ABSTRACT
Background
The incidence of radiation‐induced hypothyroidism (RIH) in patients with head and neck cancer is >50%. The purpose of this study was to assess the long‐term efficacy of free thyroid transfer (FTT) for prevention of RIH in patients with head and neck cancer.
Methods
Hemithyroid dissection was completed in 10 patients with advanced head and neck cancer undergoing ablation, radial forearm free flap (RFFF) reconstruction, and postoperative radiotherapy (RT). The hemithyroid was anastomosed to the donor site vessels in the forearm. Thyroid laboratory testing and technetium (Tc) scans were performed 6 weeks and 12 months postoperatively to establish functional integrity.
Results
FTT was successfully performed in 9 of 10 recruited patients. Postoperative Tc scans demonstrated strong Tc uptake in the forearm donor site at 6 weeks and 12 months in all patients who underwent transplantations.
Conclusion
FTT is feasible with maintenance of function, and may represent a novel strategy for prevention of RIH. © 2016 Elsevier Head & Neck Published by Wiley Periodicals, Inc. Head Neck 39: 1234–1238, 2017
Keywords: thyroid, head neck cancer, radiation‐induced hypothyroidism
INTRODUCTION
Radiation is a key component in the treatment of most advanced‐stage head and neck cancers.1, 2 A common long‐term adverse effect of head and neck irradiation is radiation‐induced hypothyroidism (RIH), which occurs in up to 53% of patients.3 Symptoms of hypothyroidism after radiotherapy (RT) are often unrecognized or misdiagnosed for a significant period of time during treatment and, as such, can significantly affect the patient's health‐related quality of life.4 Hypothyroidism requires treatment with life‐long thyroid hormone supplements, necessitating treatment compliance, which can be challenging in the head and neck cancer population. Novel strategies to reduce this adverse effect may enhance patients' recovery and limit the consequences of untreated hypothyroidism.
The concept of a gland transfer out of the radiation field for maintenance of gland function has been established previously.5 Several trials evaluating the efficacy of the submandibular gland transfer for prevention of radiation‐induced xerostomia have demonstrated a significant improvement in quality of life in patients with head and neck cancer.6, 7, 8 In a similar manner, free thyroid transfer (FTT) out of the radiation field may help prevent RIH.
Vascular supply for the free‐tissue transfer of the thyroid gland is provided by the ipsilateral superior thyroid artery and the superior or middle thyroid vein that drains directly into the internal jugular vein, as described in a previous study.9 To the authors' knowledge, free‐tissue transfer of the thyroid gland has not previously been performed for the purposes of prevention of RIH.
The purpose of this study was to determine the feasibility and long‐term functional viability of the FTT procedure in adult patients with head and neck cancer undergoing curative ablation and reconstruction followed by adjuvant RT.
MATERIALS AND METHODS
Before commencing the study, ethical approval was obtained from the University of Alberta Health Research Ethics Board.
Study design
A prospective feasibility study was performed in 10 patients with a new diagnosis of advanced head and neck cancer undergoing ablative and reconstructive surgery involving radial forearm free flap (RFFF) reconstruction. All patients received treatment at the University of Alberta Hospital and Cross Cancer Institute. Patient selection criteria are demonstrated in Table 1.
Table 1.
Patient eligibility criteria for the free thyroid transfer procedure.
| Inclusion criteria | Exclusion criteria |
|---|---|
|
• New diagnosis of stage III or IV head and neck cancer. • Receiving ablative surgery requiring RFFF r econstruction as primary treatment. • Requires postoperative adjuvant RT. |
• Received prior RT to the head or neck. • Medical history of thyroid disease or surgery. • Abnormal preoperative biochemical thyroid function laboratory work, including TSH level, free T3, or free T4 levels. • Level VI paratracheal lymph nodes that demonstrate or may be at risk for metastatic spread. • Allergy to iodine. |
Abbreviations: RT, radiotherapy; RFFF, radial forearm free flap; TSH, thyroid‐stimulating hormone.
Study procedures
Patients who were deemed eligible for the study were recruited in the Head and Neck Oncology Clinic at the University of Alberta Hospital and informed consent was obtained.
All patients included in the study underwent primary surgical treatment, including ablative surgical resection of the primary tumor, unilateral or bilateral neck dissections, and RFFF reconstruction from November 4, 2013, to December 4, 2014. In addition, all patients underwent preoperative laryngoscopy to confirm normal vocal cord movement and function before surgery, as well as preoperative ultrasonography to confirm the absence of thyroid nodules. All operations were performed with the patients under general anesthesia, and all patients underwent tracheostomy as part of the ablative and reconstructive procedure. Tracheal secretions were kept from the neck wound by placing a cuffed endotracheal tube in the stoma at 25 mm H20. All patients underwent adjuvant RT at a dose of 60 Gy.
Free thyroid transfer procedure
During the neck dissection, the primary surgeon performed an additional hemithyroidectomy through the apron neck‐dissection incision, wherein the ipsilateral superior thyroid artery was identified, dissected, and preserved as the arterial pedicle for the FTT. The superior and middle thyroid veins were identified and dissected, and the larger of the 2 venous pedicles was selected for the venous pedicle (see Figure 1). RFFF harvest was then performed. During insetting of the head and neck RFFF into the oncologic defect, the resected hemithyroid was implanted into the forearm donor site (see Figure 2). Microvascular anastomoses were performed from the resected hemithyroid to the forearm donor site radial artery and cephalic vein, respectively, using a suture technique. As is standard practice at the University of Alberta, the vascular pedicles of both the free flap and thyroid gland anastomoses were ensheathed with implantable Cook–Schwartz Dopplers to monitor the flow postoperatively.
Figure 1.
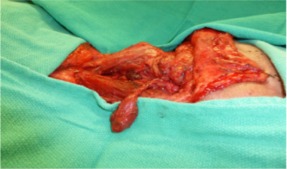
Hemithyroid dissection with preservation of arterial and venous anastomotic vessels.
Figure 2.
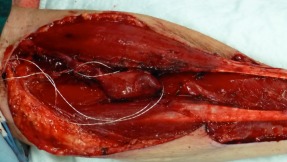
Transferred hemithyroid anastomosed to forearm donor site vessels with implantable Cook‐Schwartz Doppler probes.
The total harvest time for the FTT was calculated from the time of initiation of the hemithyroidectomy to the successful isolation of the vascular pedicles. The measured harvest time was used as a surrogate for the total additional operative time, as the microvascular anastomoses for the FTT were performed at the time of insetting. Radial forearm donor sites were skin‐grafted with split‐thickness skin grafts, which were monitored daily for seroma, hematoma, infection, and skin graft failure. Cook–Schwartz Dopplers were removed at 9 days, as is standard procedure at the University of Alberta.
Follow‐up procedures
Participants completed a diagnostic radioactive technetium (Tc) scan at the forearm donor site 6 weeks postoperatively, and at 6‐month intervals thereafter to assess iodine uptake by the transferred thyroid tissue as a measure of functional integrity. In addition, all patients had routine thyroid laboratory testing, including a thyroid‐stimulating hormone (TSH) level, free T3 level, and free T4 level at the same intervals postoperatively to ensure biochemical integrity of the implanted thyroid tissue.
RESULTS
Ten patients underwent the FTT procedure. Demographic characteristics of the patient population are demonstrated in Table 2. The mean age of the study group was 58.3 years, and the predominant cancer primary site was the oropharynx.
Table 2.
Demographic and surgical details for patients with head and neck cancer undergoing free thyroid transfer.
| Patient no. | Age, y | Sex | Site | Stage | Smoking status | Additional operative time, min |
|---|---|---|---|---|---|---|
| 1 | 59 | Male | Oral cavity | T2N2bM0 | Yes | 50 |
| 2 | 38 | Female | Oropharynx | T3N2bM0 | No | NC |
| 3 | 63 | Female | Parotid | T2N1M0 | Yes | 13 |
| 4 | 60 | Male | Hypopharynx | T4AN2CM0 | Yes | 43 |
| 5 | 53 | Female | Oropharynx | T3N2CM0 | No | 36 |
| 6 | 76 | Male | Oropharynx | T2N2BM0 | Yes | 60 |
| 7 | 64 | Male | Oropharynx | T4AN2CM0 | Yes | 42 |
| 8 | 66 | Female | Oropharynx | T2N2BM0 | No | 40 |
| 9 | 45 | Female | Oral cavity | T3N1M0 | No | 54 |
| 10 | 54 | Male | Oropharynx | T2N3M0 | Yes | 38 |
| Mean | 58.3 | – | – | – | – | 41.6 |
Abbreviation: NC, not completed.
The FTT procedure was completed in 9 patients. Anastomosis was not achieved in the second patient of the cohort because of both the small caliber of the superior and middle thyroid veins, and a short cephalic vein stump for anastomosis. Therefore, the procedure was abandoned in this patient.
A mean of 41.6 minutes of additional operative time was added to the ablative and reconstructive procedure for the 9 patients who underwent transplantations. No postoperative complications regarding seromas, hematomas, skin graft failure, or infection at the radial forearm site were observed. All Dopplers were removed at 9 days without complication. No complications regarding recurrent laryngeal nerve injury, superior laryngeal nerve injury, or transient or permanent hypocalcemia occurred.
The Tc thyroid scans were completed at 6 weeks postoperatively in 9 of 9 patients who underwent transplantations and all demonstrated strong uptake of Tc at the forearm donor site (see Figure 3). Two patients remained euthyroid at 12 months and demonstrated uptake only in the forearm, indicating the potential for development of RIH without the FTT procedure. Laboratory testing with TSH, free T4 level, and free T3 level were within normal limits for 6 of 6 patients remaining living at 6 weeks, 6 months, and 12 months postoperatively (12‐month results shown only). Laboratory results are outlined in Table 3.
Figure 3.
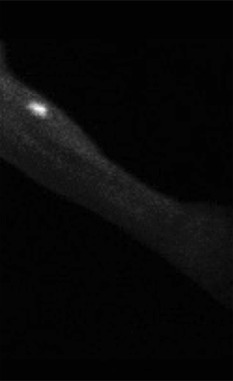
Tc uptake at the transplanted forearm site.
Table 3.
Treatment details for patients undergoing free thyroid transfer.
| Patient no. | RT, Gy | Chemotherapy | Follow‐up, mo | Deceased | Tc scan | TSH, 12 mo | Free T3, 12 mo | Free T4, 12 mo |
|---|---|---|---|---|---|---|---|---|
| 1 | 60 | No | 14.7 | Yes | F | 1.28 | 16.9 | 3.2 |
| 2 | NC | NC | NC | No | NC | NC | NC | NC |
| 3 | 60 | No | 14.8 | No | F | 2.09 | 13.9 | 4.2 |
| 4 | 60 | No | 3.7 | Yes | F | 1.49a | 11.3a | 4.1a |
| 5 | 60 | Yes | 13.0 | No | F | 2.12 | 14.8 | 3.8 |
| 6 | 60 | No | 3.7 | No | F | 1.45a | 16.8a | 3.9a |
| 7 | 60 | Yes | 4.9 | Yes | F | 0.28a | 14.1a | 5.2a |
| 8 | 60 | Yes | 12.8 | No | F | 2.78 | 11.9 | 5.0 |
| 9 | 60 | No | 26.8 | No | F | 1.80 | 12.3 | 4.4 |
| 10 | 60 | No | 19.8 | No | F | 1.40 | 15.6 | 5.3 |
Abbreviations: RT, radiotherapy; Tc, technetium; TSH, thyroid‐stimulating hormone; F, functional; NC, not completed.
Indicates last available level before death.
DISCUSSION
RIH is common in patients with head and neck cancer after ablative surgery and postoperative adjuvant RT, in particular in those patients undergoing treatment for oropharyngeal and hypopharyngeal cancers. Strategies to avoid a hypothyroid state, tedious medication titration, and arduous monitoring of compliance should be undertaken. This study illustrates the feasibility of the FTT procedure as a potential strategy in preventing RIH in patients with head and neck cancer after a minimum of 12 months' follow‐up.
Previous studies involving transfer of a gland out of the field of RT were initiated by Jha et al.5 This series of studies were first initiated with a phase I feasibility study that involved transfer of the submandibular gland out of the field of RT without the need for microvascular anastomoses, with an additional operative time of approximately 45 minutes. Subsequently, the authors described long‐term preservation of salivary flow in 83% of patients compared to 0% in the nontransfer group after a mean of 70 Gy neck treatment and a minimum of 2 years of follow‐up.10 Further studies involving submandibular gland transplantation for treatment of keratoconjunctivitis sicca with microvascular anastomoses to the superficial temporal artery and vein were completed by Yu et al11 in 2004. Several author groups went on to replicate and propagate this procedure,12, 13, 14, 15, 16 performing postoperative Tc scans, as in our study, to examine functional integrity with subjectively and objectively successful results.
This procedure should be performed by surgeons with extensive experience with thyroid surgery and head and neck microvascular free‐tissue transfer, as well as those with access to the monitoring resources provided by a tertiary care center. Potential complications of this procedure are those that would exist for a hemithyroidectomy, including superior laryngeal nerve or recurrent laryngeal nerve injury, or temporary or permanent hypocalcemia. In addition, donor site complications, including bleeding, infection, seroma, hematoma, and skin graft failure may occur. Additional potential complications may include vascular compromise of the thyroid tissue and necrosis, necessitating surgical removal. Care must be taken by the microvascular surgeon to avoid performing FTT in the setting of potential metastases to the paratracheal or pretracheal lymph node regions. In addition, careful surveillance and communication with primary care physicians is necessary in order to ensure coordinated care is provided to patients who undergo the FTT procedure.
There were several limitations present in this study. This was a pilot study describing our initial experience with a small dataset. As such, the success of this procedure and lack of complications in a small cohort should not be generalized to all patients with head and neck cancer. In addition, patient selection criteria may need to be altered if complications are observed in a larger cohort. Last, longer‐term follow‐up will be required to determine the enduring viability of the FTT procedure.
Currently, this technique is in the IDEAL stage of “Development,” and will thus require longer follow‐up to determine utility, efficacy, and consensus among experts in the field, which is ongoing.17 Interestingly, 2 of the 9 patients demonstrated functional thyroid uptake in the forearm only, implying that the transplanted tissue was the only functioning tissue after 12 months. Further study examining the functional longevity of tissue in both the neck and the forearm will be published with longer‐term results. A multidisciplinary discussion to determine patients at risk of developing RIH based on the primary site of cancer may allow for optimal patient selection for the procedure. Although only a subset of surgical candidates may ultimately benefit from this procedure, avoiding RIH in eligible patients may be feasible with this procedure. A larger study with longer‐term follow‐up is currently underway, and, if successful, a multicenter trial may be initiated.
CONCLUSION
The present feasibility study describes a novel surgical strategy that may decrease the risk of RIH in patients with head and neck cancer. A study regarding longer‐term feasibility of the FTT procedure is ongoing.
Reprinted as adapted from International Journal of Radiation Oncology Biology Physics, vol 96(1), Harris J, Almarzouki H, Barber B, et al, Free thyroid transfer: A novel procedure to prevent radiation‐induced hypothyroidism, pp. 42 ‐ 45, copyright 2016, with permission from Elsevier.
This article was published online on 15 November 2016. An error was subsequently identified. This notice is included in the online and print versions to indicate that both have been corrected 9 February 2017.
REFERENCES
- 1. Bernier J, Cooper JS, Pajak TF, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (#9501). Head Neck 2005;27:843–850. [DOI] [PubMed] [Google Scholar]
- 2. Cooper JS, Zhang Q, Pajak TF, et al. Long‐term follow‐up of the RTOG 9501/intergroup phase III trial: postoperative concurrent radiation therapy and chemotherapy in high‐risk squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys 2012;84:1198–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boomsma MJ, Bijl HP, Langendijk JA. Radiation‐induced hypothyroidism in head and neck cancer patients: a systematic review. Radiother Oncol 2011;99:1–5. [DOI] [PubMed] [Google Scholar]
- 4. Langendijk JA, Doornaert P, Verdonck–de Leeuw IM, Leemans CR, Aaronson NK, Slotman BJ. Impact of late treatment‐related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol 2008;26:3770–3776. [DOI] [PubMed] [Google Scholar]
- 5. Jha N, Seikaly H, McGaw T, Coulter L. Submandibular salivary gland transfer prevents radiation‐induced xerostomia. Int J Radiat Oncol Biol Phys 2000;46:7–11. [DOI] [PubMed] [Google Scholar]
- 6. Seikaly H, Jha N, McGaw T, Coulter L, Liu R, Oldring D. Submandibular gland transfer: a new method of preventing radiation‐induced xerostomia. Laryngoscope 2001;111:347–352. [DOI] [PubMed] [Google Scholar]
- 7. Rieger J, Seikaly H, Jha N, et al. Submandibular gland transfer for prevention of xerostomia after radiation therapy: swallowing outcomes. Arch Otolaryngol Head Neck Surg 2005;131:140–145. [DOI] [PubMed] [Google Scholar]
- 8. Jha N, Seikaly H, Harris J, et al. Phase III randomized study: oral pilocarpine versus submandibular salivary gland transfer protocol for the management of radiation‐induced xerostomia. Head Neck 2009;31:234–243. [DOI] [PubMed] [Google Scholar]
- 9. Genden EM, Gannon PJ, Smith S, Keck N, Deftereos M, Urken ML. Microvascular transfer of long tracheal autograft segments in the canine model. Laryngoscope 2002;112:439–444. [DOI] [PubMed] [Google Scholar]
- 10. Seikaly H, Jha N, Harris JR, et al. Long‐term outcomes of submandibular gland transfer for prevention of postradiation xerostomia. Arch Otolaryngol Head Neck Surg 2004;130:956–961. [DOI] [PubMed] [Google Scholar]
- 11. Yu GY, Zhu ZH, Mao C, et al. Microvascular autologous submandibular gland transfer in severe cases of keratoconjunctivitis sicca. Int J Oral Maxillofac Surg 2004;33:235–239. [DOI] [PubMed] [Google Scholar]
- 12. Geerling G, Sieg P, Bastian GO, Laqua H. Transplantation of the autologous submandibular gland for most severe cases of keratoconjunctivitis sicca. Ophthalmology 1998;105:327–335. [DOI] [PubMed] [Google Scholar]
- 13. Sieg P, Geerling G, Kosmehl H, Lauer I, Warnecke K, von Domarus H. Microvascular submandibular gland transfer for severe cases of keratoconjunctivitis sicca. Plast Reconstr Surg 2000;106:554–560; discussion 561–562. [PubMed] [Google Scholar]
- 14. Zhang L, Zhu ZH, Dai HJ, et al. Application of 99mTc‐pertechnetate scintigraphy to microvascular autologous transplantation of the submandibular gland in patients with severe keratoconjunctivitis sicca. J Nucl Med 2007;48:1431–1435. [DOI] [PubMed] [Google Scholar]
- 15. Jacobsen HC, Hakim SG, Lauer I, Dendorfer A, Wedel T, Sieg P. Long‐term results of autologous submandibular gland transfer for the surgical treatment of severe keratoconjunctivitis sicca. J Craniomaxillofac Surg 2008;36:227–233. [DOI] [PubMed] [Google Scholar]
- 16. Su JZ, Cai ZG, Yu GY. Microvascular autologous submandibular gland transplantation in severe cases of keratoconjunctivitis sicca. Maxillofac Plast Reconstr Surg 2015;37:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McCulloch P, Altman DG, Campbell WB, et al. No surgical innovation without evaluation: the IDEAL recommendations. Lancet 2009;374:1105–1112. [DOI] [PubMed] [Google Scholar]


