Abstract
Among the diverse experimental vaccines evaluated in various animal lentivirus models, live attenuated vaccines have proven to be the most effective, thus providing an important model for examining critical immune correlates of protective vaccine immunity. We previously reported that an experimental live attenuated vaccine for equine infectious anemia virus (EIAV), based on mutation of the viral S2 accessory gene, elicited protection from detectable infection by virulent virus challenge (F. Li et al., J. Virol. 77:7244-7253, 2003). To better understand the critical components of EIAV vaccine efficacy, we examine here the relationship between the extent of virus attenuation, the maturation of host immune responses, and vaccine efficacy in a comparative study of three related attenuated EIAV proviral vaccine strains: the previously described EIAVUKΔS2 derived from a virulent proviral clone, EIAVUKΔS2/DU containing a second gene mutation in the virulent proviral clone, and EIAVPRΔS2 derived from a reference avirulent proviral clone. Inoculations of parallel groups of eight horses resulted in relatively low levels of viral replication (average of 102 to 103 RNA copies/ml) and a similar maturation of EIAV envelope-specific antibody responses as determined in quantitative and qualitative serological assays. However, experimental challenge of the experimentally immunized horses by our standard virulent EIAVPV strain by using a low-dose multiple exposure protocol (three inoculations with 10 median horse infective doses, administered intravenously) revealed a marked difference in the protective efficacy of the various attenuated proviral vaccine strains that was evidently associated with the extent of vaccine virus attenuation, time of viral challenge, and the apparent maturation of virus-specific immunity.
Development of effective vaccines to animal lentiviral infections is complicated by the diverse array of immune evasion mechanisms used by these viruses to circumvent host immune surveillance. Thus far, the development of vaccines to human immunodeficiency virus type 1 has relied substantially on the use of animal lentivirus models to evaluate the efficacy of various vaccine strategies. Animal lentiviral systems used as AIDS vaccine models have included simian/human immunodeficiency virus (SHIV)-monkey, simian immunodeficiency virus (SIV)-monkey, equine infectious anemia virus (EIAV)-horse, and feline immunodeficiency virus (FIV)-cat models (1). Interestingly, the greatest level of success has been realized with live attenuated animal lentivirus vaccines that are able to drive a critical maturation of virus-specific humoral and cellular immune responses (3, 4, 15, 16, 20, 29, 36-38).
EIAV, a macrophage-tropic lentivirus, causes a persistent infection in horses and a chronic disseminated disease of worldwide importance in veterinary medicine (reviewed by Montelaro et al. [26]). EIAV is typically transmitted via blood-feeding insects or iatrogenic sources such as contaminated syringe needles. The disease, EIA, is characterized by well-defined recurring cycles of viremia at irregular intervals and is associated with clinical signs that include fever, anemia, thrombocytopenia, edema, diarrhea, and lethargy. Infected horses typically progress by 8 to 12 months postinfection to life-long inapparent carriers that lack clinical signs but continue to experience various steady-state levels of virus replication maintained by monocyte/macrophage-specific tissue reservoirs of infection (11), (13, 26). Among virulent lentiviruses, EIAV is unique in that, despite aggressive virus replication and rapid antigenic variation, >90% of infected animals progress from a chronic disease state to an inapparent carrier stage by establishing strict immunologic control over virus replication (26). The EIAV system therefore serves as a uniquely dynamic model for the natural immunologic control of lentiviral replication and disease and provides a useful and novel lentivirus system for identifying critical immune correlates of protection and ascertaining the potential for developing an effective prophylactic vaccine to protect horses from EIAV infection.
During the past 15 years, we have evaluated a number of experimental EIAV vaccines based on inactivated whole virus and on viral or recombinant envelope subunit vaccines. The results of these vaccine trials demonstrated a remarkable breadth of efficacy, ranging from protection from detectable infection and/or disease to severe enhancement of EIAV replication and disease. These data indicate that vaccine-induced immune responses are a double-edged sword that can have beneficial or deleterious effects on the outcome of virus exposure (9, 10, 12, 14, 28, 31, 32).
We previously described a genetically engineered, live attenuated proviral vaccine candidate, EIAVUKΔS2, containing a mutation of the accessory S2 gene (20). In these initial experiments we reported a comprehensive analysis of the properties of the EIAVUKΔS2 in horses experimentally immunized and challenged intravenously with single or multiple doses of the reference EIAVPV virulent strain of virus. To examine in more detail the critical parameters of attenuated EIAV vaccine efficacy, we describe here a comparative study of the virologic, immunologic, and protective efficacy of variant attenuated EIAV proviral constructs in experimentally immunized horses. The results of these studies demonstrate that vaccine efficacy is markedly affected by the specifics of the vaccine genetic engineering and reveals a critical and delicate balance between vaccine virus attenuation and the capacity to drive immune maturation sufficient to achieve optimal protective immunity. The implications for these observations with experimental attenuated EIAV vaccines for AIDS vaccine design are discussed.
MATERIALS AND METHODS
Design and production of attenuated viral vaccines.
Three distinct attenuated viral vaccines were generated for evaluation: (i) the EIAVUKΔS2 vaccine strain containing a mutation of the S2 gene of the reference virulent EIAVUK proviral clone (19), (ii) the EIAVUKΔS2/DU vaccine containing mutations in both the S2 and the DU genes of the EIAVUK provirus, and (iii) the EIAVPRΔS2 vaccine strain produced from a reference avirulent proviral clone by mutation of the viral S2 gene. The EIAVUKΔS2 proviral clone was derived from the in vivo pathogenic molecular clone, EIAVUK (6), by inserting two stop codons in the S2 reading frame. The EIAVUKΔS2/DU also derived from the in vivo pathogenic EIAVUK molecular clone a previously described 330-base nucleotide sequence deletion in the viral DU (dUTPase) gene (22). The EIAVPRΔS2 proviral clone, constructed by standard molecular biological techniques (24), was also produced from the avirulent EIAVPR provirus (30) by introduction of two termination codons at the beginning of the S2 gene, as described above for the EIAVUKΔS2 proviral clone. All of the attenuated proviral clones were sequenced to verify the required genetic mutations.
Virus stocks were prepared by harvesting the supernatant medium from Lipofectamine-mediated transfection (Gibco-BRL) of equine dermal (ED) cells (ATCC CRL 6288) with mutant proviral DNA (19). Viral stocks were assayed by a micro reverse transcriptase assay, and stock virus titers were determined in an infectious center assay in fetal equine kidney cells, as described previously (8, 22).
Experimental subjects, clinical evaluation, and sample collection.
A total of 35 outbred horses of mixed age and gender were used in the various vaccine trials. All horses were clinically monitored daily and maintained as described previously (10, 14). Daily rectal temperatures and clinical status were recorded. Samples of serum, plasma, and whole blood were collected from each horse at defined intervals and daily during apparent febrile episodes (>39°C). Plasma samples were stored at −80°C for use in quantitative or qualitative reverse transcription-PCR (RT-PCR) assays to determine the levels and identity (vaccine or challenge virus) of plasma viral RNA. Serum samples were stored at −20°C for serological assays.
Experimental vaccination and virus challenge procedures.
We examined the immunogenicity and protective efficacy of each vaccine construct (EIAVUKΔS2, EIAVUKΔS2/DU, and EIAVPRΔS2) individually in groups of eight horses experimentally inoculated with the designated attenuated virus strain. Each vaccinate was then challenged by using a low-dose multiple exposure (LDME) challenge to simulate natural exposure by horsefly bites (20). The test horses were vaccinated two times at 30-day intervals by intramuscular injection of 105 50% tissue culture infective dose(s) (TCID50) of the individual vaccine stocks (20). Six months after the initial vaccine dose, the eight vaccinated horses in each group and four naive horses were challenged by the LDME procedure with the reference virulent EIAVPV stock. The LDME protocol consisted of three sequential intravenous inoculations, at 2-day intervals, of 10 median horse infectious doses (HID50) of the virulent challenge virus, EIAVPV (HID50 determinations were made though EIAVPV titrations in 15 horses; 1 HID50 is the equivalent of ∼0.1 TCID50). The horses were monitored daily for clinical symptoms of EIA, and blood was drawn at regular intervals (weekly, daily if febrile) for assays of platelets, viral replication, and virus-specific immune responses (11, 14). The horses were observed for a total of 270 days, at which time they were euthanized.
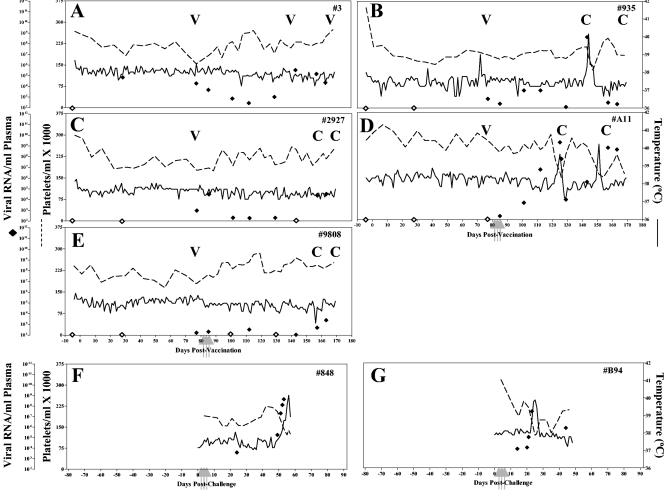
To evaluate the dynamics of the maturation and efficacy of EIAVUKΔS2 vaccine immunity in experimentally inoculated horses, a group of five horses was experimentally vaccinated with EIAVUKΔS2 as described above but then challenged at 2 months postinoculation by using the standard LDME regimen (20). The horses were monitored daily postchallenge for clinical symptoms of EIA, and blood was drawn at regular intervals (weekly, daily if febrile) for assays of platelets, viral replication, and virus-specific immune responses (11, 14). The horses were observed for a total of 170 days, at which time they were euthanized.
Quantitative and qualitative serological analyses.
Detection of serum antibody reactivity to EIAV core protein p26 was conducted by using a ViraCHEK/EIA kit according to the manufacturer's instructions (Synbiotics Laboratory, Via Frontera, San Diego, Calif.). Serum samples were also evaluated for seroreactivity by the standard agar gel immunodiffusion (AGID) procedure (2) diagnostic assay for EIA. Serum immunoglobulin G antibody reactivity to EIAV envelope glycoproteins was assayed quantitatively (endpoint titer) and qualitatively (avidity index and conformation ratio) by using our standard concanavalin A enzyme-linked immunosorbent assay (ELISA) procedures as described previously (11). The statistical significance of the differences between vaccine groups was determined by a nonparametric one-way analysis of variance (Kruskal-Wallis Test) with Dunnett's post test (GraphPad InStat, version 3.0; GraphPad Software, San Diego, Calif.). Virus neutralizing activity to the challenge virus strain EIAVPV mediated by immune sera was assessed in an indirect cell-ELISA based infectious center assay with a constant amount of infectious EIAVPV and sequential twofold dilutions of serum (10). Correlations between the presence of neutralizing antibody and protection from infection were performed by using the Spearman rank correlation test with GraphPad InStat.
Quantitative and qualitative RT-PCR of plasma virus RNA.
Plasma samples from all animals were analyzed for the levels of viral RNA per milliliter of plasma by using a previously described semiquantitative RT-PCR assay based on gag-specific amplification primers (11). The standard RNA curve was linear in the range of 102 molecules as a lower limit and 108 molecules as an upper limit. To differentiate the virion genomic RNA of the vaccine strains (EIAVUKΔS2, EIAVUKΔS2/DU, or EIAVPRΔS2) from the challenge virus (EIAVPV), virion-associated genomic RNA was extracted from plasma samples and then characterized by a nested RT-PCR and restriction digestion analysis that differentiated parental and mutated S2 gene sequences (20). This diagnostic assay is based on the introduction of a unique SpeI restriction site in a 582-bp segment of the modified S2 gene that is not present in the wild-type S2 gene segment (20). The generated SpeI diagnostic site is present in all three attenuated vaccine strains. The nested RT-PCR is highly specific and provides detection levels down to 20 RNA copies of S2. Diagnostic analyses were performed during all three periods of observation: prechallenge, postchallenge, and during immunosuppression (see Fig. 1 to 4).
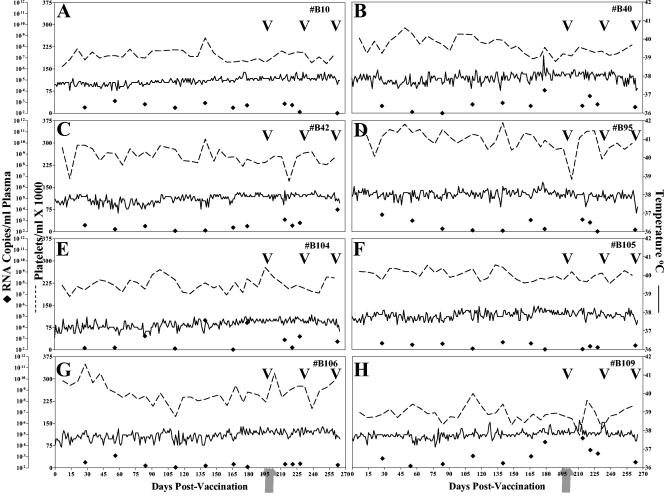
FIG. 1.
Clinical and virologic profiles of EIAVUKΔS2 experimental horses. Eight horses (A to H, respectively) were inoculated with EIAVUKΔS2 as described in Materials and Methods. Rectal temperature (solid line, right y axis) and platelet counts (dotted line, first left y axis) were monitored daily for up to 300 days (x axis) after the first vaccine dose. Quantification of the virus load (♦, second left y axis) was performed on viral RNA extracted from plasma at periodic time points prior to and after virulent virus challenge by using the LDME protocol (arrows). S2 diagnostic results for each animal are indicated in each respective panel above the date of analysis by either a V (vaccine strain) or a C (challenge strain).
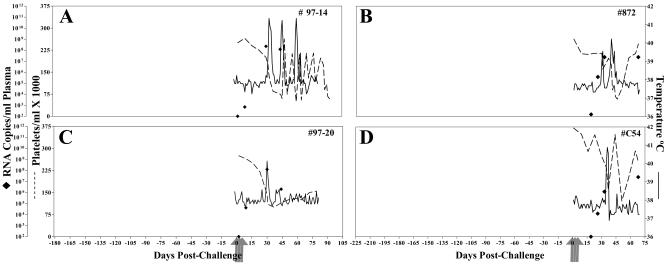
FIG. 4.
Clinical and virologic profiles of control naive horses. Four horses (A to D, respectively) were subjected to challenge with virulent EIAVPV by using the LDME protocol (see Materials and Methods). Rectal temperature (solid line, right y axis) and platelet counts (dotted line, first left y axis) were monitored daily for up to 300 days (x axis) postchallenge. Quantification of the virus load (♦, second left y axis) was performed on viral RNA extracted from plasma at periodic time points prior to and after virulent virus challenge by using the LDME protocol (arrows). Febrile episodes were defined by a rectal temperature of >39°C in conjunction with a reduction in platelets (<100,000/μl of whole blood) and other clinical symptoms of EIA.
RESULTS
Experimental vaccination with live attenuated EIAV vaccine candidates and challenge with virulent EIAV.
To assess the role of viral attenuation in vaccine efficacy, we immunized three groups of eight horses intravenously with 105 TCID50 of three different live attenuated virus stocks, EIAVUKΔS2, EIAVUKΔS2/DU, and EIAVPRΔS2. The vaccinated horses were monitored daily for clinical signs of EIA (fever, lethargy, petechiation, and diarrhea), and blood samples were taken at regular intervals for measurement of platelets, plasma virus, and EIAV-specific humoral immunity. To examine the protective efficacy of the immune responses elicited by the attenuated virus infection, we challenged all three groups of vaccinated horses with our reference virulent EIAVPV strain used in previous vaccine trials (9, 12, 20, 28, 31). To mimic the natural route of infection we utilized an LDME challenge that consists of a series of three intravenous inoculations of 10 HID50 of virulent EIAVPV administered at 2-day intervals (20). A control group of four naive horses was also challenged in an identical manner.
Prechallenge profiles of EIAVUKΔS2, EIAVPRΔS2, and EIAVUKΔS2/DU vaccinated horses.
As summarized in Fig. 1, all of the EIAVUKΔS2-vaccinated horses remained asymptomatic for EIA during the 7-month prechallenge period of observation, as characterized by a lack of febrile episodes (<39°C) and normal levels of blood platelets (≥105,000/μl whole blood), a documented measure of EIA disease (7, 11, 17, 21, 33, 39). The lack of clinical signs in the vaccinates was associated with a relatively low level of EIAVUKΔS2 replication during the first 6 months postinoculation. Viral RNA levels in plasma measured at various times postinoculation ranged from 102 to 104 copies of RNA/ml of plasma, with an overall average of ∼103 RNA copies/ml. We have previously reported that EIAV clinical episodes are associated with virus loads in excess of 106 RNA copies/ml (7, 18, 20).
Figure 2 summarizes the clinical and virologic profiles of each of the eight horses inoculated with the EIAVUKΔS2/DU provirus strain. As observed above, all of the horses inoculated with the EIAVUKΔS2/DU vaccine strain remained asymptomatic for EIA during the 7-month prechallenge observation period, as evidenced by a lack of febrile episodes and reduced blood platelet levels. The observed levels of replication of the EIAVUKΔS2/DU strain in the inoculated horses were consistently at a level of ca. 102 to 103 RNA copies/ml of plasma, representing an apparent 10-fold reduction in virus replication compared to the EIAVUKΔS2 vaccine strain described above. This reduced replication is consistent with the previous studies that defined the role of the EIAV DU protein in promoting virus replication in equine macrophage cultures in vitro and the effects of DU mutation on virus replication and virulence in vivo (22, 35).
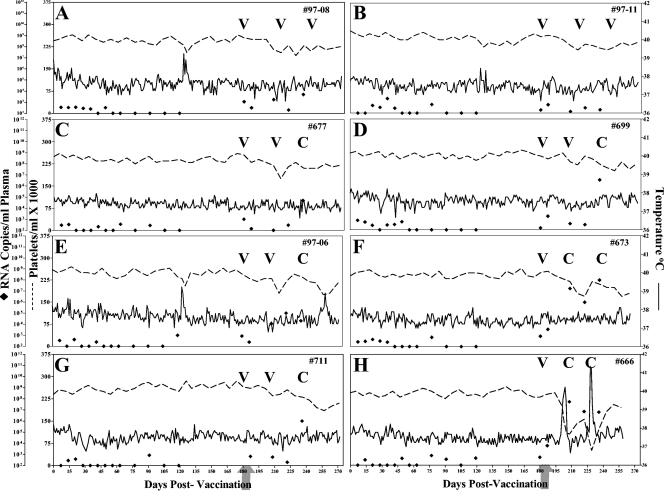
FIG. 2.
Clinical and virologic profiles of EIAVUKΔS2/DU experimental horses. Eight horses (A to H, respectively) were inoculated with EIAVUKΔS2/DU as described in Materials and Methods. Rectal temperature (solid line, right y axis) and platelet counts (dotted line, first left y axis) were monitored daily for up to 300 days (x axis) after the first vaccine dose. Quantification of the virus load (♦, second left y axis) was performed on viral RNA extracted from plasma at periodic time points prior to and after virulent virus challenge by using the LDME protocol (arrows). Febrile episodes (see panel A) were defined by a rectal temperature of >39°C in conjunction with a reduction in platelets (< 100,000/μl of whole blood) and other clinical symptoms of EIA. S2 diagnostic results for each animal are indicated in each respective panel above the date of analysis by either a V (vaccine strain) or a C (challenge strain).
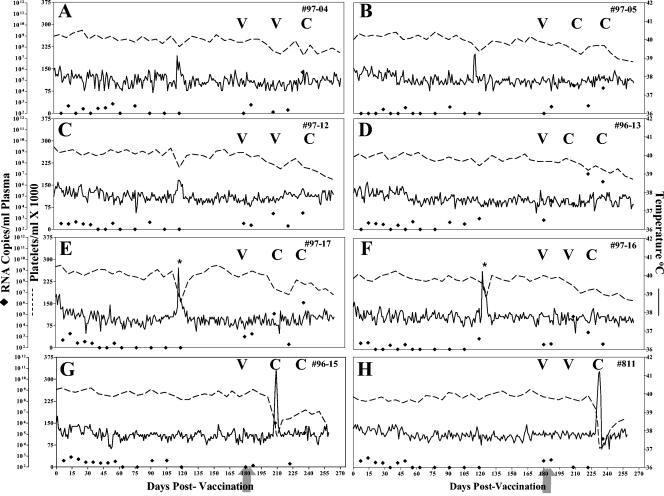
The final group of eight vaccinates received the genetically attenuated virus EIAVPRΔS2 containing a mutated S2 gene in the context of an avirulent viral backbone. As depicted in Fig. 3, six of the eight horses demonstrated no signs of EIA during the 7-month prechallenge observation period, as characterized by a lack of temperature increases and normal levels of blood platelets. However, two of the eight horses, #97-17 and #97-16 (Fig. 3G and H, respectively) experienced increased temperatures during the prechallenge period. The fevers occurred without concurrent drops in platelets. Upon further examination, these animals were diagnosed with equine herpesvirus infections, which appeared to be causing the increased temperatures. Thus, these observations were consistent with an absence of apparent virulence from the attenuated EIAV vaccine strain. Measurements of virus RNA levels in plasma in the horses inoculated with the EIAVPRΔS2 strain indicated a relatively consistent low level of vaccine virus replication, averaging less than 102 RNA copies/ml.
FIG. 3.
Clinical and virological profiles of EIAVPRΔS2 experimental horses. Eight horses (A to H, respectively) were inoculated with EIAVPRΔS2 as described in Materials and Methods. Rectal temperature (solid line, right y axis) and platelet counts (dotted line, first left y axis) were monitored daily for up to 300 days (x axis) after the first vaccine dose. Quantification of the virus load (♦, second left y axis) was performed on viral RNA extracted from plasma at periodic time points prior to and after virulent virus challenge by using the LDME protocol (arrows). Febrile episodes (E and H) were defined by a rectal temperature of >39°C in conjunction with a reduction in platelets (<100,000/μl of whole blood) and other clinical symptoms of EIA. S2 diagnostic results for each animal are indicated in each respective panel above the date of analysis by either a V (vaccine strain) or a C (challenge strain). *, EHV-associated fevers.
Taken together, these prechallenge observations demonstrated that each of the genetic modifications attenuated viral virulence such that persistent infections were asymptomatic for EIA disease, as required for an attenuated vaccine strain. In addition, the virus load measurements indicated a general trend in that the sustained replication levels of the three different proviral vaccine strains were reduced incrementally by ∼10-fold in the order EIAVUKΔS2 > EIAVUKΔS2/DU > EIAVPRΔS2.
Postchallenge clinical and virologic profiles of EIAVUKΔS2-, EIAVUKΔS2/DU-, and EIAVPRΔS2-vaccinated horses.
The four EIAV-naive control horses developed, upon challenge with EIAVPV, classic EIA signs (Fig. 4) by 40 days postchallenge, displaying concurrent fevers and declines in platelets accompanied by peaks in virus loads of 106 to 108 RNA copies/ml of plasma. In contrast, the eight EIAVUKΔS2-vaccinated animals remained asymptomatic for EIA after challenge with EIAVPV, again as monitored by a lack of fluctuation in temperature and platelet levels (Fig. 1). Virus loads remained at levels similar to the prechallenge data, ranging between 103 and 104 copies of RNA/ml of plasma. Diagnostic RT-PCR analyses of the RNA populations in plasma postchallenge detected only the mutant vaccine virus S2. The absence of detectable challenge virus S2 is consistent with an apparent protection from infection by experimental challenge, thus confirming the observations reported previously with this particular experimental vaccine (20).
The postchallenge observations for the group of horses vaccinated with the EIAVUKΔS2/DU strain are summarized in Fig. 2. Seven of these eight vaccinates remained asymptomatic for EIA after challenge with EIAVPV, as indicated by no increase in temperature or reduction in platelet levels after virulent viral challenge (Fig. 2). Virus load measurements postchallenge indicated that only two of the horses in this vaccine group (#97-08 and #97-11, Fig. 2A and B) appeared to control virus replication to the same level observed prior to virulent viral challenge, i.e., <103 RNA copies/ml. Despite the lack of clinical symptoms in the other seven vaccinates postchallenge, five of these horses did experience increases in viral loads, averaging between 105 and 106, with horse #673 peaking at 108 copies RNA/ml of plasma (Fig. 2C to G). A single animal, #666 (Fig. 2H) in the trial experienced clinical EIA, having two fever episodes postchallenge that were accompanied by platelet drops and increases in viral loads that peaked in excess of 108 RNA copies/ml of plasma. Diagnostic RT-PCR analyses of plasma virus S2 species postchallenge revealed the presence of challenge virus in all six of these vaccinates that experienced increased viral loads postchallenge, whereas only vaccine virus S2 was detected in the two horses (#97-08 and #97-11) in which no increase in virus loads postchallenge were observed.
The postchallenge observations with the group of horses inoculated with the EIAVPRΔS2 strain are summarized in Fig. 3. After challenge with EIAVPV, six of the eight EIAVPRΔS2-vaccinated animals remained asymptomatic for EIA (Fig. 3), again defined by a lack of fevers or thrombocytopenia after virulent virus inoculation. Two horses, #96-15 and #811 (Fig. 3G and H, respectively) experienced clinical EIA as indicated by marked increases in temperature and decreases in platelets. Virus load assays postchallenge revealed that all of these horses experienced increases in virus loads postchallenge; postchallenge viral loads ranged from 104 to 106 RNA copies/ml compared to < 102 RNA copies per ml prechallenge. In addition, the challenge virus S2 gene was detected in all eight of the horses in this group postchallenge.
The combined postchallenge data from the three vaccine groups reveals a spectrum of vaccine efficacy among the three experimental vaccine strains (Table 1). The EIAVUKΔS2 immunization provided 100% protection from disease and detectable infection by the virulent virus challenge. The EIAVUKΔS2/DU immunization produced substantial protection from apparent disease (87.5%) but little protection (25%) from infection by the challenge virus. Finally, the EIAVPRΔS2 immunization produced a similar protection from disease (75%) but failed to protect any of the vaccinates in this group from infection by the virulent challenge virus.
TABLE 1.
Summary of observed vaccine efficaciesa
| Vaccine strain | Dose | DOC | Plasma virus RNA at DOC | EIAV challenge strain | No. of animals protected/no. tested (%)
|
|
|---|---|---|---|---|---|---|
| Protection from clinical disease | Protection from infection | |||||
| EIAVUKΔS2 | 105 | 6 | 102-104 | PV | 8/8 (100) | 8/8 (100) |
| EIAVUKΔS2/DU | 105 | 6 | <102-103 | PV | 7/8 (87.5) | 2/8 (25) |
| EIAVPRΔS2 | 105 | 6 | <102 | PV | 6/8 (75%) | 0/8 (0) |
| EIAVUKΔS2 | 105 | 2 | 102 | PV | 3/5 (60) | 1/5 (20) |
| Control | NA | NA | NA | PV | 0/6 (0) | 0/6 (0) |
DOC, day of challenge.
Development of humoral immune responses to EIAV vaccination.
In light of the range of protective efficacy observed with the panel of attenuated EIAV strains, we next sought to evaluate the properties of the antibody responses elicited by each vaccine immunization.
The immunogenicity of each vaccine strain was first characterized by reactivity testing in standard U.S. Department of Agriculture (USDA)-approved commercial diagnostic assays for EIAV infection based on detecting antibody to the virus capsid protein, p26. These diagnostic tests included two USDA-approved assays, the reference AGID test (2) and the ELISA-based ViraCHEK assay (see Materials and Methods) (20). All experimentally immunized horses were determined to be seropositive in the ViraCHEK diagnostic assay at the day of challenge (data not shown). In contrast, the same immune serum samples were uniformly determined to be negative in the less-sensitive AGID tests (data not shown). These results illustrated that horses inoculated with EIAVUKΔS2, EIAVUKΔS2/DU, or EIAVPRΔS2 can be seropositive in diagnostic assays that are the basis of USDA and other national regulatory policies to control EIAV infection.
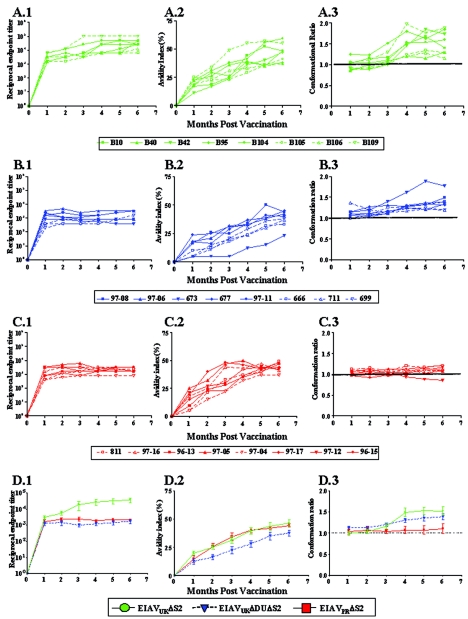
A dynamic, complex, and lengthy maturation of immune responses to the viral envelope proteins during the first 6 to 8 months postinfection appears to be a distinctive feature of lentivirus infections as the persistent infection achieves steady-state viral replication and host immunity (3, 5, 27). We have previously described, by using a novel panel of quantitative and qualitative serological assays, a characteristic maturation of EIAV envelope-specific serum antibodies in experimentally infected horses that precedes immune and virologic steady state (10, 11). These mature and immature immune responses have been further correlated with protective and nonprotective or enhancing vaccine immunity in experimental EIAV vaccines (12). Therefore, we next utilized our standard panel of quantitative (titer) and qualitative (avidity and conformation) assays to examine and compare the maturation of EIAV envelope-specific antibody responses during the prechallenge observation period of observation (Fig. 5). These data revealed a similar evolution of envelope-specific antibody response in all three vaccine groups, with only minor variations among individual horses in each vaccine group. In general, envelope-specific antibody titers increased rapidly during the first couple of months postinoculation, reaching steady-state levels that averaged 1:10,000 for the EIAVUKΔS2 vaccinates (Fig. 5A.1) and 1:1,000 for the EIAVUKΔS2/DU (Fig. 5B.1) and EIAVPRΔS2 (Fig. 5C.1) vaccinates. Overall, the EIAVUKΔS2 vaccinates (Fig. 5D.1) achieved significantly higher steady-state antibody titers (P < 0.05) compared to the other two attenuated strains.
FIG. 5.
Development of envelope-specific antibody responses to various attenuated EIAV vaccines. Longitudinal characterization of the quantitative and qualitative properties of induced EIAV envelope-specific antibodies were conducted in concanavalin A ELISAs of endpoint titer (panels 1), avidity (panels 2), and conformational dependence (panels 3) as described in Materials and Methods. Analyses were performed on allthree trial groups: EIAVUKΔS2 (A), EIAVUKΔS2/DU (B), and EIAVPRΔS2 (C). (D) Average levels for each assessment were also calculated to extricate and simplify comparative analysis. (A.1 to D.1) Antibody titers in serum for each time point are presented as the log10 of the highest reciprocal dilution yielding reactivity two standard deviations above background. (A.2 to D.2) Avidity index measurements are presented as percentages of the antibody-antigen complexes resistant to disruption with 8 M urea. (A.3 to D.3) Conformation dependence values are calculated as the ratio of serum antibody reactivity with native envelope compared to denatured envelope antigen. Conformation ratios of >1.0 indicate predominant antibody specificity for conformational determinants, whereas ratios of <1.0 indicate predominant antibody specificity for linear envelope determinants.
In contrast to the rapid establishment of steady-state antibody levels in serum, the qualitative properties of avidity and conformational dependence of the envelope-specific antibodies progressively evolved over the initial 6 months, at which time relatively steady-state levels were established (Fig. 5A.2, B.2, and C.2). In general, antibody avidity values progressed from an average of ca. 20% at 1 month postinfection to steady-state levels of ca. 40% at 6 months (Fig. 5D.2). Although the antibody avidity values observed over the first 6 months for the EIAVUKΔS2/DU vaccinates were consistently lower than those observed in the other two vaccine groups, these differences were not statistically significant (P > 0.05). Interestingly, the assays for antibody conformational dependence revealed more marked differences in the specificities of the antibodies elicited by the different attenuated vaccine strains (Fig. 5A.3, B.3, and C.3). The envelope-specific serum antibodies generated in the EIAVUKΔS2 vaccinates demonstrated an increase in conformational dependence from a relatively equal specificity of linear conformational envelope determinants (average ratio of 0.9) to a specificity for predominantly conformational determinants (average ratio of 1.5). This progression of antibody conformational properties was similar to the maturation observed in horses experimentally infected with the virulent EIAVPV (10, 11). Interestingly, the EIAVUKΔS2/DU (Fig. 5B.3) and EIAVPRΔS2 (Fig. 5C.3) inoculations were characterized by a less pronounced evolution of antibody conformational dependence compared to the EIAVUKΔS2 immunization (Fig. 3)5D). For example, the antibody conformational dependence values observed in the EIAVPRΔS2 vaccinates remained relatively constant at ∼1.0 over the 6-month observation period, which is significantly different from the steady-state levels of EIAVUKΔS2 and EIAVUKΔS2/DU (P < 0.01), indicating a lack of the characteristic immune maturation by this particular vaccination.
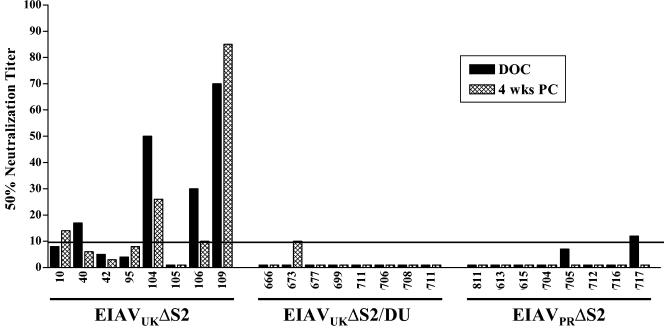
We previously reported a relatively slow development of serum neutralizing antibodies over a several-month period after experimental EIAV infection with either virulent or avirulent strains, with average maximum titers averaging 1:300 (11). To examine the ability of the various attenuated EIAV strains to elicit serum neutralizing antibody, we tested immune serum samples taken at 6 months postvaccination for their ability to inactivate the parental virulent EIAVPV infectivity in cultured fetal equine kidney cells (10), as summarized in Fig. 6. These data demonstrated that the EIAVUKΔS2 attenuated candidate generated serum antibody neutralizing titers that ranged from 1:10 to 1:90 at 6 months postinoculation. Although the level of neutralizing antibodies required to protect horses from infection or disease is not known, neutralizing antibodies were detectable in all eight vaccinates; however, only four horses developed titers above background levels by the day of challenge. In contrast, none of the horses in the EIAVUKΔS2/DU or the EIAVPRΔS2 groups displayed significant levels of serum neutralization in these standard assays. To evaluate statistically whether there was a correlation between protection from infection and increased levels of neutralizing antibodies, a Spearman rank correlation test was performed utilizing the data from all 24 animals. The calculated two-tailed P value was 0.0535 (r = 0.3989), which is considered not significant.
FIG. 6.
Characterization of virus-specific serum neutralization in horses inoculated with EIAVUKΔS2, EIAVUKΔS2/DU, or EIAVPRΔS2. The mean reciprocal dilutions of serum from vaccinated horses which neutralized 50% of input EIAVPV as measured in an infectious center assay are presented for serum samples collected at the day of challenge (DOC) and 4 weeks postchallenge (PC), as described in Materials and Methods. The line denotes the cutoff (≥10) value for valid 50% neutralization titers.
The combined serological assays described above indicate that the protective vaccine immunity did not require neutralizing antibody responses but that the vaccine efficacy was more closely associated with the extent of antibody maturation to the attenuated virus infection. The best protection was observed with the EIAVUKΔS2 inoculation, and this immunization elicited the highest values for envelope-specific antibody titer, avidity, and conformational dependence that most closely resembled steady-state antibody properties observed in long-term inapparent carriers (10, 11).
Development of vaccine protective immunity.
To more directly test the correlation of antibody maturation with vaccine protective efficacy, we next evaluated the protection provided by vaccine immunity in horses inoculated with the highly effective EIAVUKΔS2 strain but subjected to LDME challenge at only 2 months postinoculation, instead of the 6-month interval described above. As depicted in Fig. 7A to E, three of the five vaccinates were protected from disease, whereas two experienced typical EIA symptoms by about 2 months postchallenge. Virus loads prior to challenge for all five horses were ∼102 copies RNA/ml of plasma. Upon challenge with virulent EIAV, four of five horses displayed marked increases in virus loads. Virus levels peaked at an average level of 106, ranging between 105 and 108 copies of RNA/ml of plasma. In addition to the lower level of protection from disease, early challenge also resulted in four of five animals with detectable challenge virus. The two EIAV-naive control horses upon challenge with EIAVPV developed classic EIA symptoms (Fig. 7F and G) by 40 days postchallenge, displaying concurrent fevers and declines in platelets, accompanied by peaks in virus loads.
FIG. 7.
Clinical and virologic profiles of EIAVUKΔS2 vaccinated horses and naive control horses challenged at 2 months. Five horses (A to E, respectively) were inoculated with EIAVUKΔS2 and challenged at 3 months after the first vaccine dose, as described in Materials and Methods. Two naive control horses (F and G) were subjected to challenge in parallel with virulent EIAVPV by using the LDME protocol (see Materials and Methods). Rectal temperature (solid line, right y axis) and platelet counts (dotted line, first left y axis) were monitored daily for up to 170 days (x axis) after vaccination. Quantification of the virus load (♦, second left y axis, ⋄, undetectable) was performed on viral RNA extracted from plasma at periodic time points prior to and after virulent virus challenge by using the LDME protocol (arrows). S2 diagnostic results for each animal are indicated in each respective panel above the date of analysis by either a V (vaccine strain) or a C (challenge strain).
The properties of the EIAV envelope-specific antibody responses elicited by the EIAVUKΔS2 immunization by 2 months on the day of challenge are summarized in Table 2. These early antibody responses are characterized by a relatively low envelope specific antibody titer (average, 1:3,680), a lack of detectable neutralization activity, and avidity and conformational dependence values characteristic of immature immunity. Thus, these results clearly demonstrate a critical correlation between the extent of the maturation of vaccine immunity and the level of vaccine protection from virus exposure. Although the development of vaccine immunity with the attenuated virus vaccine is characterized here by a maturation of envelope-specific antibody responses, it is logical to assume a similar evolution of cellular immune responses as part of this maturation process.
TABLE 2.
Properties of envelope-specific antibody responses in EIAVUKΔS2-immunized horses at 2 months
| Animal | Endpoint titer | Avidity index | Conformation ratio | 50% Neutralization titer |
|---|---|---|---|---|
| 2927 | 3,200 | 22 | 1.09 | 0 |
| 9808 | 2,400 | 22 | 1.13 | 0 |
| 935 | 3,200 | 25 | 0.61 | 0 |
| 3 | 6,400 | 18 | 1.15 | 0 |
| A-11 | 3,200 | 25 | 0.78 | 0 |
DISCUSSION
We previously reported that inoculation of horses with an attenuated EIAV proviral vaccine, EIAVUKΔS2, induced protection from detectable virus infection and disease by experimental virulent EIAV challenge (20). The present study confirms these earlier observations with EIAVUKΔS2, supporting the ability of this vaccine to provide effective protection from virus exposure. Our observations with an engineered EIAV proviral vaccine appear to be relatively consistent with earlier reports describing an attenuated EIAV vaccine used in millions of horses in China during the 1980s and credited with controlling EIA in that country, leading to a discontinuation of vaccination against this disease (34). The Chinese EIAV vaccine was attenuated by classical long-term serial passage in donkey leukocytes, with the final vaccine being a genetically uncharacterized attenuated strain but presumably a complex mixture of genomic quasispecies. Although there has been some speculation that the efficacy of the Chinese EIAV vaccine was attributed to special but undefined properties of this virus mixture, our evaluations of the EIAVUKΔS2 proviral vaccine clearly indicate that vaccine protection against EIAV can be achieved with a homogenous vaccine virus strain engineered from a molecular proviral clone. Similar vaccine protection has been observed with attenuated provirus clones in the SIV-monkey and FIV-cat lentivirus systems (15, 16, 23, 29, 36).
However, the data presented here also demonstrate that not all attenuated EIAV proviral constructs are equally effective in eliciting protective vaccine immunity in experimentally inoculated horses. In fact, relatively minor modifications in the genetic mutations used to attenuate the virus resulted in marked differences in the efficacy of protection from disease and infection by experimental challenge (Table 1). The protective efficacy of the series of attenuated EIAV strains was inversely correlated with their extent of attenuation and steady-state replication levels in inoculated horses. We have previously reported that mutation of the S2 gene of the virulent EIAVUK provirus clone reduced in vivo steady-state virus replication levels by ∼100-fold compared to the parental virus, leading to a substantial attenuation of virulence (18). The EIAVUKΔS2 and EIAVPRΔS2 vaccine strains contain the S2 mutation but in the context of a virulent and avirulent proviral backbone, respectively. Since the EIAVUK and EIAVPR provirus clones were derived from the same parental virus stock, their genomic sequences are highly conserved, with <1.06% amino acid sequence variation in their envelope genes. However, the virulent EIAVUK provirus has been shown to establish steady-state replication levels in experimentally infected horses at levels that are 10- to 100-fold higher than those observed for the avirulent EIAVPR provirus (6). This difference in replication properties appeared to be reflected in the S2 attenuated proviruses, since the levels of EIAVUKΔS2 virus in plasma were consistently 10- to 100-fold higher than the virus levels in plasma observed with the EIAVPRΔS2 in the parallel vaccine groups. Similarly, introduction of mutations in both the S2 and DU genes of EIAVUK reduced steady-state replication levels of the EIAVUKΔS2/DU provirus by 10- to 100-fold compared to the EIAVUKΔS2 containing only the S2 mutation. Interestingly, an inverse relationship between vaccine protection and proviral attenuation has also been reported in the SIV-monkey system (16, 25). These combined observations in two lentivirus systems indicate a threshold level of persistent viral replication and presumably sustained viral antigen presentation necessary to sufficiently drive host immune responses to achieve protective immunity.
In addition to adequate levels of steady-state EIAV replication, the second critical parameter for achieving protective vaccine immunity is the length of time postinoculation with the attenuated virus. In the case of the EIAVUKΔS2 vaccine, 100% protection from detectable infection and disease was achieved at 6 months postinoculation, whereas at 2 months postinoculation the protective efficacy was reduced to 20% for infection and 60% for disease by the same attenuated virus construct and virus challenge modality. Thus, these data indicated a progressive development of protective immunity during the first 6 to 7 months postinfection, suggesting an evolution of virus-specific immune responses to the persistent attenuated EIAV infection. In studies with an attenuated SIV vaccine in monkeys, we have previously reported a protective efficacy of 50% at 3 months postinoculation compared to 100% at 8 months postinoculation (3-5). Thus, the combined EIAV and SIV attenuated virus vaccine data suggest that the prolonged process for the development of protective vaccine immunity may be a general and distinguishing property of lentiviruses.
Although the specifics of the evolution of these immune responses to persistent EIAV infection remain to be defined, the serological assays defining the development of EIAV-envelope specific antibody responses appear to reflect the progression to protective vaccine immunity. Thus, the extent of immune maturation was directly associated with the level of protective vaccine efficacy. For example, the most protective vaccine immunity elicited by the EIAVUKΔS2 strain at 6 months postinoculation was associated with mature antibody responses characterized by high titer, high avidity, and predominantly conformational dependence. In contrast, the reduced vaccine protection observed with the EIAVUKΔS2 virus at 2 months postinoculation was associated with immature antibody responses characterized by relatively low titer, low avidity, and equally linear and conformational specificity (Table 2). Similar to these early vaccine immune responses, the more severely attenuated EIAVPRΔS2 and the EIAVUKΔS2/DU vaccine strains generated relatively immature antibody responses by 6 months postinoculation. It remains to be determined whether the immune responses to these latter more attenuated vaccines can achieve maturation and higher protection, even with a substantially more extended infection time.
The association of EIAV envelope-specific antibody maturation parameters with protective efficacy of vaccine immunity provides useful insights into the properties of protective immunity but cannot with this limited number of animals reveal statistically reliable immune correlates of protection. In this regard, a more detailed characterization of the evolution of cellular immune responses to attenuated EIAV vaccines is currently in progress. However, the current studies do indicate that in vitro serum antibody neutralization activity was apparently not required for the maximum vaccine protection as produced by the EIAVUKΔS2 vaccine. In fact, horses immunized with this attenuated virus were equally protected regardless of whether low levels of neutralizing antibody titers were detected in the immune serum from the day of challenge. Correlation analysis between neutralizing antibodies and protection from infection suggested some association but failed to reach statistical significance in this data set. These observations suggest that cellular immune responses or antibody functions not measured in our in vitro assays are sufficient for protective vaccine immunity. The data do not necessarily eliminate the potential role of neutralizing antibodies in vaccine protection, if present at sufficient levels.
The achievement of protective immunity by a suitable attenuated EIAV vaccine lends support to the hypothesis that effective vaccine immunity is feasible to preclude lentivirus infections, despite the challenges that these viruses pose to the host immune system. It is important to note here that the current vaccine trials assess efficacy based on a challenge virus (EIAVPV) that is a biological clone that is closely related to the provirus vaccine envelope species. Based on the solid protection observed here, it will be of importance in future experiments to evaluate the efficacy of the EIAVUKΔS2 vaccine in protecting against a panel of EIAV envelope variants with different levels of defined envelope protein sequence variation. Thus, the attenuated EIAV vaccine model provides a unique system in which to directly test the concept that natural virus envelope heterogeneity poses a major obstacle to vaccine development, as assumed for AIDS vaccines.
Do attenuated provirus vaccines offer a practical approach to the development of a commercial EIAV vaccine that can be applied to horses worldwide? As noted above, Chinese veterinarians have reported elimination of EIAV outbreaks in that country after a compulsory inoculation of millions of horses with their attenuated EIAV vaccine (34). Due to the lack of systematic monitoring during the immunization program, however, reliable data on the current rate of EIA infections or the incidence of vaccine reversions appear to be unavailable. Although offering an important and informative lentivirus vaccine model for discerning immune correlates of protection, attenuated EIAV vaccines pose a number of practical problems for application. First, there are the theoretical concerns about potential reversion of the attenuated vaccines strain to virulence, a possibility made more likely by the persistent nature of the infection and the propensity for relentless genomic mutation during EIAV replication. To minimize the potential for reversion, attenuated vaccines could be engineered with multiple gene mutations, but the experiments described here highlight the delicate balance between the extent of attenuation and the degree of vaccine protection. Second, the persistent infection by an attenuated EIAV poses an undefined risk for in utero transmission and abortion and for virulence in immune compromised horses or foals with developing immune systems. Finally, attenuated EIAV vaccines elicit antibody responses to viral core and envelope proteins, resulting in seropositivity in current diagnostic assays used to control EIA in the United States, Europe, and other countries. In developing countries where EIAV infection is common and government regulatory policies are not defined, it is possible that the risk to benefit calculation could favor use of an attenuated EIAV vaccine for several years to break the cycle of infection and disease, as reportedly done in China.
Acknowledgments
This study was supported by NIH/NIAID grant RO1 AI25850 (R.C.M.) and by funds from the Lucille P. Markey Charitable Trust and the University of Kentucky Agricultural Experiment Station.
REFERENCES
- 1.Bogers, W. M., C. Cheng-Mayer, and R. C. Montelaro. 2000. Developments in preclinical AIDS vaccine efficacy models. AIDS 14:S141-S145. [PubMed] [Google Scholar]
- 2.Coggins, L., and N. L. Norcross. 1970. Immunodiffusion reaction in equine infectious anemia. Cornell Vet. 60:330-335. [PubMed] [Google Scholar]
- 3.Cole, K. S., J. L. Rowles, B. A. Jagerski, M. Murphey-Corb, T. Unangst, J. E. Clements, J. Robinson, M. S. Wyand, R. C. Desrosiers, and R. C. Montelaro. 1997. Evolution of envelope-specific antibody responses in monkeys experimentally infected or immunized with simian immunodeficiency virus and its association with the development of protective immunity. J. Virol. 71:5069-5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cole, K. S., J. L. Rowles, M. Murphey-Corb, J. E. Clements, J. Robinson, and R. C. Montelaro. 1997. A model for the maturation of protective antibody responses to SIV envelope proteins in experimentally immunized monkeys. J. Med. Primatol. 26:51-58. [DOI] [PubMed] [Google Scholar]
- 5.Cole, K. S., M. Murphey-Corb, O. Narayan, S. V. Joag, G. M. Shaw, and R. C. Montelaro. 1998. Common themes of antibody maturation to simian immunodeficiency virus, simian-human immunodeficiency virus, and human immunodeficiency virus type 1 infections. J. Virol. 72:7852-7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cook, R. F., C. Leroux, S. J. Cook, S. L. Berger, D. L. Lichtenstein, N. N. Ghabrial, R. C. Montelaro, and C. J. Issel. 1998. Development and characterization of an in vivo pathogenic molecular clone of equine infectious anemia virus. J. Virol. 72:1383-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Craigo, J. K., C. Leroux, L. Howe, J. D. Steckbeck, S. J. Cook, C. J. Issel, and R. C. Montelaro. 2002. Transient immune suppression of inapparent carriers infected with a principal neutralizing domain-deficient equine infectious anemia virus induces neutralizing antibodies and lowers steady-state virus replication. J. Gen. Virol. 83:1353-1359. [DOI] [PubMed] [Google Scholar]
- 8.Grund, C. H., E. R. Lechman, C. J. Issel, R. C. Montelaro, and K. E. Rushlow. 1994. Lentivirus cross-reactive determinants present in the capsid protein of equine infectious anaemia virus. J. Gen. Virol. 75(Pt. 3):657-662. [DOI] [PubMed] [Google Scholar]
- 9.Hammond, S. A., S. J. Cook, L. D. Falo, Jr., C. J. Issel, and R. C. Montelaro. 1999. A particulate viral protein vaccine reduces viral load and delays progression to disease in immunized ponies challenged with equine infectious anemia virus. Virology 254:37-49. [DOI] [PubMed] [Google Scholar]
- 10.Hammond, S. A., S. J. Cook, D. L. Lichtenstein, C. J. Issel, and R. C. Montelaro. 1997. Maturation of the cellular and humoral immune responses to persistent infection in horses by equine infectious anemia virus is a complex and lengthy process. J. Virol. 71:3840-3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hammond, S. A., F. Li, B. M. McKeon, Sr., S. J. Cook, C. J. Issel, and R. C. Montelaro. 2000. Immune responses and viral replication in long-term inapparent carrier ponies inoculated with equine infectious anemia virus. J. Virol. 74:5968-5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hammond, S. A., M. L. Raabe, C. J. Issel, and R. C. Montelaro. 1999. Evaluation of antibody parameters as potential correlates of protection or enhancement by experimental vaccines to equine infectious anemia virus. Virology 262:416-430. [DOI] [PubMed] [Google Scholar]
- 13.Harrold, S. M., S. J. Cook, R. F. Cook, K. E. Rushlow, C. J. Issel, and R. C. Montelaro. 2000. Tissue sites of persistent infection and active replication of equine infectious anemia virus during acute disease and asymptomatic infection in experimentally infected squids. J. Virol. 74:3112-3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Issel, C. J., D. W. Horohov, D. F. Lea, W. V. Adams, Jr., S. D. Hagius, J. M. McManus, A. C. Allison, and R. C. Montelaro. 1992. Efficacy of inactivated whole-virus and subunit vaccines in preventing infection and disease caused by equine infectious anemia virus. J. Virol. 66:3398-3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson, R. P., and R. C. Desrosiers. 1998. Protective immunity induced by live attenuated simian immunodeficiency virus. Curr. Opin. Immunol. 10:436-443. [DOI] [PubMed] [Google Scholar]
- 16.Johnson, R. P., J. D. Lifson, S. C. Czajak, K. S. Cole, K. H. Manson, R. Glickman, J. Yang, D. C. Montefiori, R. Montelaro, M. S. Wyand, and R. C. Desrosiers. 1999. Highly attenuated vaccine strains of simian immunodeficiency virus protect against vaginal challenge: inverse relationship of degree of protection with level of attenuation. J. Virol. 73:4952-4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leroux, C., C. Issel, and R. C. Montelaro. 1997. Novel and dynamic evolution of equine infectious anemia virus genomic quasispecies associated with sequential disease cycles in an experimentally infected pony. J. Virol. 71:9627-9639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li, F., C. Leroux, J. K. Craigo, S. J. Cook, C. J. Issel, and R. C. Montelaro. 2000. The S2 gene of equine infectious anemia virus is a highly conserved determinant of viral replication and virulence properties in experimentally infected ponies. J. Virol. 74:573-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li, F., B. A. Puffer, and R. C. Montelaro. 1998. The S2 gene of equine infectious anemia virus is dispensable for viral replication in vitro. J. Virol. 72:8344-8348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li, F., J. K. Craigo, L. Howe, J. D. Steckbeck, S. Cook, C. Issel, and R. C. Montelaro. 2003. A live attenuated equine infectious anemia virus proviral vaccine with a modified S2 gene provides protection from detectable infection by intravenous virulent virus challenge of experimentally inoculated horses. J. Virol. 77:7244-7253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lichtenstein, D. L., C. J. Issel, and R. C. Montelaro. 1996. Genomic quasispecies associated with the initiation of infection and disease in ponies experimentally infected with equine infectious anemia virus. J. Virol. 70:3346-3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lichtenstein, D. L., K. E. Rushlow, R. F. Cook, M. L. Raabe, C. J. Swardson, G. J. Kociba, C. J. Issel, and R. C. Montelaro. 1995. Replication in vitro and in vivo of an equine infectious anemia virus mutant deficient in dUTPase activity. J. Virol. 69:2881-2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lockridge, K. M., M. Chien, G. A. Dean, S. C. Kelly, R. C. Montelaro, P. A. Luciw, and E. E. Sparger. 2000. Protective immunity against feline immunodeficiency virus induced by inoculation with vif-deleted proviral DNA. Virology 273:67-79. [DOI] [PubMed] [Google Scholar]
- 24.Maniatis, T., E. F. Fritsch, and J. E. Sambrook. 1982. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 25.Mills, J., R. C. Desrosiers, E. Rud, and N. Almond. 2000. Live attenuated HIV vaccines: a proposal for further research and development. AIDS Res. Hum. Retrovir. 16:1453-1461. [DOI] [PubMed] [Google Scholar]
- 26.Montelaro, R. C., J. M. Ball, and K. Rushlow. 1993. Equine retroviruses, p. 257-360. In J. A. Levy (ed.), The retroviridae. Plenum Press, Inc., New York, N.Y.
- 27.Montelaro, R. C., K. S. Cole, and S. A. Hammond. 1998. Maturation of immune responses to lentivirus infection: implications for AIDS vaccine development. AIDS Res. Hum. Retrovir. 14(Suppl. 3):S255-S259. [PubMed] [Google Scholar]
- 28.Montelaro, R. C., C. H. Grund, M. R. Raabe, B. Woodson, R. F. Cook, S. J. Cook, and C. J. Issel. 1996. Characterization of protective and enhancing immune responses to equine infectious anemia virus resulting from experimental vaccines. AIDS Res. Hum. Retrovir. 12:413-415. [DOI] [PubMed] [Google Scholar]
- 29.Nilsson, C., B. Makitalo, R. Thorstensson, S. Norley, D. Binninger-Schinzel, M. Cranage, E. Rud, G. Biberfeld, and P. Putkonen. 1998. Live attenuated simian immunodeficiency virus (SIV)mac in macaques can induce protection against mucosal infection with SIVsm. AIDS 12:2261-2270. [DOI] [PubMed] [Google Scholar]
- 30.Payne, S. L., J. Rausch, K. Rushlow, R. C. Montelaro, C. Issel, M. Flaherty, S. Perry, D. Sellon, and F. Fuller. 1994. Characterization of infectious molecular clones of equine infectious anaemia virus. J. Gen. Virol. 75(Pt. 2):425-429. [DOI] [PubMed] [Google Scholar]
- 31.Raabe, M. L., C. J. Issel, S. J. Cook, R. F. Cook, B. Woodson, and R. C. Montelaro. 1998. Immunization with a recombinant envelope protein (rgp90) of EIAV produces a spectrum of vaccine efficacy ranging from lack of clinical disease to severe enhancement. Virology 245:151-162. [DOI] [PubMed] [Google Scholar]
- 32.Raabe, M. L., C. J. Issel, and R. C. Montelaro. 1999. In vitro antibody-dependent enhancement assays are insensitive indicators of in vivo vaccine enhancement of equine infectious anemia virus. Virology 259:416-427. [DOI] [PubMed] [Google Scholar]
- 33.Rwambo, P. M., C. J. Issel, W. V. Adams, Jr., K. A. Hussain, M. Miller, and R. C. Montelaro. 1990. Equine infectious anemia virus (EIAV) humoral responses of recipient ponies and antigenic variation during persistent infection. Arch. Virol. 111:199-212. [DOI] [PubMed] [Google Scholar]
- 34.Shen, R.-X., and Z. Wang. 1985. Development and use of an equine infectious anemia donkey leucocyte attenuated vaccine. EIAV: a national review of policies, programs, and future objectives. American Quarter Horse Association, Amarillo, Tex.
- 35.Threadgill, D. S., W. K. Steagall, M. T. Flaherty, F. J. Fuller, S. T. Perry, K. E. Rushlow, S. F. Le Grice, and S. L. Payne. 1993. Characterization of equine infectious anemia virus dUTPase: growth properties of a dUTPase-deficient mutant. J. Virol. 67:2592-2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ui, M., T. Kuwata, T. Igarashi, K. Ibuki, Y. Miyazaki, I. L. Kozyrev, Y. Enose, T. Shimada, H. Uesaka, and H. Yamamoto. 1999. Protection of macaques against a SHIV with a homologous HIV-1 Env and a pathogenic SHIV-89.6P with a heterologous Env by vaccination with multiple gene-deleted SHIVs. Virology 265:252-263. [DOI] [PubMed] [Google Scholar]
- 37.Wyand, M. S., K. H. Manson, M. Garcia-Moll, D. Montefiori, and R. C. Desrosiers. 1996. Vaccine protection by a triple deletion mutant of simian immunodeficiency virus. J. Virol. 70:3724-3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wyand, M. S., K. Manson, D. C. Montefiori, J. D. Lifson, R. P. Johnson, and R. C. Desrosiers. 1999. Protection by live, attenuated simian immunodeficiency virus against heterologous challenge. J. Virol. 73:8356-8363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng, Y. H., H. Sentsui, T. Nakaya, Y. Kono, and K. Ikuta. 1997. In vivo dynamics of equine infectious anemia viruses emerging during febrile episodes: insertions/duplications at the principal neutralizing domain. J. Virol. 71:5031-5039. [DOI] [PMC free article] [PubMed] [Google Scholar]