Abstract
Purpose
To evaluate the efficacy of photobiomodulation (PBM) treatment for patients with dry age‐related macular degeneration (AMD).
Methods
Assessments on 42 eyes with dry AMD (age related eye disease study (AREDS) 2–4) were conducted. Multiwavelength light emitting diode (LED) light comprising of yellow (590 nm), red (670 nm) and near‐infrared (790 nm) bandwidths was applied to subjects’ eyes for a treatment course of 3 weeks. Outcome measures were changes in best‐corrected visual acuity (BCVA), contrast sensitivity (CS), drusen volume and central drusen thickness.
Results
Significant improvement in mean BCVA of 5.90 letters (p < 0.001) was seen on completion of the 3‐week treatment and 5.14 letters (p < 0.001) after 3 months. Contrast sensitivity improved significantly (log unit improvement of 0.11 (p = 0.02) at 3 weeks and 3 months (log unit improvement of 0.16 (p = 0.02) at three cycles per degree. Drusen volume decreased by 0.024 mm3 (p < 0.001) and central drusen thickness was significantly reduced by a mean of 3.78 μm (p < 0.001), while overall central retinal thickness and retinal volume remained stable.
Conclusion
This is the first study demonstrating improvements in functional and anatomical outcomes in dry AMD subjects with PBM therapy. These findings corroborate an earlier pilot study that looked at functional outcome measures. The addition of anatomical evidence contributes to the basis for further development of a non‐invasive PBM treatment for dry AMD.
Keywords: age‐related macular degeneration, contrast, drusen, photobiomodulation, sensitivity, visual acuity
Introduction
Age‐related macular degeneration (AMD) is a retinal degenerative disease that causes irreversible and profound vision loss in people over the age of 60 years (Evans & Wormald 1996) with estimates of AMD patients nearing 50 million worldwide. It is emerging as one of the primary causes of vision impairment in the developed world (Gordois et al. 2012).
Age‐related macular degeneration (AMD) occurs in two major forms: exudative (wet) and atrophic (dry) AMD. Dry AMD is characterized by drusen, retinal pigment epithelial (RPE) cell atrophy and subjacent photoreceptor degeneration. Factors involved in causing RPE cell injury and dysfunction have been shown to include mitochondrial dysfunction, oxidative stress, inflammation and genetic disposition (Qin & Rodrigues 2008).
The vast majority of AMD patients suffering from dry AMD, marked by RPE dysfunction with drusen formation and eventual retinal atrophy, have no effective treatment options other than lifestyle modification and the use of vitamins (Davis et al. 2005).
A safe and globally expanding medical intervention is the use of low‐level laser (light) therapy (LLLT) that now includes diabetic wound repair, arthritis, cancer radiation protection (oral mucositis), dental, sports medicine and skeletal muscle disorders (trauma and pain). Low‐level laser therapy exerts its beneficial effects through increased blood flow and stimulation of cellular functions, a process called photobiomodulation (PBM).
Photobiomodulation (PBM) involves the use of visible to near‐infrared (NIR) light (500–1000 nm) produced by a laser or non‐coherent light sources such as light emitting diodes (LEDs) applied to the body to produce beneficial cellular effects. Light in this range penetrates tissue depending on the wavelength and stimulates cellular function via activation of photoacceptors (Rojas et al. 2008; Tata & Waynant 2010; Rojas & Gonzalaz‐Lima 2011).
Published studies demonstrate that mitochondrial cytochrome C oxidase (CCO) is a key photoacceptor of light at these wavelengths and improves blood flow and ATP formation, enhances O2 binding and reduces oxidative stress and inflammation (Karu et al. 1995; Karu & Kolyakov 2005; Wong‐Riley et al. 2005).
Although early studies identified mitochondrial CCO as an endogenous photoacceptor for PBM, the cellular and molecular mechanisms underlying PBM are better understood. Recent findings provide important new insight. First, nitric oxide has been implicated. Second, CCO, an enzyme known to reduce oxygen to water at the end of the mitochondrial respiratory chain, has been shown to have a new enzymatic activity – the reduction of nitrite to nitric oxide. This nitrite reductase activity is elevated under hypoxic conditions but also occurs under normoxia. And third, low‐intensity light regulates nitric oxide synthesis by CCO without altering its ability to reduce oxygen. From these findings, Poyton and Ball have proposed that CCO functions in PBM by regulating nitric oxide, a signalling molecule which can then function in both intra‐ and extracellular signalling pathways. They also propose that the effectiveness of PBM is under the control of tissue oxygen and nitrite levels (Poyton & Ball 2011).
Previous reports have suggested that phagocytosis is reduced by age‐related increased oxidative stress in AMD. Investigations on PBM in the human retinal pigment epithelial (ARPE‐19) cell lines demonstrate PBM‐improved phagocytosis, which is reduced under oxidative stress (Fuma et al. 2015).
Multiple preclinical animal models of ocular disease or disorders show PBM to be beneficial. These effects include reductions in damage observed in methanol toxicity, laser burn, complement factor H knockout inflammatory, bright light damage, retinitis pigmentosa and diabetic retinopathy animal models (Eells et al. 2003; Albarracin et al. 2011; Tang et al. 2013; Gkotsi et al. 2014). Photobiomodulation (PBM) can increase mitochondrial ATP, replication, density and activity and increase RNA and protein synthesis (Passarella & Karu 2014).
McDaniels has reported the effect of PBM using 590/870‐nm light on the expression of VEGF in 0‐ and 4‐week cultured human RPE cells where up to a sevenfold decrease in VEGF expression was seen. McDaniels also reported the response of PBM using 670‐nm light on the revitalization of RPE cells after an acute high‐dose blue light injury. Following blue light insult at 30 J/cm2, it was observed that 90% of RPE cells died. This is in contrast to the 5% of cells that died with blue light insult followed by red light therapy (LED photomodulation ‘reverses’ acute retinal injury, ASLMS Conference, 2006).
Ivandic and Ivandic show clinically that PBM with a laser diode aimed at the macular area significantly improves visual acuity in a case series of both dry and wet AMD subjects. Visual acuity (VA) in the control group remains unchanged. No adverse effects were observed in patients undergoing PBM therapy (Ivandic & Ivandic 2008).
Merry et al. have previously presented results of a PBM pilot study in a small cohort of dry AMD patients, The Toronto and Oak Ridge Photobiomodulation Study for Dry Age‐Related Macular Degeneration (TORPA) that demonstrated statistically significant improvements in early treatment diabetic retinopathy study (ETDRS) best‐corrected visual acuity (BCVA) and contrast sensitivity (CS) (PBM as a new treatment for dry AMD, ARVO, 2012). Further, PBM has recently been shown to reduce non‐central diabetic macular oedema in a case series (Tang et al. 2014).
Therefore, a growing body of evidence suggests that PBM treatment could have a beneficial role in dry AMD, a condition characterized by mitochondrial dysfunction, oxidative stress and inflammation within the RPE cell layer, choriocapillaris and neuroretina.
We report the findings from 42 eyes in an interventional, longitudinal case series. This study evaluated functional and pathological end‐points including retinal and drusen morphology using Spectral domain‐Optical coherence tomography (SD‐ OCT). We demonstrate that PBM has the potential to significantly improve both functional clinical and morphological outcomes in dry AMD.
Materials and Methods
Patient selection and setting
Subjects ≥50 years of age with dry AMD were eligible for study participation and were enrolled at the practices of Drs. Merry and Devenyi within a time period of 2011–2015.
Subjects were treated off‐label with available LED instruments approved for other indications by the FDA and Health Canada. Subjects who met the inclusion criteria and gave written informed consent underwent PBM with three treatment sessions per week for 3 weeks. Optical coherence tomography retinal images and fundus autofluorescence (FAF) were obtained at baseline (BL), immediately following the initial 3‐week treatment course at visit 1 and at a subsequent visit at 3 months (visit 2). Formal data collection with independent OCT and FAF analysis was conducted to evaluate both clinical and anatomical benefits of PBM. The study analysis protocol was approved by the Chesapeake Investigational Review Board and performed in adherence to the guidelines of the Declaration of Helsinki.
Inclusion criteria were dry AMD, AREDS grades (according to the American Academy of Ophthalmology) 2, 3 and 4 [geographic atrophy (GA), no choroidal neovascularization (CNV)] and a BCVA of letter score 50 (logMAR 1.0, Snellen 20/200) or better. A wide range of dry AMD categories were included as the study goal was to explore the potential benefit of PBM in varying stages and severity of AMD. Subjects with previous/active wet AMD, a history of epilepsy, other retinal diseases, significant media opacity and cataracts worse than grade 2 (LOCS III) were excluded.
PBM intervention
After carefully and extensively reviewing the available literature and the so far proposed mode of actions of the different evaluated wavelengths, the authors decided to use a multiwavelength approach as this would potentially affect different cellular targets and should therefore confer greater benefit than a single wavelength. Two separate devices were required to provide the multiple wavelengths. Both devices were FDA and Health Canada‐approved for other conditions.
The PBM intervention consisted of three distinct wavelengths in the yellow (590 nm), red (670 nm) and NIR (790 nm) range, chosen for their benefits on cellular targets involved in the disease process. We utilized LED units consisting of the Warp10 (Quantum Devices, Newark, OH, USA) and the Gentlewaves (Light Bioscience, Virginia Beach, VA, USA) instruments. The PBM treatment parameters for the Warp10 were wavelength 670 ± 15 nm delivering 50–80 mW/cm2 (4–7.68 J/cm2) for 88 ± 8 seconds, and for the Gentlewaves were wavelengths of 590 ±8 nm at 4 mW and 790 ± 60 nm at 0.6 mW, both for 35 seconds, pulsed at 2.5 Hz (250 milliseconds on, 150 milliseconds off) delivering 0.1 J/cm2/treatment.
All subjects were treated in both eyes with the two devices used sequentially at each treatment visit in nine sessions over a 3‐week period. The treatment parameters delivered to the subjects were identical at each session.
Study variables
The primary clinical efficacy end‐points were change from BL in BCVA and CS. The anatomical end‐points were change in SD‐OCT and FAF parameters.
All subjects were assessed with standardized ETDRS BCVA at a 4‐m distance (Precision Vision, Woodstock, IL, USA) recorded as correct letters scored. The letter score reported conforms to the WHO ICO report – Sydney April 2002 with a scale of 100 correct letters equivalent to logMAR: 0, decimal: 1.0 and Snellen: 20/20. Contrast sensitivity was assessed at 1.5, 3.0 and 6.0 cycles per degree (cpd) (Stereo Vision Optec 6500; functional acuity contrast test (FACT)) recorded as log CS for each cycle per degree. Subjects were tested by the same examiner at all visits under identical conditions in his private practice office.
Subjects were assessed with 20 × 20 high‐resolution SD‐OCT volume scans consisting of 25 section scans (250 μm distance between each scan, nine frames averaged) and with 488‐nm FAF (Spectralis OCT, Heidelberg Engineering, Heidelberg, Germany). Subsequent SD‐OCT scans were performed using the TruTrack™ (Heidelberg Engineering GmbH, Heidelberg, Germany) function to allow exact comparison of retina and drusen volume. Measurements included aligned mean central retinal thickness (CRT), aligned mean retinal volume (RV), GA lesion area, drusen/reticular pseudodrusen (RPD) volume and mean central drusen/RPD thickness.
Optical coherence tomography (OCT) and FAF scans were carefully reviewed by an independent imaging expert for the presence of drusen, RPD, GA, vitelliform lesions and for irregularity and disruption of the external limiting membrane (ELM), ellipsoid zone (EZ) and interdigitation zone (IZ) at each visit. Fundus autofluorescence images were reviewed for the presence of GA and vitelliform lesions.
Drusen in SD‐OCT scans were defined as focal deposits of divergent reflectivity and of variable size between the RPE and the Bruch membrane (Spaide & Curcio 2010). Drusenoid RPE detachments, defined as RPE elevations ≥350 μm, were included in the drusen volume measurements. Reticular pseudodrusen (RPD) were defined as small hyper‐reflective deposits located in the subretinal space (Spaide & Curcio 2010). Geographic atrophy on SD‐OCT was identified when there was absence/loss of the RPE, the EZ and ELM together with enhanced choroidal signal and concomitant loss of the outer plexiform layer (Sayegh et al. 2011). Vitelliform lesions were identified when there were homogenous hyper‐reflective lesions located in the subretinal space (Chowers et al. 2015).
The individual retinal layers were automatically segmented with the inbuilt software (1.9.10.0, Heidelberg Engineering, Germany) (Ctori & Huntjens 2015). The segmentation line of the Bruch's membrane and the internal limiting membrane was inspected on each scan for correct alignment and manually corrected if needed to achieve valid RV and CRT values. The automated, adjusted segmentation line of the RPE was carefully examined for exact drusen alignment in each scan and in cases of incorrect segmentation adjusted (Fig. 1). The inbuilt software then automatically calculated the total drusen volume and the mean central 1‐mm drusen thickness within the ETDRS grid between the Bruch's membrane and the fitted RPE segmentation line (Fig. 1).
Figure 1.
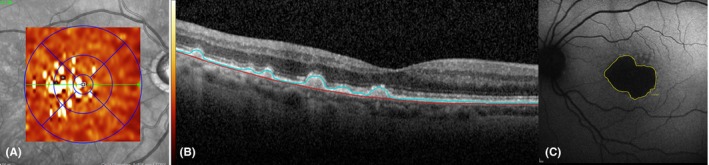
Representative images from two different patients showing characteristic pathologic changes. Semi‐automated segmentation in optical coherence tomography (OCT) and geographic atrophy (GA) assessment in fundus autofluorescence (FAF) were performed. The left image (A) displays a representative drusen volume map of an AREDS 3 patient. Colour bars indicate ascending thickness values starting with 0 μm (black). The image in the middle (B) demonstrates the drusen alignment in one representative OCT section scan of the same patient. The right image (C) illustrates the assessment of the GA area using FAF at 488 nm wavelength in a patient with central involving GA of an AREDS 4 patient. The GA is measured by manually marking the area of homogenous hypo‐autofluorescence using the inbuilt Heidelberg software (yellow line).
A 488‐nm FAF image was employed to quantitatively assess GA. The area of homogenous hypo‐autofluorescence on the FAF BL and subsequent images were manually marked using the inbuilt ‘draw’ command of the Heidelberg software. Analysis of the absolute GA lesion area as well as the square root of the area was used in order to allow growth comparison independent of initial lesion size (Fig. 1) (Yehoshua et al. 2011).
Statistical analyses
Statistical analyses were performed using r (The R Project for Statistical Computing; https://www.r-project.org) version 3.0 or higher. Linear mixed‐effects analyses were performed using the r package nlme. Graphs were generated using the r package ggplot2. Data were analysed using random intercept models with month as fixed effect, subjects as random effects and eye nested within subject. The random effects model is analogous to a paired t‐test or repeated‐measures analysis, but controls for correlation of treatment effects between eyes within subjects. We fit linear mixed‐effects models accounting that the observations of eyes are not fully independent of one another as pairs of eyes are from the same subject. Two‐sided p‐values <0.05 were considered statistically significant.
Results
The study evaluated 42 eyes from 24 subjects, 66–95 years of age (mean 78.0 ± 7.83). There were 15 females and 9 males. Baseline AMD disease demographics are presented in Table 1. Thirteen subjects (31%) presented with GA of which nine subjects (21%) had definite foveal involvement visible on SD‐OCT and FAF.
Table 1.
Baseline Disease Distribution according to AREDS Classification, and Study Outcome Measures
| Baseline disease distribution according to AREDS classification | |
|---|---|
| AREDS classification | Number of eyes (%) |
| AREDS 2 | 9 (21%) |
| AREDS 3 | 20 (48%) |
| AREDS 4 | 13 (31%) |
| GA | 13 (31%) |
| RPD | 28 (67%) |
| Baseline clinical and functional outcome measures | |
| Study variables at BL (units) | Mean ± SD |
| ETDRS (letters) | 86.29 ± 11.36 |
| CS 1.5 CPD (log CS) | 1.36 ± 0.17 |
| CS 3.0 CPD (log CS) | 1.50 ± 0.23 |
| CS 6.0 CPD (log CS) | 1.54 ± 0.20 |
| Drusen volume (mm3) | 0.46 ± 0.14 |
| Central drusen thickness (μm) | 35.12 ± 36.58 |
| GA area (mm2) | 7.01 ± 5.22 |
| CRT (μm) | 278.67 ± 47.60 |
| RV (mm3) | 8.04 ± 0.78 |
RPD = reticular pseudodrusen, SD = standard deviation, ETDRS = early treatment diabetic retinopathy study, CS = contrast sensitivity, CPD = cycles per degree, log CS = log contrast sensitivity, GA = geographic atrophy, CRT = central retinal thickness, RV = retinal volume.
Baseline values of clinical and morphological parameters are given in Table 1.
All subjects had been taking AREDS supplementation prior to the intervention, and no changes were made to their current dosing regimen during the observational period.
Functional outcome measures of BCVA and CS showed a significant positive effect immediately following the treatment (Table 2, Fig. 2). Mean ETDRS BCVA score improved by 5.9 letters immediately post‐treatment with a significant difference (p < 0.001) that remained at a statistically significant level at visit 2 (p < 0.001; Table 2, Fig. 2). A total of 11.9% of eyes treated gained more than two lines (≥11 ETDRS letters) and 59.5% of eyes achieved more than a one line increase from BL at visit 1 (Fig. 2). Contrast sensitivity was significantly improved at 1.5, 3.0 and 6.0 CPD at visit 1 immediately post‐treatment with p‐values of 0.01, 0.02 and 0.003, respectively (Table 2, Fig. 2). There was a significant correlation between CS and VA benefits (Pearson = 0.54; Spearman = 0.6).
Table 2.
Summary table of results
| Measure | Comparison | Letter score increase | p‐value |
|---|---|---|---|
| BCVA letter score | BL to V1 | +5.90 | <0.001 |
| BCVA letter score | BL to V2 | +5.14 | <0.001 |
| Measure | Comparison | Log CS increase | p‐value |
| CS 1.5 CPD | BL to V1 | +0.102 | 0.0113 |
| CS 1.5 CPD | BL to V2 | +0.080 | 0.0558 |
| CS 3.0 CPD | BL to V1 | +0.109 | 0.0224 |
| CS 3.0 CPD | BL to V2 | +0.166 | 0.0155 |
| CS 6.0 CPD | BL to V1 | +0.117 | 0.0029 |
| CS 6.0 CPD | BL to V2 | +0.10 | 0.0360 |
| Measure | Comparison | mm3 decrease | p‐value |
| Drusen volume | BL to V1 | −0.024 | <0.001 |
| Drusen volume | BL to V2 | −0.029 | 0.0206 |
| Measure | Comparison | μm change | p‐value |
| Central drusen thickness | BL to V1 | −3.78 | <0.001 |
| Central drusen thickness | BL to V2 | −0.34 | 0.8781 |
| CRT | BL to V1 | −0.36 | 0.6872 |
| CRT | BL to V2 | 3.39 | 0.1422 |
| GA area | Comparison | mm change | p‐value |
| GA square root | BL to V1 | −0.021 | 0.2043 |
| GA square root | BL to V2 | 0.026 | 0.1618 |
| Measure | Comparison | mm3 decrease | p‐value |
| RV | BL to V1 | −0.066 | 0.1133 |
| RV | BL to V2 | −0.049 | 0.4639 |
BL = baseline, GA = geographic atrophy, early treatment diabetic retinopathy study letter score (BCVA = best‐corrected visual acuity), CS = contrast sensitivity at 1.5, 3.0 and 6.0 cycles per degree (CPD, log units), drusen volume reduction (mm3), central retinal drusen thickness (μm), retinal volume (RV, mm3) and central retinal thickness (CRT, μm).
Figure 2.
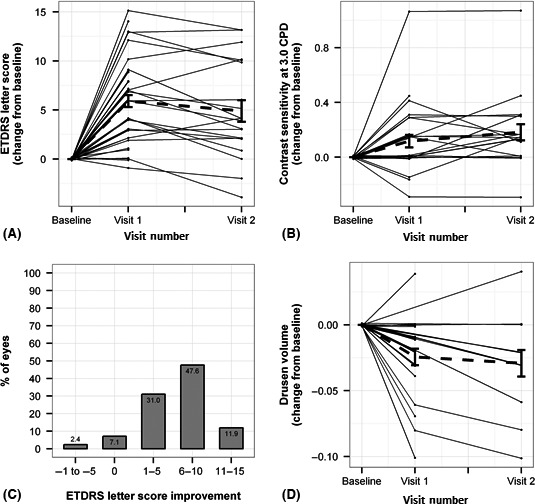
(A) Photobiomodulation (PBM) leads to significant improvement in early treatment diabetic retinopathy study (ETDRS) letter score (absolute plot – jitter) in individual subjects. The bold dotted line indicates the overall mean. (B) Contrast sensitivity (CS; 3.0 CPD) in logCS change from baseline (BL) following PBM treatment (absolute plot – jitter) in individual subjects. The bold dotted line indicates the overall mean. (C) This panel indicates the percentage of eyes presenting with one, two and three line ETDRS letter gain at visit 1 ( = immediate post‐treatment, 3 weeks after BL). (D) Drusen volume measurements in mm3 change from BL following PBM treatment (absolute plot – jitter) in individual subjects. The bold dotted line indicates the overall mean.
Further subgroup analysis of the BCVA data was employed to evaluate whether the BL score is indicative of the magnitude of change for potential improvement following PBM treatment. Although the linear regression analysis of the change in BCVA as a function of BL revealed that the BL score was not associated with the increase in visual acuity (r 2 = 0.049, p = 0.16), the majority of responders with greater than five letters improvement had a BL score of between 70 and 89 letters (Snellen equivalent of 20/80–20/32) (Fig. 3). This group also had the greatest number of responders with a 15 or more letter gain. Patients with better (≥90 letters) or lower (60–69 letters) BL scores were less likely to gain more than five letters (Fig. 4).
Figure 3.
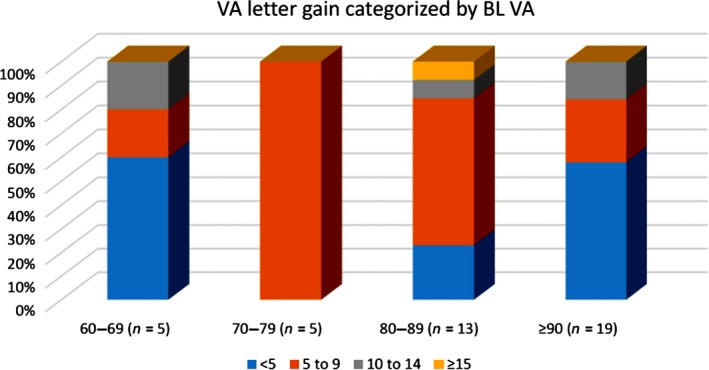
Visual acuity change in magnitude categorized by baseline (BL) VA scores. Eyes with a BL VA letter score of between 70 and 89 (Snellen equivalent of 20/80–20/32) appeared to respond to photobiomodulation treatment with a high percentage gaining greater than five letters. Eyes with lower (60–69 letters) or higher (≥90 letters) BL VA letter score were less likely to gain more than five letters.
Figure 4.
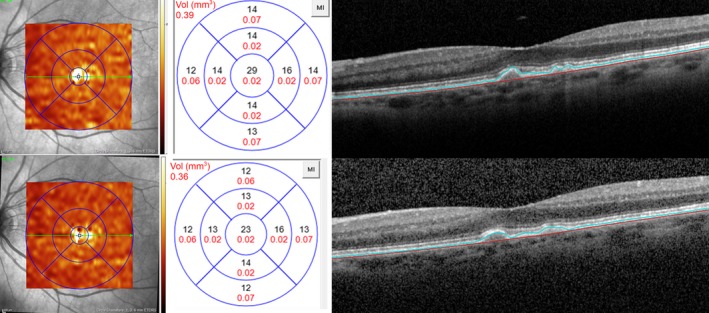
Representative example of an eye categorized as AREDS 3 with mainly convex, homogenous and low reflective drusen larger than 125 μm. Baseline (Top) shows a drusen volume of 0.39 mm3 with a mean central 1‐mm drusen with thickness of 29 μm. Black numbers indicate the mean drusen thickness of each early treatment diabetic retinopathy study subgrid and red numbers indicate the corresponding drusen volume (mm3). Bottom: Follow‐up examination at visit 1. Overall drusen volume as well as mean central drusen thickness has significantly decreased without new formation of geographic atrophy or disruption of the photoreceptor layers.
Outcome measures of drusen volume and central drusen thickness showed a significant reduction immediately following the 3‐week treatment at visit 1 (p < 0.001). At visit 2, drusen volume was still significantly reduced (p = 0.02; Table 2, Fig. 2). There were no significant changes in CRT, GA area or RV during the observational period.
Representative segmented section scans illustrating the drusen regression following therapy is given in Figs 4 and 5.
Figure 5.
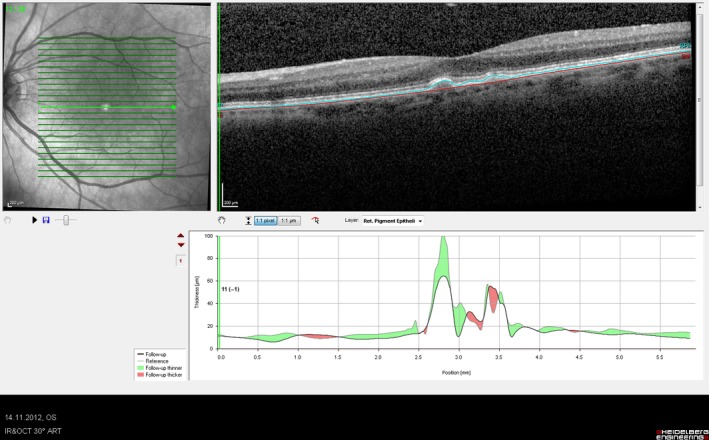
An individual cut segmented subtraction scan showing significant drusen regression at 3 months following the treatment course.
None of the patients developed new wet AMD or GA during the study analysis period of 3 months.
Discussion
The previous TORPA study was the first to show improvement in functional parameters using LED light sources for dry AMD subjects (Merry et al., PBM for dry AMD, ARVO, 2012). Significant improvement in BCVA and CS was observed immediately following PBM and was maintained at 1 year. The results from the current study further corroborate the improvement in functional measures (BCVA and CS) seen in the earlier TORPA study and demonstrate the benefits of PBM to significant improvement in anatomical end‐points of the disease. This has not been studied in any previous PBM intervention for AMD.
Best‐corrected visual acuity in dry AMD may remain stable or slowly decline without rapid vision loss. Therefore, a small visual gain in this patient cohort is clinically relevant. In this case series, BCVA improved in over 90% of subjects with 59.5% achieving better than five letters and 11.9% achieving better than 10 letters on ETDRS BCVA testing, representing significant improvement in visual function. Early treatment diabetic retinopathy study BCVA testing measurements with a logMAR score change of 0.1 (five letters) has been shown to be statistically significant and clinically relevant (Elliott & Sheridan 1988). Based on 95% confidence intervals, a change in acuity of logMAR 0.2 (10 letters) from BL is unlikely to be related to measurement variability (Beck et al. 2003).
Interestingly, it would appear that those with good to fair vision (20/30–20/80) benefited the most from PBM therapy. However, patients with BCVA both better and worse than this group at BL also showed benefit. This seems reasonable considering the ceiling effect in patients with a BCVA better than 20/32 and the fact that patients with a BL score worse than 20/80 already exhibit irreversible damage of the outer retina in the fovea. Improvements in CS were also significant and correlated to the improvements in BCVA.
The significant drusen reduction in association with functional improvement is particularly compelling. Our data clearly show a decrease in drusen volume and mean central drusen thickness. Although drusen formation and regression are in a constant process of change, we know from previous studies that the drusen area tends to increase even during a short period of time (Gregori et al. 2014). A short‐term follow‐up study analysing drusen area progression over 6 months has demonstrated a progression of 0.031 and 0.029 mm2 on SD‐OCT and CF, respectively (Gregori et al. 2014). Our data showed a mean regression of drusen volume of 0.03 mm3 over a period of 3 months. While drusen regression and resolution can leave behind GA (Suzuki et al. 2015), drusen can also collapse and vanish without contributing to further photoreceptor and RPE irregularity and loss. In this study, none of the eyes developed new onset of EZ or IZ irregularities or new formation of RPE loss, indicating that drusen regressed without contributing to new GA formation.
Increase in drusen area (Sarraf et al. 1999) has previously found to be a significant predictive factor for the onset of late‐stage AMD and the presence of RPD has been associated with a higher risk of late‐stage AMD (Marsiglia et al. 2013; Alten & Eter 2015). Patients with RPD in this case series showed similar, significant treatment responses suggesting regression of disease.
Higher power lasers as well as subthreshold lasers have been used to affect drusen regression but did not show evidence to reduce the risk of developing CNV or GA. A meta‐analysis on laser studies evaluating the functional and morphological efficacy of laser treatment in AMD has shown that laser may be effective to induce drusen reduction, but does not seem to reduce the development of CNV. A case series and a study utilizing subthreshold micropulse laser and nanosecond laser, respectively, in dry AMD patients have shown partial drusen regression but with no improvement in BCVA (Rykov et al. 2015).
In contrast to laser treatment, PBM utilizes very low energy levels causing no tissue damage. Photobiomodulation stimulates cellular processes that provide an approach to target the underlying degenerative pathology with disease‐modifying potential. A recent review paper of PBM in retinal diseases has reported that the literature supports the conclusion that the low cost and non‐invasive nature of PBM coupled with the first promising clinical reports and the numerous preclinical studies in animal models make PBM well poised to become an important player in the treatment of retinal disorders (Geneva 2016).
A randomized control group was not included as these patients were being treated in an off‐label protocol with commercial instruments that were limited in flexibility to modify or mask treatments. Consideration was given to using just one of the subjects' eyes as a control; however, this was not acceptable to patients receiving the treatment and there is a possibility of systemic signalling and improvement in remote tissues with PBM. Therefore, it is most desirable to completely separate active patients from control in future studies. Examiner bias was mitigated by rigorously ensuring identical conditions at each visit for all patients with an experienced physician. Subgroup analysis should be considered preliminary and intriguing as the subject numbers are limited; however, significant positive conclusions can be drawn on the main end‐points of ETDRS BCVA, CS and drusen reduction.
The data presented have formed the basis for a National Eye Institute partially funded grant to support a Health Canada‐ and IRB‐approved, randomized, placebo (sham treatment), double‐masked prospective, clinical trial that is currently enrolling in Toronto. The LIGHTSITE I trial is looking at similar clinical and anatomical end‐points that are presented here with the addition of microperimetry and VFQ‐25.
The present study demonstrated drusen reduction utilizing selected multiple wavelengths of LED sourced light that are non‐thermal and shown to have anti‐inflammatory, antioxidative, neuroprotective and anti‐apoptotic benefits as evident in several in vitro and in vivo ocular models (Eells et al. 2003; Albarracin et al. 2011; Tang et al. 2013; Gkotsi et al. 2014). It is the combination of anatomical changes coincident with functional improvements that is most promising for PBM as a novel treatment for dry AMD. The clinical results to date provide the foundation for a novel non‐invasive approach to the treatment of dry AMD.
The authors would like to thank Bob Schmidt for the statistical programming and analysis support and Drs. Clark Tedford, Rene Rückert and Stephanie Tedford for their review, comments and contributions to the writing of this manuscript.
References
- Albarracin R, Eells J & Valter K (2011): Photobiomodulation protects the retina from light‐induced photoreceptor degeneration. Invest Ophthalmol Vis Sci 52: 3582–3592. [DOI] [PubMed] [Google Scholar]
- Alten F & Eter N (2015): Current knowledge on reticular pseudodrusen in age‐related macular degeneration. Br J Ophthalmol 99: 717–722. [DOI] [PubMed] [Google Scholar]
- Beck RW, Moke PS, Turpin AH et al. (2003): A computerized method of visual acuity testing: adaptation of the early treatment of diabetic retinopathy study testing protocol. Am J Ophthalmol 135: 194–205. [DOI] [PubMed] [Google Scholar]
- Chowers I, Tiosano L, Audo I, Grunin M & Boon CJ (2015): Adult‐onset foveomacular vitelliform dystrophy: a fresh perspective. Prog Retin Eye Res 47: 64–85. [DOI] [PubMed] [Google Scholar]
- Ctori I & Huntjens B (2015): Repeatability of foveal measurements using spectralis optical coherence tomography segmentation software. PLoS One 10: e0129005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MD, Gangnon RE, Lee LY et al. (2005): The age‐related eye disease study severity scale for age‐related macular degeneration: AREDS report no. 17. Arch Ophthalmol 123: 1484–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eells JT, Henry MM, Summerfelt P, Wong‐Riley MT, Buchmann EV, Kane M, Whelan NT & Whelan HT (2003): Therapeutic photobiomodulation for methanol‐induced retinal toxicity. Proc Natl Acad Sci U S A 100: 3439–3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott DB & Sheridan M (1988): The use of accurate visual acuity measurements in clinical anti‐cataract formulation trials. Ophthalmic Physiol Opt 8: 397–401. [DOI] [PubMed] [Google Scholar]
- Evans J & Wormald R (1996): Is the incidence of registrable age‐related macular degeneration increasing? Br J Ophthalmol 80: 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuma S, Murase H, Kuse Y, Tsuruma K, Shimazawa M & Hara H (2015): Photobiomodulation with 670 nm light increased phagocytosis in human retinal pigment epithelial cells. Mol Vis 21: 883–892. [PMC free article] [PubMed] [Google Scholar]
- Geneva II (2016): Photobiomodulation for the treatment of retinal diseases: a review. Int J Ophthalmol 9: 145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gkotsi D, Begum R, Salt T, Lascaratos G, Hogg C, Chau KY, Schapira AH & Jeffery G (2014): Recharging mitochondrial batteries in old eyes. Near infra‐red increases ATP. Exp Eye Res 122: 50–53. [DOI] [PubMed] [Google Scholar]
- Gordois A, Cutler H, Pezzullo L, Gordon K, Cruess A, Winyard S, Hamilton W & Chua K (2012): An estimation of the worldwide economic and health burden of visual impairment. Glob Public Health 7: 465–481. [DOI] [PubMed] [Google Scholar]
- Gregori G, Yehoshua Z, Garcia Filho CA, Sadda SR, Portella NR, Feuer WJ & Rosenfeld PJ (2014): Change in drusen area over time compared using spectral‐domain optical coherence tomography and color fundus imaging. Invest Ophthalmol Vis Sci 55: 7662–7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivandic BT & Ivandic T (2008): Low‐level laser therapy improves vision in patients with age‐related macular degeneration. Photomed Laser Surg 26: 241–245. [DOI] [PubMed] [Google Scholar]
- Karu TI & Kolyakov SF (2005): Exact action spectra for cellular responses relevant to phototherapy. Photomed Laser Surg 23: 355–361. [DOI] [PubMed] [Google Scholar]
- Karu T, Pyatibrat L & Kalendo G (1995): Irradiation with He‐Ne laser increases ATP level in cells cultivated in vitro . J Photochem Photobiol, B 27: 219–223. [DOI] [PubMed] [Google Scholar]
- Marsiglia M, Boddu S, Bearelly S, Xu L, Breaux BE Jr, Freund KB, Yannuzzi LA & Smith RT (2013): Association between geographic atrophy progression and reticular pseudodrusen in eyes with dry age‐related macular degeneration. Invest Ophthalmol Vis Sci 54: 7362–7369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarella S & Karu T (2014): Absorption of monochromatic and narrow band radiation in the visible and near IR by both mitochondrial and non‐mitochondrial photoacceptors results in photobiomodulation. J Photochem Photobiol, B 140C: 344–358. [DOI] [PubMed] [Google Scholar]
- Poyton RO & Ball KA (2011): Therapeutic photobiomodulation: nitric oxide and a novel function of mitochondrial cytochrome c oxidase. Discov Med 11: 154–159. [PubMed] [Google Scholar]
- Qin S & Rodrigues GA (2008): Progress and perspectives on the role of RPE cell inflammatory responses in the development of age‐related macular degeneration. J Inflamm Res 1: 49–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas JC & Gonzalaz‐Lima F (2011): Low level light therapy of the eye and brain. Dovepress 2011: 49–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas JC, Lee J, John JM & Gonzalez‐Lima F (2008): Neuroprotective effects of near‐infrared light in an in vivo model of mitochondrial optic neuropathy. J Neurosci 28: 13511–13521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rykov S, Pasechnikova N, Suk S, & Saksonov S (2015): Prophylatic treatment of age‐related macuopathy with 577 nm subthreshold micropulse laser. Clinical Case Reports ‐ Iridex Mountainview, CA [Google Scholar]
- Sarraf D, Gin T, Yu F, Brannon A, Owens SL & Bird AC (1999): Long‐term drusen study. Retina 19: 513–519. [DOI] [PubMed] [Google Scholar]
- Sayegh RG, Simader C, Scheschy U et al. (2011): A systematic comparison of spectral‐domain optical coherence tomography and fundus autofluorescence in patients with geographic atrophy. Ophthalmology 118: 1844–1851. [DOI] [PubMed] [Google Scholar]
- Spaide RF & Curcio CA (2010): Drusen characterization with multimodal imaging. Retina 30: 1441–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Curcio CA, Mullins RF & Spaide RF (2015): Refractile drusen: clinical imaging and candidate histology. Retina 35: 859–865. [DOI] [PubMed] [Google Scholar]
- Tang J, Du Y, Lee CA, Talahalli R, Eells JT & Kern TS (2013): Low‐intensity far‐red light inhibits early lesions that contribute to diabetic retinopathy: in vivo and in vitro . Invest Ophthalmol Vis Sci 54: 3681–3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Herda AA & Kern TS (2014): Photobiomodulation in the treatment of patients with non‐center‐involving diabetic macular oedema. Br J Ophthalmol 98: 1013–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata DB & Waynant RW (2010): Laser therapy: a review of its mechanism of action and potential medical applications. Laser Photonics Rev 5: 1–12. [Google Scholar]
- Wong‐Riley MT, Liang HL, Eells JT, Chance B, Henry MM, Buchmann E, Kane M & Whelan HT (2005): Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: role of cytochrome c oxidase. J Biol Chem 280: 4761–4771. [DOI] [PubMed] [Google Scholar]
- Yehoshua Z, Rosenfeld PJ, Gregori G, Feuer WJ, Falcao M, Lujan BJ & Puliafito C (2011): Progression of geographic atrophy in age‐related macular degeneration imaged with spectral domain optical coherence tomography. Ophthalmology 118: 679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]


