Abstract
Objective
To establish the relationship between the presence of type 1 diabetes mellitus (DM) and auditory dysfunction in clinical settings by a systematic review and meta‐analysis of currently available published data.
Data Sources and Review Methods
The electronic databases PubMed, Embase, and Wanfang Data were searched for eligible relevant studies up to May 2016, and the reference lists of the retrieved articles were used for additional manual search. All the articles included in this pooled analysis were determined according to the preset inclusion and exclusion criteria. Meta‐analysis of pooled data was performed using Review Manager 5.3.
Results
A total of 15 studies were included for further combined analysis. The results showed that patients with type 1 diabetes had a significantly higher prevalence of hearing loss than controls (odds ratio = 49.08, 95% confidence interval = 12.03–200.31, P < 0.00001); standardized mean of differences (SMD) of pure tone audiometry at 4,000 Hz between diabetes and controls was 0.87 (Z = 2.22, P = 0.03, I2 = 95%); SMD of the latency time was 0.54 (Z = 2.69, P = 0.007, I2 = 78%) for waves III and 0.61 (Z = 2.38, P = 0.02, I2 = 86%) for wave V, respectively; and SMD of the interpeak latency time was 0.41 (Z = 2.84, P = 0.005, I2 = 39%) for waves I to III and 0.61 (Z = 2.67, P = 0.008, I2 = 81%) for waves I to V, respectively, between diabetics and controls.
Conclusion
Our study reveals that there is relationship between the presence of type 1 DM and an increased risk for developing mild and subclinical hearing impairment.
Level of Evidence
NA. Laryngoscope, 127:1689–1697, 2017
Keywords: Auditory function, type 1 diabetes mellitus, systematic review, meta‐analysis
INTRODUCTION
In 2014, the World Health Organization (WHO) estimated that over 360 million people (i.e., 5% of the global population) had hearing loss due to genetic factors, complications at birth, use of particular drugs, chronic ear infections, noise exposure, and aging.1 However, the relationship between diabetes mellitus (DM) and sensorineural hearing loss (SHL) has not been well established until now.2
Insulin‐dependent DM (also known as IDDM) is characterized by the presence of insulitis and β‐cell autoantibodies,3 which arise from autoimmune destruction of insulin‐producing β‐cells. The common complications of DM, such as retinopathy, nephropathy, and neuropathy, are mostly mediated through microangiopathy.4 In DM patients, the mechanism of hearing dysfunction (as with dysfunction in the retina and kidney), is not clear largely because it cannot be assessed by intravital examination. The experimental studies of cochlear structures performed in animals with induced diabetes showed that the microangiopathy associated with diabetes might affect the vascularization of the inner ear, causing degeneration of its structures by interference of nutrient transportation due to thickened capillary walls and flow reductions due to narrowed vessels.5, 6 The histopathological studies in temporal bones of the IDDM patients also showed microangiopathic changes of the cochlea, evidenced as thickening of the capillary walls in the stria vascularis and in the basilar membrane; atrophying of the stria vascularis; and loss of spiral ligament cells in upper turns.7 The retrocochlear auditory neural changes caused by demyelinating disorders and neurodegenerative diseases were associated with the progression of hearing loss.8
Horikawa et al. performed a meta‐analysis evaluating hearing loss (HL) among individuals with DM and suggested that DM patients have a higher incidence of hearing impairment compared with nondiabetic patients.9 Akinpelu et al. conducted a systematic review exploring the effects of type 2 DM on hearing function and found that patients with type 2 DM might be at greater risk for developing HL (especially mild HL) compared to control subjects.10 Some earlier studies held the opinion that pure tone audiometric thresholds were significantly higher in diabetic patients than in control subjects at all frequencies.2, 11, 12 However, others thought that there was no statistically significant difference in the thresholds for pure tones between diabetes and controls.13 Thus, in this study we sought to quantitatively analyze the currently available data of the hearing function in patients with type 1 DM compared to control groups.
MATERIALS AND METHODS
Literature Search
The electronic databases PubMed, Embase, and Wanfang Data were used to search for eligible studies from their inception until May 30, 2016. The search terms included were as follows: (hearing loss OR hearing impairment OR hearing damage OR auditory impairment OR auditory damage OR deaf) AND (insulin‐dependent diabetes mellitus OR type 1 diabetes mellitus OR type 1 diabetic). References from the retrieved articles were also analyzed for the inclusion of any missing studies relevant to our review.
Inclusion and Exclusion Criteria
The most common methods of testing hearing impairment in published literature included pure tone audiometry (PTA), auditory brainstem‐evoked response (ABR), and otoacoustic emissions (OAE). Pure tone audiometry is a hearing testing used to identify hearing threshold levels of an individual, helping determination of the degree, type, and configuration of hearing loss. Pure tone audiometry is a measurement of hearing threshold and relies on the response of patients to pure tone stimuli at various frequencies, usually ranging from 250 to 8,000 Hz. Pure‐tone threshold over 25 dB at any frequency was defined as hearing loss.14 Auditory brainstem‐evoked response is a testing method that records electrically evoked potentials over the scalp to evaluate function of the auditory pathway along the acoustic nerve: cochlear nuclei, superior olivary complex, lateral lemniscus, and inferior colliculi, represented, which are described as waves I, II, III, IV, and V, respectively.15 Otoacoustic emissions can record the acoustic energy produced by the cochlea in the outer ear canal, reflecting active mobility of the outer hair cells and revealing early or subclinical cochlear damage.16 Because the testing conditions, units, and forms for outcomes of OAE were different across all available studies, it was inappropriate to pool and analyze the relevant data. In this study, quantitative analysis of the PTA thresholds and ABR wave latencies were performed in patients with type 1 DM and age‐matched nondiabetic controls.
The inclusion criteria were as follows: 1) case‐controlled studies or randomized controlled studies; 2) the methods of hearing evaluation used had to contain PTA or ABR; 3) the type of diabetes was type 1; 4) auditory impairment without specific causes, such as presbycusis,15 noise,17, 18 and hereditary disorders19; and 5) language of reports limited to English.
The exclusion criteria included: 1) reviews, case reports, and animal studies; 2) studies focused on both type 1 and type 2 diabetes or unknown types of diabetes;13, 20, 21 3) studies without appropriate outcome measured (e.g., the means of PTA ranged from 500 to 8,000 Hz, or the latency times of ABR in any waves)22, 23, 24, 25 or statistical data with no standard deviation2, 12, 26 ; and 4) studies of elderly (aged greater than 65 years old) patients.
Study Selection
To elucidate the inclusion criteria, a protocol was written before the literature search. The first two authors (z‐p.t. and r.t.) independently screened the titles and abstracts retrieved by the electronic search to obtain a list of relevant articles. This list was jointly reviewed and a common list was generated. All relevant citations were reviewed in hard copies and as full texts to justify inclusion or exclusion, at first independently and later jointly by the first two authors. All divergence among the two reviewers was resolved by their consensus. Methodological quality was assessed by the Newcastle‐Ottawa Scale.
Data Collection and Analysis
Two researchers (z‐p.t. and r.t.) performed data extraction independently using a standard extraction form. If the eligibility of the abstract was unclear, the full article was retrieved for clarification. Any problems were solved by discussion. Data collected from the eligible studies included study characteristics (e.g., the first author's name and the year of publication), clinical characteristics of patients (e.g., the number, mean values of age, and duration of diabetics) and methods of auditory evaluation, prevalence of HL, threshold of PTA, and latency time of ABR waves.
Meta‐analysis of the data was performed using Review Manager Software version 5.3 (The Nordic Cochrane Centre). The data on the incidence of HL were entered into dichotomous formats and were obtained for the pooled odds ratio (OR) using a Mantel‐Haenszel fixed‐effects model, whereas the data of the PTA thresholds and ABR wave latencies were entered into continuous formats and were obtained for the pooled standardized mean of difference (SMD) using inverse variance and random‐effects model. The analysis model of choice was based on heterogeneity of the included studies.
Heterogeneity and Publication Bias
Statistical heterogeneity was explored using the chi squared (χ2) at the 5% significance level (P < 0.05). I2 statistic was used to quantify variation across studies results. Between‐study variance was also estimated using tau‐squared (τ2) statistic, and funnel plot was used to visually investigate publication bias.
RESULTS
Description of Included Studies
A schematic flow diagram detailing the systematic search and study selection is presented in Figure 1. First, a total of 698 studies were retrieved, and then additional five studies were obtained from the reference lists of the relevant articles. Of these initial 703 studies, 533 studies were excluded based on their titles; 135 were excluded after reviewing the abstracts (e.g. reviews, case reports, animal studies, not a case‐control association study, a study about type 2 DM); and 35 were left for detailed assessment of the full articles. After additional evaluation, 20 studies were further ruled out based on exclusion criteria, and 15 studies11, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40 were eligible for data extraction. Among them, Abd El Dayem et al.31 recruited 21 diabetes patients (disease duration < 2 years) and 32 diabetes patients (disease duration ≥ 2 years), respectively, to analyze changes in ABR in the one study. The characteristics of the eligible studies are summarized in Table 1. The quality scores of all 15 of the included studies were above six points, ranging from six to eight points (Table 2). Of those, only four studies27, 28, 29, 30 demonstrated the incidence of hearing loss in type 1 diabetes and controls; five studies11, 31, 32, 33, 34 provided the mean values of the PTA thresholds for diabetes patients and control subjects at various frequencies (500–8,000 Hz); and eight studies32, 33, 35, 36, 37, 38, 39, 40 showed the mean values of latency and interpeak latency time of waves I, III, and V, respectively, and the intervals between them for both diabetic and control subjects.
Figure 1.
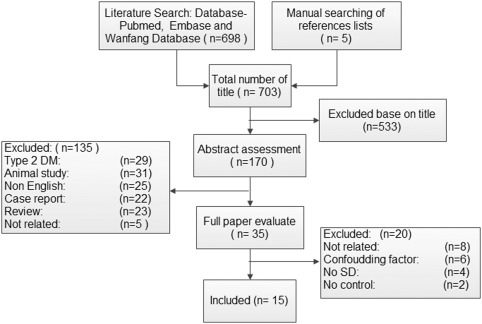
Flow diagram for the systematic search and study selection of the relationship between type 1 DM and auditory dysfunction.
DM = diabetes mellitus; SD = standard deviation.
Table 1.
Characteristics of the Eligible Studies.
| First Author (Year) |
Participants (cases/controls) |
Duration (years) |
Mean Age (years) (cases/controls) |
Hearing Test Method |
|---|---|---|---|---|
| Rance (2016)35 | 19/19 | 7.2 ± 3.7 | 13.4 ± 2.8/13.5 ± 3.0 | PTA/ABR/OAE |
| Hou (2015)28 | 50/50 | N/A | 25.56/27.56 | PTA/ABR/OAE |
| ALDajani (2015)36 | 70/30 | N/A | 9/10.8 | PTA/ABR/OAE |
| Botelho (2014)27 | 40/40 | 6.75 ± 3.64 | 14.12 ± 2.55/13.98 ± 2.48 | PTA/OAE |
| Rance (2014)39 | 10/10 | N/A | N/A | PTA/ABR/OAE |
| Abd EI Dayem (2014)31 | 40/40 | 5.3 ± 3.6 | 12.1 ± 2.9 | PTA/OAE |
| Dąbrowski (2011)32 | 31/26 | 4.6 ± 2.7 | 29.1 ± 7.1/30.3 ± 7.8 | PTA/ABR/OAE |
| Okhovat (2011)34 | 100/100 | N/A | 12.19 ± 3.1 | PTA |
| Elamin (2005)29 | 63/63 | 1.5–9.0 | N/A | PTA |
| Tóth (2003)30 | 15/15 | 23.0 ± 2.6 | N/A | PTA/BAEP |
| Ottaviania (2002)37 | 60/ 58 | 17.5 ± 8.9 | 31.0 ± 6.23/29.1 ± 5.75 | PTA/ABR/OAE |
| Lisowska (2001)38 | 41/33 | N/A | 33 | ABR/OAE |
| Seidl (1996)40, a | 32/32 | 6 ± 2.6 | 13.5 ± 2 | BARP |
| Seidl (1996)40, b | 21/ 21 | < 2 | 9.7 ± 3.5 | BARP |
| Çelik (1996)11 | 75/40 | 15 ± 7 | 45.3/43.8 | PTA |
| Virtaniemi (1994)33 | 50/50 | N/A | 25.56/27.56 | PTA/ABR/OAE |
Disease duration ≥ 2 years.
Disease duration < 2 years.
ABR = auditory brainstem evoked responses, BAEP = brainstem auditory evoked potentials; N/A = not available; OAE = otoacoustic emissions; PTA = pure‐tone audiometry.
Table 2.
The Newcastle‐Ottawa Scale for Assessing Quality of Studies.
| First Author (year) | Selection | Comparability | Exposure | Totol Quality Scores | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case Definition With Independent Validation | Consecutive or Obviously Representative Series of Cases | Community Controls | Definition of Controls Is No history of Disease (endpoint) | Study Controls for Select Most Important Factor. | Study Controls for Any Additional Factor | Secure Record/Structured Interview | Same Method of Ascertainment for Cases and Controls | Same Nonresponse Rate for Both Groups | ||
| Rance (2016)35 | * | – | – | * | * | – | * | * | * | 7 |
| Hou (2015)28 | * | * | * | * | * | * | – | * | * | 8 |
| ALDajani (2015)36 | * | – | – | * | * | * | – | * | * | 6 |
| Botelho (2014)27 | * | – | * | * | * | * | – | * | * | 7 |
| Rance (2014)39 | * | – | – | * | * | – | * | * | * | 6 |
| Abd EI Dayem (2014)31 | * | – | – | * | * | * | * | * | * | 7 |
| Dąbrowski (2011)32 | * | – | * | * | * | * | * | * | * | 8 |
| Okhovat (2011)34 | * | * | * | * | * | * | – | * | * | 8 |
| Elamin (2005)29 | * | – | * | * | * | * | – | * | * | 7 |
| Tóth (2003)30 | * | – | – | * | * | – | * | * | * | 6 |
| Ottaviania (2002)37 | * | – | – | * | * | * | * | * | * | 7 |
| Lisowska (2001)38 | * | – | – | * | * | * | * | * | * | 7 |
| Seidl (1996)40 | * | – | – | * | * | * | * | * | * | 7 |
| Çelik (1996)11 | * | * | – | * | * | * | – | * | * | 7 |
| Virtaniemi (1994)33 | * | * | * | * | * | * | – | * | – | 7 |
* = one score. – = zero score.
Prevalence of Hearing Loss
As shown in Figure 2, after 252 diabetes and 253 controls were pooled for the prevalence of HL, we found that patients with diabetes had a significantly higher prevalence than controls (OR = 49.08, 95% confidence interval = 12.03–200.31, Z = 5.43, P < 0.00001), suggesting that patients with diabetes are more likely to have hearing thresholds greater than 25 dB when compared with nondiabetic subjects.
Figure 2.
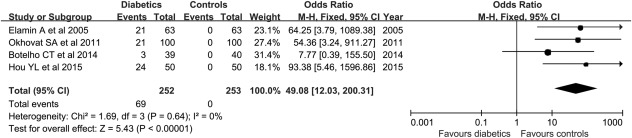
Forest plots of four studies for comparing the prevalence of hearing loss in patients with type 1 diabetics and controls. The outcome is odds ratio for the prevalence of hearing loss, with a bar denoting 95% CIs. The weights of each study in the meta‐analysis are indicated.
Analysis model = fixed‐effect; effect measure is OR.
CI = confidence interval; M‐H = Mantel‐Haenszel; OR = odds ratio.
Pure Tone Audiometry Thresholds at Different Frequencies
The frequencies of 500; 1,000; 2,000; 4,000; and 8,000 Hz, respectively, were used in these studies. The averages of pooled PTA of the 339 patients with diabetes and 288 controls at each frequency studied are summarized in Figure 3D. The mean values of PTA were significantly higher at 4,000 Hz in patients with diabetes than controls, and the SMD between patients with diabetics and controls was 0.87 (Z = 2.22, P = 0.03, I2 = 95%), but there were no significant differences at 500; 1,000; 2,000; and 8,000 Hz. It is worth noting that, although the mean PTA thresholds were higher in cases than in controls at 4,000 Hz, the PTA averages were still less than 25 dB, which did not meet the WHO definition about hearing loss.
Figure 3.
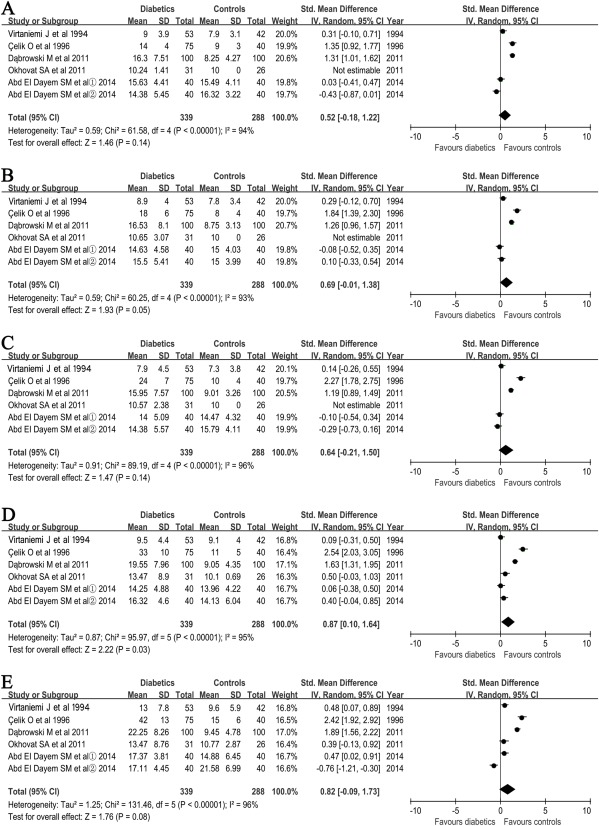
Forest plots of studies showing standardized mean difference for PTA thresholds in patients with type 1 diabetics and controls at 500 (A); 1,000 (B); 2,000 (C); 4,000 (D); and 8,000 Hz (F), respectively. The ① and ②, respectively, evaluated the mean difference for PTA thresholds of right and left ear in the same patients with diabetics or controls. Bars and diamonds denote 95% CIs. The weights of each study in the meta‐analysis are indicated.
Analysis mode = random effect; IV = inverse variance; effect measure is std. mean difference.
CI = confidence interval; PTA = pure tone audiometric; SD = standard deviation; std. = standardized.
Auditory Brainstem‐Evoked Response Wave Latencies
The pooled wave I and III latencies were performed for 280 cases and 225 controls, whereas wave V latencies were performed for 265 cases and 210 controls. Analyses were performed for the interpeak latency of waves I to III for 159 cases and 152 controls, waves III to V for 144 cases and 137 controls, and wave I to V for 200 cases and 185 controls, respectively.
The SMD of the latency time was 0.54 (Z = 2.69, P = 0.007, I2 = 78%) for waves III and 0.61 (Z = 2.38, P = 0.02, I2 = 86%) for wave V, respectively. The SMD of the interpeak latency time was 0.41 (Z = 2.84, P = 0.005, I2 = 39%) for waves I to III and 0.67 (Z = 2.67, P = 0.0008, I2 = 81%) for waves I to V, respectively, between cases and controls (Fig. 4B, C, D, F). The most significant difference was shown in waves I to V.
Figure 4.
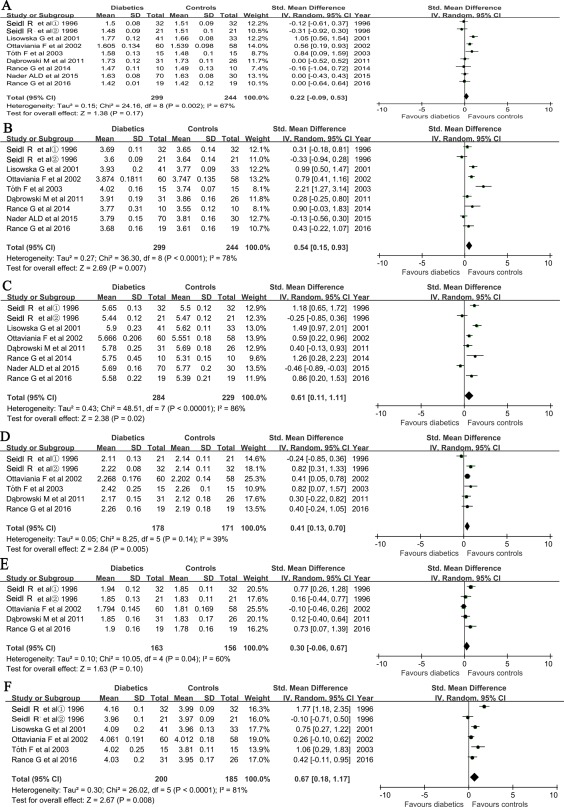
Forest plots of studies showing standardized mean of difference for auditory brainstem‐evoked responses latency times of waves I (A), III (B), and V (C), and their intervals in wave I–III (D), III–V (F) and I–V (E), respectively. ①: the disease duration ≥ 2 years ; ②: the disease duration < 2 years. Bars and diamonds denote 95% CIs. The weights of each study in the meta‐analysis are indicated. Analysis mode = random effect; IV = inverse variance; effect measure is std. mean difference. CI = confidence interval; SD = standard deviation; std. = standardized.
Publication Bias
The shapes of the funnel plots indicated that there is no significant publication bias (Appendix I). Furthermore, tau‐squared (τ2) statistic was used to statistically confirm the funnel plot symmetry. The results still did not suggest significant publication bias.
DISCUSSION
Based on the meta‐analysis, there is an association between type 1 DM and auditory organ dysfunction: the odds of hearing loss are significantly increased in the type 1 DM. Although hearing impairment is mild and subclinical, the hearing threshold has markedly increased trend at the high frequency (e.g., 4,000 Hz). The ABR wave latencies (waves III and V) and interpeak latencies (waves I–III and I–V) are prolonged. This suggests that signal transduction efficiency of the central and peripheral auditory pathways, in particular the former, may be slowed down in type 1 diabetes.
The pooled OR value of the prevalence of HL in patients with type 1 DM versus controls was 49.08, which is higher than that reported by Horikawa et al.,9 in which the type of DM was not differentiated, or that reported by Akinpelu et al.,10 in which the patients of interest were limited to type 2 DM. This phenomenon may be due to normal hearing function in age‐ and gender‐matched healthy controls. Because the highest prevalence of type 1 DM is in the pediatric group (0–4 years),41 the recruited patients and gender‐matched healthy controls are all from childhood to young adulthood (range of mean age from 12.1 ± 2.9 to 30.3 ± 7.8 years old) with normal hearing, excluding the possibility of special causes. However, the studies by Horikaw9 (without separation of the type of DM) and Akinpelu10 (a study of type 2 DM) did not explore the influence of aging, which is an important factor of hearing loss.
Moreover, we quantitatively analyzed the PTA thresholds for both diabetic and control subject at the different frequencies and found that the mean values of PTA were significantly higher at 4,000 Hz in patients with type 1 diabetes than in controls, although they did not meet the requirements of the definition of HL (pure‐tone threshold greater than 25 dB at any frequency). Abd El Dayem reported that the diabetic group recorded a significantly higher reading at the high frequencies (4,000 and 8,000 Hz) and even at increased high frequencies (16,000; 17,000; and 18,000 Hz).31 Others supported that patients with diabetes only had a higher audiometric thresholds at high frequencies (> 2,000 Hz),27, 32 and that although type 1 DM has no significant influence in the auditory system in clinical settings, it may be associated with a subclinical impairment in cochlea in the patients of interest.37, 38 This suggests that type 1 DM may be a risk for developing mild or subclinical hearing impairment, especially at a high frequency.
Finally, we found that type 1 DM is associated with a specific impairment of the auditory brainstem function in patients with patients. Analysis of ABR in our study showed that latency time of waves III and V and the interpeak latency time of waves I to III and I to V were significantly longer in patients with diabetes than in controls, with the statistically significant difference indicated for waves I to V. These outcomes supported that diabetes might be a cause of certain dysfunctions of the central and peripheral auditory pathways. Because the statistically significant difference was shown in wave I to V latencies, we could infer that the influence of type 1 DM on central auditory pathway was more severe. The detailed mechanisms of the acoustic nerve impairment in type 1 DM remain unknown, but reduced conduction efficiency may result from auditory nerve demyelization and spiral ganglion loss.39 On the other hand, the typical hearing loss in patients with diabetes is considered as a progressive, bilateral, sensorineural deafness of gradual onset, predominantly at the higher frequencies.42 This may be a result of the impairment of the basal region of the cochlea, which could be explained by more vascularized and therefore more susceptible to damage in the basal region.
There are a few limitations that could be potential sources of bias in this meta‐analysis. First, the sample number was insufficient. This may be due to exclusion of publications with important results, such as those published in other languages than English, or with a variety of hearing testing methods and various results presented in different studies, which made many data unsuitable to merge. Second, there was marked heterogeneity across the results derived from the included studies. This may be due to the number of studies included, variations in the sample sizes of the studies, differences in the populations of interest, and disparity in the proportions of diabetic to control participants in the included studies. Third, the included hearing test, such as the ABR and PTA, could only assess the function of the totality and the retrocochlear auditory pathway but was unable to evaluate the degree of damage of cochlear area. There were many studies that used OAE to reveal early or subclinical cochlear damage in type 1 DM.24 For example, Nader et al. reported that the lower amplitudes of distortion product OAE in patients with diabetics at 1,000 Hz were considered as a dysfunction of the outer hair cells in the apical portion of the cochlea.12 Elbarbary et al. pointed out that the transiently evoked OAE with suppression showed limited suppression in diabetic patients compared to control groups, particularly at high frequency and the overall suppression, implying a possible involvement of cochlear injury due to hyperglycemia.42 However, as shown above, the data of these studies have been generated by various ways and conditions, and thus the forms of manifestation and units were difficult to integrate and analyze. Fourth, we did not explore the effect of microangiopathic complications (nephropathy and retinopathy), plasma levels of glycosylated hemoglobin (HbA1C), and the duration of diabetes to auditory function on our combined analysis because there was limited use of these data. For example, Elamin et al. reported that a positive correlation existed between these microangiopathic complications and hearing loss at high frequencies (6,000–8,000 Hz).29 HbA1C was also associated with hearing loss, and outer hair cells may be damaged with the increase of HbA1C in some studies.28, 34 Several authors found that the duration of diabetes was significantly correlated with the auditory threshold at many frequencies after multiple regression analysis,8, 33 whereas other authors pointed out that the duration of diabetes did not affect hearing threshold in diabetic patients.27, 31 Finally, publication bias cannot be avoided because many researchers are less likely to report negative findings.
CONCLUSION
This meta‐analysis reveals that there is a relationship between type 1 DM and auditory dysfunction; that among type 1 diabetics, the odds of hearing loss is higher as compared to controls, although the hearing impairment may be mild and subclinical; and that signal transduction efficiency of the central and peripheral auditory pathways, in particular the former, may be slowed down in type 1 diabetes.
Supporting information
Additional supporting information may be found in the online version of this article.
Appendix I: The funnel plot of the publication bias for the included studies.
Acknowledgment
The authors thank Prof. Hong‐Guang Xie, General Clinical Research Center, Nanjing First Hospital, Nanjing Medical University, China, for critical reading of the article and language editing.
Financial Disclosure: The authors have no funding, financial relationships, or conflicts of interest to disclose.
BIBLIOGRAPHY
- 1. World Health Organization 2014 Deafness and hearing loss. Available at: http://www.who.int/pbd/deafness/estimates/en/. Accessed March 1, 2016.
- 2. Cullen JR, Cinnamond MJ. Hearing loss in diabetics. J Laryngol Otol 1993;107:179–182. [DOI] [PubMed] [Google Scholar]
- 3. Van den Driessche A, Eenkhoorn V, Van Gaal L, Block CD. Type 1 diabetes and autoimmune polyglandular syndrome: a clinical review. Neth J Med 2009;67:376–387. [PubMed] [Google Scholar]
- 4. Schalkwijk CG, Stehouwer CDA. Vascular complications in diabetes mellitus: the role of endothelial dysfunction. Clin Sci 2005;109:143–159. [DOI] [PubMed] [Google Scholar]
- 5. Raynor E, Robison WG, Garrett CG, McGuirt WT, Pillsbury HC, Prazma J. Consumption of a high‐galactose diet induces diabetic‐like changes in the inner ear. Otolaryngol Head Neck Surg 1995;113:748–754. [DOI] [PubMed] [Google Scholar]
- 6. Smith TL, Raynor E, Prazma J, Buenting JE, Pillsbury HC. Insulin‐ dependent diabetic microangiopathy in the inner ear. Laryngoscope 1995;105:236–240. [DOI] [PubMed] [Google Scholar]
- 7. Fukushima H, Cureoglu S, Schachern PA, et al. Cochlear changes in patients with type 1 diabetes mellitus. Otolaryngol Head Neck Surg 2005;133:100–106. [DOI] [PubMed] [Google Scholar]
- 8. Pudar G, Vlaski L, Filipovic D, Tanackov I. Correlation of hearing function findings in patients suffering from diabetes mellitus type 1 in regard to age and gender. Med Pregl 2009;9:395–401. [DOI] [PubMed] [Google Scholar]
- 9. Horikawa C, Kodama S, Tanaka S, et al. Diabetes and risk of hearing impairment in adults: a meta‐analysis. J Clin Endocrinol Metab 2013;98:51–58. [DOI] [PubMed] [Google Scholar]
- 10. Akinpelu OV, Mujica‐Mota M, Daniel SJ. Is type 2 diabetes mellitus associated with alterations in hearing? A systematic review and meta‐analysis. Laryngoscope 2014;124:767–776. [DOI] [PubMed] [Google Scholar]
- 11. Celik O, Yalcin S, Celebi H, Oztvrk A. Hearing loss in insulin‐dependent diabetes mellitus. Auris Nasus Larynx 1996;23:127–132. [PubMed] [Google Scholar]
- 12. Ferrer JP, Biurrun O, Lorente J, et al. Auditory function in young patients with type 1 diabetes mellitus. Diabetes Res Clin Pract 1991;11:17–22. [DOI] [PubMed] [Google Scholar]
- 13. Gibbin KP, Davis CG. A hearing survey in diabetes mellitus. Clin Otolaryngol 1981;6:345–350. [DOI] [PubMed] [Google Scholar]
- 14. Kluk K, Moore BC. Dead regions in the cochlea and enhancement of frequency discrimination: effects of audiogram slope, unilateral versus bilateral loss, and hearing‐aid use. Hear Res 2006;222:1–15. [DOI] [PubMed] [Google Scholar]
- 15. Kovai J, Lajtman Z, Oegovi I, Kneevi P, Caric T, Vlsaic A. Investigation of auditory brainstem function in elderly diabetic patients with presbycusis. Int Tinnitus J 2009;15:79–82. [PubMed] [Google Scholar]
- 16. Lamprecht‐Dinnesen A. [Otoacoustic emissions]. [Article in German]. HNO 1992;40:415–421. [PubMed] [Google Scholar]
- 17. Konrad‐Martin D, Austin DF, Griest S, McMillan GP, McDermott D, Fausti S. Diabetes‐related changes in auditory brainstem responses. Laryngoscope 2010;120:150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Austin DF, Konrad‐Martin D, Griest S, McMillan GP, McDermott D, Fausti S. Diabetes‐related changes in hearing. Laryngoscope 2009;119:1788–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Annunzio G, Minuto N, Amato E, et al. Wolfram syndrome (diabetes insipidus, diabetes, optic atrophy, and deafness): clinical and genetic study. Diabetes Care 2008;31:1743–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sunkum AJ, Pingile S. A clinical study of audiological profile in diabetes mellitus patients. Eur Arch Otorhinolaryngol 2013;270:875–879. [DOI] [PubMed] [Google Scholar]
- 21. Mozaffari M, Tajik A, Ariaei N, Ali‐Ehyaii F, Behnam AH. Diabetes mellitus and sensorineural hearing loss among non‐elderly people. East Mediterr Health J 2010;16:947–952. [PubMed] [Google Scholar]
- 22. Lisowska G, Namysłowski G, Morawski K, Strojek K. Cochlear dysfunction and diabetic microangiopathy. Scand Audiol Suppl 2001;30:199–203. [DOI] [PubMed] [Google Scholar]
- 23. Virtaniemi J, Laakso M, Nuutinen J, Karjalainen S, Vartiainen E. Acoustic‐reflex responses in patients with insulin‐dependent diabetes mellitus. Am J Otolaryngol 1994;15:109–113. [DOI] [PubMed] [Google Scholar]
- 24. Nardo W, Ghirlanda G, Paludetti G, et al. Distortion‐product otoacoustic emissions and selective sensorineural loss in IDDM. Diabetes Care 1998;21:1317–1321. [DOI] [PubMed] [Google Scholar]
- 25. Virtaniemi J, Laakso M, Nuutinen J, Karjalainen S, Vartiainen E. Tympanometry in patients with insulin‐dependent diabetes mellitus. Scand Audiol 1993;22:217–222 [DOI] [PubMed] [Google Scholar]
- 26. Pessin AB, Martins RH, Pimenta WDE P, Simoes AC, Marsiglia A, Amaral AV. Auditory evaluation in patients with type 1 diabetes. Ann Otol Rhinol Laryngol 2008;117:366–370. [DOI] [PubMed] [Google Scholar]
- 27. Botelho CT, Carvalho SA, Silva IN. Increased prevalence of early cochlear damage in young patients with type 1 diabetes detected by distortion product otoacoustic emissions. Int J Audiol 2014;53:402–408. [DOI] [PubMed] [Google Scholar]
- 28. Hou Y, Xiao X, Ren J, Wang Y, Zhao F. Auditory impairment in young type 1 diabetics. Arch Med Res 2015;46:539–545. [DOI] [PubMed] [Google Scholar]
- 29. Elamin A, Fadlallah M, Tuvemo T. Hearing loss in children with type 1 diabetes. Indian Pediatr 2005;42:15–21. [PubMed] [Google Scholar]
- 30. Toth F, Varkonyi TT, Rovo L, et al. Investigation of auditory brainstem function in diabetic patients. Int Tinnitus J 2003;9:84–86. [PubMed] [Google Scholar]
- 31. Abd El Dayem SM, Abd El Ghany SM, Beshr AE, Hassan AG, Attaya MS. Assessment of hearing in children with type 1 diabetes mellitus. J Pediatr Endocrinol Metab 2014;27:393–402. [DOI] [PubMed] [Google Scholar]
- 32. Dąbrowski M, Mielnik‐Niedzielska G, Nowakowski A. Involvement of the auditory organ in type 1 diabetes mellitus. Pol J Endocrinol 2011;62:138–144. [PubMed] [Google Scholar]
- 33. Virtaniemi J, Laakso M, Nuutinen J, Karjalainen S, Vartiainen E. Hearing thresholds in insulin‐dependent diabetic patients. J Laryngol Otol 1994;108:837–841. [DOI] [PubMed] [Google Scholar]
- 34. Okhovat SA, Moaddab MH, Okhovat SH, et al. Evaluation of hearing loss in juvenile insulin dependent patients with diabetes mellitus. J Res Med Sci 2011;16:179–183. [PMC free article] [PubMed] [Google Scholar]
- 35. Rance G, Chisari D, Edvall N, Cameron F. Functional hearing deficits in children with type 1 diabetes. Diabet Med 2016;33:1268–1274. [DOI] [PubMed] [Google Scholar]
- 36. ALDajani N, ALkurdi A, ALMutair A, ALdraiwesh A, ALMazrou KA. Is type 1 diabetes mellitus a cause for subtle hearing loss in pediatric patients? Eur Arch Otorhinolaryngol 2015;272:1867–1871. [DOI] [PubMed] [Google Scholar]
- 37. Ottaviania F, Doziob N, Cesare B. Negliaa, Riccioa F, Scavini M. Absence of otoacoustic emissions in insulin‐dependent diabetic patients Is there evidence for diabetic cochleopathy? J Diabetes Complications 2002;16:338–343. [DOI] [PubMed] [Google Scholar]
- 38. Lisowska G, Namysłowski G, Morawski K, Strojek K. Early Identification of Hearing impairment in patients with type 1 diabetes mellitus. Otol Neurotol 2001;22:316–320. [DOI] [PubMed] [Google Scholar]
- 39. Rance G, Chisari D, O'Hare F et al. Auditory neuropathy in individuals with type 1 diabetes. J Neurol 2014;261:1531–1536. [DOI] [PubMed] [Google Scholar]
- 40. Seidl R, Birnbacher R, Hauser E, Bernert G, Freilinger M, Schober E. Brainstem auditory evoked potentials and visually evoked potentials in young patients with IDDM. Diabetes Care 1996;19:1220–1224. [DOI] [PubMed] [Google Scholar]
- 41. Variation and trends in incidence of childhood diabetes in Europe. EURODIAB ACE Study Group . Lancet 2000;355:873–876. [PubMed] [Google Scholar]
- 42. Elbarbary NS, El‐Kabarity RH, Desouky ED. Cochleopathy in Egyptian adolescents with type 1 diabetes mellitus. Int J Pediatr Otorhinolaryngol 2012;76:1558–1564. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional supporting information may be found in the online version of this article.
Appendix I: The funnel plot of the publication bias for the included studies.


