Abstract
Synthetic calcite (CaCO3) particles are found in a broad range of applications. The geometry of particles produced from limestone or precipitation are versatile but limited to basic shapes. The microalga Emiliania huxleyi produces micro‐structured calcite platelets, called coccoliths. This article presents the results of an application‐orientated study, which includes characteristic values also used in the calcite industry for particle evaluation. It is demonstrated that coccoliths are significantly different from all industrial particles produced so far. Coccoliths are porous particles, mainly consisted of calcium carbonate, with further elements such as Mg, Si, Sr, and Fe often embedded in their structure. Their structure is extremely sophisticated, while the overall particle morphology and particle size distribution are homogeneous. This study gives a first inside into the potential of these exceptional objects and may set further impulses for their utilization in specific calcite particle applications.
Keywords: Biogenic calcium carbonate, Bio‐inspired materials, Coccoliths production, Emiliania huxleyi
Abbreviations
- BET
Brunauer‐Emmett‐Teller
- GCC
ground calcium carbonate
- PCC
precipitated calcium carbonate
- XRD
X‐ray diffraction
1. Introduction
Calcite (CaCO3) particles exhibit versatile and unique properties and are therefore suitable for numerous applications. The majority of industrial calcite derives directly from mined limestone, which is subsequently crushed (GCC = ground calcium carbonate). Besides GCC, synthetic calcite particles (PCC = precipitated calcium carbonate) are produced by controlled introduction of CO2 into lime milk, leading to calcite precipitation. This allows for the production of ultrafine calcite particles down to the nanometer scale. GCC and PCC particles differ mainly in particle shape, powder density, specific surface area, and absorption properties 1. Since PCCs are produced from high‐purity source materials, they are characterized by a high degree of purity, whiteness, and opacity. By varying the process parameters such as temperature, pressure, concentration, and reaction time or by adding additives, it is possible to influence the crystal morphology, for example, to create cubic or acicular particles 2, 3, 4, 5. Calcite production from limestone deposits, originating from the remains of fossil marine organisms, follows a long tradition. However, while there is an increasing interest in innovative and inimitable products, the three‐dimensional complexity of GCC and PCC products is limited. In this sense, the idea was formed to produce calcite particles de novo from cultured microalgae and to evaluate their potential for application.
Biogenic calcite differs from fossil calcite mainly by its exceptionally sophisticated three‐dimensional structure and there is a great versatility between particles of different organisms. However, there are no similar structures known for GCC and PCC and until now, they cannot be reproduced synthetically. Another interesting difference is the presence of organic components. These components form an organic matrix, which is embedded in the calcite structure. They are known to control the crystal formation during the biomineralization process 6. Furthermore, organic components can be found on the particle surface 7. The best‐known species that naturally produces calcite is the unicellular red alga Emiliania huxleyi (E. huxleyi), which belongs to the group of coccolithophores. It is found in almost every ocean, where it represents a substantial part of the phytoplankton. E. huxleyi produces calcite particles in the form of small, micro‐structured platelets, so‐called coccoliths, which cover the cell surface. A coccolith consists of two elliptical, disc‐like, grooved shields, which are connected by a central tube 8. A range of 10–15 coccoliths form a complete coccolith cover, which is referred to as coccosphere. The unique structure of E. huxleyi coccoliths derives from controlled crystal growth inside the cell and cannot be reproduced by comminution or precipitation of calcite.
It is known that coccoliths possess certain interesting chemical and physical properties. For example, coccoliths are more robust in calcium‐free saline solutions and pure water than comparable inorganic calcite particles 9. Specialized solid‐state NMR technologies demonstrated the presence of P‐ and N‐residues, which are internally integrated into the coccolith crystals 10. Despite the incorporation of these residues into the dense crystal lattice, there is no detectable disruption of the chemical or electronic structure. The only measurable effect is a slight decrease in crystal rigidity. Illumination of coccoliths from the side leads to a dynamic change in structural color, when a magnetic field of 0T and 5T is applied at the same time 11. This effect is probably caused by an alteration of the inclination angle. Furthermore, it has been suggested that ethanol can build highly organized structures on the coccolith surface 12.
Since there is in‐depth knowledge about coccolith micro‐structure and some studies about single physical and chemical properties, it is not surprising that there also has been speculation about potential applications. Suggestions include all sectors of conventional PCC and GCC applications such as bulk products like carrier material for paints‐ and lacquers, fillers for tablets, adhesives, and even cements. Also high tech‐applications such as semiconductors, lasers, optics, liquid displays, ultra‐fine surface modification, high‐quality photo papers, and self‐cleaning surfaces were already proposed in the past 13. Unfortunately, there have not been many applications that passed the stage of a rudimentary idea. Takano et al. demonstrated the immobilization of glucose oxidase and uricase onto the coccolith surface over 20 years ago 14. But, as many others, the idea has not been pursued further. What are the reasons for the limited research of coccolith usage in specific applications although there are already various ideas proposed, particularly within the PCC sector? The principal reason is probably the lack of available material quantities. Even for standard methods such as the determination of color values, which is standard for the characterization in the calcite particle industry, several grams of contamination‐free particles are necessary for one measurement.
Although it seems likely to collect coccoliths from their natural environment, it is not feasible. Even during algae blooms, the concentration of coccoliths is usually lower than 1 mg/L and samples are mixed with particles from other strains or species 15. Furthermore, shipping for marine collection is cost‐intensive and the occurrence of certain strains of E. huxleyi cannot be reliably predicted. Therefore, coccolith morphology and quality can differ substantially.
E. huxleyi can be cultivated in principal in the lab, where it also produces coccoliths 13. However, until now, there was no reported process, which yields coccoliths in high concentration. This is probably due to the fact that most research on E. huxleyi traditionally concentrates on bioecological questions. In this regard, the cultivation environments are designed to resemble natural habitats rather than support a coccolith production. Furthermore, it has been reported that coccoliths from cultivation are often malformed or partially destroyed, which may be caused by turbulence or potential toxic influence of the cultivation vessel 16. However, just recently the development of a functioning process, which delivers more than 5 g/L of coccoliths, was finally achieved 17. Although adequate quantity is the first requirement to perform material‐intensive measurements, more prerequisites have to be fulfilled in advance. First, the coccoliths have to be purified from all contaminants like cell debris and salts, prior to investigation. Secondly, the coccoliths have to remain intact during cultivation and purification.
This study uses coccoliths from cultivation, which are purified and subsequently analyzed with methods that are commonly used for industrial PCC and GCC characterization. The results might help to overcome speculation and encourage the assessment of coccoliths for suitable specific applications in the future.
2. Materials and methods
2.1. Purification of coccoliths
Coccoliths of strain E. huxleyi RCC 1216 were obtained from the Institute of Process Engineering in Life‐Sciences, Section III Bioprocess Engineering (Karlsruhe Institute of Technology), in form of culture broth in 5 L glass flasks. The coccolith concentration in the culture was approximately 1.5 g/L. For basic cell disruption, the culture broth was placed inside an incubator at 80°C for at least 48 hours. During incubation coccoliths, and bigger cell fragments settled down to the bottom of the vessel. About 90% of the supernatant was subsequently removed by using a peristaltic pump (Ismatec, IPS‐16). The remaining supernatant was suspended with the coccolith sediment, divided into 40 mL units and filled into 50 mL reaction tubes. The suspensions were centrifuged at 8.000× g for 10 minutes at 4°C (Hettich, Rotina 420R). The supernatant was then carefully removed with a pipette precisely until the 10 mL marking. The pellet was suspended with the remaining supernatant by using a vortex. For further disruption and removal of cell fragments 3.3 mL of 12% NaOCl was mixed into the suspension and incubated for 15 minutes. The reaction tube was subsequently filled up with a 6 mM NaHCO3 solution up to the 40 mL marking.
The washing procedure was initiated with a centrifugation step at 1.500× g for 6 minutes at 4°C. The supernatant was subsequently removed until the 10 mL mark. The coccoliths were suspended and subsequently filled up with fresh 6 mM NaHCO3 solution. The washing procedure was repeated at least four times until the salinity in the suspension was decreased less than 0.2. The final suspension was checked with a light microscope (Zeiss, Axio Scope) to ensure the absence of cell fragments and coccolith agglomerates. It was subsequently centrifuged at 1.500× g for 6 minutes at 4°C and the supernatant was completely removed with a pipette. The pellet was then dried at 80°C for at least 24 hours before analysis.
2.2. Coccolith analysis
The white level determination (compared to MgO) was conducted spectrophotometrically (Minolta, CM‐3610d). The proportion of organic material and CaCO3 was detected thermogravimetrically by using a simultaneous thermal analyzer (Netzsch TG 209F1). The analysis was carried out under nitrogen atmosphere in the range between 20 and 1000°C and a heating range of 5K/min. Qualitative phase analysis (XRD, X‐ray diffraction) was performed with an X‐ray diffractometer (Bragg‐Brentano‐Diffraktometer, D8 Advance von Bruker AXS). Brunauer‐Emmett‐Teller (BET) method was used for the determination of the specific surface area (Quantachrome, Quadrasorb SI). Pore size distribution was measured with the same instrument by applying the nitrogen‐adsorption‐desorption‐method (BJH method). Prior to the measurement, samples were degassed for at least 24 hours at 130°C by using a flow degasser. Sample density was measured by using a gas pycnometer (Micromeritics Multivolume Pycnometer 1305). The measurements were performed using Helium. Chemical composition analysis was conducted by using X‐ray fluorescence spectroscopy (Axios SST mAX, PANalytical). Melt compounds with lithiumtetraborate for measurements were prepared with a fusion machine (HAG 12/1500, Herzog). For a further analysis of any organic matter present in the sample a Fourier transform infrared spectroscopy of the sample was necessary (FT‐IR‐Spektrometer Nicolet 6700). Particle size distribution was determined with laser diffraction (Sympatec, Laser diffraction sensor HELOS/KR). The sample was placed in an ultrasonic bath (0, 60, 120, 180 s) prior to the measure to achieve better distribution. A 0.1% tetrasodium pyrophosphate decahydrate was used as the dispersion liquid. The results were evaluated by using the FREE Fraunhofer theory and the theoretical density of calcite (2.71 g.cm−3). As a second method, sedigraphy (Micromeretics, 5100) was used to measure the equivalence Stokes diameter of the particles. A 0.1% sodium polyphosphate solution was used for dispersion. Similar to the laser diffraction analysis the theoretical density was used to evaluate the results. Scanning electron microscopy pictures were taken with a high voltage electron microscope (Zeiss, DSM 962) at 15 kv. Samples were sputtered with a gold‐palladium layer prior to measurement.
3. Results
3.1. Coccolith appearance: Morphology, integrity, and color
Figure 1 shows scanning electron microscopy pictures of the purified coccoliths. Observation with lower magnification (A) shows that the particles are complete, homogeneous in overall size and shape and free of coccolith agglomerates. Higher magnification (B) reveals particle homogeneity regarding internal micro‐structures within the upper distal shield elements and the central tube.
Figure 1.
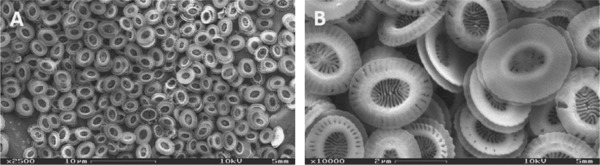
SEM images of purified coccoliths (Zeiss, DSM 962, 2500X (A) and 10000X (B)).
The sophisticated crystal units in the central area of each coccolith and the double‐disk form are sharp and clearly visible. The distal shield and the central tunnel element both exhibit gaps with a length of up to one micrometer. The particle edges appear smooth and undamaged, free of particle agglomerates and no contaminants such as cell debris and salt crystals were found in the suspension. The employed purification method is therefore suitable to provide efficient separation and cleaning of intact coccoliths from culture broth without causing any visible damage.
In comparison to coccoliths, PCC particles exhibit a very broad size distribution and although they are basically homogeneous, they are rather simple, a feature common to all industrially PCC particle variants.
When dried, coccoliths are a white, solid powder. A slight yellow shade, although hardly recognizable with the eye, was detected with color value analysis (Table 1).
Table 1.
Color value analysis of coccoliths measured spectrophotometrically and compared to MgO values
| White‐ and color value analysis | ||
|---|---|---|
| White value R 457 | Relative white value related to MgO | 91.1% |
| Brightness L* | Brightness | 98.2 |
| Color value a* | (‐)Green to (+)red/brown | 0.1 |
| Color value b* | (‐)Blue to (+)yellow | 3.2 |
| Yellow value DIN 6167 | 6.0 | |
The relative deviation from the white value of MgO, which is considered “absolute white,” suggests the presence of components other than calcium carbonate in coccoliths.
3.2. Coccolith size distribution
In order to confirm the visually observed homogeneity shown in the electron microscopy pictures, the coccoliths were further analyzed with laser diffraction and sedigraphy. The two methods presented here rely on different principles and offer a broader study of the particles size. For the laser diffraction method the size of the particles derives from the intensity of the light that is being scattered on a surface, whereas sedigraphy deals with the settling velocities of particles in a known liquid. The measured equivalence diameter was mainly distributed between 0.7 and 1.4 μm. Figure 2 gives the cumulative finer mass percent plotted against the average diameter and the results of four size distribution measurements with laser diffraction spectrometry.
Figure 2.
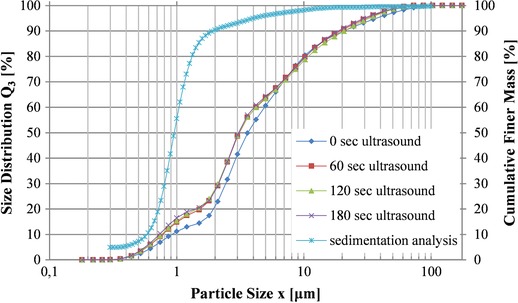
Particle size distributions of coccoliths obtained from two different methods. A sedimentation analysis (Sedigraph) with x 50,St = 0.95 μm and four laser diffraction measurements (HELOS/KR) with different ultrasound intensities with x 50 = 3.11 μm at 60 s ultrasound.
In order to break any agglomerates and disperse the material efficiently, ultrasonication was used. As can be seen in Fig. 2, three different intensities of ultrasonication were used and compared with the results of the first method were no ultrasound was applied. As expected, the measured coccoliths diameter was influenced by the ultrasound treatment and led to smaller detected particles. Although three different intensities where used, Fig. 2 verifies that the treatment for 60 s is sufficient for the sample and no further alterations are detected by increasing the duration of the treatment. There are some smaller particles detected with this analysis (x 16 = 1.09 μm), though it is difficult to know if this result reflects to broken particles or if it represents the percentage of particles that are alight infront of the laser in such a way that smaller particles are assumed to be in the sample.
The results from both methods are given in Table 2. Based on the results of the laser diffraction analysis 50% of the particles have a diameter smaller than 3.11 μm and 84% of the particles are equal or smaller than 12.67 μm. Both measurements only represent a first insight of the particles size.
Table 2.
Particle size analysis for coccoliths given by laser diffraction spectrometry and sedimentation analysis
| Particle size [μm]/Laser diffraction | Equivalence particle diameter [μm]/Sedimentation analysis | ||
|---|---|---|---|
| x 84 | 12.67 | x 84,St. | 1.43 |
| x 50 | 3.11 | x 50,St. | 0.95 |
| x 16 | 1.09 | x 16,St. | 0.67 |
3.3. Coccolith pore structure
Besides the grooves, which are part of the particle micro‐structure (Fig. 1B), the material itself exhibits mesopores in the range of 4 nm and a total pore volume of 0.05 cm³.g−1 (Table 3). The pore volume and area distribution were obtained from the BJH method proposed by Barrett et al. 18, by analyzing the nitrogen desorption isotherms. The overall geometry resulted in a specific surface area of 19 m².g−1, measured with the BET method 19. This method is widely used for determining the specific surface area of solid materials. The powder density was measured at 2.6 g.cm−3 with a gas pycnometer. This is slightly lower than the density of pure calcite, which is 2.715 g.cm−3.
Table 3.
Summary of coccolith pore and surface characteristics obtained by the BJH and BET method
| Product properties | |
|---|---|
| Total pore volume | 0.05 cm3 .g−1 |
| Most frequent pore diameter | 3.7 nm |
| Specific surface area | 19 m².g−1 |
| Powder density | 2.6 g.cm−3 |
The powder density was measured with a gas pycnometer.
3.4. Chemical composition and crystal structure
Both, the yellowish coloration and the difference between coccolith density and calcite density indicate the presence of matter other than calcite in coccoliths. This presumption could be confirmed with a material composition analysis. As coccoliths are biocomposites, the presence of organic material was anticipated before measurement. However, it was not clear to which amount it would remain in and on the surface of the coccolith after purification, especially after sodium hypochlorite treatment.
Thermogravimetry showed that the proportion of organic material was approximately 4%, whereas the coccolith consisted of 87% calcium carbonate (Fig. 3). The remaining 9% showed to be primarily Mg and Si residues (Table 4). Other elements like Fe, Al, P, Mn, and Sr were also found in minor proportions.
Figure 3.
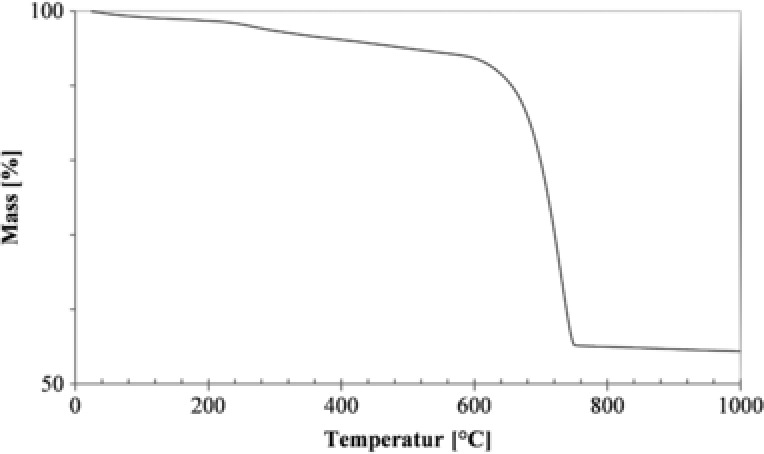
Thermogravimetric analysis of coccoliths. The decrease of weight (%) between 200°C and 600°C derives from the combustion of organic material (–5.0%). The decrease of weight (%) between 600°C and 780°C derives from the decomposition of CaCO3 (–39.7%).
Table 4.
Chemical composition of coccoliths measured with XRF‐analysis
| Chemical analysis (glowed) | |||||
|---|---|---|---|---|---|
| CaO | 85.1% | K2O | 0.006% | P2O5 | 0.392% |
| MgO | 6.4% | TiO2 | 0.004% | Mn3O4 | 0.038% |
| SiO2 | 6.6% | Na2O | 0.222% | SO4 | 0.038% |
| Fe2O3 | 0.15% | SrO | 0.606% | Σ | 100% |
| Al2O3 | 0.37% | BaO | 0.004% | CaCO3 | 86% |
XRD‐analysis revealed that calcite and aragonite are the two crystal modifications of calcium carbonate that are present in coccoliths (Fig. 4). Halite could not be detected, which again demonstrates, that all salt from the cultivation medium was removed with the chosen purification method.
Figure 4.
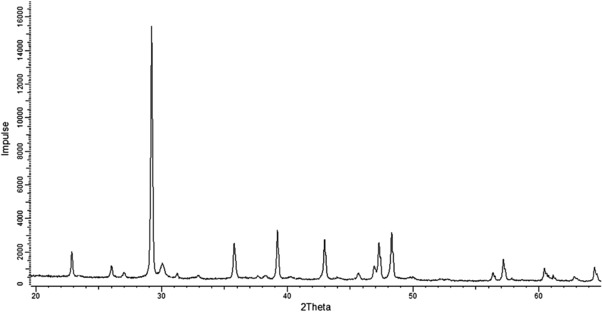
X‐ray diffraction patterns of coccoliths (XRD; Bragg‐Brentano‐Diffraktometer, D8 Advance von Bruker AXS). Calcite and Aragonite are the two crystalline phases identified in the sample.
The crystalline size of calcite from the Peak–Profile analysis with the Pawley–Fit method was measured at 153 nm and for Aragonite at 68.4 nm.
The fingerprint region of the FT‐IR analysis with the vibrational bands at 1394.02 cm−1, 871.45 cm−1, and 712.4 cm−1 confirmed that the sample is consisted of CaCO3. The sharp peak at 712.4 cm−1 and 871.45 cm−1 are characteristic for calcite. A smaller peak signal at 2515.03 cm−1 probably represents a C‐O stretch whereas the three peak signals between 2800 and 3000 cm−1 represent a C‐H stretching. Traces of hydrocarbons are detected in the sample (Fig. 5) and, as expected, the organic portion is found to be relatively low, which verifies the results of the other measurements. Further functional groups were not detected in the sample.
Figure 5.
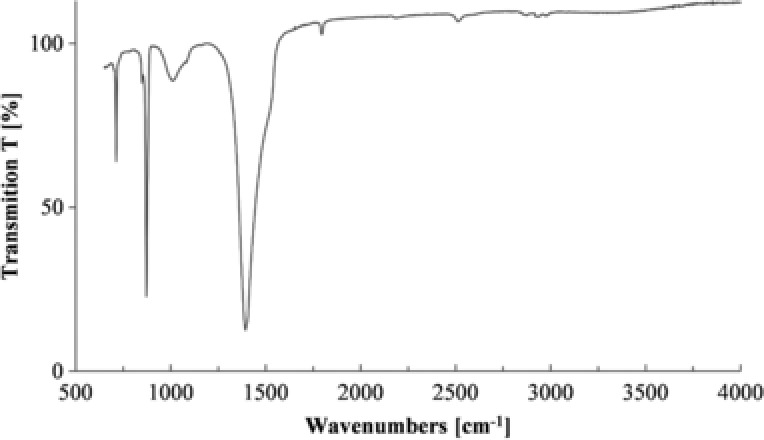
FT‐IR spectra of coccoliths (FT‐IR‐Spektrometer Nicolet 6700).
4. Discussion
4.1. Chemical composition and crystal structure
The characterization of coccoliths from the lab‐cultured strain E. huxleyi RCC1216 were performed with methods popular for PCC and GCC characterization. The form and composition of the particles, allows only some of the executed methods to be optimal for coccoliths. This is obvious in the case of particle size distribution. Both methods, sedigraphy and laser diffraction spectrometry demonstrated very narrowly distributed values for coccoliths. This result was not surprising, as the coccoliths showed to be intact in the electron micrographs. Natural deviations in coccolith size are small and are only due to different cell sizes, which depend on the cell cycle. However, we believe that both methods fail to deliver a precise picture of size distribution. As already mentioned, sedigraphy measures the sinking velocity of particles from which the diameter of an equivalent spherical body is calculated according to Stokes’ law. However, the double‐disk shaped coccoliths are nonspherical. It is not possibly to recalculate coccolith diameter from this data because we lack information about the calcification density of each particle, such as thickness or length of the individual particle crystals. The same challenge would have to be faced with electrical sensing zone techniques like the Coulter principle, which is also popular for particle size determination.
For the laser diffraction method particle size determination is based on the theory that particles scatter light at various angles and intensities relative to their particle size. Small particles will scatter light at wider angles than larger particles. Once the scattering pattern is measured, a particle size distribution is calculated using theoretical models as defined by the Fraunhofer Theory. For this sample, the particle size and the lack of concrete information are enough reasons to choose the Fraunhofer evaluation method. By applying the Mie theory the error of the results would be more evident. Similar to sedigraphy, particle size resembles diameter of a sphere, which would scatter light at the same angle and intensities. This depends on the orientation of the particle when analyzed. However, nonspherical particles like coccoliths will scatter light depending on their orientation in front of the light sensor. The laser can theoretically hit a coccolith at the distal shield, the proximal shield or even at the central tunnel element. Although it was shown that in most systems, the flow is very laminar and therefore very homogeneous for singular particles 20, our results suggest that it is difficult to ensure perfect dispersion in the case of coccoliths. Due to their double‐disk shape coccoliths tend to align themselves together, similar to their natural junction on the algal surface, which can impede the flow through the cell. Additionally, there can be measuring inaccuracies due to the sharp edges of the coccolith shields. Detection of imaginary particles at sharp edges is possible because of the large angles the instrument laser makes upon them 21. Although the chosen methods are insufficient to measure a coccolith size distribution as precise as for PCC, they provide useful information about their homogeneity. Traditional methods for marine sediment characterization include sieve analysis and size determination by manual measuring of electron micrographs 22, which are both very error‐prone and laborious. In this regard, methods like computational image analysis should be developed for coccoliths in order to determine realistic quantitative size distributions. Interestingly, a technique to determine coccolith thickness was also just recently published and could strongly support an efficient characterization and quality control of produced coccoliths 23.
4.2. Potential field of application
Although the use of coccoliths in industrial application is still at the stage of idea gathering, the results of this study can illustrate the constraints of some suggestions or even expose them as fully unsuitable. Other fields of applications are more promising. Pharmaceutical applications, for example tablets, require high‐purity calcium carbonate. It is obvious why biogenic calcite is an inappropriate choice in this case. The coccoliths produced in the lab showed to be a composite material of organic matter, calcium carbonate, and various other elements. All these elements are part of the cultivation medium, which approximates the composition of natural seawater. It is therefore comprehensible that Si‐ and Mg‐ residues are the second most abundant elements besides calcium, because they are also present in the medium in relatively large stoichiometric quantities. However, not all applications in the medical sectors must necessarily be excluded, in some cases coccolith material composition could even be an advantage. In medical implants biominerals have benefits compared to metals, because they better resemble the human bone material and possess favorable sound conduction properties 24. Therefore ideas that include the application of coccoliths in biomedical applications like artificial dental roots and artificial bone material 25 should definitely be pursued further.
Analyses revealed that coccoliths are porous with pores in the micrometer and nanometer range. High porosity is a key factor, which brings about good absorption capacity 26. In the future it will therefore be interesting to investigate their potential as substrate for paint‐ or lacquer coat or filler in adhesives or materials with enhanced porosity. Porosity is also an important factor within material science, for example the use in optically active surfaces or the design of new hybrid materials in conjunction with (bio‐) polymers. Also the research about enzyme binding on coccoliths conducted by Takano et al. 14 should definitely be followed up and also the immobilization of biopolymers or of inorganic catalysts is a realistic attempt. Despite that PCC particles with similar porosity exist on the market, there are no particles known yet with similar porosity and size distribution.
However, this study was determined with coccoliths that derived from algal cells of a single cultivation in basic artificial seawater. We know that E. huxleyi can easily be cultivated in modified medium, such as Si‐free medium, with similar coccolith production rates (unpublished results). Hence, we suggest that the elemental composition of coccoliths is influenceable by cultivation conditions. In the case of some elements like Si, this may result in a change of mechanical properties like abrasiveness. There are also elements that influence color, for example iron or other transition metals like copper. A reduction or increase of those metals could increase the white value of the coccoliths and make them more suitable for the use as pigments in paint. Combined with the birefringent properties of calcite this may lead to interesting gloss properties of the treated surfaces.
5. Concluding remarks
Although many ideas for the industrial application of coccoliths were proposed in the past, a lack of sufficient material quantities impeded any further steps toward a realization. In this study, we successfully analyzed lab produced coccoliths with standard methods of the calcium carbonate particle industry. Although gathered outside nature, these coccoliths showed to be structurally intact and homogeneous. They are not solely build of solid calcite crystals and organic surface matter but can be described as a porous biocomposite with a complex composition of various elements, which are also found in the cultivation medium. Compared to all known PCC or GCC particles, this makes them unique not only on a structural but also on a chemical level and suggests future potential for the targeted design of coccolith composition within bioprocess engineering. Although coccoliths showed to be different to all commercial calcite particles, which are producible by technical means, the characterization makes it now easier to assess the potential of some suggested ideas. This might finally encourage the start or resumption of further specialized research on a concrete application for coccoliths.
Practical application
The main goal of this research is to analyze some of the properties of coccoliths, a porous biocomposite material with a complex composition of various elements. Compared to all known PCC or GCC particles, they are unique with a distinct structure and a promising usage potential. Through bioprocess engineering the particles could be used for various applications and designs of coccoliths targeted compositions could be achieved. Many other ideas for the usage of coccoliths were proposed in the past, but a lack of sufficient material quantities impeded any further steps toward a realization. Although coccoliths showed to be different to all commercial calcite particles, which are produced by technical means, the characterization makes it now easier to assess the potential of some of the suggested ideas. With this study we hope to encourage the start or resumption of further specialized research on a concrete application for coccoliths.
The authors have declared no conflicts of interest.
Acknowledgments
The underlying research project is funded by The German Federal Ministry of Education and Research (BMBF) within the bioeconomy 2020 program. We would like to express our gratitude to all project partners of the ZeBiCa² project, especially to SCHAEFER Kalk. Particular thanks to M. Krämer and I. Diegel for the conduction of the analyses.
6 References
- 1. Heberling, F. , Bosbach, D. , Eckhardt, J. D. , Fischer, U. et al., Reactivity of the calcite–water‐interface, from molecular scale processes to geochemical engineering. Appl. Geochem. 2014, 45, 158–190. [Google Scholar]
- 2. Sangwal, K. , Additives and Crystallization Processes: Additives and Crystallization Processes: From Fundamentals to Applications, 1st edn., Wiley, Chichester, UK: 2007. [Google Scholar]
- 3. Han, Y. S. , Hadiko, G. , Fuji, M. , Takahashi, M. , Influence of initial CaCl2 concentration on the phase and morphology of CaCO3 prepared by carbonation. J. Mater. Sci. 2006, 41, 4663–4667. [Google Scholar]
- 4. Harja, M. , Cretescu, I. , Rusu, L. , Ciocinta, R. C. , The influence of experimental factors on calcium carbonate morphology prepared by carbonation. Rev. Chim. 2009, 60, 1258–1263. [Google Scholar]
- 5. Ukrainczyk, M. , Stelling, J. , Vučak, M. , Neumann, T. , Influence of etidronic acid and tartaric acid on the growth of different calcite morphologies. J. Cryst. Growth 2013, 369, 21–31. [Google Scholar]
- 6. Marsh, M. E. , Regulation of CaCO3 formation in coccolithophores. Comp. Biochem. Phys. B 2003, 136, 743–754. [DOI] [PubMed] [Google Scholar]
- 7. Godoi, R. H. , Aerts, K. , Harlay, J. , Kaegi, R. et al., Organic surface coating on Coccolithophores—Emiliania huxleyi: Its determination and implication in the marine carbon cycle. Microchem. J. 2009, 91, 266–271. [Google Scholar]
- 8. Young, J. R. , Didymus, J. M. , Bown, P. R. , Prins, B. et al., Crystal assembly and phylogenetic evolution in heterococcoliths. Nature 1992, 356, 516–518. [Google Scholar]
- 9. Hassenkam, T. , Johnsson, A. , Bechgaard, K. , Stipp, S. L. S. , Tracking single coccolith dissolution with picogram resolution and implications for CO2 sequestration and ocean acidification. Proc. Natl. Acad. Sci. USA 2011, 108, 8571–8576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gertman, R. , Ben Shir, I. , Kababya, S. , Schmidt, A. , In situ observation of the internal structure and composition of biomineralized Emiliania huxleyi calcite by solid‐state NMR spectroscopy. J. Am. Chem. Soc. 2008, 130, 13425–13432. [DOI] [PubMed] [Google Scholar]
- 11. Iwasaka, M. , Mizukawa, Y. , Magneto‐optical properties of biogenic photonic crystals in algae. J. Appl. Phys. 2014, 115, 17B501. [Google Scholar]
- 12. Pasarín, I. S. , Yang, M. , Bovet, N. , Glyvradal, M. et al., Molecular ordering of ethanol at the calcite surface. Langmuir 2012, 28, 2545–2550. [DOI] [PubMed] [Google Scholar]
- 13. Krumov, N. , Posten, C. , Nanostructured particles from coccolithophores–an undiscovered resource for application, in: Rai M., Posten C. (Eds.), Green Biosynthesis of Nanoparticles: Mechanisms and Applications, 1st edn., CABI, Oxfordshire, UK: 2013, pp. 192–215. [Google Scholar]
- 14. Takano, H. , Manabe, E. , Hirano, M. , Okazaki, M. et al., Development of a rapid isolation procedure for coccolith ultrafine particles produced by coccolithophorid algae. Appl. Biochem. Biotech. 1993, 40, 239–247. [Google Scholar]
- 15. Thierstein, H. R. , Young, J. R. , Coccolithophores: From Molecular Processes to Global Impact, 1st edn., Springer, Berlin Heidelberg, Germany: 2004. [Google Scholar]
- 16. Langer, G. , Oetjen, K. , Brenneis, T. , On culture artefacts in coccolith morphology. Helgoland Mar. Res. 2013, 67, 359–369. [Google Scholar]
- 17. Hariskos, I. , Posten, C. , Produktion und Charakterisierung von biogenen, mikrostrukturierten Calcitpartikeln ‐ ein unkonventionelles Produkt aus Mikroalgen, presented at 8. DECHEMA Bundesalgenstammtisch, 08.09.2015, Munich, Germany.
- 18. Barrett, E. P. , Joyner, L. G. , Halenda, P. , The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1954, 73, 373–380. [Google Scholar]
- 19. Brunauer, S. , Emmett, P. H. , Teller, E. , Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–318. [Google Scholar]
- 20. Berthold, C. , Klein, R. , Lühmann, J. , Nickel, K. G. , Characterization of fibres and fibre collectives with common laser diffractometers. Par. Part. Syst. Car. 2000, 17, 113–116. [Google Scholar]
- 21. Kelly, R. N. , Etzler, F. M. , What is wrong with laser diffraction?: A critical review of current laser diffraction methods for particle size analysis. Donner Technologies, http://www.donner-tech.com/whats_wrong_with_ld.pdf.
- 22. Frenz, M. , Baumann, K. H. , Boeckel, B. , Höppner, R. et al., Quantification of foraminifer and coccolith carbonate in South Atlantic surface sediments by means of carbonate grain‐size distributions. J. Sediment. Res. 2005, 75, 464–475. [Google Scholar]
- 23. Beaufort, L. , Barbarin, N. , Gally, Y. , Optical measurements to determine the thickness of calcite crystals and the mass of thin carbonate particles such as coccoliths. Nat. Prot. 2014, 9, 633–642. [DOI] [PubMed] [Google Scholar]
- 24. Behrens, P. , Jahrbuch 2005 der Braunschweigischen Wissenschaftlichen Gesellschaft: Das Feste im Leben: Das Feste im Leben: Biomineralisation—Bioinspiration—Biomaterialien, Cramer, Braunschweig, Germany: 2006. [Google Scholar]
- 25. Moheimani, N. R. , Webb, J. P. , Borowitzka, M. A. , Bioremediation and other potential applications of coccolithophorid algae: A review. Algal Res. 2012, 1, 120–133. [Google Scholar]
- 26. Trushina, D. B. , Bukreeva, T. V. , Kovalchuk, M. V. , Antipina, M. N. , CaCO₃ vaterite microparticles for biomedical and personal care applications. Mat. Sci. Eng. C 2015, 45, 644–658. [DOI] [PubMed] [Google Scholar]


