.8 (258/439) |
| Current QMG | 7.1 ± 5.5b | 7.6 ± 4.8 |
| Current MG‐QOL15‐J | 13.9 ± 11.4b | 15.2 ± 9.9 |
| MM or better at a dose ≤5 mg for ≥6 months, current status (%)a | 49.4 (123/249)c | 42.1 (185/439) |
EFT, early fast‐acting treatment; AChR‐Ab, autoantibodies against the acetylcholine receptor; MGFA, Myasthenia Gravis Foundation of America; MG‐QOL15‐J, 15‐item MG‐Specific QOL Scale—Japanese version; MM, minimal manifestations; MuSK‐Ab, autoantibodies against muscle‐specific tyrosine kinase; QMG, MGFA quantitative MG score.
Chi‐square test.
P < 0.05 vs. non‐EFT group.
P < 0.001 vs. non‐EFT group.
P < 0.0001 vs. non‐EFT group.
Table 2.
Differences in the therapies utilized between patients treated with early fast‐acting treatment (EFT) and non‐EFT strategies (Mann–Whitney U‐test)
|
EFT group (n = 249) |
Non‐EFT group (n = 439) |
|
|---|---|---|
| Oral PSL | ||
| Maximum dose of PSL (mg/day) | 25.0 ± 18.3 | 25.5 ± 20.2 |
| Current dose of PSL (mg/day) | 4.7 ± 4.7 | 4.2 ± 4.5 |
| Duration of PSL ≥20 mg/day (years) | 0.5 ± 1.5c | 1.0 ± 1.9 |
| Duration of PSL 10–19 mg/day (years) | 1.1 ± 1.7b | 2.0 ± 3.4 |
| Oral immunosuppressants other than steroids | ||
| Calcineurin inhibitor (%)a | 76.7 (191/249)b | 56.0 (246/439) |
| Azathioprine (%)a | 6.4 (16/249) | 5.2 (23/439) |
| Non‐oral fast‐acting treatments | ||
| Plasmapheresis [time (range)] | ||
| Within 6 months from starting therapy | 3.4 ± 3.0 (1–17)c | 0.2 ± 1.2 (0–8) |
| Within 1 year from starting therapy | 4.0 ± 3.8 (1–24)c | 0.5 ± 1.6 (0–8) |
| Throughout the whole course | 5.6 ± 10.0 (1–112)c | 1.5 ± 4.2 (0–27) |
| High‐dose intravenous methylprednisolone, total dose [g (range)] | ||
| Within 6 months from starting therapy | 4.8 ± 4.0 (1–24)c | 0.2 ± 0.9 (0–5) |
| Within 1 year from starting therapy | 5.5 ± 4.8 (1–24)c | 0.4 ± 1.4 (0–8) |
| Throughout the whole course | 8.0 ± 11.2 (1–106)c | 2.7 ± 6.1 (0–36) |
| Intravenous immunoglobulin 0.4 g/kg for 5 days (range) | ||
| Within 6 months from starting therapy | 0.4 ± 0.7 (0–4)c | 0.0 ± 0.1 (0–1) |
| Within 1 year from starting therapy | 0.5 ± 0.7 (0–4)c | 0.0 ± 0.2 (0–1) |
| Throughout the whole course | 1.0 ± 2.2 (0–19)c | 0.7 ± 2.6 (0–28) |
EFT, early fast‐acting treatment strategy; PSL, prednisolone.
Chi‐square test.
P < 0.05 vs. non‐EFT group.
P < 0.0001 vs. non‐EFT group.
The diagnosis of MG was based on clinical findings (fluctuating symptoms with easy fatigability and recovery after rest) that included clinical improvement after intravenous administration of anticholinesterase, decremental muscle response to a 3‐Hz train of repetitive nerve stimuli, or the presence of antibodies against skeletal muscle acetylcholine receptor (AChR‐Ab) or muscle‐specific tyrosine kinase (MuSK‐Ab). Single‐fiber electromyography was not performed systematically.
The ethics committees of each of the participating institutions approved the study protocols. Written informed consent was obtained from all patients who agreed to take part in the study.
Description of EFT and Non‐EFT Strategies
EFT
EFT was defined as the treatment strategy that attempted to achieve early MM status initially, even if long‐term stability of the status could be provided, through aggressive use of fast‐acting therapies such as plasmapheresis, often combined with HMP, HMP alone, or IVIg starting within 1 month of treatment initiation, with the improved clinical status maintained using the lowest possible dose of oral corticosteroids.11 EFT was adopted among patients with varying disease severity, with MGFA classifications II–V.8 For the EFT group, the fast‐acting treatment set generally consisted of 1 or 2 plasmapheresis sessions followed by HMP, which was performed by intravenous injection of 0.5–1.0 g methylprednisolone immediately after the plasmapheresis and often on the morning of the subsequent 1–2 days, partly with HMP alone or with IVIg, which was administered at a dose of 0.4 g/kg/day for 5 days.
After being admitted to our hospitals for the EFT procedure, patients were administered the fast‐acting treatment set, as well as oral immune therapies. If there was insufficient improvement, the fast‐acting treatment set was repeated until MM status was reached. After such treatment, patients were discharged from the hospital and followed up with the lowest possible dose of oral steroids.11 If MM status could not be maintained or a dose increase of oral steroids was needed during follow‐up, we recommended that patients undergo the treatment set again (during an additional hospital stay of 2–7 days). The fast‐acting treatment set was also performed in the non‐severe cases to achieve an early MM or better and to help reduce the dose of oral steroids. The frequencies of fast‐acting treatment and the doses given, along with the duration of oral PSL, are shown in Table 2.
HMP after plasmapheresis was omitted in some patients treated with plasmapheresis on high‐dose oral PSL. Treatment with HMP alone was actually infrequent, but, when it was performed, 0.5 g/day of methylprednisolone for 1–2 days, a lower dose than used for other autoimmune diseases, was preferentially administered. As it was only 3.5 years from the approval of IVIg for MG by the National Health Insurance in Japan (i.e., since September 2011), the frequency of IVIg use was still low. In non‐severe cases, HMP alone or IVIg were also performed in the outpatient department.
Non‐EFT
Non‐EFT mainly consisted of oral immunotherapy with oral corticosteroids. Non‐oral fast‐acting therapies, such as plasmapheresis, HMP, and IVIg, were only provided as treatment for patients in MG crisis or during an attempt to avoid crisis. Non‐EFT also was used in patients with various disease severities (MGFA classes II–V). In the non‐EFT group, various dosing regimens of oral steroids were used. Table 2 lists the frequencies of plasmapheresis, HMP, and IVIg, along with the dose and duration of oral PSL.
Non‐Steroid Immunosuppressants
Attending physicians treated patients with non‐steroid immunosuppressants in both the EFT and non‐EFT groups. The only non‐steroid immunosuppressants that have been approved for use by the National Health Insurance in Japan are calcineurin inhibitors (CNIs; cyclosporine and tacrolimus). In a few cases, azathioprine and mycophenolate mofetil were also used, but only rarely. Table 2 lists the frequency of CNI and azathioprine use in both groups.
Oral Steroid Dosing Regimens in Patients Treated with EFT
Among patients who underwent EFT, there were at least 2 distinct types of dosing regimens used for the combined oral PSL (Table 3).
Table 3.
Differences in characteristics and therapies utilized between the patients treated with EFT combined with low‐dose and high‐dose PSL dosing regimens (Mann–Whitney U‐test)
| Low‐dose PSL+EFT group (n = 97) | High‐dose PSL+EFT group (n = 73) | |
|---|---|---|
| Age (years) | 58.6 ± 18.0 | 56.1 ± 17.3 |
| Women (%)a | 72.2 (70/97) | 69.9 (51/73) |
| Duration of immune treatment (years) | 5.3 ± 4.5c | 10.2 ± 5.0 |
| Age at onset (years) | 52.0 ± 19.8 | 45.5 ± 17.5 |
| Thymectomy (%)a | 35.1 (34/97)b | 78.1 (57/73) |
| Thymoma (%)a | 24.7 (24/97) | 32.9 (24/73) |
| AChR‐Ab positivity (%) | 76.3 (74/97) | 83.6 (61/73) |
| MuSK‐Ab positivity (%)a | 4.1 (4/97) | 5.5 (4/73) |
| Worst QMG | 15.5 ± 6.9c (n = 97) | 22.0 ± 8.1 (n = 68) |
| Current QMG | 6.4 ± 4.8 | 7.8 ± 6.4 |
| Current MG‐QOL15‐J | 13.0 ± 13.8 | 14.4 ± 14.5 |
| MM or better at a dose ≤5 mg ≥6 months (current status) (%)a | 55.7 (54/97) | 56.1 (41/73) |
| Oral immunosuppressive agents | ||
| Maximum dose of PSL (mg/day) | 7.9 ± 4.0c | 49.2 ± 8.9 |
| Current dose of PSL (mg/day) | 4.4 ± 3.3 | 4.8 ± 6.6 |
| Duration of PSL ≥20 mg/day (years) | 0.0 ± 0.0c | 1.5 ± 2.4 |
| Calcineurin inhibitor use (%)a | 81.4 (79/97) | 71.2 (52/73) |
| Non‐oral fast‐acting treatments within 6 months from starting therapy | ||
| Plasmapheresis, time (range) | 3.0 ± 2.4 (1–14) | 3.8 ± 3.0 (1–12) |
| High‐dose intravenous methylprednisolone, total dose [g (range)] | 5.2 ± 3.2 (1–20)c | 2.5 ± 2.8 (0–12) |
| Intravenous immunoglobulin 0.4 g/kg for 5 days (range) | 0.4 ± 0.6 (0–3) | 0.3 ± 0.6 (0–2) |
EFT, early fast‐acting treatment strategy; AChR‐Ab, antibodies against acetylcholine receptor; MGFA, Myasthenia Gravis Foundation of America; MG‐QOL15‐J, a 15‐item MG‐specific QOL scale Japanese version; MM, minimal manifestations; MuSK, muscle‐specific tyrosine kinase; PSL, prednisolone; QMG, MGFA quantitative MG score.
Chi‐square test.
P < 0.01 vs. non‐EFT group.
P < 0.0001 vs. non‐EFT group.
EFT Combined with a Low‐Dose Regimen of Oral PSL
In the patient group treated with EFT + the low‐dose PSL regimen, improvements in MG symptoms associated with living difficulties were mainly due to the use of fast‐acting therapy sets starting during the early stages of treatment, and the use of low‐dose (≤10 mg/day) oral PSL as maintenance therapy (Table 3). If symptoms once again worsened during follow‐up, patients were readmitted for a shorter treatment period of 2–7 days, during which they underwent further fast‐acting therapy sets (1 or 2 times). In the majority of patients, it was not necessary to increase the dose of oral PSL. Patients treated with oral PSL ≥20 mg/day for >3 months were excluded from this category.
EFT Combined with a High‐Dose Regimen of Oral PSL
In the patient group treated with EFT + the high‐dose PSL regimen, MG symptoms associated with living difficulties were improved by both the fast‐acting therapy sets and high‐dose oral PSL. The oral steroids were often given using a dose‐escalation schedule until the symptoms sufficiently improved, or until a maximum dose of 50–60 mg/day was reached. These treatments were maintained at the highest dose required, after which doses were tapered. Patients who underwent this treatment regimen often received >20 mg/day of PSL over a 1‐year period (Table 3).
Statistical Analysis
Differences between the 2 groups were evaluated using the Mann–Whitney U‐test for continuous variables and the chi‐square test for categorical variables. All continuous data are expressed as mean ± standard deviation (SD) or as a range. The time course required to achieve the treatment target (MM‐or‐better‐5mg for ≥6 months) during the period up to 120 months after starting immunotherapy was analyzed by the Kaplan–Meier method. The log‐rank test and Wilcoxon test were used to compare the course between 2 patient groups undergoing different treatment strategies. Univariate and multivariate Cox regression analyses were performed to estimate the hazard ratios (HRs) and the 95% confidential intervals (CIs) of the treatment strategies and other clinical parameters associated with achievement of the treatment target, and for use in comparing the baseline cumulative hazard curves of the treatment strategies as a function of time (excluding the effects of other clinical parameters). Statistical analyses were performed using UNISTAT version 5.6 (Unistat, London, UK) statistical software.
RESULTS
Differences between the EFT and Non‐EFT Groups
Differences in Patients’ Backgrounds
Table 1 shows a comparison of characteristics between EFT and non‐EFT patients. Symptoms at the time the condition was at its worst (MGFA classification and observed bulbar symptoms) were significantly more severe, and the duration of the immune treatment was significantly shorter in the EFT group (P < 0.0001). The MGFA quantitative MG score (QMG)8 at the time the condition was at its worst was not described for a large number of the patients in the non‐EFT group (a deficit in 79 of 439 records; data not shown). Current severity (QMG) and score on the 15‐item MG‐Specific QOL Scale12 Japanese version5 (MG‐QOL15‐J) were modestly lower (P = 0.04), with the rate of patients who had currently achieved MM‐or‐better‐5mg for ≥6 months being higher (P < 0.001) in the EFT group. There was a significant correlation between the current MG‐QOL15‐J score and achieving MM‐or‐better‐5mg for ≥6 months (r = –0.52, P < 0.0001; not shown in Table 1).
Differences in Therapies Utilized
Table 2 shows the differences in the therapies between the EFT and non‐EFT groups. Although there was no difference between the 2 groups for maximum dose during the disease course and current dose of oral PSL, in the non‐EFT group there was a longer duration when taking PSL ≥20 mg (P < 0.0001) and for PSL ≥10–19 mg/day (P = 0.02). The frequency of CNI use (P = 0.001) was higher in the EFT patients. In addition, plasmapheresis, HMP, and IVIg were all performed more frequently within 6 months and within 1 year from starting the immune therapy and throughout the therapeutic course in the EFT group (P < 0.0001). These therapies were not employed in the non‐EFT group, except when attempting to treat or avoid crisis.
Differences in Time Course Required to Achieve Treatment Target
Figure 2A shows Kaplan–Meier curves for the time to first achieve treatment target (MM‐or‐better‐5mg for ≥6 months) in both the EFT and non‐EFT groups. The treatment target was achieved more frequently and earlier in the EFT group (P = 0.0004, Wilcoxon test; P = 0.0001, log‐rank test; Fig. 2A). For univariate Cox regression, utilization of EFT to achieve the treatment target resulted in an HR of 1.61 (95% CI 1.27–2.04; P = 0.0001). Furthermore, when adding the background factors that showed significant differences between the 2 groups (Table 1) to the multivariate Cox regression analysis as covariables simultaneously with the utilization of EFT, the HR and the 95% CI for each covariable were as follows: duration of the immune treatment (HR 0.97; 95% CI 0.95–0.99; P = 0.008); MGFA classification (HR 0.84; 95% CI 0.72–0.98; P = 0.02); bulbar symptoms (HR 0.71; 95% CI 0.54–0.93; P = 0.01); and CNI use (HR 0.60; 95% CI 0.46–0.78; P = 0.001), with use of EFT resulting in a significant change (P < 0.0001) in HR to 1.98 (95% CI 1.51–2.60). CNIs are effective in MG,4, 13 and frequent use of CNIs in the EFT group may have promoted achievement of the treatment target. However, the beneficial effects of CNIs were not detected in the multivariate analysis, suggesting that the effects were not strong beyond the effects of EFT.
Figure 2.
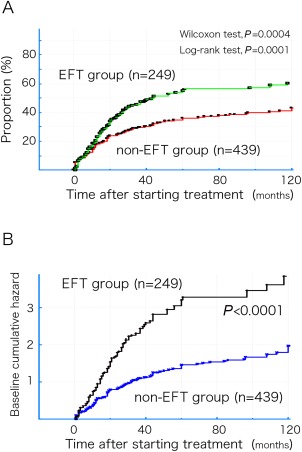
(A) Kaplan–Meier curves for the first achievement of the treatment target (MM or better‐5mg for ≥6 months) in both the EFT and non‐EFT groups. (B) Baseline cumulative hazard curves for both the EFT and non‐EFT groups, excluding the effects of the covariables as a function of time. EFT, early fast‐acting treatment; MM, minimal manifestations.
Figure 2B shows the baseline cumulative hazard curves for both the EFT and non‐EFT groups, excluding the effects of the covariables, as a function of time.
Differences between Low‐Dose and High‐Dose PSL Regimens in EFT Patients
Differences in Background and Therapies Utilized
Table 3 shows comparisons of characteristics and therapies utilized for the EFT‐treated patients when combined with either the low‐ or high‐dose PSL regimens. Symptoms at the time when conditions were at their worst (worst QMG) were more severe (P < 0.0001), the duration of immune treatment was longer (P < 0.0001), and the frequency of thymectomy was higher (P < 0.01) in the EFT‐treated patients given the high‐dose PSL regimen. Current severity (QMG), current MG‐QOL15‐J score, and percentage of patients who achieved MM‐or‐better‐5mg for ≥6 months at the present time were not different between the 2 groups. Although the maximum dose of PSL during the disease course was much higher, and the duration of taking PSL at a dose of ≥20 mg was much longer in the high‐dose PSL group (P < 0.0001), there was no difference between the 2 groups with regard to current dose of oral PSL and frequency of CNI use. In the low‐dose PSL group, only 1 patient received ≥20 mg for 3 months. Although there were no differences between groups for frequencies of plasmapheresis and IVIg within 6 months from starting the immune therapy, the dose of HMP was lower in the high‐dose PSL group (P < 0.0001).
Differences in Time Course Required to Achieve Treatment Target
Figure 3A shows the Kaplan–Meier curves for first achievement of the treatment target (MM‐or‐better‐5mg for ≥6 months) in the EFT groups with the low‐ and high‐dose PSL regimens. Achievement of the treatment target appeared to be more frequent and occur earlier in the low‐dose PSL group (P = 0.0003, Wilcoxon test; P = 0.004, log‐rank test; Fig. 3A). For the univariate Cox regression analysis, utilization of the low‐dose PSL regimen resulted in an HR of 1.81 (95% CI 1.19–2.74; P = 0.005) when compared with the high‐dose PSL regimen. However, when the background factors that showed significant differences between the 2 groups (Table 3) were added to a multivariate Cox regression analysis as covariables simultaneously with use of the low‐dose PSL regimen, the HRs and 95% CIs for each of the covariables were as follows: duration of immune treatment (HR 0.99; 95% CI 0.94–1.04; P = 0.68); thymectomy (HR 1.28; 95% CI 0.80–2.04; P = 0.30); and worst QMG (HR 0.93; 95% CI 0.91–0.97; P = 0.0001), with no significant change in HR when utilizing the low‐dose PSL regimen (HR 1.34; 95% CI 0.80–2.29; P = 0.25).
Figure 3.
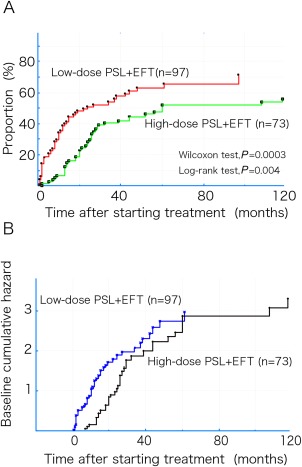
(A) Kaplan–Meier curves for first achievement of the treatment target (MM or better‐5mg for ≥6 months) in both groups of the EFT with the low‐ and high‐dose PSL regimens. (B) Time courses of the baseline cumulative hazard curves for both the low‐dose and high‐dose PSL groups as a function of time. EFT, early fast‐acting treatment; MM, minimal manifestations; PSL, prednisolone.
Figure 3B shows the time courses for the baseline cumulative hazard curves for both the low‐ and high‐dose PSL groups, excluding the effects of the covariables. Although the levels of the baseline cumulative hazard in the high‐dose PSL group appeared to belatedly increase to the same level as that observed in the low‐dose PSL group at 60 months after starting immune therapy, this change did not represent a significant difference.
DISCUSSION
Achieving a status of MM‐or‐better‐5mg probably enables patients to live a normal lifestyle without having to deal with the complications of corticosteroids.5, 6 Thus, the Japanese clinical guidelines for MG have recommended this as the first goal of treatment.9 The efficacy of using EFT against MG to achieve an early return to a normal lifestyle was first reported in 2010.11 These strategies have slowly and gradually become more common, with the procedures used in 36.1% (249 of 688) of the present immunotherapy‐treated generalized patients. The rate of patients who have achieved MM‐or‐better‐5mg in generalized MG has increased modestly from 37.4% in our previous survey in 20126 to 44.9% in this survey, which was conducted in 2015. However, further clarification is needed regarding whether utilization of EFT can actually promote early achievement of MM‐or‐better‐5mg, and whether the low‐ or high‐dose regimen would be the best choice for oral steroid therapy when combined with EFT.
Kaplan–Meier analyses of these data show that EFT was predominantly superior to non‐EFT for early achievement of MM‐or‐better‐5mg for ≥6 months in generalized MG patients. Because this was a retrospective study, there may be limitations associated with interpretation of the results. However, to avoid selection bias, all subjects enrolled were consecutive subjects. Furthermore, after excluding the effects of differences in the backgrounds between the 2 groups through the use of a multivariate analysis, we showed that EFT exhibited a rather marked significance, with an approximate 2‐fold change in the HR. Imai et al. also examined the combined use of plasmapheresis and/or IVIg, even in the absence of a crisis, and reported that it was positively associated with good outcomes in MG patients treated with oral steroids.10 Therefore, it is probable that EFT can promote early achievement of MM‐or‐better‐5mg for ≥6 months in generalized MG patients.
Plasmapheresis, IVIg, and HMP are fast‐acting therapies and probably enabled the patients to return early to MM‐or‐better‐5mg status; however, in general, plasmapheresis and IVIg are not considered treatments that can provide long‐term benefits for MG patients. Thus, the maintenance of good status for >6 months was possibly enabled by HMP to a considerable extent. There is a belief that longer term immunosuppression using higher doses of oral steroids and other immunosuppressive agents is required for MG; however, in actuality, the rate of patients who achieve long‐term remission is low, and achieving CSR is very rare, even today.4, 5, 6 Therefore, an attempt initially to achieve early MM‐or‐better‐5mg for some period (e.g., 6 months) through aggressive use of fast‐acting therapies may be more important for patients’ good HRQOL, even if long‐term stability of the status for years cannot be provided.
Although HMP is effective against generalized MG,14, 15 there can be a transient initial exacerbation, and therefore caution is required.9, 14, 15 Measures that can be taken to reduce risk include: administering the drug after oral PSL 5–10 mg/day with or without other immunosuppressive agents; reducing the dose to 500 mg; avoiding administration of the drug for consecutive days, and instead administering it once and then observing the patient for 2–5 days; and/or carrying out administration immediately after plasmapheresis.9 Therefore, HMP therapies should be carried out by a doctor who has abundant experience with the treatment, or under the supervision of a doctor with such knowledge.9 When HMP is performed as a first treatment for generalized MG without such measures or supervision, there is a risk of crisis, particularly in patients in MGFA Class III or higher, or in patients with bulbar symptoms.9 When using HMP in severe cases, it is necessary to prepare for a potential crisis and to ensure that the patient has been fully informed on the procedure and the potential side effects.9
Even when EFT was utilized, the increase in the rate of patients who achieved MM‐or‐better‐5mg for ≥6 months plateaued at around 60% at 5 years into the treatment (Fig. 2A). The Kaplan–Meier curve for the non‐EFT group also gradually changed and reached an approximate plateau as a function of time (Fig. 2A). Although immunotherapy is probably more effective against MG in the earlier stages of disease,10, 11, 13 the marked improvement observed during the first few years into treatment may lead to better long‐term results.
We found that, when oral steroid therapies were combined with EFT, there was no difference in the time course to achieve MM‐or‐better‐5mg for ≥6 months between the low‐ and the high‐dose PSL groups, after excluding the effects of background differences. Imai et al. reported that good responses to early‐stage treatment were associated with better outcomes as compared with use of a higher dose and longer treatment with steroids for MG.10 Thus, it is possible that attempts to achieve early improvement in the illness through the use of a frequent fast‐acting therapy set and dosing regimens of oral steroids (low‐dose or high‐dose) produced no difference in outcome among patients treated with EFT. Given that long‐term use of oral steroids above a certain dose can lead to a number of problems that can negatively affect the HRQOL of patients,5, 6, 7, 9 the use of oral steroids in EFT may be better when low‐dose regimens are used.
One of the advantages of using EFT is that it can lead to early improvement and early achievement of MM‐or‐better‐5mg. The disadvantages of EFT include both the labor and cost required when using this strategy, as well as potential complications.11 However, these issues may be solved on their own over the course of a few years, as the number of fast‐acting therapies required often decrease year by year.11 Even when using this type of strategy, a considerable number of generalized MG patients cannot achieve the treatment target, as seen in Figure 2A, and as reported elsewhere.11 Therefore, more effective therapies, such as molecular target drugs, are required for these patients.
In conclusion, in this analysis we have demonstrated that the advantages of EFT were early achievement of MM‐or‐better‐5mg, which is the defined treatment target, thereby enabling patients to return to a normal lifestyle, without the complications of corticosteroids, at an early time‐point. As the dosing regimens of oral steroids (low‐ or high‐dose) in EFT produced no difference in the time course required to achieve MM‐or‐better‐5mg, it appears that oral steroids are best used at a lower dose in EFT. Although there are limitations to this study due to its retrospective nature and unblinded design, we have provided useful information that may be used for planning therapeutic strategies that can help ensure an acceptable HRQOL in MG patients.
Supporting information
Additional supporting information may be found in the online version of this article.
Supporting Information
ACKNOWLEDGMENTS
The authors thank Dr. M. Motomura and Dr. H. Shiraishi (Department of Neurology, Nagasaki University Hospital) and Dr. Y. Shimizu and Dr. R. Ikeguchi (Department of Neurology, Tokyo Women's Medical University) for collection of patient data.
This work was supported by the Japan MG Registry Study Group.
REFERENCES
- 1. Pascuzzi RM, Coslett HB, Johns TR. Long‐term corticosteroid treatment of myasthenia gravis: report of 116 patients. Ann Neurol 1984;15:291–298. [DOI] [PubMed] [Google Scholar]
- 2. Grob D, Brunner N, Namba T, Pagala M. Lifetime course of myasthenia gravis. Muscle Nerve 2008;37:141–149. [DOI] [PubMed] [Google Scholar]
- 3. Gilhus NE. Autoimmune myasthenia gravis. Expert Rev Neurother 2009;9:351–358. [DOI] [PubMed] [Google Scholar]
- 4. Sanders DB, Evoli A. Immunosuppressive therapies in myasthenia gravis. Autoimmunity 2010;43:428–435. [DOI] [PubMed] [Google Scholar]
- 5. Masuda M, Utsugisawa K, Suzuki S, Nagane Y, Kabasawa C, Suzuki Y, et al The MG‐QOL15 Japanese version: validation and associations with clinical factors. Muscle Nerve 2012;46:166–173. [DOI] [PubMed] [Google Scholar]
- 6. Utsugisawa K, Suzuki S, Nagane Y, Masuda M, Murai H, Imai T, et al Health‐related quality of life and treatment targets in myasthenia gravis. Muscle Nerve 2014;50:493–500. [DOI] [PubMed] [Google Scholar]
- 7. Suzuki Y, Utsugisawa K, Suzuki S, Nagane Y, Masuda M, Kabasawa C, et al Factors associated with depressive state in patients with myasthenia gravis: a multicenter cross‐sectional study. BMJ Open 2011;1:e000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jaretzki A 3rd, Barohn RJ, Ernstoff RM, Kaminski HJ, Keesey JC, Penn AS, et al Myasthenia gravis: recommendations for clinical research standers. Task Force of the Medical Scientific Advisory Board of Myasthenia Gravis Foundation of America. Neurology 2000;55:16–23. [DOI] [PubMed] [Google Scholar]
- 9. Murai H. Japanese clinical guidelines for myasthenia gravis: putting into practice. Clin Exp Neuroimmunol 2015;6:21–31. [Google Scholar]
- 10. Imai T, Suzuki S, Tsuda E, Nagane Y, Murai H, Masuda M, et al Oral corticosteroid therapy and present disease status in myasthenia gravis. Muscle Nerve 2015;51:692–696. [DOI] [PubMed] [Google Scholar]
- 11. Nagane Y, Suzuki S, Suzuki N, Utsugisawa K. Early aggressive treatment strategy against myasthenia gravis. Eur Neurol 2011;65:16–22. [DOI] [PubMed] [Google Scholar]
- 12. Burns TM, Conaway MR, Cutter GR, Sanders DB, and the Muscle Study Group . Less is more, or almost as much: a 15‐item quality‐of‐life instrument for myasthenia gravis. Muscle Nerve 2008;38:957–963. [DOI] [PubMed] [Google Scholar]
- 13. Nagane Y, Suzuki S, Suzuki N, Utsugisawa K. Factors associated with response to calcineurin inhibitors in myasthenia gravis. Muscle Nerve 2010;41:212–218. [DOI] [PubMed] [Google Scholar]
- 14. Lindberg C, Andersen O, Lefvert AK. Treatment of myasthenia gravis with methylprednisolone pulse: a double blind study. Acta Neurol Scand 1998;97:370–373. [DOI] [PubMed] [Google Scholar]
- 15. Arsura E, Brunner NG, Namba T, Grob D. High‐dose intravenous methylprednisolone in myasthenia gravis. Arch Neurol 1985;42:1149–1153. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional supporting information may be found in the online version of this article.
Supporting Information


