Abstract
Bioactive compounds in plant‐based foods have health properties that contribute to the prevention of age‐related chronic diseases, particularly cardiometabolic disorders. Conclusive proof and understanding of these benefits in humans is essential in order to provide effective dietary recommendations but, so far, the evidence obtained from human intervention trials is limited and contradictory. This is partly due to differences between individuals in the absorption, distribution, metabolism and excretion of bioactive compounds, as well as to heterogeneity in their biological response regarding cardiometabolic health outcomes. Identifying the main factors underlying inter‐individual differences, as well as developing new and innovative methodologies to account for such variability constitute an overarching goal to ultimately optimize the beneficial health effects of plant food bioactives for each and every one of us. In this respect, this position paper from the COST Action FA1403‐POSITIVe examines the main factors likely to affect the individual responses to consumption of plant food bioactives and presents perspectives for assessment and consideration of inter‐individual variability.
Keywords: Bioavailability and metabolism, Biological responsiveness, Cardiometabolic health, Inter‐individual variation, Plant food bioactives
1. Introduction
Cardiometabolic disease, which comprises cardiovascular diseases, type 2 diabetes mellitus and their associated risk factors including metabolic syndrome and obesity, is the world's leading cause of death and a major public health concern. Observational studies provide convincing evidence that most cases of cardiometabolic disease could be avoided through dietary and lifestyle changes 1, 2. It is therefore necessary to establish effective preventive strategies targeting physical activity, smoking patterns, and dietary behaviours of populations and to drive the food industry to produce healthier foods.
A high intake of plant‐based foods has shown beneficial effects on cardiometabolic health, both in large population studies 3, 4, 5, 6 and randomized controlled trials 7, 8, 9. Plant‐based foods typically have a low caloric density and provide a range of beneficial nutrients and low‐energetic constituents, such as dietary fibres, essential vitamins and minerals, and are exclusive and rich sources of a wide variety of phytochemicals, including polyphenols, carotenoids, plant sterols, and glucosinolates. Although not strictly essential micronutrients, some phytochemicals have been described as “lifespan essential”, as they promote optimal health during ageing 10. There is increasing evidence from cohort studies and randomized controlled trials that many of these bioactive compounds may help to reduce the risk of chronic diseases, particularly of cardiometabolic diseases 11, 12, 13. Plant food bioactives are widely distributed in the diet but specific groups of compounds can be particularly abundant in some foods for which specific dietary recommendations may be developed: e.g. glucosinolates in cruciferous vegetables, isoflavones in soya, anthocyanins in berries or lycopene in tomatoes. Specific physiological effects with implications for health have been attributed to different bioactive compounds. For example, polyphenol‐rich foods and beverages can improve endothelial function, platelet function, insulin sensitivity and decrease blood pressure 14, 15, 16. In 2012, the European Food Safety Authority (EFSA) panel on Dietetic Products, Nutrition and Allergies has approved a health claim related to cocoa flavanols and maintenance of normal endothelium‐dependent vasodilation, according to regulation (EC) No 1924/2006 17. Despite intense and active research investigating the health effects of plant food bioactives over the last years, the real impact of most compounds and their mechanisms of action have not yet been fully elucidated. Plant food bioactives have been shown to exert antioxidant, anti‐inflammatory, anti‐atherogenic activities through a large panel of mechanisms, including modulation of enzyme activities, binding to cell receptors and interfering with signalling pathways and transcription factors ultimately regulating gene expression, altering DNA methylation or affecting the gut microbial population and functionality 18, 19, 20, 21.
An increasing number of studies investigating the health effects of plant food bioactives have revealed substantial between‐subject variations in response to dietary interventions, suggesting that the consumption of particular foods or bioactive constituents may benefit some individuals more than others. The major determinants responsible for the between‐subject variability may include genetic and non‐genetic factors which are only beginning to be explored and may differ depending on the compounds (Fig. 1). The aim of this position paper is to highlight the complexity of this issue and the need of increasing knowledge in the field, both to better understand the contribution of phytochemicals in the health effects of plant‐based foods and to develop effective strategies and refined dietary recommendations to optimize the beneficial effects of bioactive‐containing foods for all population groups.
Figure 1.
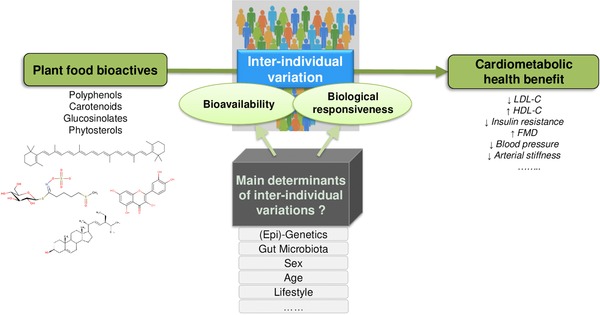
Potential factors responsible for inter‐individual variability in bioavailability and biological responsiveness to consumption of plant food bioactives in relation to cardiometabolic health.
1.1. Inter‐individual variation in metabolism and bioavailability of plant food bioactives
After decades of active research, much about the absorption, distribution, metabolism, and excretion (ADME) patterns of the main plant food bioactives is now known and has been reviewed in humans 22, 23, 24. However, key information on many of the enzymes, carrier proteins or bacterial strains critical for the absorption and metabolism of these bioactive compounds and the factors that may modulate their activities is still lacking. Some plant bioactives can be absorbed in their native form from the stomach or gut, but most of the glycosylated, polymeric, or esterified native plant compounds have to be hydrolyzed before absorption, a step partly carried out by the gut microbiota 25. The specific bacterial strains responsible for this initial hydrolysis have not been extensively studied yet. For the intestinal uptake of the bioactive compounds both passive and active mechanisms have been described, as well as efflux transporters that lower the absolute influx of bioactives through the intestinal absorption. Nevertheless, our knowledge of the carriers involved for the various compounds is still very sparse. When absorbed, hydrophilic plant food bioactives typically undergo first pass metabolism in the intestine and in the liver with phase I (oxidation/reduction reactions) and mainly phase II (β‐glucuronidation, sulfation, methylation, glutathione conjugation) biotransformations 22. A substantial enterohepatic recirculation exists for some metabolites. Lipophilic bioactive compounds may be absorbed through the lymphatic system and thereby escape the first pass metabolism by the liver. For a number of plant food bioactives, the gut microbiota in addition to the intestine and liver handles the production of a wide range of metabolites, which can be more or less specific of their precursors 26. The gut microbiota composition, specific bacteria strains and the functionalities responsible for all these metabolic biotransformations, as well as their occurrence and distribution in humans have not been extensively investigated. Further research in this specific area will be essential for a better understanding of the inter‐individual variation.
In human intervention studies that target the bioavailability of diverse plant food bioactives or in large‐scale observational studies, researchers consistently observed that the plasma concentrations and urinary excretion levels of plant bioactives or their derived metabolites can markedly differ between individuals upon a similar dietary intake. Several examples of clinical intervention studies that demonstrated inter‐individual variability in bioavailability of different families of plant food bioactives are presented in Table 1 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41. However, a systematic analysis of the magnitude of this inter‐individual variability has not been performed so far. Between‐subject differences could arise due to variances in absorption, metabolism, tissue distribution and turnover, excretion or a combination of these parameters. This has been clearly demonstrated for carotenoids and has led researchers to propose that some subjects could be considered “non‐responders” to carotenoids on the basis of their low plasma concentration after intake 42. Several studies recently demonstrated an association between the carotenoid status and variants of genes encoding proteins involved in the absorption and metabolism of carotenoids. These include SR‐BI (Scavenger Receptor Class B, Member 1) and CD36 (CD36 Molecule, Thrombospondin Receptor) both involved in the uptake of carotenoids by intestinal cells, as well as BCO1 (Beta‐Carotene Oxygenase 1) which catalyzes the oxidative cleavage of carotenoids into vitamin‐A 43, 44.
Table 1.
Clinical intervention studies demonstrating interindividual variability in bioavailability of plant food bioactives
| Bioactives/food rich in bioactives | Study products | Study type | Nb volunteers | Volunteers | Objectives | Results | Reference |
|---|---|---|---|---|---|---|---|
| Carotenoids | Watermelon juice providing 20 mg lycopene, 2.5 mg b‐carotene (Treatment 1) or 40 mg lycopene + 5 mg b‐caroten (Treatment 2) or Tomato juice providing 18 mg lycopene + 0.6 mg b‐carotene (Treatment 3) Daily intake of each treatment for 3 weeks | Chronic parrallel study | N = 10 for Treatment 1 N = 23 for treatment 2 N = 12 for treatment 3 | Healthy non‐smokers | Use of cluster analysis to examine individual temporal response of plasma carotenoids and provide basis for classifying subjects as strong or weak responders | Various carotenoids can produce various plasma responses for a given subject. Individual responsiveness was associated with genetic variants of the carotenoid metabolizing enzyme b‐caroten 15,15′ monoxygenase 1. | 41 |
| Lutein | Meal 1: providing lutein as a supplement (15 mg) Meal 2: providing the same amount of lutein as tomato puree | Acute cross‐over study | N = 39 | Healthy non‐obese, non‐smoker men | Characterization of the interindividual variability in lutein bioavailability by measuring lutein in plasma chylomicrons sampled over 8h postprandially and identification of SNPs involved | The CV of the postprandial lutein response was 75% after lutein supplement and 137% after tomato puree consumption. Most of this variance was explained by 29 SNPs in 15 genes related to both lutein and chylomicron metabolism. | 29 |
| Lycopene | Test meal containing 100 g tomato puree providing l9.7 mg all‐trans Lycopene | Acute study | N = 39 | Healthy non‐obese, non‐smoker men | Characterization of the interindividual variability in lycopene bioavailability by measuring lycopene in plasma chylomicrons sampled over 8h postprandially and identification of SNPs involved | The CV of the postprandial concentrations of lycopen in plasma chylomicrons was of 70%. 72% of this variance was explained by 28 SNPs in 16 genes involved in lycopen and lipid metabolism. | 28 |
| Cocoa flavanols | 5.3 and 10.7 mg total Cocoa Flavanols (CF)/kg BW | Acute parralel study | N = 20 young (25–35 yrs) N = 20 elderly (65–80 yrs) | Healthy men | Follow‐up of concentrations in total structurally‐related EC metabolites (SREM) and of individual metabolites in blood and urine over 24 h | The interindividual variations in total SREM were 38 and 39% in AUC (0–6 h) and Cmax, respectively without effect of age. Small differences observed between young and elderly for some specific metabolites. | 34 |
| Orange juice ‐ Flavanones | 400 mL fresh, homogenized and pasteurized orange juice | Acute cross‐over study | N = 18 | Healthy subjects (20–50 yrs) | Effect of orange juice processing on flavanone bioavailability as measured in 24 h urine | High excretors (N = 4), medium excretors (N = 7) and low excretors (N = 7) had mean flavanone excretion values of 15, 9, and 3% respectively after fresh juice intake. Type of food processing had an impact in high excretors but not in medium and low excretors | 40 |
| Hop prenylflavonoids ‐ 8 prenylnaringenin | 3 hop‐derived supplements per day for 5 days | Short‐term study | N = 50 | Healthy post‐menopausal women (46–74 yrs) | Examine the extent of interindividual variation in urinary excretion of hop‐derived prenylflavonoids | Subjects were classified into poor (60%), moderate (25%) and strong (15%) 8‐prenylnaringenin (8‐PN) producers based on urinary excretion and in vitro microbial bioactivation capacity (convertion of isoxanthohumol to 8‐PN). | 27 |
| Soy Isoflavones | Soymilk challenge (240 ml twice daily for 3.5 days) | Short‐term study | N = 159 (89 from USA and 70 from Australia) | Healthy adults | Follow‐up of urinary excretion of S‐equol (24h urine) to identify equol producers and search for association between equol producer status and dietary factors | The observed overall frequency of equol producer is 29.6% without any significant difference between USA and australian participants. Subtle effects of some dietary factors (PUFAs, maltose, VitA, Vit E). | 36 |
| Soy Isoflavones | Soy protein bar (containing 38 mg Daidzein) per day on 3 consecutive days | Short‐term study | N = 91 Korean American (KA) and N = 222 Caucasian American (CA) | Healthy Women and girls | Use a soy challenge test to confirm results from observational studies suggesting that the prevalence of equol producers is higher in Asian than in Western populations | Prevalence of Equol‐producer phenotype higher in KA (51%) than in CA (36%), of ODMA‐producer phenotype lower in KA (84%) than in CA (92%). No differences in dietary habits between equol and non‐equol producers. | 38 |
| Soy Isoflavones | Soymilk challenge (250 ml twice daily) on 3 consecutive days | Short‐term study | N = 41 including 29 vegetarians and 12 nonvegetarians | Healthy adults | Comparison of the frequency of equol producers in the two dietary patterns | The frequency of equol producers was higher in vegetarians (59%) than in nonvegetarians adults (25%). | 37 |
| Lignans‐ enterolactone | Purified secoisolariciresinol diglucoside (SDG) (1,31 micromol/kg BW) | Acute study | N = 12 | Healthy men and women | Pharmacokinetics study of enterolignans (enterodiol (END), enterolactone (ENL)) in volunteers after consumption of their plant precursor | Substantial variation observed among subjects in plasma concentration and urinary excretion of enterolignans: In 5 subjects AUC of ENL was twice that of END; in other 5 subjects AUC of ENL was only 1–2 times that of END; In 2 subjects AUC of END exceeded that of ENL. No differences between men and women. | 32 |
| Rapsberries‐Ellagitannins | 300 g rapsberries | Acute study | N = 10 | Healthy subjects (25–48 yrs) | Follow‐up of plasma (24h kinetics) and urine (48h kinetics) concentrations of ellagic acid and its microbial metabolites urolithins | Large interindividual variation in timing, quantity and type of urolithins‐O‐glucuronides excreted in urine. One subject produced no urolithins, whereas the other nine excreted quantities ranging from 1.3 to 44 μmol. Only one subject produced urolithin B‐glucuronide. | 31 |
| Walnuts and pomegranate extract ‐Ellagitannins | Two interventions: 1. 30 g walnuts per day for 3 days; 2. pomegranate extract 0.9g per day for 3 days | Short‐term study | 1. N = 20 2. N = 49 | 1. Healthy subjects (21–55 yrs) 2. Healthy, overweight subjects (40‐65 yrs) | Characterization of the interindividual variability in urolithin production assessed by measuring derivatives in 24 h urine; Analysis of fecal microbiota composition | Three metabotypes identified: metabotype A subjects (1. 65%; 2. 60%) excreted only urolithin A metabolites, metabotype B subjects (1. 20%; 2. 30%) in addition to UA also excreted urolithin B and isourolithin A, metabotype 0 subjects (1. 15%; 2. 10%) did not excrete urolithins. Higher level of Gordonibacter were found in metabotype A individuals. Metabotype B prevailed in overweight‐obese versus normoweight subjects. | 35 |
| Standard broccoli and high‐glucosinolate broccoli | 3 test meals (150 ml): broccoli soup, super broccoli soup, or water | Acute study | N = 16 | Healthy subjects | Investigate the impact of GSTM1 genotypes on sulphoraphane metabolism and excretion | Significant increase in plasma AUC and urinary excretion of total sulforaphane metabolites observed in GSTM1‐null subjects compared with the GSTM1‐positive subjects. | 30 |
| broccoli‐ Isothiocyanates | Test meal consisting in 2.5 g broccoli/kg BW with pasta | Acute study | N = 114 | Healthy subjects (18–50 yrs) | Investigate the effect of GSTM1 genotypes on sulforaphane metabolism and excretion | 62% of GSTM1 null individuals had high ITC excretion whereas only 39% of individuals with the GSTM present genotype were high ITC excretors. | 39 |
| broccoli‐ glucosinolates | Standardized meal containing 200 g cooked broccoli | Acute study | N = 23 | Healthy subjects | Examine the association between glucosinolate metabolism and gut bacterial community composition | Differences in gut microbiota composition contribute to observed variation in glucosinolate metabolism in low‐ (N = 5) vs high‐ITC excreters (N = 5). | 33 |
BW, body weight; CV, coefficient of variation; SNP, single nucleotide polymorphism; CF, cocoa flavanols; EC, epicatechin; PUFA, poly‐unsaturated fatty acids; ODMA, O‐desmethylangolensin, AUC, area under the curve; Cmax, peak plasma concentration; GSTM1, glutathione S‐transferase M1; ITC, isothiocyanate.
In addition, it is most likely that host genetic and epigenetic variations in metabolizing enzymes can be a major determinant of individual phenotypes. Pharmacogenomics studies have demonstrated that for particular drugs, including some phytochemicals of the alkaloid family like codeine, individuals can be categorized into poor, intermediate or extensive and ultra‐rapid metabolizers and dosing has to be adapted to achieve equivalent plasma or tissue concentration of the active metabolite 45. Differences have been attributed to specific genes and enzymes of the xenobiotic metabolism pathways and the genetic variants associated have been compiled in online databases such as the Pharmacogenomics knowledgebase (PharmGKB) 46 and on the FDA Table of Pharmacogenomic Biomarkers in Drug Labeling (http://www.fda.gov/Drugs/ScienceResearch/ResearchAreas/Pharmacogenetics/ucm083378.htm, accessed 26/08/16). Most plant food bioactives are absorbed and metabolized through the same polymorphic carriers and enzymatic systems as some of these drugs, and their pharmacokinetics is thus also likely to depend on genetic background. Caffeine is a plant bioactive for which the importance of genetic polymorphisms has been demonstrated. It is mainly metabolized by CYP1A2 in the liver. Subjects with the CYP1A2*1F variant (carriers of the ‐163 C allele) associated with a low enzyme inducibility are considered to be slow caffeine metabolizers in comparison with the rapid caffeine metabolizers carrying the wild‐type allele 47. Slow caffeine metabolizers have been shown to be at increased risk of hypertension and myocardial infarction, whereas for rapid caffeine metabolizers it might be more safe to drink coffee 48. Plant food bioactives are also extensively metabolized by phase II conjugation enzymes. Several variants exist for genes encoding glutathione S‐transferases (GSTs), which play an important role in the metabolism of glucosinolates present in cruciferous vegetables. After broccoli consumption, the bioavailability of isothiocyanates was shown to be 20% higher in GSTM1 null subjects than in GSTM1 positive subjects 30. This is of significance as the prevalence of the null allele for GSTM1 has been estimated to be 40–60% 49. Variants of UDP‐glucuronosyltransferases, sulfotransferases and catechol‐O‐methyltransferase, may also contribute to variability in plant food bioactives metabolism, clearance and efficacy although it is less documented 50. The tissue:blood partition and the excretion kinetics of the metabolites produced, for example glucuronides, is likely to be different from that of the native compounds due to differences in compound polarity. The modulation of phase II enzymes may thus affect the duration of exposure to bioactive metabolites and consequently the biological response.
Gut microbiota obviously plays an important role in inter‐individual variation in the metabolism of some plant bioactives. A well‐known example is the conversion of the soy isoflavones daidzin/daidzein into equol by the gut microbiota. Only 25–35% of the Western population and up to 50–70% of the vegetarian and Asian populations possess the ability to produce equol from these precursors 36, and producers have been reported to experience more beneficial health effects from soy consumption than equol non‐producers 51, 52, 53. The equol‐producing bacteria identified so far include Adlercreutzia, Eggerthella, Paraeggerthella, and Slackia species all of them belonging to the family Coriobacteriaceae, a family that has been associated with beneficial metabolic processes for obesity and diabetes 54. Different combinations of bacteria can perform the successive steps of the conversion of soy isoflavones into equol, but the determinants that govern the daidzein‐metabolizing phenotype are not elucidated yet. Identification of these determinants may lead to the development of intervention strategies or food processes to stimulate equol production in equol non‐producers. The gut microbiota also play an important role in explaining the variability in the metabolism of other plant food bioactives, in particular lignans 55 and ellagitannins 56, 57. Recent advances in metagenomics, and increased knowledge of the intestinal microbiota, open up new perspectives to further investigate the implication of the microbiota in the inter‐individual variation of the bioavailability of plant food bioactives. The European MetaHit project (FP7, http://www.metahit.eu/) proposed that the microbial communities could be classified into three main enterotypes characterised by the predominant bacterial population: Bacteroides (enterotype 1), Prevotella (enterotype 2) and Ruminococcus (enterotype 3) 58. While it is nowadays clear that the enterotype concept follows a continuum rather than discrete values, it must be emphasized that a functional stratification, rather than a phylogenetic stratification in microbiome profile also needs to be made 59. Enterotypes as well as functionality differences in microbiota (putatively grouped in “metabotypes”) may be both important for explaining the inter‐individual variation in plant bioactives ADME and putative health effects as well as for stratifying subjects 60. Furthermore, the extent to which dietary interventions can modulate the gut microbiota and consequently affect the metabolism of plant food bioactives will have to be determined.
Besides genetic background and gut microbiota composition, other factors such as sex, age, dietary habits, may affect the bioavailability of plant food bioactives. For example, sex‐dependent differences in the glucuronidation of resveratrol, a polyphenol present in grapes and wine, may be explained by sex‐specific UDP‐glucuronosyltransferase isoenzyme expression profiles regulated by sex hormones 61. Important pharmacokinetic changes are known to occur with aging, for example the volume of distribution is reduced for polar compounds resulting in higher plasma concentrations 62. How these changes may affect plant food bioactive ADME needs to be investigated in details. Dietary habits are known to influence microbiota diversity and activity 63. Furthermore, some dietary phytochemicals such as polyphenols and glucosinolates themselves can induce or inhibit the activity of phase I and phase II enzymes 50.
As illustrated by the examples above, there is a body of evidence that various factors can control the bioavailability and metabolism of plant food bioactives in individuals. These factors must now be studied in a more systematic and integrative way to determine their relative importance for each family of plant food bioactives.
1.2. Inter‐individual variation in biological response regarding cardiometabolic health outcomes
Compared with the inter‐individual variation in ADME and bioavailability, the inter‐individual variation in efficacy of plant food bioactives to modulate physiological outcomes has been less studied. The etiological basis for this variability has rarely been considered and individual data are seldom published. The biological effects of plant food bioactives that might be occurring only in a subgroup of subjects can be overlooked when data are averaged for an entire sample population without an appropriate stratification of the participants. Several clinical intervention studies describing inter‐individual variability in the bioactivity of plant food bioactives and the associated determinants are presented in Table 2. For example, in post‐menopausal women, consumption of soy isoflavones resulted in decreased systolic and diastolic blood pressure and improvement of the endothelial function, but only in 30% of the volunteers taking part in the study and who were also found to be equol producers 64. The effect would not have been observed when just considering average values. Similarly, a recent clinical trial which prospectively recruited male equol producers and non‐equol producers with moderate cardiovascular disease risk, provided evidence of the importance of the equol phenotype, and thereby of the gut microbial activity in mediating the positive effect of dietary isoflavones on arterial stiffness 53. This would suggest a clear link between bioavailability and efficacy of plant bioactives but this is not necessarily the case. Indeed, a recent controlled clinical trial showed that consumption of flavan‐3‐ol‐enriched dark chocolate resulted in decreased platelet aggregation in males but not females volunteers, however this effect could not be correlated with plasma or urine concentrations of flavan‐3‐ols metabolites 65. This observation suggests that inter‐individual variation in biological responses is not solely due to variation in the bioavailability of plant food bioactives but can depend on other factors, such as sex.
Table 2.
Clinical intervention studies with plant food bioactives demonstrating factors of interindividual variability in their bioactivity related to cardiometabolic outcomes
| Bioactives/food rich in bioactives | Quantity (per day) | Duration | Number of volunteers | Volunteers | Biomarkers presenting interindividual variability | Factors of inter‐individual variability | Reference |
|---|---|---|---|---|---|---|---|
| Isoflavones | 99 mg | 12 months | 202 | Postmenopausal women | Systolic/diastolic blood pressure and endothelial function | Equol producers | 64 |
| Isoflavones | 80 mg | Acute | 28 | Men at moderate cardiovascular risk | Arterial stiffness | Equol producers | 53 |
| Cocoa Flavanols | 907 mg | Acute | 42 | Healthy subjects | Platelet function | Gender | 65 |
| Cocoa Flavanols | 450 mg | 14 days | 42 | Young and elderly healthy men | Systolic blood pressure and arterial stiffness | Age | 67 |
| Cocoa Flavanols | 821 mg | 4–6 days | 34 | Healthy young and elderly subjects | Blood pressure and endothelial function | Age | 68 |
| Quercetin | 150 mg | 6 weeks | 93 | Overweight or obese young and elderly subjects | Systolic blood pressure | Age and diseases state (hypertension) | 69 |
| Plant sterols | 2–3.2 g | 4‐8 weeks | 67 | Men and women with normal or increased blood cholesterol levels | Total cholesterol | CYP7A1 polymorphism | 72 |
Age is the strongest independent cardiovascular risk factor for CVD and is also associated with increased vascular stiffness, endothelial dysfunction and systolic hypertension 66. So far, few studies have investigated the impact of age on the cardiometabolic effects of food bioactives (Table 2). Consumption of a flavanol‐rich drink reversed age‐related increase in systolic blood pressure together with aortic augmentation index (a measure of vascular stiffness) in healthy elderly but not in young men 67. In this study, the plasma levels of flavanol metabolites were not significantly different between young and elderly individuals, suggesting that differences in bioavailability could not explain the differences observed in biological response 34. In another intervention study, the effect of the consumption of a flavanol‐rich cocoa drink on blood pressure was also higher in the elderly than in young volunteers 68. An age‐dependent effect of quercetin on blood pressure was also reported 69.
Inter‐individual variability in the response to the consumption of plant food bioactives has also been described for other phytochemicals (Table 2). For example, despite the abundance of data of clinical trials demonstrating the cholesterol‐lowering action of plant sterol supplements, substantial variability in efficacy exists in responsiveness among individuals 70. Genetic polymorphisms of genes associated with cholesterol trafficking processes may be responsible for this inter‐individual variability. For example, a significant inter‐individual variation in the LDL‐cholesterol lowering response to plant sterol consumption has been associated with a polymorphism in the ABCG8 gene. The subjects carrying the A allele of the gene were reported to get a greater benefit from plant sterol intake 71. The bioactivity of plant sterols was also associated with polymorphism in cholesterol 7 alpha‐hydroxylase (CYP7A1) gene with ‐204A>C variant presenting greater CYP7A1 activity 72.
Environmental factors, including dietary habits, can also affect our genetic imprint through a range of epigenetic mechanisms that can impact gene transcription and the subsequent cellular responses. These epigenetic mechanisms involving DNA methylation, histone modifications, and non‐coding RNAs (microRNAs, Long noncoding RNAs) regulation emerge as important players in cardiovascular diseases 73, 74, 75. Recent review papers reported that dietary polyphenols induced epigenetic changes that may promote cardiovascular health benefit 19, 76. For example, consumption of a cocoa extract for two weeks significantly reduced the global DNA methylation levels of peripheral leukocytes in subjects with cardiovascular risk factors 77. Another study demonstrated that one‐year supplementation with a grape extract containing resveratrol modulated the expression of microRNAs involved in the regulation of the inflammatory response in circulating immune cells of hypertensive male patients with type 2 diabetes 78. However, the role of the individual epigenome in the variation of the responses to plant food bioactives is still unexplored.
Recently, modest progress has been made toward a better understanding of the role of genetic polymorphisms in the variability of the response to diet. For example, the benefit of the Mediterranean diet (MedDiet) on cardiovascular‐related outcomes was shown to depend on genetic variants of the Transcription factor 7‐like 2 (TCF7L2) gene 79. This gene encodes a protein acting as a transcription factor, and the TCF7L2‐rs7903146 polymorphism is known as a strong genetic determinant of type‐2 diabetes risk and fasting glucose concentrations 80. The prevalence of the 7903146T allele is associated with higher type‐2 diabetes risk. In their study, Corella et al. showed that an intervention with the MedDiet was more beneficial toward cardiovascular risk factors and stroke incidence for individuals with the 7903146T allele 79. However, it has to be noted that most nutrigenetics studies thus far are association studies and results have not been confirmed in studies.
The development of high‐throughput “omics” technologies (epigenomics, metagenomics, transcriptomics, proteomics, metabolomics) and bioinformatic tools has enabled researchers to go deeper in the analysis of the complex mechanisms that are involved in the way the organism responds to plant food bioactives and ultimately impact human health and well‐being. For example, the consumption of hesperidin from orange juice or of a resveratrol‐containing grape extract have been shown to change leukocyte gene expression toward anti‐atherogenic and anti‐inflammatory profiles in healthy men 81 and in patients with stable coronary artery disease 82, respectively. The inter‐individual variation in the overall expression profile of genes involved in the regulation of cardiometabolic health in response to plant food bioactives has not been evaluated in humans. However preliminary evidence of a large between‐subject variability in the expression levels of genes and microRNAs related to inflammation and cardiovascular diseases in humans has been reported 78. Nutrigenomics, particularly transcriptomic studies, will help to reveal genes and non‐coding RNA expression profiles that may be used as new biomarkers of responsiveness to plant food bioactives. Furthermore, investigations focused on the identification of the molecular mechanisms of action in response to plant food bioactives are still necessary to identify genes and (or) non‐coding RNAs underlying the health effects of these compounds. These genes may become candidates for future nutrigenetic studies aiming to identify gene variants involved in inter‐individual variability in responsiveness to plant bioactive intake.
In summary, a range of studies have indicated that inter‐individual variation in biological responsiveness to plant food bioactives exists and that it may be influenced by several factors including bioavailability and metabolism, gut microbiome composition, (epi)‐genetic profiles, age, or sex.
1.3. How to tackle the complexity of the inter‐individual variability through a multi‐dimensional approach?
Research in the field of inter‐individual variation in response to consumption of plant bioactives and the determinants involved is presently highly fragmented and many research gaps remain. Addressing this question requires the participation of experts from a range of disciplines. The first initiative to this end is the creation of a dedicated Action called “FA1403‐POSITIVe” (https://www6.inra.fr/cost‐positive) within the framework of the European Cooperation in Science and Technology (COST). This open network gathers nutritionists, clinicians, geneticists, epidemiologists, microbiologists, experts in nutrigenomics, bioinformaticians, molecular biologists and biochemists from more than 70 research institutions in 31 countries. Their common goal is to analyse in a systematic way the available knowledge to evaluate the extent of inter‐individual variation for some of the main families of plant food bioactives and to identify the key determinants of the inter‐individual variation regarding both bioavailability and bioactivity related to cardiometabolic health outcomes. A major expected outcome of this COST Action will be to define strategies and methods to stratify populations into subgroups according to their ability to respond to plant food bioactives.
From the findings of the POSITIVe network and of other complementary initiatives, the relative contribution and possible interactions of the main determinants of inter‐individual variability identified should be further validated through dedicated randomized controlled trials and large‐scale prospective studies for the different families of plant food bioactives. The power of these studies will have to be calculated to allow different ‘responsive’ subgroups analyses and/or wise targeted recruitments must be performed based on the factors likely to affect the individual response. In upcoming studies assessing the health effects of plant foods and their bioactives, publication of individual data should be strongly recommended and as far as possible the volunteers should be phenotyped for the determinants that have already been identified as important.
Because of the inter‐individual variation in ADME, the internal exposure to plant food bioactive compounds and (or) derived metabolites is not directly correlated to the dietary intake and will have to be characterized in depth in future studies. To date in the vast majority of epidemiological studies, health outcomes were related to food or nutrient intake data, estimated from dietary questionnaires and tables of food composition without taking into account the bioavailability. In intervention studies, the background diet and the supplementation are usually standardized but this does not guarantee equal internal exposures among volunteers due to the inter‐individual variability in absorption and metabolizing capacity. Known metabolites can be quantified in biofluids such as plasma and urine or in tissue biopsies using targeted methods of analysis based on UV or mass spectrometry detection. However, the scope of targeted methods of analysis is usually restricted to a limited number of known metabolites of a particular family of compounds. Non‐targeted metabolomics with high‐resolution mass spectrometry (Q‐TOF and Orbitrap instruments) has emerged as a holistic and sensitive approach to analyse in human biofluids the food metabolome which encompasses all the metabolites derived from food intake and digestion, including the plant food bioactive metabolites 83, 84. Comprehensive profiles of metabolites present in urine, plasma or other biospecimens, including still unknown metabolites of plant food bioactives, can be obtained and compared in nutritional intervention studies for subjects consuming the same diet but stratified according to factors such as age, sex, gut microbiota composition, and genotype. The extent and the nature of the differences existing between the metabolomic profiles of the study groups, for example, between males and females after the same plant food intake is likely to provide valuable new insights for a better understanding of the inter‐individual variation.
Some technological and methodological improvements will have to be implemented to facilitate the use of metabolomics for plant food bioactive research. First initiatives have been launched in the framework of the COST Action POSITIVe with the development of a standardized combination of methods ensuring a wide coverage of plant food metabolites, the sharing of standards of rare metabolites, and the compilation of metabolism and analytical data in the online database PhytoHub dedicated to dietary phytochemicals (www.phytohub.eu). The objective is to make possible the use of comprehensive annotated profiles of plant food bioactive metabolites in intervention trials and large‐scale observational studies such as wide‐association studies.
Furthermore, despite the knowledge accumulated on the metabolism of plant bioactives in humans, and while most plant biosynthetic pathways of these compounds are accessible on‐line at KEGG (http://www.genome.jp/kegg/pathway.html) or MetaCyc (http://metacyc.org/) databases, their metabolic pathways in humans or mammals have not yet been published in online databases. Up to now, caffeine is the only plant food bioactive for which the metabolism in humans is described in the KEGG database (http://www.genome.jp/kegg/pathway/map/map00232.html). As modern biology increasingly relies on bioinformatics, there is an urgent need to fill this gap. We need to compile existing knowledge in online resources, with data on proteins, genes, polymorphisms, gut bacteria involved as well as to design new research projects aiming to find out the missing information. In this way, the updated and complete human metabolic pathways of the plant bioactive compounds will be made available for systems biology approaches to study the inter‐individual variability in an integrative way.
Well‐designed intervention and prospective studies, integrating omics approaches for a better phenotyping of individuals will show the relative contribution of various factors to control the bioavailability of different families of plant food bioactives and the individual biological responsiveness to their consumption. A major challenge will be to develop methods and tools to phenotype and stratify individuals based on their ability to respond to plant food bioactives intake. An in‐depth characterisation of the subjects, including genotyping, gut microbiota analysis, food metabolome profiling has become achievable, although still quite expensive, making feasible the search for correlations between information‐rich datasets and health outcomes in intervention and prospective studies. New bioactives as well as new molecular targets in population subgroups may be identified. However, for the majority of studies investigating the health effects of plant foods and for clinical applications, the subject stratification methods should be more cost‐effective and easy to handle. Several approaches can be foreseen, including modeling and challenge tests. Models may be built from the acquired knowledge to predict the internal exposure of individuals based on their declared food intake and their status for the main factors controlling inter‐individual variation in ADME (e.g. genotyping and gut microbiota). These models will be improved as new data and knowledge are generated and could be validated through measurement of actual exposure in controlled intervention studies. Together with models aiming to predict the internal exposure, more complex ones should also be developed to predict biological responsiveness to plant food bioactives intake and thus estimate the personal health benefits that an individual can gain from different bioactives compounds. This estimation would be made by taking into account the bioavailability of bioactives together with other factors identified as involved in the inter‐individual variability in responsiveness, such as age, sex, specific genetic polymorphisms or physiological status. Before obtaining enough knowledge to develop such models, another option for stratification of the population into responders and non‐responders may be considered. This could consist of designing challenge tests in which subjects would consume a standardized supplement containing various bioactives. The plasma concentrations and/or urinary excretion of key metabolites would be monitored according to a precise sampling schedule over a defined timeframe, as it has already been proposed for equol‐production capacity 85. In addition, standardized postprandial nutritionally challenging conditions could be defined to induce disturbances impacting cardiometabolic outcomes to assess the individual capacity to respond to plant food bioactives intake. This would be done by testing the ability of subjects receiving specific bioactives to attenuate/counterbalance the induced disturbances by measuring the impact on well‐established and emerging biomarkers of cardiometabolic risk. This challenge test approach will be helpful both to strengthen the scientific knowledge of the determinants of inter‐individual variability and to stratify responders/non‐responders.
2. Conclusion and perspective
Within the context of improving the health of populations and promoting sustainable economic growth of the food sector, research policies emphasize the importance of developing high quality and healthy foods and refining dietary recommendations to decrease the burden of diet‐related chronic diseases. The challenge is to stimulate both the consumers to select foods that fit into a healthy diet and the agro‐food sector to develop healthier foods. Dietary patterns rich in plant foods have been largely shown to be healthier than those rich in animal products 86. Consequently, high consumption of fruits, vegetables and whole‐grains is widely encouraged in many countries through national public health policies. However, thus far the recommendations are applied to the general population in a “one‐size‐fits‐all approach” without considering the possible high between‐subject variations in response to plant food bioactives. As a consequence, this approach does not necessarily ensure adequate exposure to protective compounds for all individuals.
Improving our knowledge of the factors (such as age, sex, (epi)genotype and gut microbiota) that influence whether plant food bioactives are more or less effective in individuals, together with the development of methods to identify responsiveness profiles will be invaluable to progress in the development of effective and innovative solutions leading to health improvements. There is a high potential for the development of new functional foods or optimized traditional foods with higher effectiveness for general or targeted populations. For example, adapting processing methods may improve the bioavailability of key bioactive compounds for individuals identified as poor absorbers/metabolizers. This research will also provide the scientific evidence to help the public health bodies to elaborate a new generation of nutritional recommendations for improving the prevention of cardiometabolic diseases in the long run. In particular, it may lead to refined recommendations toward foods particularly rich in specific bioactives and to public health advice targeting specific populations. As recently demonstrated in the European FP7‐Food4me project, personalized dietary advice is more efficient to positively affect dietary behaviours (see white paper at: www.food4me.org/). In a global context of an increasing prevalence of low intake of fruits and vegetables, with less than 25% of the population consuming at least five portions per day (400 g) in most countries, more personalized messages regarding this food group could be of high benefit 87. Acquired knowledge might also be integrated for individual counseling along personal profiles of determinants affecting the bioavailability and responsiveness to plant food bioactives.
Addressing the inter‐individual variation in response to plant food bioactives perfectly fits one of the main European research priorities identified for foods and diets toward 2050, which is to contribute to the understanding of human variation in response to nutrition factors and to better translate this knowledge into innovative individualised food and nutrition solutions to improve citizens’ health 88. This position paper elaborated in the framework of the COST POSITIVe Action proposes some priorities for tackling the challenging research question of inter‐individual variation, with the ultimate goal of ensuring that the cardiometabolic health‐promoting effects associated with bioactives present in plant foods are applicable for everyone.
The authors have declared no conflict of interest.
Acknowledgments
The authors would like to acknowledge networking support by the COST Action FA 1403 POSITIVe (Inter‐individual variation in response to consumption of plant food bioactives and determinants involved), supported by COST (European Cooperation in Science and Technology).
The authors' responsibilities were as follows: Ch.M., C.M., D.M., F.T.B. contributed to the conception and design of the manuscript; Ch.M., C.M., and D.M. equally participate in writing the manuscript and Ch.M. had primary responsibility for final content. All of the authors critically reviewed and edited the manuscript. All of the authors read and approved the final manuscript.
Manach C., Milenkovic D., Rodriguez‐Mateos A., Garcia‐Conesa M. T., Landberg R., Gibney E. R., Heinonen M., Tomás‐Barberán F., Morand C., Mol. Nutr. Food Res. 2017, 61, 1600557.
Colour Online: See the article online to view Fig. 1 in colour.
3 References
- 1. Willett, W. C. , Koplan, J. P. , Nugent, R. , Dusenbury, C. et al., in: Jamison D. T., Breman J. G., Measham A. R., Alleyne G., et al (Eds.), Disease Control Priorities in Developing Countries, World Bank. The International Bank for Reconstruction and Development/The World Bank Group, Washington: (DC: ) 2006. [PubMed] [Google Scholar]
- 2. Boeing, H. , Bechthold, A. , Bub, A. , Ellinger, S. et al., Critical review: vegetables and fruit in the prevention of chronic diseases. Eur. J. Nutr. 2012, 51, 637–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carter, P. , Gray, L. J. , Troughton, J. , Khunti, K. et al., Fruit and vegetable intake and incidence of type 2 diabetes mellitus: systematic review and meta‐analysis. BMJ (Clinical research ed.) 2010, 341, c4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nothlings, U. , Schulze, M. B. , Weikert, C. , Boeing, H. et al., Intake of vegetables, legumes, and fruit, and risk for all‐cause, cardiovascular, and cancer mortality in a European diabetic population. J. Nutr. 2008, 138, 775–781. [DOI] [PubMed] [Google Scholar]
- 5. Wang, X. , Ouyang, Y. , Liu, J. , Zhu, M. et al., Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose‐response meta‐analysis of prospective cohort studies. BMJ (Clinical research ed.) 2014, 349, g4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Crowe, F. L. , Roddam, A. W. , Key, T. J. , Appleby, P. N. et al., Fruit and vegetable intake and mortality from ischaemic heart disease: results from the European Prospective Investigation into Cancer and Nutrition (EPIC)‐Heart study. Eur. Heart. J. 2011, 32, 1235–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. John, J. H. , Ziebland, S. , Yudkin, P. , Roe, L. S. et al., Effects of fruit and vegetable consumption on plasma antioxidant concentrations and blood pressure: a randomised controlled trial. Lancet 2002, 359, 1969–1974. [DOI] [PubMed] [Google Scholar]
- 8. Macready, A. L. , George, T. W. , Chong, M. F. , Alimbetov, D. S. et al., Flavonoid‐rich fruit and vegetables improve microvascular reactivity and inflammatory status in men at risk of cardiovascular disease–FLAVURS: a randomized controlled trial. Am. J. Nutr. 2014, 99, 479–489. [DOI] [PubMed] [Google Scholar]
- 9. McCall, D. O. , McGartland, C. P. , McKinley, M. C. , Patterson, C. C. et al., Dietary intake of fruits and vegetables improves microvascular function in hypertensive subjects in a dose‐dependent manner. Circulation. 2009, 119, 2153–2160. [DOI] [PubMed] [Google Scholar]
- 10. Holst, B. , Williamson, G. , Nutrients and phytochemicals: from bioavailability to bioefficacy beyond antioxidants. Curr. Opin. Biotechnol. 2008, 19, 73–82. [DOI] [PubMed] [Google Scholar]
- 11. Howes, M. J. , Simmonds, M. S. , The role of phytochemicals as micronutrients in health and disease. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 558–566. [DOI] [PubMed] [Google Scholar]
- 12. Ponzo, V. , Goitre, I. , Fadda, M. , Gambino, R. et al., Dietary flavonoid intake and cardiovascular risk: a population‐based cohort study. J. Transl. Med. 2015, 13, 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Dam, R. M. , Naidoo, N. , Landberg, R. , Dietary flavonoids and the development of type 2 diabetes and cardiovascular diseases: review of recent findings. Curr. Opin. Lipidol. 2013, 24, 25–33. [DOI] [PubMed] [Google Scholar]
- 14. Habauzit, V. , Morand, C. , Evidence for a protective effect of polyphenols‐containing foods on cardiovascular health: an update for clinicians. Ther. Adv. Chronic Dis. 2012, 3, 87–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hooper, L. , Kay, C. , Abdelhamid, A. , Kroon, P. A. et al., Effects of chocolate, cocoa, and flavan‐3‐ols on cardiovascular health: a systematic review and meta‐analysis of randomized trials. Am. J. Clin. Nutr. 2012, 95, 740–751. [DOI] [PubMed] [Google Scholar]
- 16. Hooper, L. , Kroon, P. A. , Rimm, E. B. , Cohn, J. S. et al., Flavonoids, flavonoid‐rich foods, and cardiovascular risk: a meta‐analysis of randomized controlled trials. Am. J. Clin. Nutr. 2008, 88, 38–50. [DOI] [PubMed] [Google Scholar]
- 17. EFSA , Scientific opinion on the substantiation of a health claim related to cocoa flavanols and maintenance of normal endothelium‐dependent vasodilatation pursuant to Article 13(5) of Regulation (EC) No 1924/200. EFSA J. 2012, 10, 2809. [Google Scholar]
- 18. Andriantsitohaina, R. , Auger, C. , Chataigneau, T. , Etienne‐Selloum, N. et al., Molecular mechanisms of the cardiovascular protective effects of polyphenols. Brit. J. Nutr. 2012, 108, 1532–1549. [DOI] [PubMed] [Google Scholar]
- 19. Declerck, K. , Szarc vel Szic, K. , Palagani, A. , Heyninck, K. et al., Epigenetic control of cardiovascular health by nutritional polyphenols involves multiple chromatin‐modifying writer‐reader‐eraser proteins. Curr. Topics Med. Chem. 2016, 16, 788–806. [DOI] [PubMed] [Google Scholar]
- 20. Garcia‐Conesa, M. T. , Dietary polyphenols against metabolic disorders: how far have we progressed in the understanding of the molecular mechanisms of action of these compounds? Crit. Rev. Food. Sci. Nutr. 2015, DOI: 10.1080/10408398.2014.980499. [DOI] [PubMed] [Google Scholar]
- 21. Ozdal, T. , Sela, D. A. , Xiao, J. , Boyacioglu, D. et al., The reciprocal interactions between polyphenols and gut microbiota and effects on bioaccessibility. Nutrients 2016, 8, 1–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Del Rio, D. , Rodriguez‐Mateos, A. , Spencer, J. P. , Tognolini, M. et al., Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxidants Redox Signaling 2013, 18, 1818–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maiani, G. , Caston, M. J. , Catasta, G. , Toti, E. et al., Carotenoids: actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol. Nutr. Food Res. 2009, 53(Suppl 2), S194–218. [DOI] [PubMed] [Google Scholar]
- 24. Verkerk, R. , Schreiner, M. , Krumbein, A. , Ciska, E. et al., Glucosinolates in Brassica vegetables: the influence of the food supply chain on intake, bioavailability and human health. Mol. Nutr. Food Res. 2009, 53(Suppl 2), S219. [DOI] [PubMed] [Google Scholar]
- 25. Manach, C. , Scalbert, A. , Morand, C. , Remesy, C. et al., Polyphenols: food sources and bioavailability. Am. J. Clinical Nutr. 2004, 79, 727–747. [DOI] [PubMed] [Google Scholar]
- 26. Duda‐Chodak, A. , Tarko, T. , Satora, P. , Sroka, P. , Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: a review. Eur. J. Nutr. 2015, 54, 325–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bolca, S. , Possemiers, S. , Maervoet, V. , Huybrechts, I. et al., Microbial and dietary factors associated with the 8‐prenylnaringenin producer phenotype: a dietary intervention trial with fifty healthy post‐menopausal Caucasian women. Brit. J. Nutr. 2007, 98, 950–959. [DOI] [PubMed] [Google Scholar]
- 28. Borel, P. , Desmarchelier, C. , Nowicki, M. , Bott, R. , Lycopene bioavailability is associated with a combination of genetic variants. Free Radic. Biol. Med. 2015, 83, 238–244. [DOI] [PubMed] [Google Scholar]
- 29. Borel, P. , Desmarchelier, C. , Nowicki, M. , Bott, R. et al., Interindividual variability of lutein bioavailability in healthy men: characterization, genetic variants involved, and relation with fasting plasma lutein concentration. Am. J. Clinical Nutr. 2014, 100, 168–175. [DOI] [PubMed] [Google Scholar]
- 30. Gasper, A. V. , Al‐Janobi, A. , Smith, J. A. , Bacon, J. R. et al., Glutathione S‐transferase M1 polymorphism and metabolism of sulforaphane from standard and high‐glucosinolate broccoli. Am. J. Clinical Nutr. 2005, 82, 1283–1291. [DOI] [PubMed] [Google Scholar]
- 31. Gonzalez‐Barrio, R. , Borges, G. , Mullen, W. , Crozier, A. , Bioavailability of anthocyanins and ellagitannins following consumption of raspberries by healthy humans and subjects with an ileostomy. J. Agricultural Food Chem. 2010, 58, 3933–3939. [DOI] [PubMed] [Google Scholar]
- 32. Kuijsten, A. , Arts, I. C. , Vree, T. B. , Hollman, P. C. , Pharmacokinetics of enterolignans in healthy men and women consuming a single dose of secoisolariciresinol diglucoside. J. Nutr. 2005, 135, 795–801. [DOI] [PubMed] [Google Scholar]
- 33. Li, F. , Hullar, M. A. , Beresford, S. A. , Lampe, J. W. , Variation of glucoraphanin metabolism in vivo and ex vivo by human gut bacteria. Brit. J. Nutr. 2011, 106, 408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rodriguez‐Mateos, A. , Cifuentes‐Gomez, T. , Gonzalez‐Salvador, I. et al., Influence of age on the absorption, metabolism, and excretion of cocoa flavanols in healthy subjects. Mol. Nutr. Food Res. 2015, 59, 1504–1512. [DOI] [PubMed] [Google Scholar]
- 35. Selma, M. V. , Romo‐Vaquero, M. , Garcia‐Villalba, R. , Gonzalez‐Sarrias, A. et al., The human gut microbial ecology associated with overweight and obesity determines ellagic acid metabolism. Food Funct. 2016, 7, 1769–1774. [DOI] [PubMed] [Google Scholar]
- 36. Setchell, K. D. , Brown, N. M. , Summer, S. , King, E. C. et al., Dietary factors influence production of the soy isoflavone metabolite s‐(‐)equol in healthy adults. J. Nutr. 2013, 143, 1950–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Setchell, K. D. , Cole, S. J. , Method of defining equol‐producer status and its frequency among vegetarians. J. Nutr. 2006, 136, 2188–2193. [DOI] [PubMed] [Google Scholar]
- 38. Song, K. B. , Atkinson, C. , Frankenfeld, C. L. , Jokela, T. et al., Prevalence of daidzein‐metabolizing phenotypes differs between Caucasian and Korean American women and girls. J. Nutr. 2006, 136, 1347–1351. [DOI] [PubMed] [Google Scholar]
- 39. Steck, S. E. , Gammon, M. D. , Hebert, J. R. , Wall, D. E. et al., GSTM1, GSTT1, GSTP1, and GSTA1 polymorphisms and urinary isothiocyanate metabolites following broccoli consumption in humans. J. Nutr. 2007, 137, 904–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tomas‐Navarro, M. , Vallejo, F. , Sentandreu, E. , Navarro, J. L. et al., Volunteer stratification is more relevant than technological treatment in orange juice flavanone bioavailability. J. Agricultural Food Chem. 2014, 62, 24–27. [DOI] [PubMed] [Google Scholar]
- 41. Wang, T. T. , Edwards, A. J. , Clevidence, B. A. , Strong and weak plasma response to dietary carotenoids identified by cluster analysis and linked to beta‐carotene 15,15'‐monooxygenase 1 single nucleotide polymorphisms. J. Nutritional Biochem. 2013, 24, 1538–1546. [DOI] [PubMed] [Google Scholar]
- 42. Faulks, R. M. , Southon, S. , Challenges to understanding and measuring carotenoid bioavailability. Biochim. Biophys. Acta 2005, 1740, 95–100. [DOI] [PubMed] [Google Scholar]
- 43. Borel, P. , Genetic variations involved in interindividual variability in carotenoid status. Mol. Nutr. Food Res. 2012, 56, 228–240. [DOI] [PubMed] [Google Scholar]
- 44. Ferrucci, L. , Perry, J. R. , Matteini, A. , Perola, M. et al., Common variation in the beta‐carotene 15,15'‐monooxygenase 1 gene affects circulating levels of carotenoids: a genome‐wide association study. Am. J. Hum. Genet. 2009, 84, 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Filipski, K. K. , Pacanowski, M. A. , Ramamoorthy, A. , Feero, W. G. et al., Dosing recommendations for pharmacogenetic interactions related to drug metabolism. Pharmacogenet Genomics 2016, 26, 334–339. [DOI] [PubMed] [Google Scholar]
- 46. Hewett, M. , Oliver, D. E. , Rubin, D. L. , Easton, K. L. et al., PharmGKB: the Pharmacogenetics Knowledge Base. Nucleic Acids Res. 2002, 30, 163–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cornelis, M. C. , El‐Sohemy, A. , Kabagambe, E. K. , Campos, H. , Coffee, CYP1A2 genotype, and risk of myocardial infarction. JAMA 2006, 295, 1135–1141. [DOI] [PubMed] [Google Scholar]
- 48. Palatini, P. , Ceolotto, G. , Ragazzo, F. , Dorigatti, F. et al., CYP1A2 genotype modifies the association between coffee intake and the risk of hypertension. J. Hypertension 2009, 27, 1594–1601. [DOI] [PubMed] [Google Scholar]
- 49. Riso, P. , Brusamolino, A. , Moro, M. , Porrini, M. , Absorption of bioactive compounds from steamed broccoli and their effect on plasma glutathione S‐transferase activity. Int. J. Food Sci. Nutr. 2009, 60(Suppl 1), 56–71. [DOI] [PubMed] [Google Scholar]
- 50. Lampe, J. W. , Interindividual differences in response to plant‐based diets: implications for cancer risk. Am. J. Clinical Nutr. 2009, 89, 1553S–1557S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Liu, Z. M. , Ho, S. C. , Chen, Y. M. , Liu, J. et al., Cardiovascular risks in relation to daidzein metabolizing phenotypes among Chinese postmenopausal women. PLoS One 2014, 9, e87861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang, X. , Gao, Y. T. , Yang, G. , Li, H. et al., Urinary isoflavonoids and risk of coronary heart disease. Internat. J. Epidemiol. 2012, 41, 1367–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hazim, S. , Curtis, P. J. , Schar, M. Y. , Ostertag, L. M. et al., Acute benefits of the microbial‐derived isoflavone metabolite equol on arterial stiffness in men prospectively recruited according to equol producer phenotype: a double‐blind randomized controlled trial. Am. J. Clinical Nutr. 2016, 103, 694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Clavel, T. , Desmarchelier, C. , Haller, D. , Gerard, P. et al., Intestinal microbiota in metabolic diseases: from bacterial community structure and functions to species of pathophysiological relevance. Gut Microbes 2014, 5, 544–551. [DOI] [PubMed] [Google Scholar]
- 55. Hullar, M. A. , Lancaster, S. M. , Li, F. , Tseng, E. et al., Enterolignan‐producing phenotypes are associated with increased gut microbial diversity and altered composition in premenopausal women in the United States. Cancer Epidemiol. Biomarkers Prev. 2015, 24, 546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Espin, J. C. , Larrosa, M. , Garcia‐Conesa, M. T. , Tomas‐Barberan, F. , Biological significance of urolithins, the gut microbial ellagic acid‐derived metabolites: the evidence so far. Evidence‐based Complementary Alternative Med. 2013, 2013, 270418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Landete, J. M. , Updated knowledge about polyphenols: functions, bioavailability, metabolism, and health. Crit. Rev. Food Sci. Nutr. 2012, 52, 936–948. [DOI] [PubMed] [Google Scholar]
- 58. Arumugam, M. , Raes, J. , Pelletier, E. , Le Paslier, D. et al., Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tomas‐Barberan, F. A. , Andres‐Lacueva, C. , Polyphenols and health: current state and progress. J. Agricultural Food Chem. 2012, 60, 8773–8775. [DOI] [PubMed] [Google Scholar]
- 60. Bolca, S. , Van de Wiele, T. , Possemiers, S. , Gut metabotypes govern health effects of dietary polyphenols. Curr. Opin. Biotechnol. 2013, 24, 220–225. [DOI] [PubMed] [Google Scholar]
- 61. Dellinger, R. W. , Garcia, A. M. , Meyskens, F. L., Jr. , Differences in the glucuronidation of resveratrol and pterostilbene: altered enzyme specificity and potential gender differences. Drug Metab. Pharmacokinet. 2014, 29, 112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mangoni, A. A. , Jackson, S. H. , Age‐related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br. J. Clin. Pharmacol. 2004, 57, 6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Moco, S. , Martin, F. P. , Rezzi, S. , Metabolomics view on gut microbiome modulation by polyphenol‐rich foods. J. Proteome Res. 2012, 11, 4781–4790. [DOI] [PubMed] [Google Scholar]
- 64. Kreijkamp‐Kaspers, S. , Kok, L. , Bots, M. L. , Grobbee, D. E. et al., Randomized controlled trial of the effects of soy protein containing isoflavones on vascular function in postmenopausal women. Am. J. Clinical Nutr. 2005, 81, 189–195. [DOI] [PubMed] [Google Scholar]
- 65. Ostertag, L. M. , Kroon, P. A. , Wood, S. , Horgan, G. W. et al., Flavan‐3‐ol‐enriched dark chocolate and white chocolate improve acute measures of platelet function in a gender‐specific way–a randomized‐controlled human intervention trial. Mol. Nutr. Food Res. 2013, 57, 191–202. [DOI] [PubMed] [Google Scholar]
- 66. Wilson, P. W. , D'Agostino, R. B. , Levy, D. , Belanger, A. M. et al., Prediction of coronary heart disease using risk factor categories. Circulation. 1998, 97, 1837–1847. [DOI] [PubMed] [Google Scholar]
- 67. Heiss, C. , Sansone, R. , Karimi, H. , Krabbe, M. et al., Impact of cocoa flavanol intake on age‐dependent vascular stiffness in healthy men: a randomized, controlled, double‐masked trial. Age (Dordr) 2015, 37, 9794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Fisher, N. D. , Hollenberg, N. K. , Aging and vascular responses to flavanol‐rich cocoa. J. Hypertension 2006, 24, 1575–1580. [DOI] [PubMed] [Google Scholar]
- 69. Egert, S. , Bosy‐Westphal, A. , Seiberl, J. , Kurbitz, C. et al., Quercetin reduces systolic blood pressure and plasma oxidised low‐density lipoprotein concentrations in overweight subjects with a high‐cardiovascular disease risk phenotype: a double‐blinded, placebo‐controlled cross‐over study. Brit. J. Nutr. 2009, 102, 1065–1074. [DOI] [PubMed] [Google Scholar]
- 70. Jones, P. J. , Inter‐individual variability in response to plant sterol and stanol consumption. J AOAC Int. 2015, 98, 724–728. [DOI] [PubMed] [Google Scholar]
- 71. Rideout, T. C. , Harding, S. V. , Mackay, D. S. , Metabolic and genetic factors modulating subject specific LDL‐C responses to plant sterol therapy. Canadian J. Physiol. Pharmacol. 2012, 90, 509–514. [DOI] [PubMed] [Google Scholar]
- 72. De Castro‐Oros, I. , Pampin, S. , Cofan, M. , Mozas, P. et al., Promoter variant ‐204A >C of the cholesterol 7alpha‐hydroxylase gene: association with response to plant sterols in humans and increased transcriptional activity in transfected HepG2 cells. Clin. Nutr. 2011, 30, 239–246. [DOI] [PubMed] [Google Scholar]
- 73. Boon, R. A. , Jae, N. , Holdt, L. , Dimmeler, S. , Long Noncoding RNAs: from clinical genetics to therapeutic targets? J. Am. College Cardiol. 2016, 67, 1214–1226. [DOI] [PubMed] [Google Scholar]
- 74. Samanta, S. , Balasubramanian, S. , Rajasingh, S. , Patel, U. et al., MicroRNA: a new therapeutic strategy for cardiovascular diseases. Trends Cardiovasc. Med. 2016, 26, 407–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Voelter‐Mahlknecht, S. , Epigenetic associations in relation to cardiovascular prevention and therapeutics. Clin. Epigenetics 2016, 8, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Milenkovic, D. , Jude, B. , Morand, C. , miRNA as molecular target of polyphenols underlying their biological effects. Free. Radic. Biol. Med. 2013, 64, 40–51. [DOI] [PubMed] [Google Scholar]
- 77. Crescenti, A. , Sola, R. , Valls, R. M. , Caimari, A. et al., Cocoa consumption alters the global DNA methylation of peripheral leukocytes in humans with cardiovascular disease risk factors: a randomized controlled trial. PLoS One 2013, 8, e65744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tome‐Carneiro, J. , Larrosa, M. , Yanez‐Gascon, M. J. , Davalos, A. et al., One‐year supplementation with a grape extract containing resveratrol modulates inflammatory‐related microRNAs and cytokines expression in peripheral blood mononuclear cells of type 2 diabetes and hypertensive patients with coronary artery disease. Pharmacol. Res. 2013, 72, 69–82. [DOI] [PubMed] [Google Scholar]
- 79. Corella, D. , Carrasco, P. , Sorli, J. V. , Estruch, R. et al., Mediterranean diet reduces the adverse effect of the TCF7L2‐rs7903146 polymorphism on cardiovascular risk factors and stroke incidence: a randomized controlled trial in a high‐cardiovascular‐risk population. Diabetes. Care. 2013, 36, 3803–3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Palmer, N. D. , Hester, J. M. , An, S. S. , Adeyemo, A. et al., Resequencing and analysis of variation in the TCF7L2 gene in African Americans suggests that SNP rs7903146 is the causal diabetes susceptibility variant. Diabetes. 2011, 60, 662–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Milenkovic, D. , Deval, C. , Dubray, C. , Mazur, A. et al., Hesperidin displays relevant role in the nutrigenomic effect of orange juice on blood leukocytes in human volunteers: a randomized controlled cross‐over study. PLoS One 2011, 6, e26669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Tome‐Carneiro, J. , Gonzalvez, M. , Larrosa, M. , Yanez‐Gascon, M. J. et al., Grape resveratrol increases serum adiponectin and downregulates inflammatory genes in peripheral blood mononuclear cells: a triple‐blind, placebo‐controlled, one‐year clinical trial in patients with stable coronary artery disease. Cardiovasc. Drugs Ther. 2013, 27, 37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Manach, C. , Hubert, J. , Llorach, R. , Scalbert, A. , The complex links between dietary phytochemicals and human health deciphered by metabolomics. Mol. Nutr. Food Res. 2009, 53, 1303–1315. [DOI] [PubMed] [Google Scholar]
- 84. Scalbert, A. , Brennan, L. , Manach, C. , Andres‐Lacueva, C. et al., The food metabolome: a window over dietary exposure. Am. J. Clinical Nutr. 2014, 99, 1286–1308. [DOI] [PubMed] [Google Scholar]
- 85. Franke, A. A. , Lai, J. F. , Halm, B. M. , Pagano, I. et al., Equol production changes over time in postmenopausal women. J. Nutritional Biochem. 2012, 23, 573–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Katz, D. L. , Meller, S. , Can we say what diet is best for health? Annu. Rev. Public. Health. 2014, 35, 83–103. [DOI] [PubMed] [Google Scholar]
- 87. Hall, J. N. , Moore, S. , Harper, S. B. , Lynch, J. W. , Global variability in fruit and vegetable consumption. Am. J. Preventive Med. 2009, 36, 402–409.e405. [DOI] [PubMed] [Google Scholar]
- 88. Bock, A. , Maragkoudakis, P. , Wollgast, J. , Caldeira, S. et al., Joint Research Center Foresight Study ‐ Tomorrow's Healthy Society ‐ Research Priorities for Foods and Diets (Final Report). Publications Office European Union 2014, 1–66, DOI: 10.2788/14108. [Google Scholar]


