Abstract
Objectives
To estimate outcomes according to attained blood pressure (BP) in the oldest adults treated for hypertension in routine family practice.
Design
Cohort analysis of primary care inpatient and death certificate data for individuals with hypertension.
Setting
Primary care practices in England (Clinical Practice Research Datalink).
Participants
Individuals aged 80 and older taking antihypertensive medication and free of dementia, cancer, coronary heart disease, stroke, heart failure, and end‐stage renal failure at baseline.
Measurements
Outcomes were mortality, cardiovascular events, and fragility fractures. Systolic BP (SBP) was grouped in 10‐mmHg increments from less than 125 to 185 mmHg or more (reference 145–154 mmHg).
Results
Myocardial infarction hazards increased linearly with increasing SBP, and stroke hazards increased for SBP of 145 mmHg or greater, although lowest mortality was in individuals with SBP of 135 to 154 mmHg. Mortality of the 13.1% of patients with SBP less than 135 mmHg was higher than that of the reference group (Cox hazard ratio=1.25, 95% confidence interval=1.19–1.31; equating to one extra death per 12.6 participants). This difference in mortality was consistent over short‐ and long‐term follow‐up; adjusting for diastolic BP did not change the risk. Incident heart failure rates were higher in those with SBP less than 125 mmHg than in the reference group.
Conclusion
In routine primary care, SBP less than 135 mmHg was associated with greater mortality in the oldest adults with hypertension and free of selected potentially confounding comorbidities. Although important confounders were accounted for, observational studies cannot exclude residual confounding. More work is needed to establish whether unplanned SBPs less than 135 mmHg in older adults with hypertension may be a useful clinical sign of poor prognosis, perhaps requiring clinical review of overall care.
Keywords: hypertension, outcomes, mortality, oldest old, primary care
Hypertension is the most common chronic condition in older people,1 yet there is debate about treatment targets and longer‐term adverse event rates, especially in the oldest adults. Current guidelines for adults aged 80 and older recommend upper systolic blood pressure (SBP) treatment targets varying from 150 mmHg2, 3, 4, 5 to 140 mmHg.6 Although there is accumulating evidence on the efficacy of antihypertensive treatment in older adults from randomized trials, less information is available on the overall prognosis for the oldest adults with hypertension treated under current guidelines, especially over the long term.7
The current study used electronic medical record data from a large, nationally representative population registered with primary care practices in England in the Clinical Practice Research Datalink (CPRD) database8 to estimate overall prognosis according to attained SBP in the oldest adults in routine family practice, working under national guidance to achieve a SBP less than 150 mmHg. Associations between attained SBP and all‐cause mortality, cardiovascular events, and fragility fractures were estimated in older adults undergoing treatment for hypertension. The goal was to estimate prognosis in individuals without comorbidities who might require specialized treatment or bias results (aiming to minimize confounding); thus, participants were free of dementia, recent cancer, stroke, heart failure, coronary heart disease, or end‐stage renal failure at baseline. An extensive set of sensitivity analyses was also performed to examine the effects of suggested additional confounders.
Methods
Data Source
The CPRD is the English National Health Service (NHS) observational data service, with more than 11.3 million patients from 674 U.K. primary care practices.8 CPRD data from primary care linked to Hospital Episode Statistics (HES) data for hospital admissions and diagnosis and Office for National Statistics (ONS) death certificate data were used. The data include information on individuals living in institutionalized care settings, as well as community‐dwelling older people, and registration with primary care is very near complete for the older population in England.8 The data have been shown to be representative of the U.K. population in terms of age and sex.8
Study Population
All adults in CPRD aged 80 and older with diagnosed hypertension who had at least three blood pressure (BP) measurements during a 3‐year lead‐in period and were prescribed at least one class of antihypertensive medication (alpha‐blockers, angiotensin‐converting enzyme inhibitors, angiotensin receptor antagonists, beta‐blockers, calcium channel blockers, centrally active antihypertensives, diuretics, renin inhibitors) before the lead‐in period and during the follow‐up period were included. The distribution of the classes of antihypertensive medication prescribed according to attained blood SBP is shown in Table S1. The earliest start date for the lead‐in period was January 1, 2000. Primary care Read (diagnostic) codes as defined by NHS Quality of Outcomes Framework rules9 and International Classification of Diseases, Tenth Revision (ICD‐10) codes in HES10 were used to identify individuals with hypertension.
Individuals with comorbidities that require specialized treatment or might introduce confounding (reverse causation with the comorbidity reducing BP) were excluded. Diagnoses excluded at baseline were dementia, cancer, stroke, heart failure, coronary heart disease, and end‐stage renal failure (diagnosis of chronic kidney disease Stage 5 from CPRD or HES or dialysis code in CPRD, HES, or Office of Population Censuses and Surveys Classification of Interventions and Procedures version 4) (Figure S1)10, 11. Sensitivity analyses on the effect of excluding individuals with diabetes mellitus or chronic obstructive pulmonary disease (conditions that might particularly affect management of hypertension in their late stages) on all‐cause mortality did not significantly alter results, so such individuals were not excluded (Table S2).
BP Data
BP was measured during routine general practitioner (GP) visits and recorded by the GP, nurse, or other practice staff,8 normally in a sitting position at rest.4 Measurements were excluded if they did not record SBP and diastolic BP (DBP). Individual measurements with extreme values (<0.15 and >99.85 centile) (SBP: <85 mmHg and > 224 mmHg; DBP: <46 mmHg and >120 mmHg) were excluded. The median of BP measurements recorded during the lead‐in period were used to estimate stable treated baseline SBP and DBP; the median was used to avoid biases from extreme measures during acute clinical events. The average number of BP measurements according to SBP category varied from 7.2 for less than 125 mmHg to 13.4 for 165 to 174 mmHg (Table S3); 15,265 individuals diagnosed with and treated for hypertension had fewer than three BP measurements (Figure S1). This excluded group had a higher prevalence of dementia and heart failure at baseline, which would have triggered exclusion anyway. (This group may have fewer BPs recorded in primary care because of greater specialist input in secondary care.) Median SBP was categorized as less than 125 mmHg, 125 to 134 mmHg, 135 to 144 mmHg, 145 to 154 mmHg, 155 to 164 mmHg, 165 to 174 mmHg, 175 to 184 mmHg, and 185 mmHg and greater.
Covariates
Sex, age at beginning of follow‐up, quintile of 2010 English Index of Multiple Deprivation for England (based on GP's postcode, as a proxy for socioeconomic status), and smoking status (from recorded GP Read terms, classified as current or recent smoker, exsmoker, and never smoker over the 10 years before study entry) were adjusted for in the statistical modelling. Adjusting for year of beginning of follow‐up did not significantly affect estimates, so it was not included in the final models. Sensitivity analyses of the effect of comorbidity (Charlson Comorbidity Index12), major weight loss (a history of weight loss of ≥10% in the 5 years before baseline), body mass index (BMI), and exclusion of individuals in institutional settings on the association between SBP and all‐cause mortality did not significantly alter the results. Individuals in institutional settings during the 3‐year lead‐in period of analysis were identified through recorded contacts in residential or nursing homes with doctors or other practice staff. When testing for confounding, stratified survival analyses were selected when adjustment for the tested covariate violated hazard proportionality.13 Therefore, because this analysis aimed to estimate overall prognosis for the defined complete group of typical patients, individuals were not excluded based on these factors. Neither were these factors further adjusted for in the main analyses, because that might have adjusted away intermediate pathology on the causal pathway to outcomes, potentially introducing bias, and would have made estimates difficult to interpret for routine clinical practice.14
Outcomes
The outcomes of interest for this analysis were all‐cause mortality, cardiovascular events (ischemic stroke, myocardial infarction (MI), heart failure), and fragility fractures. Outcomes were considered if the event occurred while the participant was registered with a practice and the practice data quality was classified as up‐to standard according to CPRD. Fragility fractures are pathological fractures that occur as part of normal activities such as falling from a standing height and are often associated with osteoporosis.15 Only the commonest fragility fractures were included, to increase specificity: hip, vertebrae, humerus, distal radius, pelvis or pubic ramus, and ankle. Data for outcomes were obtained from participant HES data. For MI and fragility factors, ICD‐10 codes were supplemented with procedural coding from Office of Population Censuses and Surveys Classification of Interventions and Procedures version 4 10, 11.
Statistical Analysis
Cox proportional hazards models were used to estimate associations between BP and mortality outcomes. Competing‐risk survival models were used for incident stroke, MI, heart failure, and fragility fractures, with all‐cause mortality as a competing risk.16 For all survival analyses, the attained SBP of 145 to 154 mmHg (with midpoint 150 mmHg) was used as the reference category, because it lies within most international BP targets for older people.17 For all outcomes, the numbers needed to treat or harm were calculated using the Altman and Anderson method.18 All statistical analyses were conducted using Stata version 13 (Stata Corp., College Station, TX). In the main analyses, 12,854 individuals were excluded because of missing information on smoking habits, and an additional 32 were excluded for missing information on Index of Multiple Deprivation (Figure S1).
Ethical and Scientific Approval
The CPRD has been granted multiple research ethics committee approval (05/MRE04/87) to undertake purely observational studies, with external data linkages including HES and ONS mortality data. The work of CPRD is also covered by National Information Governance Board for Health and Social Care Ethics and Confidentiality Committee approval ECC 5–05 (a) 2012.
Results
Data were from 79,376 individuals aged 80 and older who met inclusion criteria for the analysis. Mean age was 82.1 ± 3.3, and 30.5% were men (Table 1). The average number of BP measurements according to SBP category varied from 7.2 for SBP less than 125 mmHg to 13.4 for SBP of 165 to 174 mmHg (Table S3). There were trends in covariates across SBP (e.g., current or recent smoking 24% at SBP <125 mmHg and 18% at SBP >185 mmHg) (Table 1). Maximum follow‐up was 11.9 years (overall mean 4.4 ± 2.9 years), with mean follow‐up times somewhat shorter in the low SBP groups.
Table 1.
Cohort Characteristics and Frequency of Outcomes According to Achieved Median Systolic Blood Pressure
| Characteristics and Outcomes | Systolic Blood Pressure, mmHg | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| <125, n = 2,141 | 125–134, n = 8,198 | 135–144, n = 23,949 | 145–154, n = 22,824 | 155–164, n = 13,257 | 165–174, n = 5,726 | 175–184, n = 2,305 | ≥185, n = 976 | Total, N = 79,376 | |
| Demographic characteristics | |||||||||
| Age, mean ± standard deviation | 81.7 ± 3.6 | 81.4 ± 3.0 | 81.6 ± 3.1 | 82.1 ± 3.3 | 82.6 ± 3.5 | 83.0 ± 3.7 | 83.5 ± 3.9 | 83.6 ± 4.0 | 82.1 ± 3.3 |
| Male, % | 39.1 | 36.5 | 33.5 | 30.2 | 26.7 | 22.8 | 19.7 | 18.8 | 30.5 |
| Follow‐up, years, mean | 3.1 | 3.3 | 3.9 | 4.6 | 5.3 | 5.5 | 5.6 | 5.5 | 4.4 |
| Diastolic blood pressure, mmHg, % | |||||||||
| <70 | 31.0 | 16.7 | 9.9 | 6.6 | 4.5 | 2.9 | 2.6 | 1.4 | 8.5 |
| 70–84 | 67.9 | 79.7 | 80.4 | 73.0 | 63.7 | 56.3 | 44.6 | 35.3 | 71.7 |
| ≥85 | 1.1 | 3.7 | 9.7 | 20.4 | 31.9 | 40.7 | 52.8 | 63.3 | 19.8 |
| Smoking status, % | |||||||||
| Never | 45.4 | 46.0 | 48.1 | 51.6 | 54.8 | 57.3 | 60.9 | 61.5 | 51.1 |
| Current | 23.9 | 22.7 | 21.8 | 19.2 | 17.7 | 17.0 | 16.1 | 18.2 | 20.0 |
| Exsmoker | 30.6 | 31.3 | 30.1 | 29.1 | 27.5 | 25.7 | 23.0 | 20.3 | 28.9 |
| Most deprived (1 or 2), %a | 48.6 | 49.1 | 49.2 | 50.1 | 47.7 | 48.4 | 47.7 | 44.0 | 49.0 |
| Carlson Comorbidity Index score, %b | |||||||||
| 0 | 75.3 | 75.1 | 77.3 | 77.5 | 76.8 | 76.2 | 77.0 | 76.33 | 76.9 |
| 1–2 | 12.5 | 13.9 | 12.9 | 13.2 | 13.1 | 13.9 | 13.1 | 12.7 | 13.2 |
| ≥3 | 12.3 | 11.0 | 9.8 | 9.3 | 10.1 | 9.9 | 10.0 | 11.0 | 9.9 |
| Outcomes | |||||||||
| Death | |||||||||
| n | 534 | 1804 | 5,723 | 7,116 | 5,584 | 2,888 | 1,296 | 598 | 25,543 |
| Per 1,000 person‐yearsc | 81.0 | 66.2 | 61.9 | 67.4 | 79.7 | 91.6 | 100.4 | 111.3 | |
| Cardiovascular death | |||||||||
| n | 17 | 72 | 292 | 384 | 299 | 192 | 94 | 45 | 1,395 |
| Per 1,000 person‐yearsc | 2.6 | 2.6 | 3.2 | 3.6 | 4.3 | 6.1 | 7.3 | 8.4 | |
| Stroke | |||||||||
| n | 56 | 212 | 695 | 883 | 631 | 340 | 197 | 84 | 3,098 |
| Per 1,000 person‐yearsc | 8.6 | 7.9 | 7.6 | 8.5 | 9.2 | 11.0 | 15.6 | 16.1 | |
| Myocardial infarction | |||||||||
| n | 36 | 178 | 635 | 850 | 642 | 375 | 154 | 84 | 2,954 |
| Per 1,000 person‐yearsc | 5.5 | 6.6 | 6.9 | 8.2 | 9.3 | 12.2 | 12.1 | 16.0 | |
| Heart failure | |||||||||
| n | 170 | 566 | 1,765 | 2,148 | 1,718 | 908 | 407 | 200 | 7,882 |
| Per 1,000 person‐yearsc | 26.4 | 21.4 | 19.6 | 21.0 | 25.5 | 30.1 | 33.0 | 39.6 | |
| Fragility fracture | |||||||||
| n | 127 | 541 | 1,740 | 2,219 | 1,545 | 738 | 309 | 141 | 7,360 |
| Per 1,000 person‐yearsc | 19.9 | 20.8 | 19.7 | 22.2 | 23.3 | 25.0 | 25.6 | 28.1 | |
Index of multiple deprivation: 1 highest deprivation to 5 lowest deprivation
Lower scores indicate better health.
Adjusted for age at beginning of follow‐up and sex.
All‐cause Mortality According to Attained SBP
During follow‐up, 25,543 patients died (Table 1). Mortality hazards showed a U‐shaped pattern (Figure 1, Table 2), with lowest mortality in individuals with SBP of 135 to 154 mmHg (hazard ratio (HR) = 1.03, confidence interval (CI) = 0.99–1.06, risk is essentially the same as that of the comparison group of SBP 145–154 mmHg) and greater mortality for higher levels of SBP. The risk of mortality was greater for SBP less than 135 mmHg (combining the two lowest SBP categories, 13.1% of participants) (HR=1.25, 95% CI=1.19–1.31). This excess risk is equivalent to one extra death for every 13 individuals aged 80 and older with SBP less than 135 than for the comparison group based on number needed to harm estimation. Mortality hazard was estimated separately in 2‐year follow‐up segments to check for stability between short‐ and longer‐term outcomes (Table S4). Lower attained SBP (<135 mmHg) was associated with greater mortality in all follow‐up periods, although estimates for the first 2 years were higher (HR = 1.38, 95% CI = 1.26–1.51) than, for example, at 6 to 8 years follow‐up (HR = 1.19, 95% CI = 1.07–1.32), albeit with overlapping confidence intervals. Central estimates of mortality hazard were higher again from 8 years to end of follow‐up (HR = 1.29, 95% CI = 1.09–1.53), although in small numbers of participants.
Figure 1.
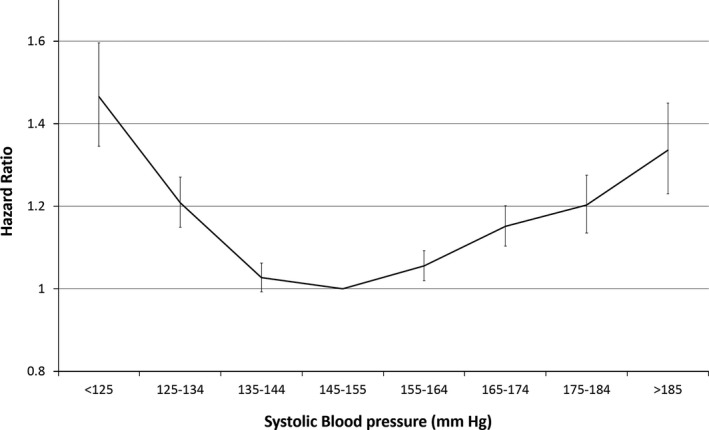
Risk of all‐cause mortality according to systolic blood pressure.
Table 2.
Risk of All‐Cause Mortality and Incident Disease According to Systolic Blood Pressure (Reference 145–154 mmHg)
| Outcome | <125 | 125–134 | 135–144 | 155–164 | 165–174 | 175–184 | ≥185 |
|---|---|---|---|---|---|---|---|
| Hazard Ratio (95% Confidence Interval) | |||||||
| All‐cause mortality | 1.46 (1.34–1.60)a | 1.20 (1.14–1.26)a | 1.03 (0.99–1.06) | 1.06 (1.02–1.10)a | 1.15 (1.10–1.20)a | 1.21 (1.14–1.28)a | 1.34 (1.23–1.46)a |
| Stroke | 0.95 (0.72–1.25) | 0.93 (0.80–1.08) | 0.91 (0.83–1.01) | 1.04 (0.94–1.15) | 1.21 (1.06–1.37)a | 1.67 (1.43–1.95)a | 1.67 (1.33–2.09)a |
| Myocardial infarction | 0.60 (0.43–0.84)a | 0.78 (0.67–0.92)a | 0.85 (0.76–0.94)a | 1.12 (1.01–1.24)a | 1.44 (1.27–1.63)a | 1.41 (1.19–1.68)a | 1.80 (1.44–2.26) |
| Heart failure | 1.20 (1.02–1.40)a | 1.04 (0.95–1.14) | 0.96 (0.90–1.02) | 1.15 (1.08–1.23)a | 1.32 (1.22–1.43)a | 1.39 (1.25–1.55)a | 1.63 (1.40–1.89)a |
| Fragility fracture | 0.88 (0.74–1.05) | 0.97 (0.88–1.06) | 0.92 (0.87–0.98)a | 0.99 (0.93–1.05) | 0.99 (0.92–1.08) | 0.95 (0.85–1.07) | 1.02 (0.86–1.20) |
| Cancer | 0.93 (0.81–1.06) | 0.99 (0.92–1.06) | 0.94 (0.89–0.99)a | 0.99 (0.94–1.04) | 1.01 (0.94–1.08) | 0.85 (0.75–0.95)a | 0.81 (0.68–0.96)a |
P<.05.
To test for modifying effects of DBP, DBP was grouped into 85 mmHg and higher, 70 to 84 mmHg, and less than 70 mmHg (estimates with more‐extreme groups were underpowered). The U‐shaped pattern of systolic mortality hazard was similar for all three DBP groups (Table 3, Figure S2). The modifying effect of pulse pressure on all‐cause mortality was also tested by adjusting the model for five levels of pulse pressure (<50 mmHg, 50–59 mmHg (reference), 60–69 mmHg, 70–79 mmHg, ≥80 mmHg). A significantly greater risk of all‐cause mortality remained in those with low BP even when adjusted for pulse pressure (<125 mmHg: HR = 1.33, 95% CI = 1.2–1.48, P < .001; 125–134 mmHg: HR = 1.15, 95% CI = 1.08–1.22, P < .001).
Table 3.
Risk of All‐Cause Mortality According to Systolic Blood Pressure (SBP) (Reference 145–154 mmHg) and Diastolic Blood Pressure (DBP)
| DBP, mmHg | SBP, mmHg | ||||||
|---|---|---|---|---|---|---|---|
| <125 | 125–134 | 135–144 | 155–164 | 165–174 | 175–184 | >185 | |
| <70 | 1.40 (1.16–1.70)a | 1.25 (1.07–1.46)a | 1.06 (0.93–1.21) | 1.12 (0.95–1.32) | 1.20 (0.94–1.54) | 1.49 (1.05–2.10)a | 1.58 (0.82–3.07) |
| 70–84 | 1.50 (1.35–1.66)a | 1.20 (1.13–1.27)a | 1.04 (1.00–1.08) | 1.05 (1.00–1.09)a | 1.13 (1.07–1.20)a | 1.24 (1.14–1.35)a | 1.38 (1.21–1.58)a |
| ≥85 | 1.05 (0.47–2.34) | 1.36 (1.11–1.66)a | 1.00 (0.91–1.09) | 1.05 (0.99–1.12) | 1.15 (1.07–1.24)a | 1.13 (1.03–1.23)a | 1.27 (1.13–1.42)a |
Survival analysis of all‐cause mortality according to SBP level stratified according to DBP.
P<.05.
An interaction term and analysis stratified according to sex (Table S5) demonstrated that differences according to sex were not significant. An analysis of similarly selected individuals with one or two recorded blood pressure measures (n = 5,012) in the 3 years before baseline resulted in a similar risk curve to that of individuals with 3 or more measurements (Table S6). Sensitivity analyses adjusted for and stratified according to level of comorbidity (Charlson Comorbidity Index12), major weight loss (≥10% in 5 years before baseline), or BMI or excluding individuals with consultations in institutional settings during the baseline period; in all cases, mortality at low SBP (<135 mmHg) remained significantly greater. Analyses adjusting for and excluding individuals with diabetes mellitus and with chronic obstructive pulmonary disease had similar results (Table S2).
Incident Disease
There was an approximately linear increase in risk of incident MI across the SBP range (Figure 2), with lower SBP associated with lower risk (<125 mmHg: HR = 0.6, 95% CI = 0.43–0.84, number needed to treat 9.8; 125–134 mmHg: HR = 0.78, 95% CI = 0.67 to 0.92, number needed to treat 25.8) than the SBP 145 to 154 mmHg (Table 2, Table S7). Risk of incident stroke was greater at SBP above 145 to 154 mmHg (Figure 2).
Figure 2.
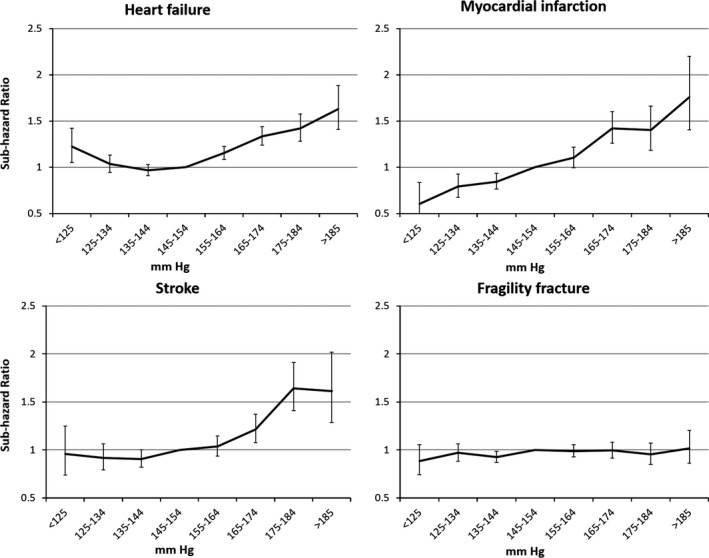
Risk of all‐cause mortality and systolic blood pressure according to diastolic blood pressure.
During follow‐up, 7,882 individuals developed heart failure (Table 1). Competing subhazards for incident heart failure rose progressively at SBP above 145 to 154 mmHg, but there was also greater risk for lower SBP (e.g., SBP <125 mmHg: HR = 1.2, 95% CI = 1.02–1.4, number needed to harm 28.6) (Figure 2, Table 2).
During follow‐up, 7,360 individuals were admitted to hospital with a fragility fracture or had surgical intervention for a hip fracture (follow‐up mean 4.4 ± 2.9 years). Risk of fracture was not different for different SBP, with no indication of greater risk with low SBP.
Confounding of the association between SBP less than 135 mmHg and greater mortality might theoretically have been due to undiagnosed cancer present at baseline, but cancer incidence during the 11.9 years of follow‐up was not significantly different (and tended to be lower) in those with (e.g., HR = 0.93, 95% CI = 0.81–1.06) SBP less than 125 mmHg. The highest cancer incidence was in the reference group of SBP 145 to 154 mmHg, and cancer incidence was significantly lower at SBP greater than 175 mmHg (e.g., HR = 0.85, 95% CI = 0.75–0.95) for SBP of 175 to 184 mmHg. Although there were no data on why cancer incidence was lower in the low and high SBP categories, an analysis for all‐cause mortality adjusted for incident cancer, and separate analyses for individuals with and without incident cancer, did not significantly alter results (Table S8).
Discussion
A large cohort of the oldest adults in primary care taking antihypertensive medication over the long term was studied to estimate prognosis according to attained BP level in routine primary care practice working under national guidance to achieve a SBP less than 150 mmHg in older adults with hypertension. Individuals with major comorbidities at baseline that might have posed specialist treatment challenges or confounded estimates were excluded. The analysis included 49.7% of individuals aged 80 and older in the complete population‐based analysis. It was found, as expected, that all‐cause mortality and risk of cardiovascular disease rose with increasing SBP above 145 to 154 mmHg. Linearly increasing risk of myocardial infarction was found with increasing SBP, although greater mortality was also found in those with SBP of less than 135 mmHg. Confounding is typically evident in the first few years of follow‐up, but the excess mortality at SBP less than 135 mmHg was consistent, even over the longest follow‐up period (8–11.9 years) Table S4. The sensitivity analyses on comorbidity, BMI, major weight loss, incident cancer, and exclusion of those seen by practice staff in residential or nursing homes also had little effect on estimates. Results were also similar in individuals with diabetes mellitus (Table S2).
These results reflect outcomes of individuals aged 80 and older receiving ongoing treatment for hypertension under currently recommended targets; at the time the data were recorded in electronic clinical records, intensive treatment to reduce SBP below 140 mmHg in individuals aged 80 and older was not indicated. Observational studies of prognosis in the above context are not comparable with studies that randomize participants to intensive treatment to achieve lower target BP. If intensive treatment were instituted to achieve SBP substantially lower than 150 mmHg, those with low SBP might be expected to be taking more antihypertensive medications than those with higher attained BP, but the group with lower attained SBP (<125 mmHg) had slightly fewer prescribed medications (mean 1.84) than the reference group (mean 1.99 for SBP 145–154 mmHg). Also, the participants being in routine care, the medications prescribed may have differed from those used in recent trials aiming to achieve lower SBP. This analysis has other limitations, including inaccuracies in routine data recording. Although routine practice is to measure BP more than once for each clinical review, the available data contain only one recorded BP at each consultation, although it seems likely that the BPs recorded in clinical records are those used as a basis for management. Inaccuracies in estimated BPs were minimized by using median of recorded BPs across consultations during a 3‐year lead‐in period, avoiding “outliers” (perhaps recorded during acute events) that might distort mean values, and the mean number of measurements studied varied from 7.2 for the group with SBP less than 125 mmHg to 13.4 for those with SBP of 165 to 174 mmHg (Table S3). The studied BPs were used in monitoring antihypertensive treatment and thus have validity for the purpose of estimating prognosis in routine practice. Other aspects of BP, including orthostatic hypotension, have been associated with adverse events and may have contributed to the results observed,19 alghouth no data are available on orthostatic hypotension in the dataset. Although assessment of orthostatic hypotension is thought to be common, the lack of specific data on these measures limits this analysis. Given the “typical care” rather than etiological context, BP measured during follow‐up (e.g., accounting for regression dilution)20 was not accounted for because subsequent BP measurement were not available at treatment review in practice. Antihypertensive medication data are from real‐time prescribing records; no measure of adherence was available,8 although nonadherence should be associated with higher BP and was unlikely to bias estimates of greater mortality at low SBP. Cause‐specific mortality was not studied, because death certification is thought to lack precision in the oldest adults, especially for cardiac outcomes. Records of lower SBP were more common in the later part of the period (2000–2014), with shorter mean follow‐up times for individuals with low SBP (Table 1). This may have been the result of incentive payments to improve hypertension management introduced from 2004, but adjusting for year of baseline BP measurement did not significantly alter results.21 Elaborate adjustment of models was also avoided, with potentially confounding conditions excluded instead and easily interpretable estimates of prognosis in routine primary care provided. It is also possible that there was some undiagnosed disease at baseline that reduced BP and increased mortality, but low SBP was associated with excess mortality even in the 8 years to the end of follow‐up (ignoring the first 8 years of follow‐up), making this explanation less likely. The Charlson Comorbidity Index was used to account for comorbidities, but this does not fully capture functional status or frailty in this population; better measurement of functional status or frailty (e.g., gait speed) might correlate with the poor outcomes in the lower SBP group, although the study subjects were selected to be free of dementia, cancer, stroke, heart failure, coronary heart disease, and end‐stage renal disease, and adjustment for Charlson comorbidities altered the results only slightly, as did any of the other sensitivity analyses.
In the United Kingdom, 98% of the population are registered with a GP, and the CPRD sample was found to be broadly representative of English population based on the 2011 Census, particularly in older age groups.8 Longer follow‐up was included than in existing studies in adults aged 80 and older.22 Randomized trials provide the most robust evidence of causation by tested treatments, observational population representative studies are also useful for estimating overall clinical prognosis (combination of antihypertensive effects and all other factors, measured and unmeasured). Observational studies are useful for investigating applicability to typical patient groups, longer follow‐up times, and adverse outcomes.23, 24
Comparison with Previous Evidence
These results are restricted to the oldest adults undergoing antihypertensive treatment and focus on clinical prognosis in routine care. The finding of J‐shaped associations for some outcomes is broadly consistent with previous evidence for individuals aged 80 and older in studies of individuals with high cardiovascular risk.25, 26, 27 The Clinical research using LInked Bespoke studies and Electronic health Records (CALIBER) study also used CPRD GP medical records and linked data to study cardiovascular outcomes in individuals taking antihypertensive medications. In their subgroup aged 80 to 95 taking antihypertensive medications (with no exclusions for non‐CVD comorbidities, Table S15), increasing SBP was similarly associated with a linear increase in incident myocardial infarction, plus a J‐shaped association for heart failure and a flat association with ischemic stroke for SBP less than 159 mmHg.23 CALIBER did not study all‐cause mortality or fragility fracture outcomes. CALIBER reported “unheralded” CHD mortality based on death certification in people without a history of cardiovascular disease and found markedly higher mortality at low SBP in those aged 80 to 95 (SBP 100–115 mmHg: HR = 1.27, 95% CI = 1.09–1.48; SBP 140–159 mmHg: HR = 0.60, 95% CI = 0.43–0.84). The Prospective Studies Group pooled volunteer cohorts from the 1960s to the 1980s and found a linear association between BP (irrespective of treatment) and vascular and overall mortality in adults aged 80 and older (published 2002), but it is uncertain how these findings relate to older adults with treated hypertension in the current treatment era.24
The observational data of outcomes in routine care focus on a different concern and are not comparable with data from randomized controlled trials, although the HYpertension in the Very Elderly Trial—a randomized controlled trial of hypertension management in adults aged 80 and older—showed lower all‐cause mortality28 with a target BP of 150/80 mmHg and an achieved mean sitting BP of 145.0/76.6 mmHg in the active treatment group at the start of the open‐label extension. The Systolic Blood Pressure Intervention Trial in Patients Age 75 and Older (SPRINT 75+) aimed “To evaluate the effects of intensive (<120 mmHg) compared with standard (<140 mmHg) SBP targets in persons aged 75 years or older with hypertension but without diabetes”.29 SPRINT included 1,317 cases and 1,319 controls aged 75 and older (37.9% female) free of diabetes mellitus, stroke, a recent cardiovascular event or heart failure, dementia, medical conditions limiting life expectancy to less than 3 years (including cancer), and factors that the clinic team judged to be likely to limit adherence to therapy, or residence in a nursing home. SPRINT 75+ concluded that “intensive treatment resulted in significantly lower rates of fatal and nonfatal major cardiovascular events” over a median follow‐up of 3.14 years. There were also nonsignificant trends toward higher rates of some adverse events, except for injurious fracture, which was nonsignificantly less common in the intensive treatment group (4.9% vs 5.5%).
More work is needed to include measures of orthostatic hypotension and to identify the reasons for the apparently poor prognosis of individuals with SBP less than 135 mmHg in routine care. It may be that unplanned low attained SBP may be a useful clinical sign to trigger general clinical review of all comorbidities. Better measurement of frailty and functioning may be informative.
Conclusions
In a complete population of individuals with hypertension in primary care aged 80 and older treated under guidance to achieve SBP less than 150 mmHg, greater mortality was found over 11.9 years of follow‐up in those with SBP of less than 135 mmHg. More work is needed to establish whether unplanned SBP of less than 135 mmHg in older adults with hypertension may be a useful clinical sign of poorer prognosis requiring overall clinical review of care.
Supporting information
Figure S1. Cohort definition and exclusions.
Figure S2. Risk of all‐cause mortality according to systolic blood pressure stratified according to diastolic blood pressure (DBP) in individuals aged 80 and older.
Table S1. Antihypertensive Medication Class According to Attained Systolic Blood Pressure (SBP)
Table S2. Sensitivity Analysis of Effect of Prior Diagnosis of Diabetes Mellitus and Chronic Obstructive Pulmonary Disease (COPD) on All‐Cause Mortality According to Systolic Blood Pressure (SBP; Reference 145–154 mmHg)
Table S3. Number of Recorded Blood Pressure Measurements During Lead‐In Period According to Systolic Blood Pressure (SBP)
Table S4. All‐Cause Mortality According to Years of Follow‐Up and Systolic Blood Pressure (SBP; Reference 145–154 mmHg)
Table S5. Sensitivity Analyses of Effect of Sex on All‐Cause Mortality According to Systolic Blood Pressure (SBP; Reference 145–154 mmHg)
Table S6. Risk of All‐Cause Mortality According to Systolic Blood Pressure (SBP; Reference 145–154 mmHg) for Individuals Ineligible for the Analysis Because of Incomplete Data on Blood Pressure
Table S7. Number Needed to Treat (NNT)‐Based Estimates of Mortality or Incident Disease According to Systolic Blood Pressure (SBP; Reference 145–154 mmHg)
Table S8. Sensitivity Analyses of Effect of Incident Cancer on All‐Cause Mortality According to Systolic Blood Pressure (SBP; Reference 145–154 mmHg)
Acknowledgments
Financial Disclosure: This work was supported in part by the National Institute for Health Research (NIHR) School for Public Health Research Ageing Well programme. School for Public Health Research is a partnership between the universities of Sheffield, Bristol, and Cambridge; University College of London; the London School for Hygiene and Tropical Medicine; the University of Exeter Medical School; the LiLaC collaboration between the Universities of Liverpool and Lancaster and Fuse; and the Centre for Translational Research in Public Health, a collaboration between Newcastle, Durham, Northumbria, Sunderland and Teesside Universities.
Conflict of Interest: Drs. Delgado, Masoli, and Ble have grant support from the NIHR for the submitted work. Dr Strain reports personal fees from Pfizer, Boehringer Ingelheim, and MSD; grants and personal fees from Novartis and Novo Nordisk outside the submitted work. The other authors report no disclosures.
Author Contributions: Dr. Delgado and Professor Melzer had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Masoli, Delgado, Ble, and Melzer: Study concept and design; All authors: Acquisition, analysis, or interpretation of data; Masoli, Delgado, Ble and Melzer: Drafting of the manuscript. All authors: Critical revision of the manuscript for important intellectual content. Delgado, Bowman, Masoli, Ble and Melzer: Statistical analysis.
Sponsor's Role: The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Melzer D, Tavakoly B, Winder RE et al. Much more medicine for the oldest old: Trends in UK electronic clinical records. Age Ageing 2015;44:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mancia G, Fagard R, Narkiewicz K et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013;34:2159–2219. [DOI] [PubMed] [Google Scholar]
- 3. James PA, Oparil S, Carter BL et al. 2014 evidence‐based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014;311:507–520. [DOI] [PubMed] [Google Scholar]
- 4. National Institute for Health and Care Excellence . Hypertension: Guidance and Guidelines. London, UK: National Institute for Health and Care Excellence, 2013. [Google Scholar]
- 5. Weber MA, Schiffrin EL, White WB et al. Clinical practice guidelines for the management of hypertension in the community: A statement by the American Society of Hypertension and the International Society of Hypertension. J Hypertens 2014;32:3–15. [DOI] [PubMed] [Google Scholar]
- 6. Aronow WS, Fleg JL, Pepine CJ et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly. J Am Soc Hypertens 2011;5:259–352. [DOI] [PubMed] [Google Scholar]
- 7. Chobanian AV. SPRINT results in older patients: How low to go? J Am Geriatr Soc 2016;315:2669–2670. [DOI] [PubMed] [Google Scholar]
- 8. Herrett E, Gallagher AM, Bhaskaran K et al. Data resource profile: Clinical practice research datalink (CPRD). Int J Epidemiol 2015;44:827–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. HSCIC . QOF Implementation Dataset and Business Rules ‐ Hypertension (HYP) Indicator Set [Internet], 2013; 1–25. Available from: https://www.pcc-cic.org.uk/sites/default/files/articles/attachments/hypertension_ruleset_v25_1.pdf, Last accessed: 08 December 2016. [Google Scholar]
- 10. (WHO) WHO . ICD‐10 Version:2015 [Internet]. 2015. [cited 2016 Dec 5]. Available from: http://apps.who.int/classifications/icd10/browse/2015/en Accessed December 08, 2016.
- 11. NHS. OPCS Classification of Interventions and Procedures [Internet] . NHS digital. 2016; 1. Available from: http://www.datadictionary.nhs.uk/web_site_content/supporting_information/clinical_coding/opcs_classification_of_interventions_and_procedures.asp Accessed December 08, 2016. [Google Scholar]
- 12. Khan NF, Perera R, Harper S et al. Adaptation and validation of the Charlson Index for Read/OXMIS coded databases. BMC Fam Pract 2010;11:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schemper M. Cox analysis of survival data with non‐proportional hazard functions. the Statistician 1992;41:455–465. [Google Scholar]
- 14. Schisterman EF, Cole SR, Platt R. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology 2009;20:488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. National Institute for Health and Care Excellence . Osteoporosis: Assessing the Risk of Fragility Fracture: Guidance and Guidelines. London, UK: National Institute for Health and Care Excellence, 2012. [Google Scholar]
- 16. Jason P, Fine RJG. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. [Google Scholar]
- 17. Benetos A, Rossignol P, Cherubini A et al. Polypharmacy in the aging patient. JAMA 2015;314:170. [DOI] [PubMed] [Google Scholar]
- 18. Altman DG, Andersen PK. Calculating the number needed to treat for trials where the outcome is time to an event. BMJ 1999;319:1492–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Feldstein C, Weder AB. Orthostatic hypotension : A common, serious and underrecognized problem in hospitalized patients. J Am Soc Hypertens 2012;6:27–39. [DOI] [PubMed] [Google Scholar]
- 20. Clarke R, Shipley M, Lewington S et al. Underestimation of risk associations due to regression dilution in long‐term follow‐up of prospective studies. Am J Epidemiol 1999;150:341–353. [DOI] [PubMed] [Google Scholar]
- 21. Health and Social Care Information Centre . National Quality and Outcomes Framework Statistics for England—2004–05 [on‐line]. Available at http://www.hscic.gov.uk/catalogue/PUB0194620 Accessed June 29, 2016. [Google Scholar]
- 22. Musini V, Tejani A, Bassett K et al. Pharmacotherapy for hypertension in the elderly. Cochrane Database Syst Rev 2009;4:CD000028. [DOI] [PubMed] [Google Scholar]
- 23. Rapsomaniki E, Timmis A, George J et al. Blood pressure and incidence of twelve cardiovascular diseases: Lifetime risks, healthy life‐years lost, and age‐specific associations in 1·25 million people. Lancet 2014;383:1899–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Prospective Studies Collaboration . Age‐specific relevance of usual blood pressure to vascular mortality: A meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet 2002;360:1903–1913. [DOI] [PubMed] [Google Scholar]
- 25. Mancia G, Grassi G. Aggressive blood pressure lowering is dangerous: The J‐curve: Pro side of the argument. Hypertension 2014;63:29–35. [DOI] [PubMed] [Google Scholar]
- 26. Messerli FH. Dogma disputed: Can aggressively lowering blood pressure in hypertensive patients with coronary artery disease be dangerous? Ann Intern Med 2006;144:884. [DOI] [PubMed] [Google Scholar]
- 27. Panjrath GS, Chaudhari S, Messerli FH. The J‐point phenomenon in aggressive therapy of hypertension: New insights. Curr Atheroscler Rep 2012;14:124–129. [DOI] [PubMed] [Google Scholar]
- 28. Beckett NS, Peters R, Fletcher AE et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008;358:1887–1898. [DOI] [PubMed] [Google Scholar]
- 29. Williamson JD, Supiano MA, Applegate WB et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥ 75 years: A randomized clinical trial. JAMA 2016;315:2673–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Cohort definition and exclusions.
Figure S2. Risk of all‐cause mortality according to systolic blood pressure stratified according to diastolic blood pressure (DBP) in individuals aged 80 and older.
Table S1. Antihypertensive Medication Class According to Attained Systolic Blood Pressure (SBP)
Table S2. Sensitivity Analysis of Effect of Prior Diagnosis of Diabetes Mellitus and Chronic Obstructive Pulmonary Disease (COPD) on All‐Cause Mortality According to Systolic Blood Pressure (SBP; Reference 145–154 mmHg)
Table S3. Number of Recorded Blood Pressure Measurements During Lead‐In Period According to Systolic Blood Pressure (SBP)
Table S4. All‐Cause Mortality According to Years of Follow‐Up and Systolic Blood Pressure (SBP; Reference 145–154 mmHg)
Table S5. Sensitivity Analyses of Effect of Sex on All‐Cause Mortality According to Systolic Blood Pressure (SBP; Reference 145–154 mmHg)
Table S6. Risk of All‐Cause Mortality According to Systolic Blood Pressure (SBP; Reference 145–154 mmHg) for Individuals Ineligible for the Analysis Because of Incomplete Data on Blood Pressure
Table S7. Number Needed to Treat (NNT)‐Based Estimates of Mortality or Incident Disease According to Systolic Blood Pressure (SBP; Reference 145–154 mmHg)
Table S8. Sensitivity Analyses of Effect of Incident Cancer on All‐Cause Mortality According to Systolic Blood Pressure (SBP; Reference 145–154 mmHg)


