Abstract
An open‐label, randomized, multicenter study was conducted to evaluate the safety and efficacy of long‐term use of 2.5% and 5% benzoyl peroxide (BPO) gels administrated once daily for 52 weeks to Japanese patients with acne vulgaris. The efficacy of the study drugs was evaluated by counting inflammatory lesions and non‐inflammatory lesions. Safety was evaluated based on adverse events, local skin tolerability scores and laboratory test values. In total, 458 subjects were included in the efficacy and safety analyses. The total lesion count, the efficacy end‐point, was similarly changed both in the 2.5% and 5% BPO groups over the course of the study. The median rates of reduction from baseline to week 12 were approximately 65%. Thereafter, the counts were maintained at a reduced level without increasing until week 52. The median rates at week 52 were approximately 80%. Similar trends were observed for inflammatory and non‐inflammatory lesion counts. Bacteriological evaluation indicated similar distribution of the minimum inhibitory concentration of each of the antibacterial drugs against Propionibacterium acnes between the values at baseline and at week 52, suggesting that long‐term use did not result in changes in the drug sensitivity. The incidence of adverse events was 84.0% in the 2.5% BPO group and 87.2% in the 5% BPO group. Many of the adverse events occurred within the first month and were mild or moderate in severity and transient. The results suggest that both 2.5% and 5% BPO gels are effective and safe for long‐term treatment of patients with acne vulgaris.
Keywords: acne vulgaris, benzoyl peroxide, long‐term treatment, open‐label, randomized study
Introduction
Acne vulgaris is a chronic inflammatory skin disease that develops on the face, chest and back in adolescents, starting as comedones in hair follicles.1 The Japanese medical guideline strongly recommends topical administration of antimicrobials to treat inflammatory lesions, but it also advises that long‐term use should be avoided due to the risk of developing antibiotic‐resistant bacteria.2
The products used in this study were gels containing either 2.5% or 5% of benzoyl peroxide (BPO). Topical medications containing BPO are widely recognized as a standard treatment for acne vulgaris in Europe and the USA.3, 4 The antiseptic effect of BPO is a result of its oxidizing property5 and therefore suggests a low possibility that antibiotic‐resistant bacteria may develop, and we therefore expect that it is safe to use long term for acne treatment. Aiming to evaluate the safety and efficacy of long‐term BPO use, we performed an open‐label, randomized, multicenter study by administration of 2.5% or 5% BPO gel once daily for 52 weeks to patients with acne vulgaris. To our knowledge, this is the first report on efficacy and safety of long‐term treatment with BPO monotherapy for up to 1 year.
This article is based on a study that was first reported in Japanese in the Journal of Clinical Therapeutics and Medicine (“An open‐label, randomized, multicenter, phase III study to evaluate the safety and efficacy of benzoyl peroxide gel in long‐term use in patients with acne vulgaris,” 2014; 30: 669–689), and this secondary publication in English is made with permission of the journal.
Methods
Study design
This long‐term phase III study, conducted between May 2012 and September 2013, was a multicenter (25 centers), randomized, open‐label study that evaluated the safety and efficacy of long‐term use of BPO 2.5% and 5% gels when applied once daily for 52 weeks in Japanese patients with acne vulgaris. This study was registered at the Japan Pharmaceutical Information Center (JapicCTI‐121909). Enrolled patients were randomized (1:1) by a computer randomization system (four patients within each block) to receive BPO 2.5% gel or BPO 5% gel. Patients were assessed at baseline (week 0/day 1) and weeks 2, 4, 8, 12, 16, 20, 24, 28, 32, 36, 40, 44, 48 and 52 (end of study). The final evaluation was at week 52 or discontinuation (Table S1).
The study was conducted in accordance with the International Conference on Harmonisation Good Clinical Practice and the ethical principles outlined in the Declaration of Helsinki 2008. The protocol and other relevant study documents were approved by the relevant institutional review boards. Prior to the start of the study, fully informed and written consent was obtained from adult patients and parents or legal guardians of patients under 20 years of age.
Patients
Male and female outpatients aged 12–49 years were eligible if they had facial acne (excluding periocular and lip lesions) characterized by five to 40 inflammatory lesions (IL, erythematous papules and pustules excluding pustules for bacteriological evaluation) and one to 100 non‐inflammatory lesions (non‐IL, open and closed comedones) and two or fewer nodules/cysts at baseline (Table S2).
Patients were excluded if they had complications of other facial acne than acne vulgaris, rosacea, or skin diseases that may produce a facial rash (such as atopic dermatitis), started or restarted the use of topical retinoids or retinoid‐like agents within 4 weeks prior to the initiation of the treatment, participated in another clinical study within 4 months prior to the initiation of the treatment, had exhibited hypersensitivity or previous allergic reactions to any component of the study drugs, had serious complications (including systemic disease) which precluded participation in the study, or were pregnant, breast‐feeding or hoping to become pregnant.
Treatment regimen
Enrolled patients were instructed to apply a sufficient amount of the assigned study gel to cover the entire face (excluding periocular and lip lesions) once daily at night for 52 weeks.
Systemic antibiotics (p.o. or by injection) were prohibited during the treatment, although the use of antibiotics was permitted for a total of up to 3 days between two consecutive visits from the beginning of the treatment to week 4 and for up to 7 days between two visits from week 4 to week 52 if the purpose was not related to acne treatment. The use of BPO‐containing products and cosmetic products, topical antibiotics, retinoid and retinoid‐like agents on the face were also prohibited. In case patients had used retinoid or retinoid‐like agents at the start of the study, continued use of these was permitted. Prohibited therapies were chemical peeling, laser therapy, phototherapy and comedone suction or extraction on the face.
Assessments
Efficacy end‐points included absolute and percentage changes in TL, IL and non‐IL counts from baseline to weeks 2, 4, 8, 12, 16, 20, 24, 28, 32, 36, 40, 44, 48 and 52.
Bacteriological evaluation was performed at baseline and the final evaluation to test the susceptibility (minimum inhibitory concentration [MIC]) of clinical isolates (sampling from pustules) to antibiotics, and then assessed the percentage change in IL count from baseline to the final evaluation by MIC.
Safety was assessed based on adverse events (AE), local skin tolerability scores (scaling and erythema) and laboratory tests (hematology, blood chemistry and urine analysis). The local skin tolerability scores were evaluated on a four‐grade scale (0 = none, 1 = mild, 2 = moderate, 3 = severe; Table S3) by the investigators at each visit. An increase in the score over the baseline level was recorded as an AE.
Statistical analysis
To assess the long‐term efficacy and safety of BPO, a sample size of 450 (225 both for 2.5% and 5% BPO), considering dropout rate, was expected to provide 300 subjects or more who complete the treatment for 28 weeks, and 100 subjects or more for 52 weeks.
The population for efficacy and safety assessment included patients who received a study drug at least once and were evaluated for efficacy or safety at least once. For efficacy assessment, summary statistics and the corresponding two‐sided 95% confidence intervals (CI) were calculated by group. All AE were coded using the Medical Dictionary for Regulatory Activities (MedDRA version 15.0) and summary statistics were calculated for each treatment group by the System Organ Class and Preferred Term, and by causal relationships with the study treatments.
Results
Disposition of enrolled patients and their profiles
Figure 1 shows the disposition of enrolled patients. In total, 458 participants of the randomized 459 received the study drugs, and 393 participants completed the study (198 for 2.5% BPO gel and 195 for 5% BPO gel). The number of patients who withdrew and the reasons for withdrawal were similar in each group (Table S4). All 458 patients were included in the safety and efficacy analysis.
Figure 1.
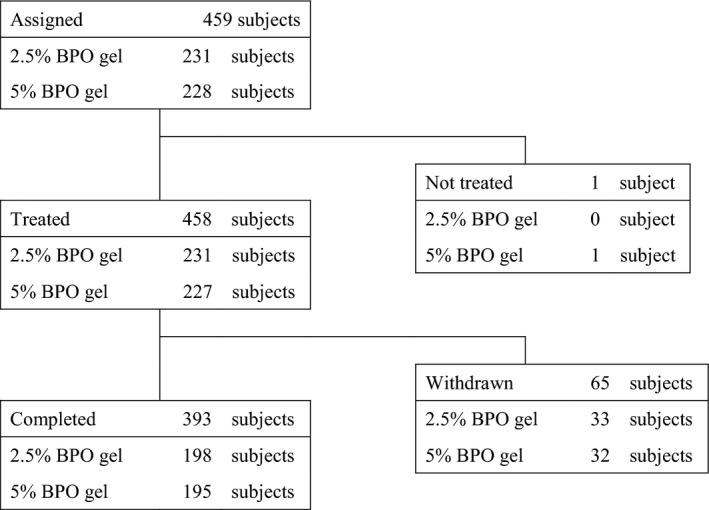
Disposition of participants.
Baseline demographics and disease characteristics of the patients included in the efficacy analysis is shown in Table 1. In both groups, participants were of similar age and primarily female. The age of most participants was between 16 and 20 years, followed by 21–25 years and then 12–15 years. These three groups accounted for approximately 70% of all participants. There was no bias between the two groups regarding IL and non‐IL counts at baseline, history of hypersensitivity and concomitant drugs or combination therapy.
Table 1.
Baseline demographic and disease characteristics of participants (population for efficacy analysis)
| 2.5% BPO gel | 5% BPO gel | |
|---|---|---|
| No. of cases (%) | No. of cases (%) | |
| No. of subjects analyzed | 231 | 227 |
| Sex | ||
| Male | 72 (31.2) | 72 (31.7) |
| Female | 159 (68.8) | 155 (68.3) |
| Age (years) | ||
| 12–15 | 34 (14.7) | 34 (15.0) |
| 16–20 | 75 (32.5) | 62 (27.3) |
| 21–25 | 56 (24.2) | 62 (27.3) |
| 26–30 | 22 (9.5) | 34 (15.0) |
| 31–35 | 27 (11.7) | 20 (8.8) |
| 36–40 | 13 (5.6) | 8 (3.5) |
| 41–45 | 1 (0.4) | 5 (2.2) |
| 46–49 | 3 (1.3) | 2 (0.9) |
| Average | 22.9 | 23.0 |
| SD | 7.3 | 7.5 |
| History of hypersensitivity | ||
| Yes | 14 (6.1) | 22 (9.7) |
| No | 217 (93.9) | 205 (90.3) |
| Concomitant drugs | ||
| Yes | 187 (81.0) | 181 (79.7) |
| No | 44 (19.0) | 46 (20.3) |
| Unknown | 0 | 0 |
| Concomitant therapies | ||
| Yes | 36 (15.6) | 31 (13.7) |
| No | 193 (83.5) | 193 (85.0) |
| Unknown | 2 (0.9) | 3 (1.3) |
| No. of IL at baseline | ||
| Median | 12.0 | 11.0 |
| Range | 5–39 | 5–40 |
| No. of TL at baseline | ||
| Median | 35.0 | 37.0 |
| Range | 6–127 | 7–130 |
| No. of non‐IL at baseline | ||
| Median | 21.0 | 21.0 |
| Range | 1–99 | 1–97 |
| No. of nodules/cysts at baseline | ||
| Median | 0.0 | 0.0 |
| Range | 0–1 | 0–2 |
BPO, benzoyl peroxide; IL, inflammatory lesions; SD, standard deviation; TL, total lesions.
Rate of compliance
Patients with less than 70% compliance rate with treatment from the start to completion of the study was 2.2% in the 2.5% BPO gel group (5/231) and 3.5% in the 5% group (8/227), namely, 2.2% (5/231) and 3.5% (8/227) from week 0/day 1 to week 12, 0.9% (2/221) and 2.3% (5/214) from week 12–28, 2.4% (5/211) and 1.9% (4/206) from week 28–40, and 1.0% (2/201) and 1.5% (3/199) from week 40–52 in the 2.5% and 5% BPO groups, respectively.
Efficacy
Total lesion count
The number of total lesions (TL) was similarly reduced over time in both groups (Fig. 2, Table S5). The median percentage reductions in TL in the 2.5% and 5% BPO gel group were 30.6% and 35.6% at week 2, 43.8% and 46.4% at week 4, 55.7% and 60.3% at week 8, and 62.1% and 66.9% at week 12, respectively. The median number of TL at week 12 was 11 in both groups. The median reduction in TL from baseline to week 12 was 22 in the 2.5% BPO group and 23 in the 5% BPO group. TL were continuously reduced after week 12, and at week 52, the percentage reduction was 75.3% in the 2.5% BPO group and 80.4% in the 5% BPO group, with reductions of 25 and 27, resulting in a median TL count of eight and seven, respectively.
Figure 2.
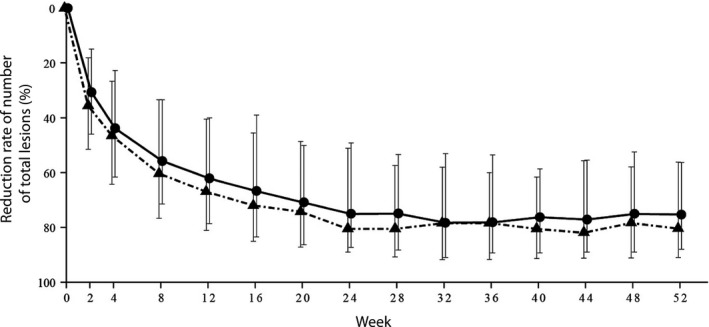
Percentage reduction in number of total lesions (median, interquartile range) over time. Circles and triangles represent reduction in the 2.5% and 5% benzoyl peroxide gel cohorts, respectively.
Inflammatory lesion counts
The percentage reduction in IL count at each observation point in the 2.5% and 5% BPO groups shifted in a similar manner over time (Fig. 3, Table S6). The median percentage reduction in the 2.5% and 5% BPO groups was 33.3% versus 40.0% at week 2, 51.7% versus 57.1% at week 4, 63.3% versus 66.7% at week 8 and 68.2% versus 72.7% at week 12. The median reduction in the number of IL at week 12 was eight in both groups, and the median IL number was four in the 2.5% BPO group and three in the 5% group. The number of IL continuously decreased after week 12, achieving 75.0% (2.5% BPO) and 83.3% (5% BPO) reduction. At week 52, the median absolute reduction was eight versus nine, and the median IL number was three versus two in the 2.5% and 5% groups, respectively.
Figure 3.
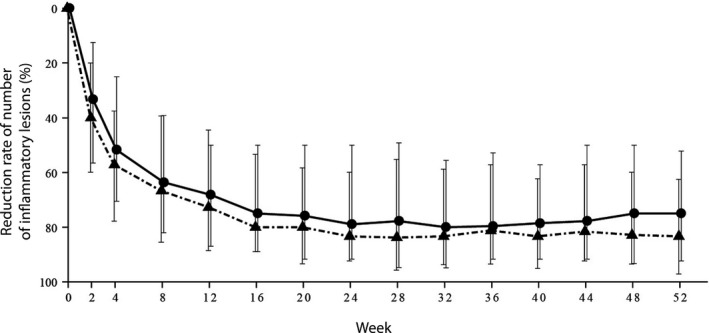
Percentage reduction in the number of inflammatory lesions (median, interquartile range) over time. Circles and triangles represent that in the 2.5% and 5% benzoyl peroxide gel groups, respectively.
Non‐inflammatory lesion counts
Median percentage reduction in non‐IL count over time shifted in a similar manner in both the 2.5% and 5% BPO groups (Fig. 4, Table S7): 29.3% and 30.4% at week 2, 40.0% and 42.9% at week 4, 48.2% and 57.3% at week 8, and 61.8% and 64.6% at week 12, respectively. The median reduction in non‐IL numbers at week 12 was 12 for 2.5% BPO versus 14.5 for 5% BPO, and the median non‐IL number was eight and 6.5. The reduction in non‐IL number was subsequently maintained. At week 52, the percentage reduction in non‐IL reached 76.6% and 80.0%, the median reductions were 15 and 16, and the non‐IL numbers were five and four, respectively.
Figure 4.
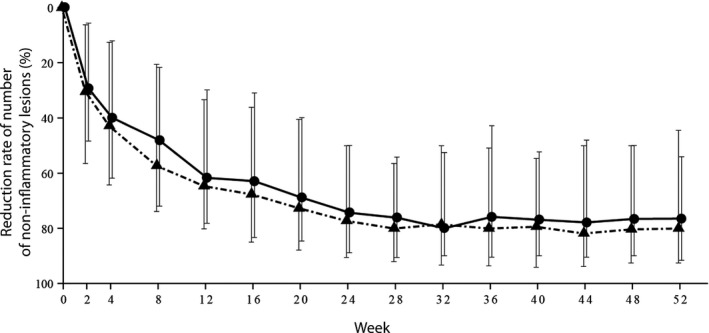
Percentage reduction in number of non‐inflammatory lesions (median, interquartile range) over time. Circles and triangles represent that in the 2.5% and 5% benzoyl peroxide (BPO) gel groups, respectively.
Microbiological evaluation
Profile of detected bacteria
Microbial assay was performed in 238 of 458 patients on the day this study started. Propionibacterium acnes was detected in 179 participants, and Staphylococcus epidermidis in 111 participants. At week 52, assay was performed in 87 of 393 patients, and P. acnes was detected in 65 and S. epidermidis in 39.
Antimicrobial susceptibility of isolated bacteria
Baseline MIC of antibacterial drugs
The range of the baseline MIC of each antibacterial drug against P. acnes was 0.12–16 μg/mL for nadifloxacin (NDFX), 0.06 or less to more than 128 μg/mL for clindamycin (CLDM), 0.06 or less to more than 128 μg/mL for erythromycin (EM), 1–32 μg/mL for gentamycin (GM), 0.25–32 μg/mL for tetracycline (TC), 0.06 or less to 2 μg/mL for minocycline (MINO) and 0.06 μg/mL or less for feropenem (FRPM). Large differences between MIC50 and MIC90 were observed for CLDM and EM, compared with other antibiotics. The percentages of patients who showed a higher MIC against P. acnes than the maximum concentration of 128 μg/mL were 1.7% (3/177) for CLDM and 13.6% (24/177) for EM, suggesting the existence of P. acnes samples with low sensitivity to CLDM and EM. There was no difference between baseline MIC in the percentage reduction in IL count at the end of the study (Tables S8,S9).
The range of baseline MIC against S. epidermidis was 0.06 or less to more than 64 μg/mL for NDFX, 0.06 or less to more than 128 μg/mL for CLDM, 0.12 to more than 128 μg/mL for EM, 0.06 or less to more than 128 μg/mL for GM, 0.12 to more than 64 μg/mL for TC, 0.06 or less to 16 μg/mL for MINO and 0.06 or less to more than 128 μg/mL for FRPM. Some samples showed a higher MIC than the maximum concentration (128 μg/mL): 44.1% (49 of 111) for CLDM and 45.9% (51 of 111) for EM. This result suggests the existence of S. epidermidis samples with low sensitivity to these antibiotics.
MIC of each antibiotic at baseline and after 52‐week treatment
The MIC50 of NDFX against P. acnes was 0.5 μg/mL at week 0/day 1 and 0.25 μg/mL at week 52 in both the 2.5% and 5% BPO gel groups. On the other hand, the MIC90 was 0.5 μg/mL at both time points in both groups. The MIC50 of CLDM against P. acnes was 0.12 μg/mL at both week 0/day 1 and week 52 in the 2.5% BPO group, and 0.25 μg/mL at week 0/day 1 and 0.12 μg/mL at week 52 in the 5% BPO group. The MIC90 in the 2.5% BPO group was 4 μg/mL at baseline and 1 μg/mL at week 52, and that in the 5% BPO group was 16 μg/mL at baseline and 4 μg/mL at week 52. With respect to other antibiotics, the MIC50 and MIC90 at week 52 were equivalent to or lower than those at baseline. None of the antibiotics showed higher than fourfold concentrations (Table S10).
The MIC50 of NDFX against S. epidermidis was 0.06 μg/mL or less at both week 0/day 1 and week 52 in both the 2.5% and 5% BPO groups. The MIC90 in the 2.5% BPO group was 2 μg/mL at baseline and 0.06 μg/mL or less at week 52, and that in the 5% BPO group was 2 μg/mL at both time points of baseline and week 52. The MIC50 of CLDM against S. epidermidis was 0.12 μg/mL both at week 0/day 1 and week 52 in the 2.5% BPO group, and 0.25 μg/mL at baseline and 0.12 μg/mL at week 52 in the 5% BPO group. The MIC90 was more than 128 μg/mL at baseline and week 52 in both groups. Regarding other antibiotics, their MIC50 and MIC90 at week 52 were equivalent to or lower than those at baseline. None of the antibiotics showed higher than fourfold concentrations. A comparison of the MIC of various antibiotics at baseline and the end of the study against P. acnes is shown in Table S10, and that against S. epidermidis in Table S11.
Safety
Adverse events
The incidence of AE is summarized in Table 2. The percentage of patients who experienced AE was 84% (194/231) in the 2.5% BPO group, 87.2% (198/227) in the 5% BPO group and 85.6% (392/458) in total. Among them, AE with a possible causal relation with the study drugs was 49.4% (114/231) for 2.5% BPO, 55.1% (125/227) for 5% BPO and 52.2% (239/458) in total. The majority of AE were mild in severity.
Table 2.
Adverse event profiles
| No causal relation with BPO | Possible causal relation with BPO | |||||
|---|---|---|---|---|---|---|
| 2.5% BPO gel | 5% BPO gel | 2.5% + 5% | 2.5% BPO gel | 5% BPO gel | 2.5% + 5% | |
| No. of cases (%) | No. of cases (%) | No. of cases (%) | No. of cases (%) | No. of cases (%) | No. of cases (%) | |
| No. of subjects analyzed | 231 | 227 | 458 | 231 | 227 | 458 |
| No. of adverse events | 194 (84.0) | 198 (87.2) | 392 (85.6) | 114 (49.4) | 125 (55.1) | 239 (52.2) |
| Mild | 191 (82.7) | 195 (85.9) | 386 (84.3) | 111 (48.1) | 123 (54.2) | 234 (51.1) |
| Moderate | 17 (7.4) | 14 (6.2) | 31 (6.8) | 5 (2.2) | 5 (2.2) | 10 (2.2) |
| Severe | 1 (0.4) | 1 (0.4) | 2 (0.4) | 0 | 0 | 0 |
| Very severe | 3 (1.3) | 2 (0.9) | 5 (1.1) | 0 | 0 | 0 |
| Noteworthy reactions | 7 (3.0) | 12 (5.3) | 19 (4.1) | 6 (2.6) | 11 (4.8) | 17 (3.7) |
BPO, benzoyl peroxide.
Relatively frequent adverse events (>2% incidence)
Table 3 shows a summary of AE that occurred in 2% or more of participants in either group. Relatively frequent AE with a possible causal relation with BPO application were skin exfoliation in 18.2% (42/231) in the 2.5% BPO group and 23.3% (52/227) in the 5% BPO group, and application site irritation in 19.0% (44/231) and 20.3% (46/227), application site erythema in 13.9% (32/231) and 18.1% (41/227), application site dryness in 13.0% (30/231) and 16.7% (38/227), application site pruritus in 6.1% (14/231) and 5.7% (13/227), and contact dermatitis in 3.0% (7/231) and 1.8% (4/227). These were known AE from previous short‐term clinical studies. Application site erythema, application site dryness and skin exfoliation were more frequent in the 5% BPO gel group compared with the 2.5% BPO group.
Table 3.
Frequently observed adverse events with or without a causal relation with the study drugs (>2% incidence)
| More than 2% incidence | No causal relation with BPO | Possible causal relation with BPO | ||||
|---|---|---|---|---|---|---|
| System organ class | 2.5% BPO | 5% BPO | 2.5% + 5% | 2.5% BPO | 5% BPO | 2.5% + 5% |
| Preferred term | No. cases (%) | No. cases (%) | No. cases (%) | No. cases (%) | No. cases (%) | No. cases (%) |
| No. of subjects analyzed | 231 | 227 | 458 | 231 | 227 | 458 |
| Eye disorders | – | – | – | – | – | – |
| Blepharitis | 3 (1.3) | 6 (2.6) | 9 (2.0) | 1 (0.4) | 0 | 1 (0.2) |
| Gastrointestinal disorders | – | – | – | – | – | – |
| Diarrhea | 7 (3.0) | 5 (2.2) | 12 (2.6) | 0 | 0 | 0 |
| General disorders and administration site conditions | – | – | – | – | – | – |
| Application site irritation | 44 (19.0) | 46 (20.3) | 90 (19.7) | 44 (19.0) | 46 (20.3) | 90 (19.7) |
| Application site erythema | 32 (13.9) | 41 (18.1) | 73 (15.9) | 32 (13.9) | 41 (18.1) | 73 (15.9) |
| Application site dryness | 30 (13.0) | 38 (16.7) | 68 (14.8) | 30 (13.0) | 38 (16.7) | 68 (14.8) |
| Application site pruritus | 14 (6.1) | 14 (6.2) | 28 (6.1) | 14 (6.1) | 13 (5.7) | 27 (5.9) |
| Immune system disorders | – | – | – | – | – | – |
| Seasonal allergy | 4 (1.7) | 8 (3.5) | 12 (2.6) | 0 | 0 | 0 |
| Infections and infestations | – | – | – | – | – | – |
| Nasopharyngitis | 59 (25.5) | 74 (32.6) | 133 (29.0) | 0 | 0 | 0 |
| Influenza | 7 (3.0) | 12 (5.3) | 19 (4.1) | 0 | 0 | 0 |
| Gastroenteritis | 8 (3.5) | 5 (2.2) | 13 (2.8) | 0 | 0 | 0 |
| Oral herpes | 4 (1.7) | 6 (2.6) | 10 (2.2) | 0 | 0 | 0 |
| Injury, poisoning and procedural complications | – | – | – | – | – | – |
| Excoriation | 2 (0.9) | 7 (3.1) | 9 (2.0) | 0 | 0 | 0 |
| Arthropod sting | 4 (1.7) | 5 (2.2) | 9 (2.0) | 0 | 0 | 0 |
| Laboratory test | – | – | – | – | – | – |
| White blood cell count increased | 27 (11.7) | 26 (11.5) | 53 (11.6) | 0 | 1 (0.4) | 1 (0.2) |
| White blood cell count decreased | 13 (5.6) | 12 (5.3) | 25 (5.5) | 0 | 1 (0.4) | 1 (0.2) |
| Alanine aminotransferase increased | 5 (2.2) | 16 (7.0) | 21 (4.6) | 0 | 0 | 0 |
| Aspartate aminotransferase increased | 4 (1.7) | 14 (6.2) | 18 (3.9) | 0 | 0 | 0 |
| Blood cholesterol decreased | 11 (4.8) | 5 (2.2) | 16 (3.5) | 0 | 0 | 0 |
| Blood urea decreased | 11 (4.8) | 5 (2.2) | 16 (3.5) | 0 | 0 | 0 |
| Blood bilirubin increased | 5 (2.2) | 10 (4.4) | 15 (3.3) | 0 | 0 | 0 |
| Blood cholesterol increased | 5 (2.2) | 10 (4.4) | 15 (3.3) | 0 | 0 | 0 |
| γ‐Glutamyltransferase increased | 6 (2.6) | 9 (4.0) | 15 (3.3) | 0 | 0 | 0 |
| Blood creatinine decreased | 5 (2.2) | 3 (1.3) | 8 (1.7) | 0 | 0 | 0 |
| Neoplasms benign, malignant and unspecified (incl. cysts and polyps) | – | – | – | – | – | – |
| Skin papillomas | 6 (2.6) | 7 (3.1) | 13 (2.8) | 0 | 0 | 0 |
| Nervous system disorders | – | – | – | – | – | – |
| Headache | 6 (2.6) | 5 (2.2) | 11 (2.4) | 0 | 0 | 0 |
| Skin and subcutaneous tissue disorders | – | – | – | – | – | – |
| Skin exfoliation | 43 (18.6) | 54 (23.8) | 97 (21.2) | 42 (18.2) | 53 (23.3) | 95 (20.7) |
| Eczema | 16 (6.9) | 21 (9.3) | 37 (8.1) | 0 | 3 (1.3) | 3 (0.7) |
| Dermatitis | 15 (6.5) | 12 (5.3) | 27 (5.9) | 2 (0.9) | 1 (0.4) | 3 (0.7) |
| Contact dermatitis | 13 (5.6) | 9 (4.0) | 22 (4.8) | 7 (3.0) | 4 (1.8) | 11 (2.4) |
| Urticaria | 5 (2.2) | 2 (0.9) | 7 (1.5) | 0 | 0 | 0 |
Medical Dictionary for Regulatory Activities (MedDRA version 15.0).
Profile of adverse events incidence by terms
Based on the aggregate data analysis of AE observed throughout this study of 85.6% (392/458), 46.7% (214/458) occurred within a month after initiation of the treatment and 62.0% (284/458) within 3 months. The occurrence dramatically decreased after 3 months, namely, to 13.1% (57/435) at 4–6 months, 6.4% (27/421) at 7–9 months and 5.9% (24/408) at 10–12 months. The incidence of AE with a possible causal relation with the study drug (application site irritation, application site erythema, application site pruritus, application site dryness, skin exfoliation and contact dermatitis) also showed a similar trend (Fig. 5). Some of these AE occurred only after 3 months from the initiation of the treatment, but all of them were local. Reactions observed between 4 and 6 months were xeroderma (three cases), blepharitis, erythema of eyelid, application site urticaria, intertrigo and dry skin (one case each), eyelid exfoliation (one case) between 7 and 9 months, and application site eczema (one case) after 10 months. All of them were mild, except for one case of moderate xeroderma. Among these, erythema of eyelid and eyelid exfoliation resolved spontaneously, and blepharitis, application site urticaria, intertrigo, xeroderma and dry skin recovered by application of moisturizers or topical steroids.
Figure 5.
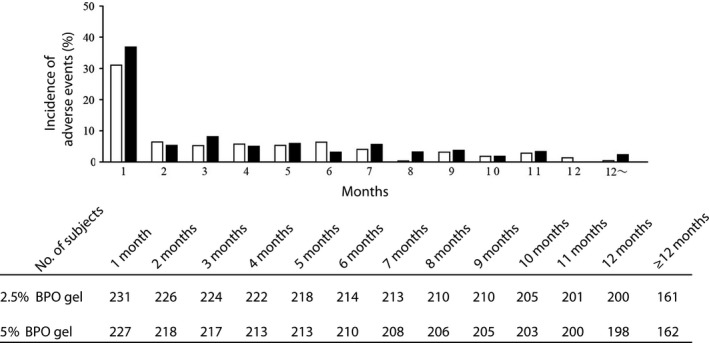
Reduction in the incidence of adverse reaction with a possible causal relation with the study drug over time. The incidence of new adverse events including repetitions that occurred in each period (1 month, 30 days) was graphed, and was represented as white (2.5% benzoyl peroxide [BPO] gel) and black (5% BPO gel) bars. Adverse events included here were irritation, erythema, pruritus and dryness at the application sites, skin exfoliation and contact dermatitis.
Effect of concomitant drugs on the incidence of adverse events
The incidence rate of AE when patients used concomitant drugs was higher than with BPO monotherapy, namely, 89.9% (311/368) versus 67.8% (61/90). Adapalene, which is indicated for acne, was often used as a concomitant drug. Therefore, we further analyzed cases that used adapalene. The incidence rate of patients with concomitant adapalene use was 91.7% (33/36) in the 2.5% BPO group and 97.3% (36/37) in the 5% BPO group, compared with 82.6% (161/195) and 85.3% (162/190) in patients who received monotherapy with BPO. The incidence rates of AE with a possible causal relation with the study drug in patients with concomitant adapalene use were 66.7% (24/36) in the 2.5% BPO group and 70.3% (26/37) in the 5% BPO group. On the other hand, those in patients without concomitant drug use were 46.2% (90/195) and 52.1% (99/190), respectively.
Deaths and other severe or noteworthy adverse events
There was one case of death in this study, in which a patient in the 2.5% BPO group committed suicide attributed to electrocution. Therefore, a causal relation with the study drug was denied. Other severe AE were reported in four participants: gastric ulcer and pneumothorax in the 2.5% BPO group, and road traffic accident and ulcerative colitis in the 5% BPO group. However, none of these had a causal relation with the study drug.
The number of patients who discontinued the study due to AE was 19 in total (seven in the 2.5% BPO group and 12 in the 5% BPO group), all of these AE were mild to moderate. In the 2.5% BPO group, contact dermatitis (three cases), application site irritation, application site urticaria, application site erythema and sebaceous gland overactivity (one case each) were observed. A causal relation of these AE with the study drug was not denied, except for sebaceous gland overactivity. In the 5% BPO group, application site irritation and contact dermatitis (three cases each), application site erythema (two cases), and ulcerative colitis, application site pruritus, dermatitis and dermatitis allergic (one case each) were reported. Except for ulcerative colitis, a causal relation between these AE and the study drug was not denied.
Evaluation of local skin tolerability scores for scaling and erythema and laboratory test values
Most patients in both the 2.5% and 5% groups scored 0 throughout the study. Participants with scores for scaling of 1 or 2 were slightly more at weeks 2 and 4 in the 2.5% BPO group and at week 2 in the 5% BPO group than at other evaluation time points. Patients with erythema scores of 1 or 2 were the most at week 2 in both groups (Tables S12,S13).
None of the patients showed a significant change in clinical laboratory tests after the initiation of treatment in both the 2.5% and 5% BPO groups. Regarding individual cases, there were no tendencies in changes seen in multiple patients, suggesting that the study drug had no effect on the clinical laboratory tests.
Based on these results, we concluded that long‐term (52 weeks) application of 2.5% and 5% BPO gel was safe.
Discussion
Aiming to evaluate the safety and efficacy of long‐term use of benzoyl peroxide for acne vulgaris, we performed an open‐label, randomized, multicenter study. The counts of TL, IL and non‐IL were reduced over time during the first 12 weeks in both the 2.5% and 5% BPO gel groups, and were maintained thereafter, suggesting the possibility to apply 2.5% and 5% BPO long‐term without a risk for the development of drug‐resistant P. acnes.
The baseline MIC assays of various antibiotics against P. acnes indicated that the differences between MIC50 and MIC90 of CLDM and EM were larger than those of other antibiotics. The fact that some samples showed a higher MIC than 128 μg/mL (the maximum concentration tested) confirmed other reports that CLDM‐ or EM‐resistant P. acnes was increasing among Japanese patients.6 However, there was not a significant difference in the reduction rate of IL depending on the MIC values, indicating that the sensitivity of P. acnes to CLDM or EM does not affect the efficacy of BPO. It has been reported that BPO is effective against P. acnes that has developed resistance to antibiotics with long‐term use.7 Therefore, BPO can be used even if patients have antibiotic‐resistant P. acnes.
The incidence of AE with a possible causal relation with the study drugs was 49.9% (114/231) in the 2.5% group and 55.1% (125/227) in the 5% group. Among these, relatively frequent AE were skin exfoliation, contact dermatitis, application site irritation, application site erythema, application site dryness and application site pruritus. Most of these were mild.
Adapalene increased the incidence of AE with a possible causal relation with BPO, to 66.7% (24/36) in the 2.5% BPO group and 70.3% (26/37) in the 5% BPO group. It has been reported that the occurrence rate of adverse drug reactions caused by long‐term use of adapalene was 84.0% (373/444),8 indicating that the incidence of AE in concomitant treatment with adapalene and BPO is similar to adapalene monotherapy.
Investigation of the occurrence of AE revealed that most of them were manifested during the time period from the initiation of the study to 3 months, and that they were especially frequent during the first month, but very few events occurred after 3 months. AE that were observed after 3 months and had a possible causal relation with the study drug were xeroderma, blepharitis, erythema of eyelid, application site urticaria, intertrigo, dry skin, eyelid exfoliation and application site eczema, and they resolved spontaneously or by application of moisturizer or topical steroids. These results suggested that long‐term use of the study drug is fairly safe.
In conclusion, we confirmed that 2.5% and 5% BPO gel may be valuable as a treatment for Japanese patients with acne vulgaris.
Conflict of Interest
Maruho covered all expenses of this study. All drugs used for this study were provided by Maruho (Kyoto, Japan). M. K. received fees for consultation, lectures and writing from Maruho. T. N. and T. K. are employees of Maruho.
Supporting information
Table S1 Schedule for investigations and observations
Table S2 Definitions of Inflammatory and non‐inflammatory lesions
Table S3 Grading criteria for local skin tolerability scores
Table S4 Profiles of withdrawals
Table S5 Shift in the number of total lesions over time
Table S6 Shift in the number of inflammatory lesions over time
Table S7 Shift in the number of non‐inflammatory lesions over time
Table S8 Reduction rate in the number of inflammatory lesions depending on the minimum inhibitory concentration of clindamycin (against Propionibacterium acnes)
Table S9 Reduction rate in the number of inflammatory lesions depending on the minimum inhibitory concentration of erythromycin (against Propionibacterium acnes)
Table S10 Minimum inhibitory concentration of each antibiotic against Propionibacterium acnes at baseline and week 52
Table S11 Minimum inhibitory concentration of each antibiotic against Staphylococcus epidermidis at baseline and week 52
Table S12 Shift in the local skin tolerability scores for scaling over time
Table S13 Shift in the local skin tolerability scores for erythema over time
Acknowledgments
We would like to acknowledge the contributions of 41 investigators including the following principal investigators (in alphabetical order of centers): Yuki Horiuchi, Akihabara Skin Clinic; Kyoko Kaminishi, Eda Dermatology Clinic; Akira Eguchi, Eguchi Hifuka; Yuriko Egami, Ekihigashi Hifuka Allergy Clinic; Takahiro Gyotoku, Gyotoku Dermatological Clinic; Akitoshi Hatamoto, Hatamoto Dermatology Clinic; Takeaki Hiramoto, Hiramoto Dermatology Clinic; Sachiko Hirota, Hirota Skin Clinic; Ken Hohki, Hohki Dermatology Clinic; Masaru Igarashi, Igarashi Dermatology Clinic; Shiomi Kawano, Iidabashi Skin Clinic; Ryoji Kanda, Kanda Skin Clinic; Hiroto Kitahara, Kitahara Dermatology Clinic; Satoko Shibata‐Kikuchi and Sho Miake, Kyushu Central Hospital of the Mutual Aid Association of Public School Teachers; Tetsuo Matsuda, Matsuda Dermatology Clinic For Skin, Hair, Nail Diseases; Keizo Matsuo, Matsuo Clinic; Ichiro Kurokawa, Meiwa Hospital; Nobuaki Morishita, Miu Skin Clinic; Keisuke Morita, Morita Dermatology and Allergy Clinic; Keiji Ohkubo, Okubo Skin Care and Clinic; Hisashi Kokuba, Sakurazaka Dermatology Clinic; Akio Taneda, Taneda Dermatology Clinic; Tomoko Matsuda, Tomoko Matsuda Dermatological Clinic; Nobukazu Hayashi, Toranomon Hospital; Kenichi Tsutsui, Tsutsui Skin and Plastic Surgery Clinic.
This is a secondary publication of an article originally published in Japanese in the Journal of Clinical Therapeutics and Medicines (“An open‐label, randomized, multicenter, phase III study to evaluate the safety and efficacy of benzoyl peroxide gel in long‐term use in patients with acne vulgaris”, 2014; 30: 669–689). Our submission for secondary publication in English was approved by the Editor‐in‐Chief of the Journal of Clinical Therapeutics and Medicines.
References
- 1. Kurokawa I, Nishijima S. Acne vulgaris. Compr Handb Clin Dermatol 2002; 17: 117–130. (in Japanese) [Google Scholar]
- 2. Hayashi N, Akamatsu H, Iwatsuki K et al Guideline for acne vulgaris. J Dermatol 2008; 118: 1893–1923. (in Japanese) [Google Scholar]
- 3. Strauss JS, Krowchuk DP, Leyden JJ et al Guidelines of care for acne vulgaris management. J Am Acad Dermatol 2007; 56: 651–663. [DOI] [PubMed] [Google Scholar]
- 4. Nast A, Dréno B, Bettoli V et al European evidence‐based (S3) guidelines for the treatment of acne. J Eur Acad Dermatol Venereol 2012; 26 (Suppl 1): 1–29. [DOI] [PubMed] [Google Scholar]
- 5. Decker LC, Deuel DM, Sedlock DM. Role of lipids in augmenting the antibacterial activity of benzoyl peroxide against Propionibacterium acnes. Antimicrob Agents Chemother 1989; 33: 326–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nakase K, Nakaminami H, Noguchi N, Nishijima S, Sasatsu M. First report of high levels of clindamycin‐resistant Propionibacterium acnes carrying erm (X) in Japanese patients with acne vulgaris. J Dermotol 2012; 39: 794–796. [DOI] [PubMed] [Google Scholar]
- 7. Eady EA, Farmery MR, Ross JI, Cove JH, Cunliffe WJ. Effects of benzoyl peroxide and erythromycin alone and in combination against antibiotic‐sensitive and ‐resistant skin bacteria from acne patients. Br J Dermatol 1994; 131: 331–336. [DOI] [PubMed] [Google Scholar]
- 8. Kawashima M, Harada S, Philippe A, Miyachi Y. One‐year efficacy and safety of adapalene gel 0.1% gel in Japanese patients with acne vulgaris. Skin Res 2007; 6: 504–512. (in Japanese) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Schedule for investigations and observations
Table S2 Definitions of Inflammatory and non‐inflammatory lesions
Table S3 Grading criteria for local skin tolerability scores
Table S4 Profiles of withdrawals
Table S5 Shift in the number of total lesions over time
Table S6 Shift in the number of inflammatory lesions over time
Table S7 Shift in the number of non‐inflammatory lesions over time
Table S8 Reduction rate in the number of inflammatory lesions depending on the minimum inhibitory concentration of clindamycin (against Propionibacterium acnes)
Table S9 Reduction rate in the number of inflammatory lesions depending on the minimum inhibitory concentration of erythromycin (against Propionibacterium acnes)
Table S10 Minimum inhibitory concentration of each antibiotic against Propionibacterium acnes at baseline and week 52
Table S11 Minimum inhibitory concentration of each antibiotic against Staphylococcus epidermidis at baseline and week 52
Table S12 Shift in the local skin tolerability scores for scaling over time
Table S13 Shift in the local skin tolerability scores for erythema over time


