Abstract
Aim
Although it is well established that an external (EF) compared to an internal (IF) or neutral focus of attention enhances motor performance, little is known about the underlying neural mechanisms. This study aimed to clarify whether the focus of attention influences not only motor performance but also activity of the primary motor cortex (M1) when executing identical fatiguing tasks of the right index finger (first dorsal interosseous). Transcranial magnetic stimulation (TMS) at intensities below motor threshold was applied over M1 to assess and compare the excitability of intracortical inhibitory circuits.
Methods
In session 1, 14 subjects performed an isometric finger abduction at 30% of their maximal force to measure the time to task failure (TTF) with either an IF or EF. In session 2, the same task was performed with the other focus. In sessions 3 and 4, subthreshold TMS (subTMS) and paired‐pulse TMS were applied to the contralateral M1 to compare the activity of cortical inhibitory circuits within M1 during EF and IF.
Results
With an EF, TTF was significantly prolonged (P = 0.01), subTMS‐induced electromyographical suppression enhanced (P = 0.001) and short‐interval intracortical inhibition (SICI) increased (P = 0.004).
Conclusion
The level of intracortical inhibition was previously shown to influence motor performance. Our data shed new light on the ability to instantly modulate the activity of inhibitory circuits within M1 by changing the type of attentional focus. The increased inhibition with EF might contribute to the better movement efficiency, which is generally associated with focusing externally.
Keywords: cognitive manipulation, motor cortex, movement control, short‐interval intracortical inhibition, time to task failure, transcranial magnetic stimulation
The theory of attentional foci has received considerable attention in the movement and sport sciences literature over the past 15 years (Wulf 2012). Today, it is well established that an external focus of attention (EF) – compared with an internal (IF) or no imposed focus of attention – enhances motor performance and motor learning. Studies indicated benefits in balance (Oliveira et al. 1997, Landers et al. 2005, Wulf et al. 2009), accuracy (Perkins‐Ceccato et al. 2003, Marchant et al. 2007), jumping performance (Wulf & Dufek 2009, Wulf et al. 2010, Keller et al. 2015, Wälchli et al. 2015), force production (Wulf & Dufek 2009, Marchant 2011), movement speed (Fasoli et al. 2002) and oxygen consumption during running (Schücker et al. 2009, 2013). In addition and closely related to this study, research has demonstrated that an EF contrasted to an IF improves performance during a fatiguing task (Lohse & Sherwood 2011).
Although behavioural outcomes of using an EF strategy are well investigated, the underlying neural mechanisms remain poorly understood. A relatively consisting finding describes reduced electromyographical (EMG) activity of the agonist (Vance et al. 2004, Zachry et al. 2005, Marchant et al. 2009, Lohse et al. 2010, Wulf et al. 2010, Wälchli et al. 2015) or the antagonist muscle (Lohse et al. 2011) when adopting an EF. This may be considered as an improved neuromuscular efficiency leading to a more economic motor output; that is, the same task is performed with less energy expended (Lohse et al. 2010). However, the underlying brain mechanisms that are responsible for the reduced and/or more efficient muscular activity are not known.
In an fMRI study, Binkofski et al. (2002) evaluated brain activity for different attentional situations. The authors showed an impact of attention on brain activity. They demonstrated an altered activity of the posterior part of M1 (Brodmann's area 4p) with different attentional situations. However, this study did not evaluate brain activation under EF and IF conditions; rather, it showed in general that attention has an impact on the activity of M1. Similarly, the load of attention was shown to influence the susceptibility of the primary motor cortex in response to paired associative stimulation and intermittent theta‐burst stimulation (Kamke et al. 2012). In another fMRI experiment, Zimmermann et al. (2012) investigated the neural correlates of switching attentional foci. Results revealed that switching from a trained IF to an unfamiliar EF elicited a greater activation of the left lateral premotor cortex. On the other side, switching from a trained EF to an unfamiliar IF increased activation of the left primary somatosensory cortex and intraparietal lobule. However, in that study, participants trained a certain task and then switched to an untrained task. Thus, there is a serious drawback when comparing EF and IF as it is not clear whether the changes in brain activation were caused by switching from an EF to an IF (or vice versa) or by switching from a trained to an untrained task. Finally, in another fMRI study (Zentgraf et al. 2009), participants were trained to tap finger sequences on a keyboard. The participants had to concentrate either on their finger movements (IF) or on targeting the keys (EF). Results displayed a greater activation in motor cortex, primary somatosensory cortex and insular region when executing the task in an EF condition compared with an IF condition. In that study, it was hypothesized that adopting an EF (focusing on the task‐related environment without visual feedback) enhances tactile input to somatosensory brain areas that intimately connect to motor areas. However, the main limitation of this study is that two different groups of participants were compared (between‐groups design) so that one group adopted an EF, whereas the other group applied an IF. Furthermore, fMRI studies present an undeniable limitation. Using intrinsic blood–tissue contrasts (Kwong et al. 1992), this imaging technique is not suitable to distinguish between excitatory and inhibitory neural activity (Arthurs & Boniface 2002).
The present work therefore aimed to (i) confirm that the type of instruction (cognitive manipulation) influences motor performance when executing identical fatiguing tasks of the right first dorsal interosseous (FDI) muscle and (ii) outline differences in the activity of intracortical inhibitory circuits within M1 during the two different focus of attention conditions (EF vs. IF).
For this purpose and in contrast to previous research, we used a repeated‐measures design to evaluate whether motor cortical activity differs in an EF compared with an IF condition. Single‐pulse transcranial magnetic stimulation at intensities below the motor threshold (subTMS) and paired‐pulse TMS inducing short‐interval intracortical inhibition (SICI) were applied to the contralateral M1 to measure and compare the excitability of inhibitory circuits within M1 during the two attentional focus conditions. These techniques were selected as they are assumed to reflect the responsiveness of GABAA inhibitory intracortical circuits, without affecting spinal structures (Davey et al. 1994, Di Lazzaro et al. 1998). SubTMS elicits a suppression of the ongoing EMG activity, which can be compared in terms of duration and amount as shown in previous research (Lauber et al. 2012, 2013, Papegaaij et al. 2016). Additionally, intracortical inhibition can also be demonstrated by a paired‐pulse TMS paradigm that uses a conditioning stimulus below the motor threshold to reduce the size of a suprathreshold test stimulus response elicited at interstimulus intervals (ISI) of 1–5 ms (Kujirai et al. 1993, Wassermann et al. 1996, Di Lazzaro et al. 1998, Chen 2004). This so‐called SICI is expressed as the ratio of conditioned to test motor‐evoked potential (MEP) peak‐to‐peak amplitudes.
According to previous research, we assumed that in the fatiguing task, the time to task failure (TTF) would increase when performing the sustained contraction of the FDI in an EF condition compared with an IF.
With respect to the neural control of this finger abduction task, we expected focus‐dependent activity in M1 as cortical neurones controlling the hand and fingers occupy the large central core of M1 and regulate the activity of hand and finger muscles mostly by monosynaptic projection from M1 onto spinal motor neurones (Kalaska & Rizzolatti 2013). Additionally, M1 is essential in voluntary movement control (Scott 2003, 2004, Lemon 2008) and is part of the transcortical (reflex) loop (Shemmell et al. 2009). Moreover, it has been shown that M1 is modulated differently by different attentional situations (Binkofski et al. 2002) and that it is sensitive to different attentional strategies during a motor task (Zentgraf et al. 2009). Thus, we predicted that M1 processes EF and IF in different ways, even during the execution of identical motor tasks. More precisely, we expected more intracortical inhibition as indexed by an increased subTMS‐induced EMG suppression and an enhanced level of SICI when adopting an EF. Indeed, it has been suggested that poor development of intracortical inhibition impairs motor function (Heise et al. 2013), which might be associated with the impaired motor performance when adopting an IF.
Material and methods
Study participants
Fourteen subjects (22–33 years; six women) participated in the experiment. All subjects were right‐handed and free from any known neurological or orthopaedic disorders. They gave their written informed consent to the experiment. The study was approved by the local ethics committee and is in accordance with the Declaration of Helsinki.
Experimental design and set‐up
All subjects participated in a total of four laboratory sessions that were separated by at least 72 h (see Fig. 1). The first two sessions aimed to outline differences between an EF and an IF in the TTF of a submaximal sustained index finger abduction. The third and fourth sessions aimed to compare the activity of M1 during the same two focus of attention conditions by means of subTMS and paired‐pulse TMS. The sessions are described in detail below.
Figure 1.
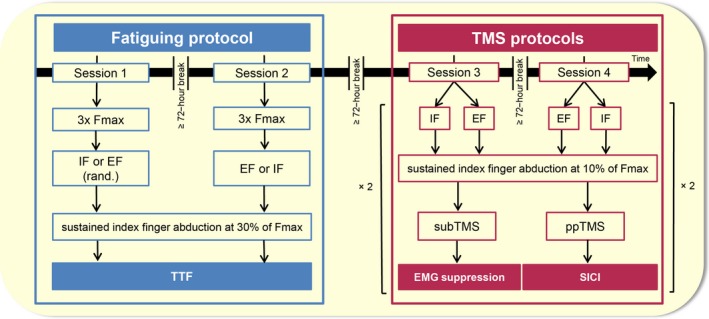
Time course of the four laboratory sessions. The first two sessions (sessions 1 and 2) aimed to outline differences in the time to task failure (TTF) of a submaximal sustained index finger abduction at 30% of F max between an external focus of attention (EF) and an internal focus of attention (IF). In one session, participants were asked to adopt an IF by concentrating on the muscle and finger, while in the other session, they were asked to adopt an EF by concentrating on the goniometer angle. The order of sessions was randomized. Sessions 3 and 4 aimed to compare the activity of M1 during the same two focus of attention conditions by means of subthreshold TMS (subTMS) and paired‐pulse TMS to assess intracortical inhibition; subTMS‐induced electromyographical (EMG) suppression and short‐interval intracortical inhibition (SICI) respectively. The participants performed the same motor task as in sessions 1 and 2 but at only 10% of F max to prevent the effect of fatigue. TMS, transcranial magnetic stimulation.
During all sessions, subjects were seated in an upright position in an adjustable chair facing a monitor placed 1 m in front of them (see Fig. 2a). The right arm was in a pronated position and fixed in a custom‐made splint to restrict degrees of freedom. Thus, any abduction and adduction movements were limited to the metacarpophalangeal joint of the index finger (see Fig. 2b). The left arm rested in a relaxed and comfortable position. During the tasks, subjects pushed with their index finger against a lever whose axis of rotation was aligned with that of the finger joint. A goniometer was fixed to the lever that measured its angle. The position signal of the goniometer was displayed on the monitor in form of a red line that became thicker when subjects moved their finger away from the target position (neutral position). The splint position was recorded for each participant to perform all sessions in the same position.
Figure 2.
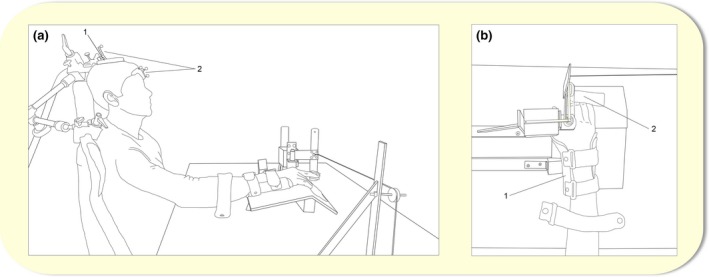
(a) Illustration of the experimental set‐up during the subTMS and paired‐pulse TMS protocol in a sagittal plane. The stimulator coil (1) was mounted with a coil tracker (2), and markers were attached to the participant's forehead (2) as shown in the picture. (b) Illustration of a closer look of the experimental set‐up used during all experimental sessions in a transverse plane. The arm was held in a pronated position by a splint (1) so that the finger movements were restricted to only allow abduction and adduction of the right index finger. Electromyographical (EMG) electrodes were placed on the right first dorsal interosseous (FDI) (not illustrated). TMS, transcranial magnetic stimulation.
Fatiguing task (sessions 1 and 2)
At the beginning of sessions 1 and 2, subjects performed three maximal isometric abductions of the index finger to determine their maximal force (F max). For this purpose, subjects pushed the lever against a force transducer (MC3A‐500; Advanced Mechanical Technologies Inc., Watertown, MA, USA) without any instruction about the focus of attention. After the maximal contractions, the force transducer was removed to allow free movement of the index finger in the transverse plane (adduction–abduction). For the fatiguing task, a weight representing 30% of F max was attached to the lever, pulling the finger into adduction. The same weight representing 30% of F max obtained in the first session was used in both sessions. The second F max measure served as a control that the F max had not changed between sessions. The fatiguing task consisted in holding the finger in the target position by counteracting the weight until task failure. Task failure was determined as a deviation of more than 10° from the target position.
In one session, participants were asked to adopt an IF by concentrating on the muscle and finger, while in the other session, they were asked to adopt an EF by concentrating on the goniometer angle. The order of sessions was randomized. The IF and EF instructions were formulated as similar as possible. The instruction for the IF condition was ‘Concentrate on the position of your finger. Hold this position for as long as possible. When the position of your finger changes, the thickness of the red line on the screen changes. Correct the position of your finger by contracting the muscle until the red line is thin again’. The instruction for the EF condition was ‘Concentrate on the position of the goniometer. Hold this position for as long as possible. When the position of the goniometer changes, the thickness of the red line on the screen changes. Correct the position of the goniometer until the red line is thin again’. Every 30 s, the subjects were reminded to ‘contract and concentrate on their finger muscles’ (IF) or ‘control and concentrate on the position of the goniometer’ (EF).
Transcranial magnetic stimulation (sessions 3 and 4)
EMG recordings
Electromyographical recordings were obtained from the FDI muscle of the right hand. After skin preparation, Ag/AgCl bipolar surface electrodes (BlueSensor P; Ambu A/S, Ballerup, Denmark) were attached to the skin with 1 cm interelectrode distance. The reference electrode was placed on the phalanx of the digitus medius. EMG recordings were amplified (×1000), bandpass‐filtered (Butterworth 10–1000 Hz) and sampled at 4 kHz. All data were recorded and stored on a computer for offline analysis using imago record software (Pfitec Biomedical Systems, Endingen, Germany).
Stimulation
Transcranial magnetic stimuli were delivered over the left M1 using a MagVenture Pro stimulator (MagVenture A/S, Farum, Denmark) with a 95‐mm focal figure of eight coils (MagVenture D‐B80). The initial stimulation point was set approx. 0.5 cm anterior to the vertex and over the midline. The TMS coil was oriented 45° towards the contralateral forehead to ensure that the induced current flow is approximately perpendicular to the central sulcus (Rossini et al. 2015). Induced current was in the reverse (posterior to anterior directed currents) mode, and the waveform was monophasic in all conditions. The optimal position of the coil for eliciting MEPs in the FDI with minimal intensity was determined by moving the coil anterior and left from the vertex, while the MEP size was monitored. This position was recorded and constantly controlled with a neuronavigation system (Polaris Spectra; Northern Digital, Waterloo, ON, Canada and Localite TMS Navigator Version 2.0.5; LOCALITE GmbH, Sankt Augustin, Germany). The active motor threshold (aMT) was determined, while subjects maintained a contraction of 10% of their individual F max. It was defined as the minimal stimulation intensity that elicited MEPs of at least 100 μV peak‐to‐peak amplitude in three of five trials. One hundred microvolt was chosen to minimize the error of identifying background EMG activity as a TMS‐related MEP.
Protocols
Throughout the stimulation protocols, participants held a weight representing 10% of their F max. This lighter weight compared to the fatiguing tasks was chosen to prevent effects of fatigue (Seifert & Petersen 2010). Two different TMS protocols were completed during both focus of attention conditions with the same counterbalanced order of the conditions as in the first two sessions and with a 5‐min break between series. Also, the same verbal instructions were given to the participants. FDI background EMG obtained in a time window of 100 ms before each stimulus (subTMS, control MEP and paired‐pulse TMS) was analysed to compare muscular activity between conditions.
The first protocol (session 3) was a subTMS protocol (see Fig. 1). The stimulator output was successively diminished in steps of 2% (from the aMT intensity defined previously) to find the intensity that induced the greatest amount of EMG suppression without any preceding MEP (see below for details on calculation). Once this stimulation intensity was determined, two series of 40 trials with and 40 trials without stimulation (total of 80 trials with and 80 trials without stimulation) with randomized ISIs from 0.8 to 1.1 s were recorded for each condition. The same stimulation intensity was used in both conditions.
During the fourth session (see Fig. 1), a paired‐pulse TMS paradigm composed of a conditioning stimulus (0.8 aMT) followed by a suprathreshold control stimulus (1.2 aMT) at ISI of 2.5 ms was used to assess SICI over the motor cortical representation of the FDI. The ISI was chosen based on the literature (Roshan et al. 2003). The interval between single‐pulse and paired‐pulse stimuli was set at 0.25 Hz. Subjects underwent 4 × 20 stimuli, two times in each condition. One set of 20 stimuli was composed of 10 control MEPs (single‐pulses with 1.2 aMT) and 10 conditioned MEPs (paired‐pulses with 2.5 ms ISI between the sub‐ (0.8 aMT) and the suprathreshold (1.2 aMT) stimulus). For the final analysis, the magnitude of the SICI was expressed as percentage using the following formula: 100 − (conditioned MEP/control MEP × 100). Additionally, control MEP peak‐to‐peak amplitudes in millivolts were also compared between both conditions.
Calculation of subTMS‐induced EMG suppression
Electromyographical signals were rectified and averaged before analysis. The onset of the EMG suppression was defined as the instant when the difference between the trials with and those without stimulation (EMGDiff = EMGWithout − EMGWith) was negative for at least 4 ms in a time window from 20 to 50 ms after the stimulation. The end of the suppression was determined as the point where the EMGDiff presented a clear facilitation. The amount of suppression was calculated by integrating (cumulative trapezoidal numerical integration) EMGDiff from the onset to the end of the suppression. Importantly, to determine this inhibition, the average of all trials with stimulation was subtracted from the average of all trials without stimulation. This method of quantifying and comparing subTMS‐induced EMG suppression has been used previously (Zuur et al. 2010, Lauber et al. 2012, 2013, Papegaaij et al. 2016). The onset, the duration and the amount of the EMG suppression were computed in matlab (R2014b; MathWorks, Natick, MA, USA) using a custom script and used for the statistical analysis.
Statistics
Before the analyses, normal distribution of the data was tested. Unless indicated otherwise, data are reported as mean ± standard deviation. For the analysis of behavioural data, paired Student's t‐tests were performed to assess differences in the TTF between the two focus of attention conditions and in F max between the two sessions.
Separate paired Student's t‐tests were performed for each output parameter of the TMS protocols (subTMS and paired‐pulse TMS) to compare the two conditions (EF vs. IF). To compare the background EMG 100 ms prior brain stimulations during the paired‐pulse TMS protocol, a two‐way anova was computed. Pearson correlation coefficients were computed to assess the association between the difference in TTF and the difference in intracortical inhibition within M1 (subTMS‐induced EMG suppression and SICI). The level of significance was set at P ≤ 0.05. r version 3.2.3 software (R Foundation for Statistical Computing, Vienna, Austria) was used for all statistical analyses.
Results
The TTF was significantly longer (+18.5%, t 13 = −2.73, P = 0.01) with an EF (146.73 ± 38.88 s) compared with an IF (123.84 ± 34.37 s) during the fatiguing task (see Fig. 3). Importantly, F max were comparable in both conditions (t 13 = −1.17, P = 0.25; session EF = 25.33 ± 10.48 N, session IF = 27.29 ± 13.11 N). This shows that subjects were not generally fitter in one test session compared to the other.
Figure 3.
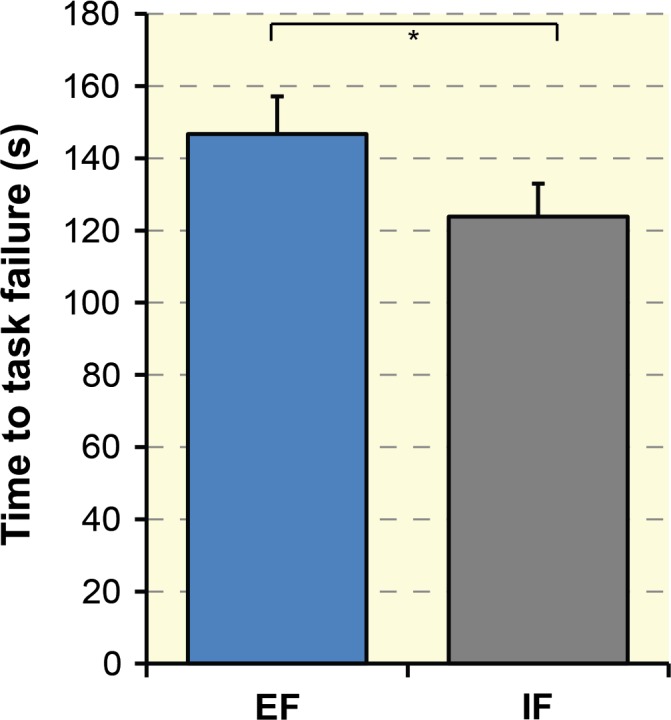
Group data (n = 14) of the time to task failure (TTF) during both attentional focus conditions. The TTF was significantly longer when adopting an external focus of attention (EF) contrasted to an internal focus of attention (IF). *P < 0 .05. Error bars represent the standard error of the mean.
Four subjects had to be excluded from the TMS protocols as they showed no clear and reproducible EMG suppression after subTMS. In the 10 remaining participants, subTMS resulted in a clear suppression of the FDI muscle EMG. The mean TMS intensity to elicit EMG suppression in the FDI was 77.85 ± 4.32% of aMT. The FDI background EMG recorded in the 100‐ms time interval before subthreshold stimulation (subTMS) was comparable in all trials with EF and IF (t 9 = 0.32, P = 0.76), and the onset of subTMS‐induced EMG suppression was comparable in both conditions (t 9 = 0.82, P = 0.42, 31.82 ± 11.88 ms in EF and 29.92 ± 4.87 ms in IF; see Fig. 4). Adopting an EF increased the amount of subTMS‐induced EMG suppression by around 74% compared to an IF (t 9 = 4.32, P = 0.001, EF = 0.40 ± 0.09 mV*ms, IF = 0.23 ± 0.11 mV*ms; see Figs 4 and 5a). No significant difference (P = 0.19) in the duration of the suppression was found between conditions (see Fig. 5b).
Figure 4.
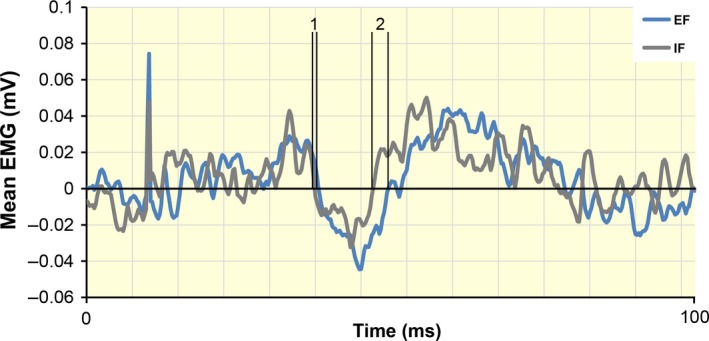
Group data of the mean electromyographical (EMG) activity (n = 10) during a sustained contraction of the right first dorsal interosseous (FDI) (10% of F max). The curves were obtained by subtracting the rectified EMG of the trials with subthreshold TMS from that of the trials without stimulation. The horizontal dashed line represents the mean level of background EMG. The vertical lines represent the onset of EMG suppression (1) and the end of EMG suppression (2). The amount of EMG suppression was significantly greater (P = 0.001) with an external focus of attention (EF, blue line) than with an internal (IF, grey line). No difference between the two foci was found for the onset and the duration of the suppression. TMS, transcranial magnetic stimulation.
Figure 5.
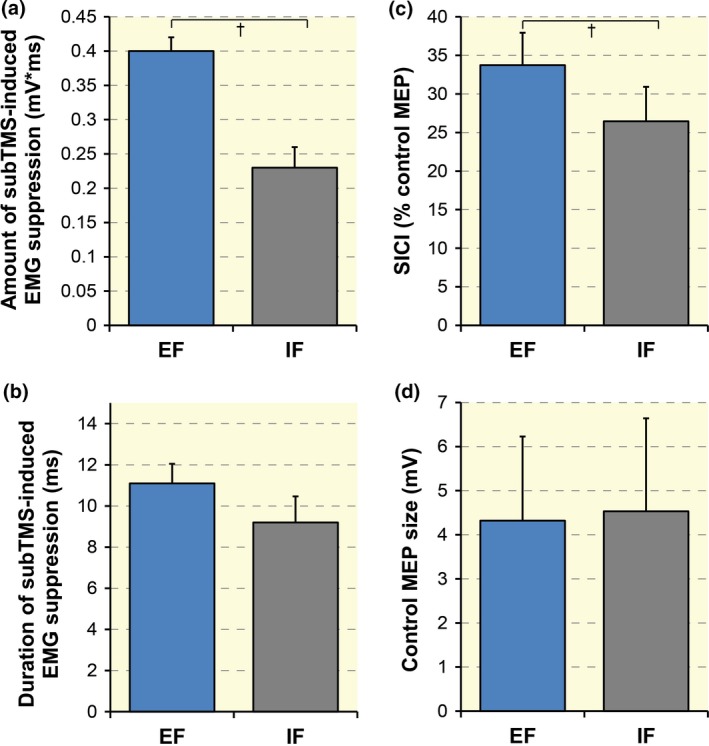
Group data (n = 10) of the amount (a) and the duration (b) of subTMS‐induced electromyographical (EMG) suppression in first dorsal interosseous (FDI) under two focus of attention conditions (EF = 11.1 ± 3.00 ms, IF = 9.2 ± 4.01 ms). The amount of EMG suppression was significantly greater with an external (EF) than with an internal focus of attention (IF). No significant difference was found for the duration. (c) When adopting an EF, the short‐interval intracortical inhibition (SICI) expressed as percentage of control motor‐evoked potential (MEP) in FDI was significantly enhanced contrasted to an IF. (d) Control MEP at 1.2 aMT peak‐to‐peak amplitudes during both attentional conditions. No significant difference was found between the two conditions. † P < 0.01. Error bars represent the standard error of the mean. aMT, active motor threshold; EF, external focus; TMS, transcranial magnetic stimulation.
In session 4, the same 10 participants underwent the paired‐pulse TMS protocol that revealed an increase in SICI acting on the FDI by around 7% during the EF (see Fig. 5c) contrasted to the IF condition (t 9 = 3.75, P = 0.004; EF = 33.72 ± 13.32%, IF = 26.45 ±14.12%). When comparing the suprathreshold control MEPs (control MEPs at 1.2 aMT; see Fig. 5d), no statistically significant differences were found (t 9 = −0.78, P = 0.45; EF = 4.32 ± 1.91 mV, IF = 4.53 ±2.11 mV). The FDI background EMG recorded in the 100‐ms time interval before brain stimulations was comparable between both conditions (F 1,36 = 0.02, P = 0.88, ω 2 = −0.006) and between stimulation types (single vs. paired TMS; F 1,36 = 0.11, P = 0.73, ω 2 = 0.07). There was no significant interaction effect (conditions × stimulation type; F 1,36 < 0.001, P = 0.99, ω 2 = −0.02).
To determine whether differences in intracortical inhibition (subTMS‐induced EMG suppression and SICI) measured between the two focus of attention conditions could be directly related to differences in TTF, we performed correlation analyses. Results showed no significant correlation between the difference in TTF and the amount of subTMS‐induced EMG suppression (r = −0.39, P = 0.25), nor between the difference in TTF and the difference in SICI (r = 0.13, P = 0.71). In addition, no significant correlation was found between the difference in the amount of subTMS‐induced EMG suppression and the difference in SICI (r = 0.41, P = 0.23).
Discussion
We examined attention‐related changes in the TTF and activity of M1 during submaximal sustained contractions. The main findings were an increase in TTF associated with an increase in subTMS‐induced EMG suppression and an increase in SICI when adopting an EF compared with an IF.
Does the focus of attention influence motor behaviour?
Previous research on the focus of attention during fatiguing tasks showed that adopting an EF increases TTF and reduces perceived exertion (Lohse & Sherwood 2011). In two other studies, Schücker et al. (2009, 2013) demonstrated that adopting an EF during running led to lower oxygen consumption; thus, movement efficiency was enhanced.
To explain the benefits of focusing externally, the ‘constrained action hypothesis’ was postulated (Wulf et al. 2001, McNevin et al. 2003), which stipulates that adopting an EF allows more automatic modes of motor control, using fast and unconscious control processes. The assumption of an improved motor efficiency with an EF was further strengthened by studies showing that EMG activity of the agonist (Vance et al. 2004, Zachry et al. 2005, Marchant et al. 2009, Lohse et al. 2010, Wulf et al. 2010, Wälchli et al. 2015) or antagonist muscle (Lohse et al. 2011) is reduced despite better performance during an EF. Thus, it seems well established that an EF enhances performance by increasing the efficiency of the movement execution. Our finding of a prolonged TTF as soon as subjects focused externally is therefore well in line with previous studies. However, little is known about the underlying neural mechanisms at the supraspinal level, and the question remains how this increased movement efficiency is organized from a motor cortical point of view. Based on the reduced efficiency with an IF, we hypothesized that an IF may lead to attenuation of inhibitory processes.
Does the focus of attention change inhibitory activity within M1?
Cortical activity is influenced by the balance between inhibitory and excitatory circuits (Chen 2004). It is suggested that interactions between excitatory and intracortical inhibitory processes within M1 are essential for motor control (Hummel et al. 2009). For example, elderly subjects (Papegaaij et al. 2014) or children (Mall et al. 2004, Walther et al. 2009, van de Laar et al. 2012) show reduced levels of intracortical inhibition. At the same time, these age groups demonstrate reduced coordinative abilities compared to healthy young adults. For instance, elderly subjects displayed an increased cocontraction resulting in reduced movement efficiency when executing motor tasks (Macaluso et al. 2002). Besides, compared to healthy peers, 8‐year‐old children born preterm demonstrated impaired visual‐motor integration and displayed reduced (or even absent) intracortical inhibition (Flamand et al. 2012). At the same time, variability of corticomotor excitability was enhanced. Thus, there seems to be a close interrelation of intracortical inhibitory processes and motor performance when considering different populations. However, not only across age groups or different populations but also within age groups, corticospinal inhibitory processes seem to strongly influence motor function, such as interlimb coordination (Fujiyama et al. 2012) or dexterity (Heise et al. 2013). Thus, the level of intracortical inhibition seems to strongly influence motor control in general.
In a previous fMRI study, Zentgraf et al. (2009) investigated brain activity associated with different foci of attention (EF vs. IF). The authors observed greater activation in M1, in primary somatosensory and insular cortices when participants performed a finger sequence in an EF condition compared with an IF condition. On the first view, these results may look contradictory to our findings. However, given the fact that fMRI uses intrinsic blood–tissue contrasts (Kwong et al. 1992), this technique is not able to distinguish between excitatory and inhibitory neural activity (Arthurs & Boniface 2002). Thus, the larger BOLD activation of M1 in the EF condition found in the Zentgraf et al. study (2009) may have been related to an increased inhibitory activity.
In contrast to fMRI that provides only an estimate about the overall neural activity, TMS can provide also information about activity of intracortical inhibitory circuits. As the cortical inhibitory interneurones have a lower threshold to TMS than excitatory neurones (Davey et al. 1994, Ziemann et al. 1996, Petersen et al. 2001, Butler et al. 2007, Ortu et al. 2008), transcranial magnetic stimuli at intensities lower than the aMT can be used to inhibit motor cortical output without affecting spinal structures (Davey et al. 1994, Di Lazzaro et al. 1998).
It has been suggested that the mechanism of subTMS‐induced EMG suppression is the result of inhibition of the ongoing activity of fast‐conducting corticospinal cells (Roy 2009). This means that an increased excitability of intracortical inhibitory circuits would consequently result in more subTMS‐induced EMG suppression (Papegaaij et al. 2016).
Similar to subTMS‐induced EMG suppression, the excitability of intracortical inhibitory circuits can be assessed by paired‐pulse TMS with short ISIs. The measure of SICI reflects the excitability of inhibitory GABAergic neurones (Kujirai et al. 1993, Ziemann et al. 1996, Di Lazzaro et al. 2000). Importantly, a positive correlation between the amount of SICI and cerebral blood flow in the motor cortex was shown by means of positron emission tomography (Strafella & Paus 2001). Thus, the present results of increased intracortical inhibition with an EF are in no way contradictory to the observations of increased blood flow with an EF by means by fMRI.
As the motor tasks and background EMG prior to stimulation were identical in both conditions, it seems reasonable to assume that attention was indeed the dominant modulatory influence on the excitability of the intracortical inhibitory cells projecting to the FDI corticomotoneurones. Thus, as both the amount of subTMS‐induced EMG suppression and SICI were significantly larger in the EF condition in the present study, we suggest that intracortical circuits, in all likelihood inhibitory GABAergic neurones (Classen & Benecke 1995), are modulated differently within M1 when adopting an EF. This would be in line with previous research showing that M1 is not only an executive structure but also sensitive to differential attentional situations (Binkofski et al. 2002).
The new finding of the present study is that intracortical inhibition may be modulated instantly in one and the same person depending on the attentional strategy adopted during the motor task. This would nicely explain on a neural level the reduced efficiency of an IF compared to an EF and might therefore constitute (one of) the underlying mechanism(s) of the constrained action hypothesis.
Limitations and further research
In the present study, EMG activity was not recorded during the fatiguing task as this protocol was only foreseen to prove the feasibility of the motor task. We wanted to ensure that subjects indeed increased performance, that is, TTF, with an EF during this simple finger contraction task to outline differences in cortical activity. Apart from this, future studies are needed to examine the effect of practice with different foci of attention on brain activity in the long‐term, as indicated, for example, by connectivity of brain motor networks (Wu et al. 2008).
Conclusion
Our data shed further light on the neural mechanisms underlying attentional foci. Directing attention externally led not only to an improved motor performance in the endurance task but was also accompanied by a larger subTMS‐induced EMG suppression and SICI. Our results therefore suggest that focusing internally or externally results in a differential organization and integration within M1. In addition to previous research outlining attention‐specific activity within M1, we further specify that modulation of intracortical inhibitory circuits probably contributes to an enhanced motor efficiency when adopting an EF.
Conflict of interest
There are no conflicts of interests.
See Editorial Commentary: Marinovic, W. 2016. Focus of attention changes intracortical excitability in the primary motor cortex. Acta Physiol (Oxf) 220, 179–180.
References
- Arthurs, O.J. & Boniface, S. 2002. How well do we understand the neural origins of the fMRI BOLD signal? Trends Neurosci 25, 27–31. [DOI] [PubMed] [Google Scholar]
- Binkofski, F. , Fink, G.R. , Geyer, S. , Buccino, G. , Gruber, O. , Shah, N.J. , Taylor, J.G. , Seitz, R.J. , Zilles, K. & Freund, H.J. 2002. Neural activity in human primary motor cortex areas 4a and 4p is modulated differentially by attention to action. J Neurophysiol 88, 514–519. [DOI] [PubMed] [Google Scholar]
- Butler, J.E. , Larsen, T.S. , Gandevia, S.C. & Petersen, N.T. 2007. The nature of corticospinal paths driving human motoneurones during voluntary contractions. J Physiol 584, 651–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, R. 2004. Interactions between inhibitory and excitatory circuits in the human motor cortex. Exp Brain Res 154, 1–10. [DOI] [PubMed] [Google Scholar]
- Classen, J. & Benecke, R. 1995. Inhibitory phenomena in individual motor units induced by transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol 97, 264–274. [DOI] [PubMed] [Google Scholar]
- Davey, N.J. , Romaiguère, P. , Maskill, D.W. & Ellaway, P.H. 1994. Suppression of voluntary motor activity revealed using transcranial magnetic stimulation of the motor cortex in man. J Physiol 477, 223–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro, V. , Restuccia, D. , Oliviero, A. , Profice, P. , Ferrara, L. , Insola, A. , Mazzone, P. , Tonali, P. & Rothwell, J.C. 1998. Magnetic transcranial stimulation at intensities below active motor threshold activates intracortical inhibitory circuits. Exp Brain Res 119, 265–268. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro, V. , Oliviero, A. , Meglio, M. , Cioni, B. , Tamburrini, G. , Tonali, P. & Rothwell, J.C. 2000. Direct demonstration of the effect of lorazepam on the excitability of the human motor cortex. Clin Neurophysiol 111, 794–799. [DOI] [PubMed] [Google Scholar]
- Fasoli, S.E. , Trombly, C.A. , Tickle‐Degnen, L. & Verfaellie, M.H. 2002. Effect of instructions on functional reach in persons with and without cerebrovascular accident. Am J Occup Ther 56, 380–390. [DOI] [PubMed] [Google Scholar]
- Flamand, V.H. , Nadeau, L. & Schneider, C. 2012. Brain motor excitability and visuomotor coordination in 8‐year‐old children born very preterm. Clin Neurophysiol 123, 1191–1199. [DOI] [PubMed] [Google Scholar]
- Fujiyama, H. , Hinder, M.R. , Schmidt, M.W. , Garry, M.I. & Summers, J.J. 2012. Age‐related differences in corticospinal excitability and inhibition during coordination of upper and lower limbs. Neurobiol Aging 33, 1484.e1–1484.e14. [DOI] [PubMed] [Google Scholar]
- Heise, K.‐F. , Zimerman, M. , Hoppe, J. , Gerloff, C. , Wegscheider, K. & Hummel, F.‐C. 2013. The aging motor system as a model for plastic changes of GABA‐mediated intracortical inhibition and their behavioral relevance. J Neurosci 33, 9039–9049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel, F.C. , Steven, B. , Hoppe, J. , Heise, K. , Thomalla, G. , Cohen, L.G. & Gerloff, C. 2009. Deficient intracortical inhibition (SICI) during movement preparation after chronic stroke. Neurology 72, 1766–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaska, J.F. & Rizzolatti, G. 2013. Voluntary movement: the primary motor cortex In: Kandel E.R., Schwartz J.H., Jessell T.M., Siegelbaum S.A. & Hudspeth A.J. (eds) Principles of neural science, 5th edn, pp. 843 McGraw‐Hill Medical, New York, USA. [Google Scholar]
- Kamke, M.R. , Hall, M.G. , Lye, H.F. , Sale, M.V. , Fenlon, L.R. , Carroll, T.J. , Riek, S. & Mattingley, J.B. 2012. Visual attentional load influences plasticity in the human motor cortex. J Neurosci 32, 7001–7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller, M. , Lauber, B. , Gottschalk, M. & Taube, W. 2015. Enhanced jump performance when providing augmented feedback compared to an external or internal focus of attention. J Sports Sci 33, 1067–1075. [DOI] [PubMed] [Google Scholar]
- Kujirai, T. , Caramia, M.D. , Rothwell, J.C. , Day, B.L. , Thompson, P.D. , Ferbert, A. , Wroe, S. , Asselman, P. & Marsden, C.D. 1993. Corticocortical inhibition in human motor cortex. J Physiol 471, 501–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong, K.K. , Belliveau, J.W. , Chesler, D.A. , Goldberg, I.E. , Weisskoff, R.M. , Poncelet, B.P. , Kennedy, D.N. , Hoppel, B.E. , Cohen, M.S. & Turner, R. 1992. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci U S A 89, 5675–5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Laar, M.C. , van den Wildenberg, W.P. , van Boxtel, G.J. , Huizenga, H.M. & van der Molen, M.W. 2012. Lifespan changes in motor activation and inhibition during choice reactions: a Laplacian ERP study. Biol Psychol 89, 323–334. [DOI] [PubMed] [Google Scholar]
- Landers, M. , Wulf, G. , Wallmann, H. & Guadagnoli, M. 2005. An external focus of attention attenuates balance impairment in patients with Parkinson's disease who have a fall history. Physiotherapy 91, 152–158. [Google Scholar]
- Lauber, B. , Leukel, C. , Gollhofer, A. & Taube, W. 2012. Time to task failure and motor cortical activity depend on the type of feedback in visuomotor tasks. PLoS ONE 7, e32433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauber, B. , Keller, M. , Leukel, C. , Gollhofer, A. & Taube, W. 2013. Specific interpretation of augmented feedback changes motor performance and cortical processing. Exp Brain Res 227, 31–41. [DOI] [PubMed] [Google Scholar]
- Lemon, R.N. 2008. Descending pathways in motor control. Annu Rev Neurosci 31, 195–218. [DOI] [PubMed] [Google Scholar]
- Lohse, K.R. & Sherwood, D.E. 2011. Defining the focus of attention: effects of attention on perceived exertion and fatigue. Front Psychol 2, 332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohse, K.R. , Sherwood, D.E. & Healy, A.F. 2010. How changing the focus of attention affects performance, kinematics, and electromyography in dart throwing. Hum Mov Sci 29, 542–555. [DOI] [PubMed] [Google Scholar]
- Lohse, K.R. , Sherwood, D.E. & Healy, A.F. 2011. Neuromuscular effects of shifting the focus of attention in a simple force production task. J Mot Behav 43, 173–184. [DOI] [PubMed] [Google Scholar]
- Macaluso, A. , Nimmo, M.A. , Foster, J.E. , Cockburn, M. , McMillan, N.C. & De Vito, G. 2002. Contractile muscle volume and agonist‐antagonist coactivation account for differences in torque between young and older women. Muscle Nerve 25, 858–863. [DOI] [PubMed] [Google Scholar]
- Mall, V. , Berweck, S. , Fietzek, U.M. , Glocker, F.X. , Oberhuber, U. , Walther, M. , Schessl, J. , Schulte‐Monting, J. , Korinthenberg, R. & Heinen, F. 2004. Low level of intracortical inhibition in children shown by transcranial magnetic stimulation. Neuropediatrics 35, 120–125. [DOI] [PubMed] [Google Scholar]
- Marchant, D.C. 2011. Attentional focusing instructions and force production. Front Psychol 1, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant, D.C. , Clough, J.C. & Crawshaw, M. 2007. The effects of attentional focusing strategies on novice dart throwing performance and their task experiences. Int Rev Sport Exerc Psychol 5, 291–303. [Google Scholar]
- Marchant, D.C. , Greig, M. & Scott, C. 2009. Attentional focusing instructions influence force production and muscular activity during isokinetic elbow flexions. J Strength Cond Res 23, 2358–2366. [DOI] [PubMed] [Google Scholar]
- McNevin, N. , Shea, C.H. & Wulf, G. 2003. Increasing the distance of an external focus of attention enhances learning. Psychol Res 67, 22–29. [DOI] [PubMed] [Google Scholar]
- Oliveira, R.M. , Gurd, J.M. , Nixon, P. , Marshall, J.C. & Passingham, R.E. 1997. Micrographia in Parkinson's disease: the effect of providing external cues. J Neurol Neurosurg Psychiatry 63, 429–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortu, E. , Deriu, F. , Suppa, A. , Tolu, E. & Rothwell, J.C. 2008. Effects of volitional contraction on intracortical inhibition and facilitation in the human motor cortex. J Physiol 586, 5147–5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papegaaij, S. , Taube, W. , Baudry, S. , Otten, E. & Hortobagyi, T. 2014. Aging causes a reorganization of cortical and spinal control of posture. Front Aging Neurosci 6, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papegaaij, S. , Taube, W. , van Keeken, H.G. , Otten, E. , Baudry, S. & Hortobagyi, T. 2016. Postural challenge affects motor cortical activity in young and old adults. Exp Gerontol 73, 78–85. [DOI] [PubMed] [Google Scholar]
- Perkins‐Ceccato, N. , Passmore, S.R. & Lee, T.D. 2003. Effects of focus of attention depend on golfers’ skill. J Sports Sci 21, 593–600. [DOI] [PubMed] [Google Scholar]
- Petersen, N.T. , Butler, J.E. , Marchand‐Pauvert, V. , Fisher, R. , Ledebt, A. , Pyndt, H.S. , Hansen, N.L. & Nielsen, J.B. 2001. Suppression of EMG activity by transcranial magnetic stimulation in human subjects during walking. J Physiol 537, 651–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roshan, L. , Paradiso, G.O. & Chen, R. 2003. Two phases of short‐interval intracortical inhibition. Exp Brain Res 151, 330–337. [DOI] [PubMed] [Google Scholar]
- Rossini, P.M. , Burke, D. , Chen, R. , Cohen, L.G. , Daskalakis, Z. , Di Iorio, R. , Di Lazzaro, V. , Ferreri, F. , Fitzgerald, P.B. , George, M.S. et al 2015. Non‐invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin Neurophysiol 126, 1071–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy, F.D. 2009. Suppression of EMG activity by subthreshold paired‐pulse transcranial magnetic stimulation to the leg motor cortex. Exp Brain Res 193, 477–482. [DOI] [PubMed] [Google Scholar]
- Schücker, L. , Hagemann, N. , Strauss, B. & Völker, K. 2009. The effect of attentional focus on running economy. J Sports Sci 27, 1241–1248. [DOI] [PubMed] [Google Scholar]
- Schücker, L. , Anheier, W. , Hagemann, N. , Strauss, B. & Völker, K. 2013. On the optimal focus of attention for efficient running at high intensity. Sport Exerc Perform Psychol 2, 207–219. [Google Scholar]
- Scott, S.H. 2003. The role of primary motor cortex in goal‐directed movements: insights from neurophysiological studies on non‐human primates. Curr Opin Neurobiol 13, 671–677. [DOI] [PubMed] [Google Scholar]
- Scott, S.H. 2004. Optimal feedback control and the neural basis of volitional motor control. Nat Rev Neurosci 5, 532–546. [DOI] [PubMed] [Google Scholar]
- Seifert, T. & Petersen, N.C. 2010. Changes in presumed motor cortical activity during fatiguing muscle contraction in humans. Acta Physiol (Oxf) 199, 317–325. [DOI] [PubMed] [Google Scholar]
- Shemmell, J. , An, J.H. & Perreault, E.J. 2009. The differential role of motor cortex in the stretch reflex modulation induced by changes in environmental mechanics and verbal instruction. J Neurosci 29, 13255–13263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strafella, A.P. & Paus, T. 2001. Cerebral blood‐flow changes induced by paired‐pulse transcranial magnetic stimulation of the primary motor cortex. J Neurophysiol 85, 2624–2629. [DOI] [PubMed] [Google Scholar]
- Vance, J. , Wulf, G. , Töllner, T. , McNevin, N. & Mercer, J. 2004. EMG activity as a function of the performer's focus of attention. J Mot Behav 36, 450–459. [DOI] [PubMed] [Google Scholar]
- Wälchli, M. , Ruffieux, J. , Bourquin, Y. , Keller, M. & Taube, W. 2015. Maximizing performance: augmented feedback, focus of attention, and/or reward? Med Sci Sports Exerc 48, 714–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther, M. , Berweck, S. , Schessl, J. , Linder‐Lucht, M. , Fietzek, U.M. , Glocker, F.X. , Heinen, F. & Mall, V. 2009. Maturation of inhibitory and excitatory motor cortex pathways in children. Brain Dev 31, 562–567. [DOI] [PubMed] [Google Scholar]
- Wassermann, E.M. , Samii, A. , Mercuri, B. , Ikoma, K. , Oddo, D. , Grill, S.E. & Hallett, M. 1996. Responses to paired transcranial magnetic stimuli in resting, active, and recently activated muscles. Exp Brain Res 109, 158–163. [DOI] [PubMed] [Google Scholar]
- Wu, T. , Chan, P. & Hallett, M. 2008. Modifications of the interactions in the motor networks when a movement becomes automatic. J Physiol 586, 4295–4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulf, G. 2012. Attentional focus and motor learning: a review of 15 years. Int Rev Sport Exerc Psychol 6, 77–104. [Google Scholar]
- Wulf, G. & Dufek, J.S. 2009. Increased jump height with an external focus due to enhanced lower extremity joint kinetics. J Mot Behav 41, 401–409. [DOI] [PubMed] [Google Scholar]
- Wulf, G. , McNevin, N. & Shea, C.H. 2001. The automaticity of complex motor skill learning as a function of attentional focus. Q J Exp Psychol A 54, 1143–1154. [DOI] [PubMed] [Google Scholar]
- Wulf, G. , Landers, M. , Lewthwaite, R. & Töllner, T. 2009. External focus instructions reduce postural instability in individuals with Parkinson disease. Phys Ther 89, 162–168. [DOI] [PubMed] [Google Scholar]
- Wulf, G. , Dufek, J.S. , Lozano, L. & Pettigrew, C. 2010. Increased jump height and reduced EMG activity with an external focus. Hum Mov Sci 29, 440–448. [DOI] [PubMed] [Google Scholar]
- Zachry, T. , Wulf, G. , Mercer, J. & Bezodis, N. 2005. Increased movement accuracy and reduced EMG activity as the result of adopting an external focus of attention. Brain Res Bull 67, 304–309. [DOI] [PubMed] [Google Scholar]
- Zentgraf, K. , Lorey, B. , Bischoff, M. , Zimmermann, K. , Stark, R. & Munzert, J. 2009. Neural correlates of attentional focusing during finger movements: a fMRI study. J Mot Behav 41, 535–541. [DOI] [PubMed] [Google Scholar]
- Ziemann, U. , Rothwell, J.C. & Ridding, M.C. 1996. Interaction between intracortical inhibition and facilitation in human motor cortex. J Physiol 496, 873–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann, K. , Bischoff, M. , Lorey, B. , Stark, R. , Munzert, J. & Zentgraf, K. 2012. Neural correlates of switching attentional focus during finger movements: an fMRI study. Front Psychol 3, 555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuur, A.T. , Lundbye‐Jensen, J. , Leukel, C. , Taube, W. , Grey, M.J. , Gollhofer, A. , Nielsen, J.B. & Gruber, M. 2010. Contribution of afferent feedback and descending drive to human hopping. J Physiol 588, 799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]


