Abstract
Aim
To assess the spatial‐temporal dynamics of primary succession following deglaciation in soil‐dwelling lichen communities.
Location
European Alps (Austria, Switzerland and Italy).
Methods
Five glacier forelands subjected to relevant glacier retreat during the last century were investigated. In each glacier foreland, three successional stages were selected at increasing distance from the glacier, corresponding to a gradient of time since deglaciation between 25 and 160 years. In each successional stage, soil‐dwelling lichens were surveyed within five 1 × 1 m plots. In addition to a classical ecological framework, based on species richness and composition, we applied a functional approach to better elucidate community assembly mechanisms.
Results
A positive relationship was found between species richness and time since deglaciation indicating that richer lichen communities can be found at increasing terrain ageing. This pattern was associated with compositional shifts, suggesting that different community assemblages can be found along the successional stages. The analysis of β‐diversity revealed a significant nested pattern of species assemblages along the gradient (i.e. earlier successional stages hosted a subset of the species already established in older successional stages), while the turnover component was less relevant. Considering functional groups, we found contrasting patterns in relation to time since deglaciation: the incidence of species with a cyanobacterial photobiont and those reproducing by spores decreased, while that of species reproducing by vegetative propagules increased.
Main conclusions
This study reveals that community assembly patterns of soil‐dwelling lichens in alpine glacier forelands are ruled by mechanisms of directional species accumulation and trait selection that involve a trade‐off between different functional strategies. Functional traits that reflect the dispersal and adaptation capability of the species underpin the colonization success of soil‐dwelling lichens in glacier forelands.
Keywords: dispersal traits, glacier forelands, photobiont type, primary succession, spatial‐temporal patterns, species accumulation, species richness and composition, trait selection, β‐diversity
Introduction
Glacial retreat is accelerating in most mountain regions of the world, and consequently, newly exposed land surfaces are available for biotic colonization (Pepin et al., 2015). In this framework, glacier forelands provide an interesting experimental case to better comprehend the spatial‐temporal dynamics of primary biological succession (e.g. Whittaker, 1993; Walther et al., 2002; Tscherko et al., 2005). Previous studies on plant dynamics indicated a directional pattern of increasing species richness along primary succession that is usually associated with compositional shifts (e.g. Raffl et al., 2006; Cannone et al., 2008). Most of these studies suggest that community dynamics are generally shaped by two main mechanisms (e.g. Jones & Henry, 2003; Caccianiga et al., 2006): (1) a directional replacement starting from species‐poor communities composed of few pioneer species, and (2) a directional species accumulation, leading to a nested community structure along the spatial‐temporal gradient.
A promising avenue to better comprehend community dynamics in primary succession involves the integration of functional traits with traditional approaches (e.g. Walker et al., 1986; Huston & Smith, 1987; Chapin et al., 1994; Caccianiga et al., 2006; Erschbamer & Mayer, 2011). Species functional traits are expected to directly link to environmental factors (Webb et al., 2010) providing mechanistic insights on the ecological processes ruling community assembly along environmental gradients (Diaz & Cabido, 2001; De Bello et al., 2013; Dainese et al., 2015). In particular, variation in species strategies throughout a succession is expected to be explained by a trade‐off between traits (Kneitel & Chase, 2004). For example, Löbel & Rydin (2010) suggested a trade‐off between dispersal and establishment ability on successful colonization of new habitats in bryophytes, indicating that species reproducing asexually have a higher ability to establish, but a lower long‐distance dispersal ability than species with sexual reproduction. Chapin et al. (1994) revealed that plant life history traits related to dispersal ability, as well as to nitrogen fixation, were critical to early succession dynamics. Similar results have been recently reported by Erschbamer & Mayer (2011), who elucidated the importance of several functional traits governing the colonization process and the progress of plant succession.
While many studies have focused on the dynamics of vascular plant communities in glacier retreat areas (e.g. Whittaker, 1993; Vetaas, 1994; Tscherko et al., 2005; Raffl et al., 2006; Cannone et al., 2008; Burga et al., 2010), a surprising knowledge gap still exists about soil‐dwelling lichens (but see e.g. Favero‐Longo et al., 2012). These organisms contribute considerably to the biodiversity of high elevation alpine environments (Nascimbene et al., 2012), underpinning relevant ecological functions and ecosystem services (Elbert et al., 2012; Zedda & Rambold, 2015). Lichens are a complex symbiotic system based on the interaction between a fungus (mycobiont) and a photosynthetic partner (photobiont), also hosting hyperdiverse microbial communities (Grube et al., 2009). The photobiont may improve the capacity of the species to establish and develop in extreme environments like glacier forelands (Haugland & Beatty, 2005), thus facilitating ecological succession (Breen & Lévesque, 2008). In particular, cyanobacterial photobionts can fix atmospheric nitrogen (Rikkinen, 2015) and hence contribute to the biogeochemical cycle of this element that is critical in newly exposed, nutrient‐poor soils (Schmidt et al., 2008). Besides the photobiont type, successful dispersal is the precondition for occupying new habitats (Scheidegger & Werth, 2009), such as newly exposed areas after glacier retreat. Lichens have contrasting reproduction strategies, i.e. sexual or asexual reproduction, that influence their dispersal and, consequently, colonization rates (Scheidegger & Werth, 2009; Johansson et al., 2012).
Here, we examined communities of soil‐dwelling lichens in five glacier forelands across the European Alps, along a gradient of distance from the glacier edge that reflected a chronosequence of the glacier forelands. In addition to a classical ecological framework, based on species richness and composition analyses, we applied a functional trait approach to better elucidate and interpret community assembly mechanisms. As substrate stability, nutrient availability, and time for colonization increase (Hodkinson et al., 2003; Bradley et al., 2014), we hypothesize an increasing species richness with succession time. We expect that this pattern would also be associated with compositional shifts according to the hypothesis that sites more recently deglaciated host a subset of the older communities. This should be reflected by a nested community pattern along the chronosequence gradient. Specifically, communities are expected to progressively recruit from a limited pool of effectively dispersed species (Hodkinson et al., 2003) that can rapidly reach and adapt under limiting environmental conditions onto newly available habitat patches. This pattern should be related to a trade‐off between dispersal and colonization ability of the species similar to that found in epiphytic bryophyte communities (Löbel & Rydin, 2010). Species with sexual reproduction are assumed to have better long‐distance dispersal and then should have more chances to rapidly reach recently deglaciated moraines (Löbel et al., 2006). Instead, species reproducing asexually are assumed to be better adapted to local dispersal suggesting that recently deglaciated moraines are poorly accessible for these species (Löbel et al., 2009). Hence, we expect a contrasting relationship between time since deglaciation and the two functional groups. Also, the photobiont may have a key role, mainly in the earlier colonization stages, mediating the adaptation of lichens on such extreme conditions. In this case, we expect that lichens with cyanobacterial photobionts would show a greater contribution to community assembly on more recently deglaciated sites due to their capability to fix atmospheric nitrogen that is crucial in nutrient‐poor, recently deglaciated soils.
Materials and methods
Study areas
Five glacier forelands distributed across Switzerland, Italy and Austria (central‐eastern Alps), which have been subjected to glacier retreat during the last century, were investigated (Fig. 1)
Figure 1.
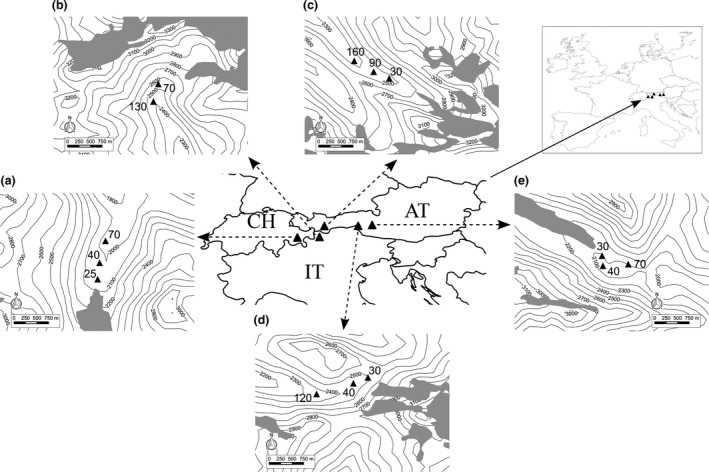
Location of the five glacier forelands distributed across Switzerland (CH), Italy (IT) and Austria (AT) in Central‐Eastern European Alps. For each glacier foreland, the three lichen sampling sites are marked with black triangles and a number indicating the age since deglaciation. Contour lines indicate elevation. Grey areas indicate the glaciers. Glacier forelands: (a) Morteratsch (Switzerland); (b) Matscherferner (Italy); (c) Gaisbergferner (Austria); (d) Rötkees (Italy); (e) Pasterze (Austria).
Morteratsch glacier – this is the third largest and by volume the most massive glacier in the central‐eastern Alps, situated in the Bernina Range in the canton Graubünden, Switzerland. Since 1878, the glacier has retreated almost continuously (average decrease between 1878 and 1998: 16.2 m per year), only interrupted by few periods of slight advances. The climate is continental, the bedrock consists of siliceous rocks, and the soils in the foreland area are weakly developed and have a maximum age of 150 years.
Matscherferner – this glacier is situated in the valley “Matschertal” in South Tyrol, Italy. This is a side valley of the Vinschgau (Val Venosta), running from south‐west to north‐east for about 20 km and covering an area of about 100 km2. The Matscherferner has retreated continuously in the period between 1927 and 2012 (total decrease of c. 570 m; average decrease between 1993 and 2012: 15 m per year). The climate is continental, the bedrock consists of siliceous rocks without calcareous rock, and thus the soils have an acidic character.
Gaisbergferner – this is a valley glacier situated in the valley “Gaisbergtal”, which is an alpine side valley of the Gurgler Tal in the Ötztal Alps of Tyrol, Austria. The glacier has retreated almost continuously for the last 70 years, only interrupted by a short period of slight increase in the 1980s (average decrease between 1969 and 2013: 7.2 m per year). The climate is continental, and the dominant rock types mainly include paragneiss, mica‐schists, amphibolites and marble.
Rötkees – this glacier is situated in the high valley “Röttal”, which runs from north‐west to south‐east and is a side valley of the Ahrntal (Valle Aurina) in South Tyrol, Italy. The Rötkees has retreated continuously with a total decrease of c. 300 m between 1932 and 2011 (average decrease between 1979 and 2011: 9.5 m per year). The climate is continental, and the bedrock of the glacier foreland mainly consists of lime‐containing mica‐schists.
Pasterze – with a current length of about 8 km, this is the longest glacier in the eastern Alps, situated within the High Tauern mountain range in Carinthia, Austria. The glacier has retreated almost continuously since the end of the Little Ice Age to 2014 with a total retreat of c. 2.2 km, only interrupted by few periods of stagnation or slight advances (average decrease between 1969 and 2013: 21.5 m per year). The Tauern valleys are relatively dry, even if the continental character of the climate becomes weaker with increasing elevation. The dominant rock types mainly include calcareous mica‐schists and green‐schists (= prasinite).
Further information on the study areas can be found in Bilovitz et al. (2014a,b,c, 2015a,b).
Sampling design
In each glacier foreland, three successional stages (sampling sites) were selected at increasing distance from the glacier on the main ground moraine structures, corresponding to a passage of time since deglaciation between 25 and 160 years (Table 1). The first successional stage corresponded to the distance/time since deglaciation at which the first lichen thallus was found. The second stage corresponded to the distance/time since deglaciation at which vegetation is still dominated by cryptogams, while the third stage was placed in areas dominated by vascular plants (grasses and forbs; small trees and shrubs were only present at Morteratsch). In Matscherferner, the stage closest to the glacier was omitted due to logistic constraints. In each successional stage, lichens on soil and plant debris or decaying terricolous bryophytes were surveyed within five 1 × 1 m plots that were selected in the field without any preconceptions on lichen communities. Areas with aquatic habitats and large rocks were avoided. Distance between plots was between 30 and 50 m. In each plot, we recorded the number of 10 × 10 cm subplots in which each species occurred. As local conditions could also contribute to community patterns (Rydgren et al., 2014), for each plot we recorded other meaningful predictors, such as elevation, slope, and aspect that were accounted for in the analyses.
Table 1.
Characteristics of the sampling sites in the five glacier forelands in the central‐eastern Alps, Europe. For each glacier foreland, the number of lichen species, the range of elevation, slope and aspect of the three sampling sites based on values recorded in the field are reported, as well as the distance (m) from the glacier edge and time (years) since deglaciation
| Glacier foreland | Number of lichen species | Elevation (m) | Slope (°) | Aspect (°) | Distance from the glacier (m)/time since deglaciation (y) | ||
|---|---|---|---|---|---|---|---|
| Site 1 | Site 2 | Site 3 | |||||
| Morteratsch (CH) 46°25′–26′N/09°55′–56''E | 15 | 1965–2020 | 0–20 | 0–140 | 300/25 | 800/40 | 1500/70 |
| Matscherferner (IT) 46°46′N/10°41′E | 34 | 2390–2560 | 5–40 | 90–180 | ‐ | 800/70 | 1400/130 |
| Gaisbergferner (AT) 46°50′N/11°02′–03′E | 41 | 2350–2460 | 5–40 | 0–330 | 600/30 | 1000/90 | 1500/160 |
| Rötkees (IT) 47°01′–02′N/12°10′–11′E | 32 | 2340–2490 | 7–30 | 150–310 | 300/30 | 600/40 | 1500/120 |
| Pasterze (AT) 47°04′N/12°44′–45′E | 35 | 2075–2090 | 0–40 | 80–300 | 600/30 | 1000/40 | 1300/70 |
CH, Switzerland; IT, Italy; AT, Austria.
Species identification and species traits
When possible, lichens were identified in the field. Additionally, in each plot we collected specimens of each species for a more accurate identification in the laboratory. The specimens were identified mainly with the aid of Wirth et al. (2013), using routine light microscopy techniques. Some of the identifications required verification using standardized thin‐layer chromatography (TLC), following the protocols of White & James (1985) and Orange et al. (2001). The nomenclature mainly follows Wirth et al. (2013). Reproductive trait and photobiont type of the species were retrieved from Nimis & Martellos (2008). In particular, species were classified according to their main reproduction strategy as (1) sexually reproducing by spores (size c. 10–100 μm), or (2) asexually reproducing by different types of vegetative propagules (size from 50–200 μm to centimetres). Species with cyanobacteria (cyanolichens) included both bipartite species in which cyanobacteria are the main photobiont and tripartite species in which cyanobacteria are secondary photobiont incorporated in specific structures (cephalodia).
Statistical analyses
Species richness
Linear mixed models (LMMs) were used to test the effect of the successional stage (time since deglaciation) and local conditions (elevation, slope, aspect) on plot‐level lichen species richness. Four response variables were tested: (1) total number of species, (2) number of species with cyanobacteria, (3) number of species reproducing by ascospores, and (4) number of species reproducing by vegetative diasporas. The nested sampling design was accounted for in the analysis by including the following random factors: glacier foreland identity and sampling site identity within glacier foreland. LMMs were implemented using the ‘lme4’ package in R (R Development Core Team 2015). We used an information‐theoretic model selection procedure to evaluate alternative competing models using second‐order Akaike's information criterion (AICc) (Burnham & Anderson, 2002). For each parameter, we used model averaging to incorporate model selection uncertainty into our parameter estimates (Burnham & Anderson, 2002). Individual predictor variables that had an Akaike weight > 0.75 or model‐averaged confidence intervals that did not include 0 were considered as the most important predictors. Model comparison was implemented using the ‘MuMIn’ package in R.
Species composition and β‐diversity
We applied canonical ordination in combination with forward selection to test whether the successional stage (time since deglaciation) and local site conditions affect variation in species composition based on species frequencies. For this analysis, we built two matrices: a species‐by‐plot matrix and a plot‐by‐environmental predictors matrix. As a preliminary detrended correspondence analysis (DCA) showed a total inertia 4 standard unit, unimodal ordination (CCA, canonical correspondence analysis) was considered suitable for the data set. To identify which explanatory variables significantly predict species composition, we applied a forward selection based on permutation tests (n = 999) and an inclusion threshold of α = 0.05 using the ordistep function in the ‘vegan’ package in R. A specific randomization scheme was applied to take into account the hierarchical structure of the sampling design by restricting permutations of samples to each glacier. All the explanatory variables recorded were included in the CCA diagram to provide an overview of the relationships.
We used the method by Baselga (2010) to estimate both the turnover and nestedness‐resultant components of β‐diversity. This method decomposes the overall β‐diversity (measured using the Sørensen dissimilarity index) into two additive fractions describing the species temporal turnover (βsim, the dissimilarity due to species replacement) and the variation in species composition due to richness difference in nested patterns (βnes). Pairwise β‐diversity measures were calculated using the ‘beta.pair’ function in the ‘betapart'package in R (Baselga & Orme, 2012). Then, we used regression on distance matrices (MRM) (Lichstein, 2007) to examine the relationships between the matrices of turnover and nestedness‐resultant dissimilarities and the Euclidean distance matrix of time since deglaciation (difference in time). We tested both linear and quadratic terms by permutation test (n = 9999). The MRM analysis was carried out with the MRM function in the ‘ecodist’ package in R (Goslee & Urban, 2007).
Because βnes is a measure of the compositional variation due to nestedness‐resultant dissimilarity rather than a true metric of nestedness (Baselga, 2012), we assessed whether its results were consistent with those provided by NODF (nestedness based on overlap and decreasing fill), an explicit metric of nestedness (Almeida‐Neto et al., 2008). NODF is based on the difference between columns (species) and rows (plots) and the paired matching of species occurrences (Almeida‐Neto et al., 2008). NODF analysis yields a nestedness score ranging from 0 (non‐nested) to 100 (perfectly nested) and can be computed between species (NODFspecies), sites (NODFsites) or both (NODF). We evaluated the statistic using a matrix with the plots (rows) ordered by increasing time since deglaciation and the species ordered by increasing frequencies. We tested the hypothesis that sampling sites more recently deglaciated host a subset of the species already established in sites where glacier retreat occurred over a longer period (‘directional process of species accumulation’), using only the NODFsites on the matrix described above. The significance of the observed nested pattern was tested against 999 null matrices using the null model described in Patterson & Atmar (1986) that maintains observed column totals but allows row totals to vary randomly. This null model preserves species richness per site but allows species composition to vary randomly and equiprobably (Ulrich & Gotelli, 2007). As bedrock type and geographic location may have a strong effect on lichen communities (i.e. the resulting turnover may be larger than that measured in each glacier foreland separately), we repeated the NODF analysis considering the five glaciers separately. NODF analysis was performed using the ‘vegan’ package in R.
Functional groups incidence
For each sampling plot, we computed the incidence (% of species) of the three selected functional groups: (1) species with cyanobacteria, (2) species reproducing by ascospores, and (3) species reproducing by vegetative propagules. Then, the incidence of the selected functional groups was used as the response variable in LMMs by including nested random structure (glacier foreland identity and site identity within glacier foreland). The main aim of these analyses was to test whether the proportion of lichens with different dispersal strategy of and cyanolichens changed along the successional stages of the spatial‐temporal gradient. We also accounted for the local site conditions in the models using the same information‐theoretic approach that was applied in the species richness model.
Results
In total, 85 lichen species were found (see Appendix S1 in Supporting Information). The total number of lichen species per glacier foreland ranged from 15 to 41 species (Table 1). The average number of species (mean ± SE) in the three successional stage was 7.0 ± 1.8 in earliest stages, 15.6 ± 3.0 in intermediate stages, and 27.2 ± 3.5 in oldest stages. At the plot level, the average number ± SE of species was instead 3.15 ± 0.36 in more recently deglaciated plots, 6.56 ± 0.58 in intermediate plots, and 11.28 ± 0.97 in the oldest plots. Total species richness was best predicted by time since deglaciation (summed Akaike weights, Σw i = 1.00), while other factors had low Σw i (< 0.60 and 95% CIs including zero) (Table 2). The same result was found for models considering the species richness of functional groups (Table 2). Overall, species richness increased with increasing time since deglaciation for all the functional groups (Fig. 2a–c).
Table 2.
Standardized model‐averaged regression coefficients (β) and unconditional 95% confidence intervals (CIs) derived from multi‐model inference analysis. The sum of model weights (Σw i) indicates the relative importance of covariate i based on summing weights across the entire model set. Values in bold indicate parameter estimates whose confidence intervals do not overlap zero
| Time | Elevation | Slope | Northness | |||||
|---|---|---|---|---|---|---|---|---|
| Σw i | β (CIs) | Σw i | β (CIs) | Σw i | β (CIs) | Σw i | β (CIs) | |
| Species richness | ||||||||
| All species | 1.00 | 0.752 (0.587, 0.917) | 0.25 | 0.081 (−0.277, 0.439) | 0.25 | 0.029 (−0.136, 0.193) | 0.51 | 0.108 (−0.032, 0.249) |
| With cynobacteria | 0.88 | 0.302 (0.067, 0.537) | 0.99 | 0.566 (0.292, 0.840) | 0.23 | 0.053 (−0.161, 0.267) | 0.24 | 0.022 (−0.175, 0.219) |
| Reproducing by ascospores | 1.00 | 0.676 (0.390, 0.961) | 0.24 | 0.097 (−0.384, 0.579) | 0.43 | −0.127 (−0.289, 0.035) | 0.26 | 0.051 (−0.095, 0.196) |
| Reproducing by vegetative diasporas | 1.00 | 0.709 (0.466, 0.952) | 0.24 | −0.074 (−0.540, 0.391) | 0.27 | −0.072 (−0.242, 0.098) | 0.36 | 0.091 (−0.056, 0.238) |
| Functional groups incidence | ||||||||
| With cynobacteria | 1.00 | −0.497 (−0.729, 0.266) | 0.25 | −0.065 (−0.398, 0.268) | 0.32 | −0.106 (−0.337, 0.124) | 0.24 | 0.024 (−0.181, 0.229) |
| Reproducing by ascospores | 0.93 | −0.345 (−0.593, −0.096) | 0.32 | 0.159 (−0.160, 0.477) | 0.24 | 0.005 (−0.258, 0.268) | 0.25 | 0.042 (−0.185, 0.269) |
| Reproducing by vegetative diasporas | 0.99 | 0.435 (0.197, 0.673) | 0.36 | −0.212 (−0.565, 0.142) | 0.24 | −0.034 (−0.275, 0.207) | 0.27 | −0.060 (−0.273, 0.153) |
Figure 2.
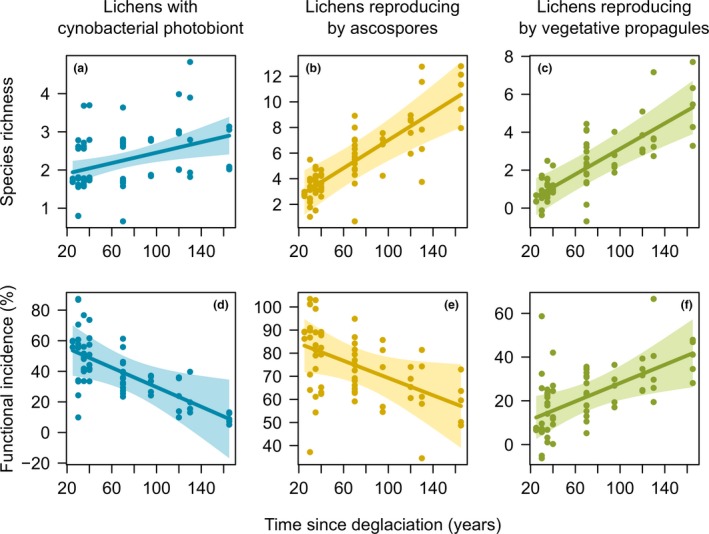
Model estimates and 95% confidence interval from linear mixed models testing time since deglaciation on (a–c) species richness and (d–f) functional group incidence for lichens (a, d) with cynobacterial photobiont, (b, e) reproducing by ascopores, and (c, f) reproducing by vegetative propagules separately, in soil‐dwelling lichen communities in five glacier forelands of the European Alps. The points represent the 70 plots and show the partial residuals from the best plausible model.
The forward selection confirmed that species composition was significantly affected by time since deglaciation (P < 0.05) while local site conditions did not show any significant effect (P > 0.05). In the CCA ordination diagram, the community assemblages differed along the spatial‐temporal gradient (Fig. 3, Appendix S2). Species turnover (βsim) showed a weak negative association with difference in time since deglaciation (MRM, P = 0.021, R 2 = 0.02) (Fig. 4a). On the contrary, we found a strong positive relationship between compositional dissimilarity due to nestedness (βnes) and difference in time since deglaciation (MRM, P < 0.001, R 2 = 0.10), i.e. βnes is larger between plots with different successional stages than between plots with similar time since deglaciation (Fig. 4b). Nestedness analysis confirmed a significant nested pattern of species assemblages (NODFsites = 44.106; P = 0.001) along the gradient. The same result was found considering the five glaciers separately (Morteratsch, NODFsites = 68.079; P = 0.001; Matscherferner, NODFsites = 44.815; P = 0.001; Gaisbergferner, NODFsites = 51.798; P = 0.001; Rötkees, NODFsites = 41.367; P = 0.001; Pasterze, NODFsites = 59.462; P = 0.001). This analysis indicated that earlier successional stages host a subset of the species already established in older successional stages. In particular, the average proportion (mean ± SD) of the species found in the earliest stages also occurred in the intermediate (74.5 ± 20%) and oldest stages (75.3 ± 23%) of the same glacier, respectively (differences not significant), while 76 ± 18% of those occurring in the intermediate stages were also found in the oldest ones.
Figure 3.
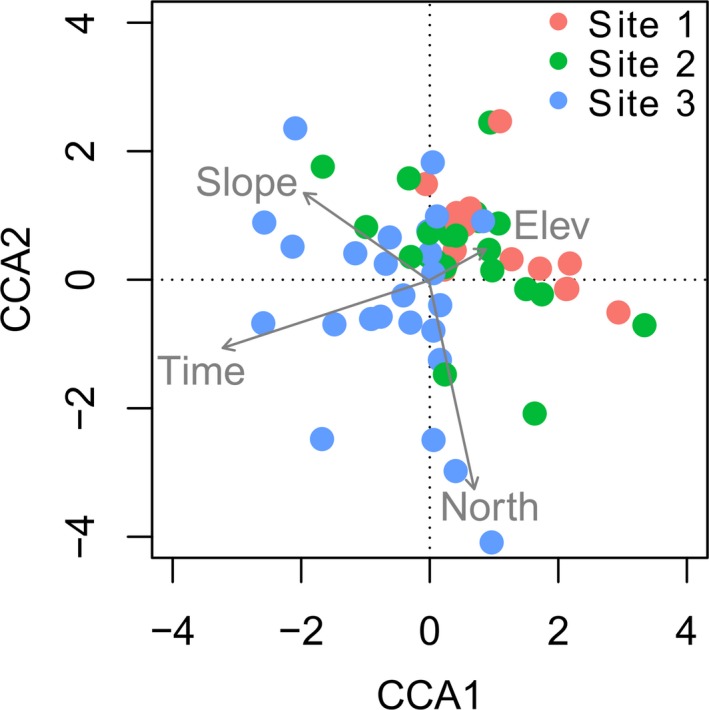
CCA ordination diagram of the 70 plots of soil‐dwelling lichen communities in glacier forelands of the European Alps against the first two canonical axes. Site scores are grouped according to their site position in the five glacier forelands (see Table 1). Axes 1 and 2 explain 44.0% and 27.4% of the total variance, respectively.
Figure 4.
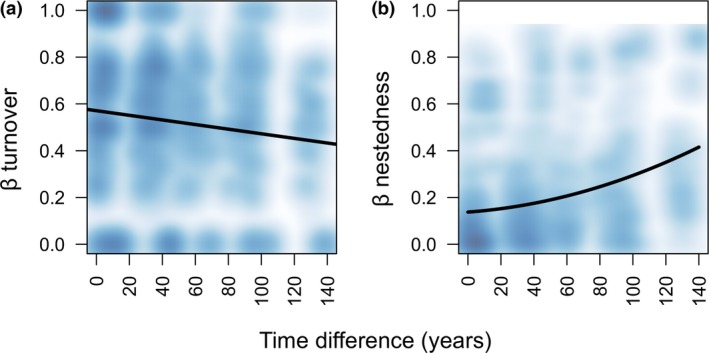
Scatterplot of the relationship for (a) species turnover and (b) nestedness‐resultant dissimilarity versus difference in time since deglaciation in soil‐dwelling lichen communities of five glacier forelands of the European Alps. The fitted line indicates a linear or quadratic regression on distance matrices regression (MRM). The scatterplot is a smoothed shade density representation obtained through a kernel density (function ‘smoothScatter’ in the ‘graphics’ package in R).
Considering functional groups, all the models were best predicted by time since deglaciation (Σw i ≥ 0.90), while local conditions had low Σw i (< 0.50) (Table 2). However, we found contrasting patterns in relation to time since deglaciation. The incidence of species with cyanobacterial photobiont and those reproducing by ascospores (good dispersers over long distances) decreased with time since deglaciation (Fig. 2d–e), while that of species reproducing by vegetative propagules (poor dispersers over long distances and good colonization ability) had a positive trend, increasing with time since deglaciation (Fig. 2f).
Discussion
The results confirm our initial hypotheses that community assembly patterns in glacier forelands of the European Alps are mainly ruled by mechanisms of directional species accumulation and trait selection that involve a trade‐off between different functional strategies (Fig. 5). Communities recruit from a pool of effectively dispersed species (Hodkinson et al., 2003) that can rapidly reach and colonize recently deglaciated moraines.
Figure 5.
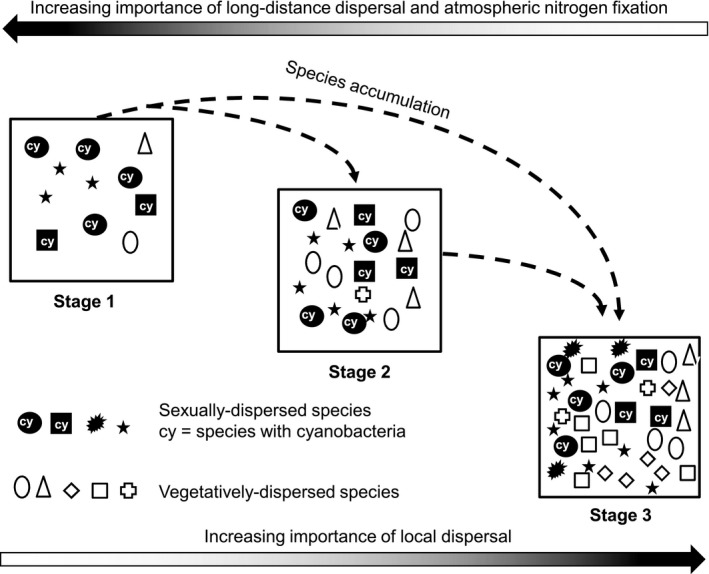
Potential mechanisms that shape the soil‐dwelling lichen assemblages in glacier forelands of the European Alps. Changes of community richness and composition are likely mainly ruled by a directional mechanism of species accumulation reflecting a nested pattern. Our results indicate that species turnover plays only a minor role. The contrasting patterns of spore‐dispersed and vegetatively dispersed species suggest a decreasing importance of dispersal over local recruitment moving from younger to older moraines. On the other hand, such pattern suggests a better establishment ability for species reproducing asexually along the gradient. The pattern of lichens with cyanobacterial photobionts suggests an increasing importance of the nitrogen fixation capacity in younger moraines.
Species richness and composition
We observed a positive species‐time since deglaciation relationship indicating that richer lichen communities can be found at increasing terrain ageing. This relationship is similar to that described in alpine forest ecosystems along a chronosequence of forest age (Nascimbene et al., 2010) and likely reflects a combined effect of time per se (i.e. time available for colonization) and chemical–physical changes in substrate conditions associated with terrain ageing (e.g. increased surface availability, substrate stability, nutrient availability). To disentangle the effects of these factors is out of the scope of this study and would require a different design with contrasting substrate conditions at the same age.
As in forest succession (e.g. Nascimbene et al., 2010), the increase of species richness along the age gradient is associated with compositional shifts, showing that different community assemblages of soil‐dwelling lichens can be found along the gradient of time since deglaciation. However, our results indicate that community assembly is mainly shaped according to a nested structure along the gradient, in which sites more recently deglaciated host a subset of the species established in sites where glacier retreat occurred over a longer period. The contribution of species turnover to community assembly pattern seems to be less relevant, as indicated by our results on β‐diversity. Species turnover could gain importance when local habitat conditions are more heterogeneous (Nascimbene et al., 2013).
Functional groups
As expected, the analysis of functional groups further fostered a more mechanistic view of community dynamics. While the number of species of each functional group reflects the positive species‐richness time since deglaciation relationship (see previous paragraph), the directional pattern of reproduction strategy and photobiont type incidence along the gradient of time since deglaciation supports the hypothesis that community dynamics are mediated by trait selection and involve both the dispersal capability and the adaptive response of the species to environmental conditions related to glacier retreat dynamics. In particular, the contrasting pattern of the functional groups incidence for spore‐dispersed and vegetatively dispersed species supports the hypothesis of a trade‐off between dispersal and colonization ability also for soil‐dwelling lichens. These patterns suggest a decreasing importance of long‐range dispersal over local recruitment moving from younger to older terrains (Marini et al., 2014; Jones et al., 2015) and an increasing importance of establishment ability of species along the gradient. The pattern of lichens incidence with cyanobacterial photobionts suggests instead a greater importance of the nitrogen fixation capacity in younger terrains, reflecting an adaptation to extreme substrate conditions.
Considering lichen dispersal capability, our results indicate that species dispersed by spores seem to have more chances to reach recently deglaciated moraines rapidly. Lichens produce thousands of small ascospores (rough length range 10–100 micron) that are easily dispersed. Once the spores arrive on these newly available substrates, the formation of the lichen symbiosis can be sustained by the diverse communities of cyanobacteria and green algae, already established in these primitive environments (Schmidt et al., 2008; Frey et al., 2013). Conversely, recently deglaciated moraines are poorly accessible for dispersal‐limited species, such as lichen species reproducing by vegetative propagules. This group of lichens is more effective in local dispersal (Löbel et al., 2009), given that the relatively small number and the large size of these propagules can limit long‐distance dispersal (Walser, 2004; Scheidegger & Werth, 2009). On the other hand, vegetative propagules seem to have a higher probability of successful colonization than sexual spores (but see Wornik & Grube, 2010) since the photobiont is dispersed with the fungus. Spores arriving on a new substrate must acquire a compatible photobiont to re‐establish the lichen symbiosis. Also, vegetative propagules are probably less adapted to persist in harsh conditions (Nimis & Martellos, 2003), showing a better adaptation to older, more stable sites. These findings highlight the potential for a rapid recruitment of new thalli for species reproducing by vegetative propagules that is likely to allow these species to successfully compete for space with vascular plants in the late phases of the lichen succession.
Considering the photobiont type, our results indicate that lichens with cyanobacteria are particularly adapted to develop viable populations on recently deglaciated sites. Indeed, their capability to fix atmospheric nitrogen, coupled with high rates of carbon fixation (Rikkinen, 2015), emphasises their functional role in acquiring nutrients and, therefore, facilitating ecological succession in glacier forelands (Breen & Lévesque, 2008). An example is the tripartite lichen Stereocaulon alpinum, the most abundant species found in our recently deglaciated sites. This species likely plays a key role in transforming the local habitat conditions due to the high rates of metabolic activity of cyanobacterial photobionts encapsulated in external cephalodia (Rikkinen, 2015).
Conclusions
The nested structure of lichen communities found along our spatial‐temporal gradient corroborates the hypothesis that species accumulation, rather than species replacement, shape lichen community assembly in glacier forelands of the Alps. Moreover, this study reveals that functional groups, reflecting the dispersal and adaptation capability of the species, underpin the colonization success and community assembly patterns of soil‐dwelling lichens in alpine glacier forelands. In particular, a crucial role in colonization dynamics is played by the photobiont, especially in the early stage of establishment, supporting the view that this trait is among the most relevant in mediating the response of lichens to environmental factors (Marini et al., 2011; Matos et al., 2015). While this study contributes to fill the knowledge gap on primary successions of soil‐dwelling lichens in alpine glacier forelands, we are aware that further research is needed to better clarify community dynamics and the role of species functional traits. For example, similarly to studies on epiphytic lichens (Löbel et al., 2006, 2009), a meta‐population approach could help to better elucidate the roles of dispersal and photobionts in shaping lichen dynamics in these environments.
Biosketch
Juri Nascimbene is a plant ecologist with a strong focus on conservation biology in terrestrial ecosystems. He investigates the impact of local and climatic factors on lichen diversity in forest and alpine ecosystems. His research interests in biogeography include the study of lichen diversity patterns along wide environmental gradients.
Supporting information
Appendix S1 List of the species.
Appendix S2 CCA ordination diagram.
Acknowledgements
This study was performed as part of the project “Lichens of the Alps: diversity and climate change” funded by the Austrian Science Fund (FWF project P25078‐B16). We would like to thank the following individuals and institutions for their support: Martin Aebli, Teuvo Ahti, Benno Baumgarten, Othmar Breuss, Roberto Dinale, Brigitta Erschbamer, Josef Hafellner, Anja Hüsler, Peter Kosnik, Eleonore Mayrhofer, Raquel Pino‐Bodas, Christian Scheuer, Herwig Teppner, Günther Unterthiner, Elisa Varolo, Vito Zingerle, the Amt für Geologie und Baustoffprüfung and the Hydrographisches Amt Autonome Provinz Bozen, the administration of the National Park High Tauern Carinthia, the Amt der Kärntner Landesregierung (Abteilung 8), the Bezirkshauptmannschaft Spittal an der Drau (Bereich 10) and the University of Graz. Special thanks for Veronika Tutzer and Anja Wallner.
Editor: Hans‐Peter Comes
References
- Almeida‐Neto, M. , Guimarães, P. , Guimarães, P.R. Jr , Loyola, R.D. & Ulrich, W. (2008) A consistent metric for nestedness analysis in ecological systems: reconciling concept and measurement. Oikos, 117, 1227–1239. [Google Scholar]
- Baselga, A. (2010) Partitioning the turnover and nestedness components of beta diversity. Global Ecology and Biogeography, 19, 134–143. [Google Scholar]
- Baselga, A. (2012) The relationship between species replacement, dissimilarity derived from nestedness, and nestedness. Global Ecology and Biogeography, 21, 1223–1232. [Google Scholar]
- Baselga, A. & Orme, C.D.L. (2012) betapart: an R package for the study of beta diversity. Methods in Ecology and Evolution, 3, 808–812. [Google Scholar]
- Bilovitz, P.O. , Nascimbene, J. , Tutzer, V. , Wallner, A. & Mayrhofer, H . (2014a) Terricolous lichens in the glacier forefield of the Rötkees (Eastern Alps, South Tyrol, Italy). Phyton, (Horn, Austria), 54, 245–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilovitz, P.O. , Tutzer, V. , Wallner, A. , Nascimbene, J. & Mayrhofer, H. (2014b) Terricolous lichens in the glacier forefield of the Matscherferner (Eastern Alps, South Tyrol, Italy). Acta Zoo Bot Austria, 150/151, 197–202. [PMC free article] [PubMed] [Google Scholar]
- Bilovitz, P.O. , Wallner, A. , Tutzer, V. , Nascimbene, J. & Mayrhofer, H . (2014c) Terricolous lichens in the glacier forefield of the Gaisbergferner (Eastern Alps, Tyrol, Austria). Phyton, (Horn, Austria), 54, 235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilovitz, P.O. , Nascimbene, J. & Mayrhofer, H . (2015a) Terricolous lichens in the glacier forefield of the Morteratsch glacier (Eastern Alps, Graubünden, Switzerland). Phyton, (Horn, Austria), 55, 193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilovitz, P.O. , Wallner A., Tutzer V. & Nascimbene J., Mayrhofer H . (2015b) Terricolous lichens in the glacier forefield of the Pasterze (Eastern Alps, Carinthia, Austria). Phyton, (Horn, Austria), 55, 201–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley, J.A. , Singarayer, J.S. & Anesio, A.M. (2014) Microbial community dynamics in the forefield of glaciers. Proceedings of the Royal Society B: Biological Sciences, 281, 20140882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breen, K. & Lévesque, E. (2008) The influence of biological soil crusts on soil characteristics along a high arctic glacier foreland, Nunavut, Canada. Arctic, Antarctic, and Alpine Research, 40, 287–297. [Google Scholar]
- Burga, C.A. , Krüsi, B. , Egli, M. , Wernli, M. , Elsener, S. , Ziefle, M. , Fischer, T. & Mavris, C. (2010) Plant succession and soil development on the foreland of the Morteratsch glacier (Pontresina, Switzerland): straight forward or chaotic? Flora, 205, 561–576. [Google Scholar]
- Burnham, K. & Anderson, D. (2002) Model selection and multimodel inference: a practical information‐theoretic approach. Springer‐Verlag, New York. [Google Scholar]
- Caccianiga, M. , Luzzaro, A. , Pierce, S. , Ceriani, R.M. & Cerabolini, B. (2006) The functional basis of a primary succession resolved by CSR classification. Oikos, 112, 10–20. [Google Scholar]
- Cannone, N. , Diolaiuti, G. , Guglielmin, M. & Smiraglia, C. (2008) Accelerating climate change impacts on alpine glacier forefield ecosystems in the European Alps. Ecological Applications, 18, 637–648. [DOI] [PubMed] [Google Scholar]
- Chapin, F.S., III , Walker, L.R. , Fastie, C.L. & Sharman, L.C. (1994) Mechanisms of primary succession following deglaciation at Glacier Bay, Alaska. Ecological Monographs, 64, 149–175. [Google Scholar]
- Dainese, M. , Lepš, J. & de Bello, F. (2015) Different effects of elevation, habitat fragmentation and grazing management on the functional, phylogenetic and taxonomic structure of mountain grasslands. Perspectives in Plant Ecology, Evolution and Systematics, 17, 44–53. [Google Scholar]
- de Bello, F. , Lavorel, S. , Lavergne, S. , Albert, C.H. , Boulangeat, I. , Mazel, F. & Thuiller, W. (2013) Hierarchical effects of environmental filters on the functional structure of plant communities: a case study in the French Alps. Ecography, 36, 393–402. [Google Scholar]
- Diaz, S. & Cabido, M. (2001) Vive la difference: plant functional diversity matters to ecosystem processes. Trends in Ecology and Evolution, 16, 646–655. [Google Scholar]
- Elbert, W. , Weber, B. , Burrows, S. , Steinkamp, J. , Büdel, B. , Andreae, M.O. & Pöschl, U. (2012) Contribution of cryptogamic covers to the global cycles of carbon and nitrogen. Nature Geoscience, 5, 459–462. [Google Scholar]
- Erschbamer, B. & Mayer, R. (2011) Can successional species groups be discriminated based on their life history traits? A study from a glacier foreland in the Central Alps. Plant Ecology & Diversity, 4, 341–351. [Google Scholar]
- Favero‐Longo, S.E. , Worland, M.R. , Convey, P. , Lewis Smith, R.I. , Piervittori, R. , Guglielmin, M. & Cannone, N. (2012) Primary succession of lichen and bryophyte communities following glacial recession on Signy Island, South Orkney Islands, Maritime Antarctic. Antarctic Science, 24, 323–336. [Google Scholar]
- Frey, B. , Bühler, L. , Schmutz, S. , Zumsteg, A. & Furrer, G. (2013) Molecular characterization of phototrophic microorganisms in the forefield of a receding glacier in the Swiss Alps. Environmental Research Letters, 8, 015033. [Google Scholar]
- Goslee, S.C. & Urban, D.L. (2007) The ecodist package for dissimilarity‐based analysis of ecological data. Journal of Statistical Software, 22, 1–19. [Google Scholar]
- Grube, M. , Cardinale, M. , de Castro, Vieira , Jr, J. , Müller, H. & Berg, G. (2009) Species‐specific structural and functional diversity of bacterial communities in lichen symbioses. The ISME Journal, 3, 1105–1115. [DOI] [PubMed] [Google Scholar]
- Hanski, I. (1982) Dynamics of regional distribution: the core and satellite species hypothesis. Oikos, 38, 210–221. [Google Scholar]
- Haugland, J.E. & Beatty, S.W. (2005) Vegetation establishment, succession and microsite frost disturbance on glacier forelands within patterned ground chronosequences. Journal of Biogeography, 32, 145–153. [Google Scholar]
- Hodkinson, I.D. , Coulson, S.J. & Webb, N.R. (2003) Community assembly along proglacial chronosequences in the high Arctic: vegetation and soil development in north‐west Svalbard. Journal of Ecology, 91, 651–663. [Google Scholar]
- Huston, M. & Smith, T. (1987) Plant succession: life history and competition. The American Naturalist, 130, 168–198. [Google Scholar]
- Johansson, V. , Ranius, T. & Snäll, T. (2012) Epiphyte metapopulation dynamics are explained by species traits, connectivity, and patch dynamics. Ecology, 93, 235–241. [DOI] [PubMed] [Google Scholar]
- Jones, G.A. & Henry, G.H.R. (2003) Primary plant succession on recently deglaciated terrain in the Canadian High Arctic. Journal of Biogeography, 30, 277–296. [Google Scholar]
- Jones, N.T. , Germain, R.M. , Grainger, T.N. , Hall, A. , Baldwin, L. & Gilbert, B. (2015) Dispersal mode mediates the effect of patch size and patch connectivity on metacommunity diversity. Journal of Ecology, 103, 935–944. [Google Scholar]
- Kneitel, J.M. & Chase, J.M. (2004) Trade‐offs and community ecology: linking spatial scales and species coexistence. Ecology Letters, 7, 69–80. [Google Scholar]
- Lichstein, J.W. (2007) Multiple regression on distance matrices: a multivariate spatial analysis tool. Plant Ecology, 188, 117–131. [Google Scholar]
- Löbel, S. & Rydin, H. (2010) Trade‐offs and habitat constraints in the establishment of epiphytic bryophytes. Functional Ecology, 24, 887–897. [Google Scholar]
- Löbel, S. , Snäll, T. & Rydin, H. (2006) Metapopulation processes in epiphytes inferred from patterns of regional distribution and local abundance in fragmented forest landscapes. Journal of Ecology, 94, 856–868. [Google Scholar]
- Löbel, S. , Snäll, T. & Rydin, H. (2009) Mating system, reproduction mode and diaspore size affect metacommunity diversity. Journal of Ecology, 97, 176–185. [Google Scholar]
- Marini, L. , Nascimbene, J. & Nimis, P.L. (2011) Large‐scale patterns of epiphytic lichen species richness: photobiont‐dependent response to climate and forest structure. Science of the Total Environment, 409, 4381–4386. [DOI] [PubMed] [Google Scholar]
- Marini, L. , Öckinger, E. , Bergman, K.‐O. , Jauker, B. , Krauss, J. , Kuussaari, M. , Pöyry, J. , Smith, H.G. , Steffan‐Dewenter, I. & Bommarco, R. (2014) Contrasting effects of habitat area and connectivity on evenness of pollinator communities. Ecography, 37, 544–551. [Google Scholar]
- Matos, P. , Pedro, P. , Gregorio, A. , Isabel, M. , Alice, N. , Amilcar, S. & Cristina, B. (2015) Lichen traits responding to aridity. Journal of Ecology, 103, 451–458. [Google Scholar]
- Nascimbene, J. & Marini, L. (2015) Epiphytic lichen diversity along elevational gradients: biological traits reveal a complex response to water and energy. Journal of Biogeography, 42, 1222–1232. [Google Scholar]
- Nascimbene, J. , Marini, L. & Nimis, P.L. (2010) Epiphytic lichen diversity in old‐growth and managed Picea abies stands in Alpine spruce forests. Forest Ecology and Management, 260, 603–609. [Google Scholar]
- Nascimbene, J. , Thor, G. & Nimis, P.L. (2012) Habitat types and lichen conservation in the Alps: perspectives from a case study in the Stelvio National Park (Italy). Plant Biosystems, 146, 428–442. [Google Scholar]
- Nascimbene, J. , Benesperi, R. , Brunialti, G. , Catalano, I. , Dalle Vedove, M. , Grillo, M. et al (2013) Patterns and drivers of β‐diversity and similarity of Lobaria pulmonaria communities in Italian forests. Journal of Ecology, 101, 493–505. [Google Scholar]
- Nimis, P.L. & Martellos, S. (2003) On the ecology of sorediate lichens in Italy. Bibliotheca Lichenologica, 86, 393–406. [Google Scholar]
- Nimis, P. & Martellos, S . (2008) ITALIC–The Information System on Italian Lichens. Version 4.0. University of Trieste, Department of Biology, IN4. 0/1. http://dbiodbs.univ.trieste.it/
- Orange, A. , James, P.W. & White, F.J. (2001) Microchemical methods for the identification of Lichens. British Lichen Society, London. [Google Scholar]
- Patterson, B.D. & Atmar, W. (1986) Nested subsets and the structure of insular mammalian faunas and archipelagos. Biological Journal of the Linnean Society, 28, 65–82. [Google Scholar]
- Pepin, N. , Bradley, R.S. , Diaz, H.F. et al (2015) Elevation‐dependent warming in mountain regions of the world. Nature Climate Change, 5, 424–430. [Google Scholar]
- R Development Core Team . (2015) R: a language and environment for statistical computing. R Foundation for Statistical Computing.
- Raffl, C. , Mallaun, M. , Mayer, R. & Erschbamer, B. (2006) Vegetation succession pattern and diversity changes in a glacier valley, central Alps, Austria. Arctic, Antarctic, and Alpine Research, 38, 421–428. [Google Scholar]
- Rees, M. , Condit, R. , Crawley, M. , Pacala, S. & Tilman, D. (2001) Long‐term studies of vegetation dynamics. Science, 293, 650–655. [DOI] [PubMed] [Google Scholar]
- Rikkinen, J. (2015) Cyanolichens. Biodiversity and Conservation, 24, 973–993. [Google Scholar]
- Rydgren, K. , Halvorsen, R. , Töpper, J.P. & Njøs, J.M. (2014) Glacier foreland succession and the fading effect of terrain age. Journal of Vegetation Science, 25, 1367–1380. [Google Scholar]
- Scheidegger, C. & Werth, S. (2009) Conservation strategies for lichens: insights from population biology. Fungal Biology Reviews, 23, 55–66. [Google Scholar]
- Schmidt, S.K. , Reed, S.C. , Nemergut, D.R. , Grandy, A.S. , Cleveland, C.C. , Weintraub, M.N. , Hill, A.W. , Costello, E.K. , Meyer, A.F. , Neff, J.C. & Martin, A.M. (2008) The earliest stages of ecosystem succession in high‐elevation (5000 metres above sea level), recently deglaciated soils. Proceedings of the Royal Society, Series B – Biological Sciences, 275, 2793–2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tscherko, D. , Hammesfahr, U. , Zeltner, G. , Kandeler, E. & Böcker, R. (2005) Plant succession and rhizosphere microbial communities in a recently deglaciated alpine terrain. Basic and Applied Ecology, 6, 367–383. [Google Scholar]
- Ulrich, W. & Gotelli, N.J. (2007) Null model analysis of species nestedness patterns. Ecology, 88, 1824–1831. [DOI] [PubMed] [Google Scholar]
- Vetaas, O.R. (1994) Primary succession of plant assemblages on a glacier foreland‐Bodalsbreen, southern Norway. Journal of Biogeography, 21, 297–308. [Google Scholar]
- Walker, L.R. , Zasada, J.C. & Chapin, F.S., III (1986) The role of life history processes in primary succession a an Alaskan floodplain. Ecology, 67, 1243–1253. [Google Scholar]
- Walser, J.‐C. (2004) Molecular evidence for limited dispersal of vegetative propagules in the epiphytic lichen Lobaria pulmonaria. American Journal of Botany, 91, 1273–1276. [DOI] [PubMed] [Google Scholar]
- Walther, G. , Post, E. , Convey, P. , Menzel, A. , Parmesan, C. , Beebee, T.J.C. et al (2002) Ecological responses to recent climate change. Nature, 416, 389–395. [DOI] [PubMed] [Google Scholar]
- Webb, C.T. , Hoeting, J.A. , Ames, G.M. , Pyne, M.I. & Poff, N.L. (2010) A structured and dynamic framework to advance traits‐based theory and prediction in ecology. Ecology Letters, 13, 267–283. [DOI] [PubMed] [Google Scholar]
- White, F.J. & James, P.W. (1985) A new guide to microchemical techniques for the identification of lichen substances. Bulletin of the British Lichen Society, 57, 1–41. [Google Scholar]
- Whittaker, R.J. (1993) Plant population patterns in a glacier foreland succession: pioneer herbs and later‐colonizing shrubs. Ecography, 16, 117–136. [Google Scholar]
- Wirth, V. , Hauck, M. & Schultz, M. (2013) Die Flechten Deutschlands. Ulmer, Stuttgart. [Google Scholar]
- Wornik, S. & Grube, M. (2010) Joint dispersal does not imply maintenance of partnerships in lichen symbioses. Microbial Ecology, 59, 150–7. [DOI] [PubMed] [Google Scholar]
- Zedda, L. & Rambold, G . (2015) The diversity of lichenised fungi: ecosystem functions and ecosystem services Recent advances in Lichenology (eds Upreti D.K., Divakar P.K., Shukla V. and Bajpai R.), pp. 121–145. Springer India, New Delhi. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 List of the species.
Appendix S2 CCA ordination diagram.


