Abstract
Aims
To examine the efficacy and safety of add‐on ipragliflozin in Japanese patients with type 2 diabetes in the early stage of insulin therapy.
Methods
Patients treated with insulin (bolus component <30% of total daily dose) with/without a dipeptidyl peptidase‐4 (DPP‐4) inhibitor were randomized to receive placebo (n = 87) or ipragliflozin (n = 175) for 16 weeks. The primary endpoint was the change in glycated haemoglobin (HbA1c) from baseline. Secondary endpoints included changes in fasting plasma glucose (FPG) and metabolic hormones. Safety endpoints were also examined.
Results
The changes in HbA1c were 0.27% and −0.79% (2.9 and −8.7 mmol/mol) in the placebo and ipragliflozin groups, respectively (baseline: 8.62% vs 8.67% [70.8 vs 71.2 mmol/mol]), corresponding to an adjusted mean difference of −1.07% (95% confidence interval −1.24, −0.91) or −11.7 mmol/mol (−13.5, −9.9), p < .001. Ipragliflozin reduced FPG and serum C‐peptide levels and body weight (all p < .001), and increased serum adiponectin levels (p = .022). There was a statistically significant interaction for use/non‐use of a DPP‐4 inhibitor × treatment group for the change in HbA1c (p = .042). Hypoglycaemia was the only treatment‐related adverse event reported in >5% of patients (14.9% vs 29.1%). Events consistent with urinary tract infection (placebo 1.1% vs ipragliflozin 2.3%) or genital infection (0.0% and 4.0%, respectively) occurred in <5% of patients.
Conclusion
Ipragliflozin was well tolerated and effective in insulin‐treated patients, especially when used with a DPP‐4 inhibitor.
Keywords: type 2 diabetes, insulin therapy, SGLT2 inhibitor, DPP-4 inhibitor
1. INTRODUCTION
Basal insulin and premixed insulin represent an effective treatment option for patients with inadequate glycaemic control with oral antidiabetic drugs1, 2, 3; however, some patients are still unable to achieve glycaemic targets for a variety of reasons.4 Other treatment strategies are then required, such as intensifying the insulin regimen or adding an(other) oral antidiabetic drug; however, patients are often reluctant to intensify their insulin regimen because it is restrictive to their daily life and might cause hypoglycaemia and weight gain. Novel oral antidiabetic agents are therefore required to facilitate further improvement in glycaemic control in patients with type 2 diabetes in the early stages of insulin therapy, defined as patients treated with long‐acting, intermediate‐acting or premixed insulin.
Ipragliflozin, a selective sodium‐glucose co‐transporter 2 (SGLT2) inhibitor, was recently approved in Japan for the treatment of type 2 diabetes as monotherapy or in combination with other oral antidiabetic drugs on the basis of clinical trials performed in Japan.5, 6, 7 Considering the beneficial effects of ipragliflozin on glycaemic control observed in these trials and because of the insulin‐independent nature of ipragliflozin action and its low risk of hypoglycaemia, adding ipragliflozin to insulin‐treated patients is expected to be efficacious and well tolerated without increasing the risk of hypoglycaemia and weight gain. Dipeptidyl peptidase‐4 (DPP‐4) inhibitors are commonly used in Japanese patients, and are frequently administered together with insulin, because this was reported to be an effective combination.8, 9 It is important, therefore, to determine whether DPP‐4 inhibitors affect the efficacy of ipragliflozin in insulin‐treated patients.
As part of the clinical development of ipragliflozin, the aim of the present study was to examine the efficacy and safety of administering ipragliflozin compared with placebo over 16 weeks in addition to ongoing insulin therapy in Japanese patients with type 2 diabetes, with or without a DPP‐4 inhibitor.
2. MATERIALS AND METHODS
2.1. Study design
The present study consisted of a 16‐week, randomized, double‐blind, placebo‐controlled period (superiority trial) performed between March 2014 and March 2015, and a 36‐week, open‐label extension period, which is ongoing. This report describes the results of the initial 16‐week treatment period. A total of 43 sites in Japan participated in the study, which was approved by the institutional review board at each site. The study complied with Good Clinical Practice, the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Guidelines, and all applicable laws and regulations. It was registered at ClinicalTrials.gov (identifier: NCT02175784). All patients provided written informed consent.
2.2. Patients
Patients aged ≥20 years diagnosed with type 2 diabetes ≥12 weeks before enrolment were eligible if they had been prescribed a stable dose/regimen of insulin (8‐40 units/day) for ≥6 weeks, alone or in combination with other oral antidiabetic drugs, had a glycated haemoglobin level (HbA1c) of ≥7.5% to ≤10.5% (≥58 to ≤91 mmol/mol), a maximum change in HbA1c of 1% (10.9 mmol/mol) during the 4‐week screening period, and a body mass index of 20.0‐45.0 kg/m2. Patients in the early stage of insulin therapy to support basal insulin secretion were eligible if they were prescribed fixed doses of mixed insulin (providing the rapid‐acting or ultra‐rapid‐acting insulin component was not >30% of the total daily dose), intermediate‐acting insulin or long‐acting insulin alone. Patients using a DPP‐4 inhibitor were eligible if they had been prescribed the same drug at a fixed dose for ≥6 weeks before enrolment and its dose was continued throughout the study. Exclusion criteria are listed in File S1.
2.3. Treatments
After a 4‐week screening period, patients entered a 2‐week single‐blind placebo run‐in period, then were randomized to receive 50 mg ipragliflozin or placebo, stratified by DPP‐4 inhibitor use. The dose of ipragliflozin remained unchanged throughout the treatment period. Patients using oral antidiabetic drugs other than DPP‐4 inhibitors entered an additional 4‐week washout period before screening. The randomization list was prepared by the patient registration centre and patients were allocated to study groups at a 2:1 ratio by the investigator.
To reduce hypoglycaemia risk, patients were instructed to measure their fasting blood glucose (FBG) level using self‐monitored blood glucose (SMBG) values every morning and to record whether they felt symptoms associated with hypoglycaemia (values were recorded in patient diaries).
Insulin type was unchanged during the treatment period. The dose of insulin was to continue unchanged during the treatment period, unless dose changes were required for safety reasons. The insulin dose could be reduced at the investigator's discretion if FBG concentrations were <3.89 mmol/L (<70 mg/dL) on two consecutive days and the patient was suspected of having hypoglycaemic symptoms. The insulin dose could be increased if FBG (measured by SMBG) was >11.10 mmol/L (>200 mg/dL) on two consecutive days or if the investigator considered it necessary to increase the insulin dose for safety. The insulin dose could be adjusted by a maximum of four units throughout the study, regardless of the number of dose adjustments. If the insulin dose was changed by >4 units, the patient discontinued the study and was prescribed an appropriate treatment regimen.
The patients’ diet and exercise therapies at enrolment were continued unchanged throughout the study. Concomitant use of antidiabetic drugs other than insulin and DPP‐4 inhibitors used at enrolment was prohibited. Corticosteroids, immunosuppressants, glucagon and glucose could be administered temporarily, but continuous use of these agents was prohibited.
2.4. Endpoints and assessments
The primary efficacy endpoint was change in HbA1c from baseline to end of the 16‐week treatment period. Secondary endpoints included changes in fasting plasma glucose (FPG) and glycoalbumin levels, SMBG values recorded by patient diary, body weight, waist circumference, and glucagon, leptin and adiponectin levels. SMBG was performed at baseline and at the end of treatment at the following times: before breakfast (FBG), 1 hour after breakfast, before lunch, 1 hour after lunch, before dinner, 1 hour after dinner, and at bedtime. Changes in HbA1c and FPG were examined in patients, stratified by use/non‐use of DPP‐4 inhibitors in prespecified analyses.
Safety endpoints included vital signs, treatment‐emergent adverse events (TEAEs), and laboratory tests. TEAEs were classified according to system organ class and preferred terms [Medical Dictionary for Regulatory Activities (MedDRA) version 16.1], and their relationship to the study drug, seriousness and severity were evaluated. We examined several TEAEs of special interest, including those related to hypoglycaemia, urinary tract infection, genital infection, body fluid volume and electrolytes. TEAEs related to body fluid volume and electrolytes included cerebral infarction, dehydration, haemorrhagic cerebral infarction and thirst.
2.5. Statistical analysis
Efficacy analyses were carried out using the full analysis set, defined as all patients who received at least one dose of the study drug and in whom at least one efficacy variable was measured during the treatment period. Safety analyses were performed using the safety analysis set, defined as all patients who received at least one dose of the study drug during the treatment period.
Baseline characteristics are presented descriptively as mean ± standard deviation (s.d.) and n (%) of patients for continuous and categorical variables, respectively. The end of treatment was defined as end of the randomized double‐blind treatment period. For efficacy analyses, data obtained after changing insulin dose were excluded from analyses.
The primary efficacy endpoint (change in HbA1c from baseline to week 16, analysed using the last observation carried forward method to impute missing data) was evaluated by analysis of covariance (ancova) with the baseline value as a covariate, and use/non‐use of a DPP‐4 inhibitor and treatment group as fixed effects. The same method was used for subgroup analyses. We performed ancova with interaction terms [baseline HbA1c × treatment group] and [use/non‐use of a DPP‐4 inhibitor × treatment group] to evaluate the significance of interactions. In the prespecified subgroup analysis of use/non‐use of a DPP‐4 inhibitor, this variable was excluded from ancova. Similar statistical methods were applied to other efficacy endpoints.
Numbers and percentages of patients with TEAEs, serious adverse events, TEAEs leading to permanent discontinuation of the study drug or TEAEs of special interest (TEAEs related to hypoglycaemia, urinary tract infection, genital infection, and effects on body fluid volume and electrolytes) were calculated for each treatment group.
No interim analyses were performed. There were no changes to the statistical analysis plan after unblinding, except for adding the efficacy analysis of C‐peptide concentrations.
3. RESULTS
3.1. Patients
A total of 262 patients were randomized and treated with placebo (n = 87) or ipragliflozin (n = 175; Figure 1). Of these, 245 patients (placebo, n = 76; ipragliflozin, n = 169) completed the study. Table 1 shows no significant differences in patient characteristics between the groups. The mean patient age was 59.2 ± 9.3 years in the placebo group and 58.7 ± 11.1 years in the ipragliflozin group, and 27.6% of patients in the placebo group and 34.5% in the ipragliflozin group were aged ≥65 years. At baseline, HbA1c was 8.62% ± 0.86% (70.8 ± 9.5 mmol/mol) and 8.67% ± 0.77% (71.2 ± 8.4 mmol/mol) in the placebo and ipragliflozin groups, respectively. Insulin preparation types were as follows: 27.6% of the placebo group and 29.8% of the ipragliflozin group were treated with mixed insulin, 6.9% of the placebo group and 6.5% of the ipragliflozin group were on intermediate‐acting insulin, and 65.5% of the placebo group and 63.7% of the ipragliflozin group were on long‐acting insulin only (glargine 40.4%, detemir 3.1%, degludec 20.8%). Approximately 50% of patients in each group were current or former smokers, and just over half currently or formerly consumed alcohol.
Figure 1.
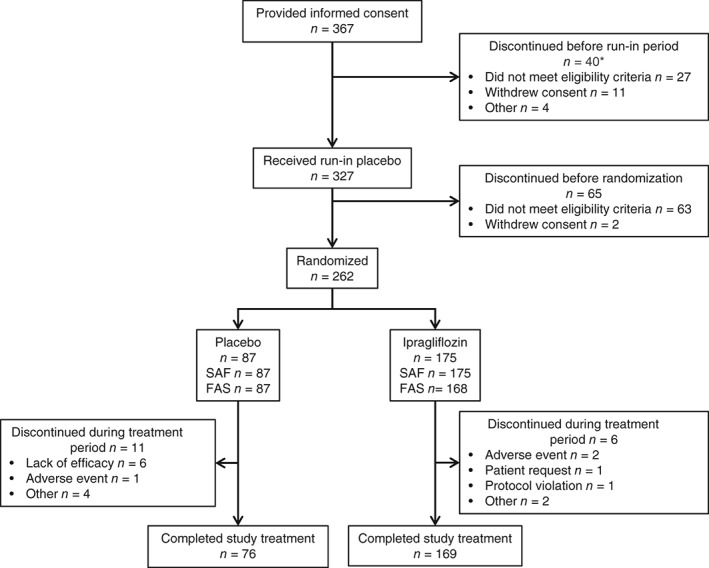
Patient disposition. *Some patients discontinued for more than one reason. FAS, full analysis set; SAF, safety analysis set.
Table 1.
Baseline characteristics
| Placebo | Ipragliflozin | p‐value | |
|---|---|---|---|
| N = 87 | N = 168 | ||
| Male | 51 (58.6) | 105 (62.5) | .589 1 |
| Age (years) | 59.2 ± 9.3 | 58.7 ± 11.1 | .723 2 |
| Age ≥ 65 years | 24 (27.6) | 58 (34.5) | .322 1 |
| Body weight, kg | 70.32 ± 12.17 | 69.05 ± 11.61 | .415 2 |
| Height, cm | 163.03 ± 9.74 | 164.08 ± 8.86 | .384 2 |
| BMI, kg/m2 | 26.42 ± 3.81 | 25.61 ± 3.53 | .089 2 |
| SBP, mm Hg | 131.6 ± 14.7 | 131.2 ± 14.0 | .838 2 |
| DBP, mm Hg | 77.0 ± 11.0 | 76.8 ± 10.1 | .910 2 |
| Duration of diabetes mellitus, months | 171.4 ± 102.5 | 151.1 ± 93.5 | .115 2 |
| Patients who entered a washout period before the observation period | 24 (27.6) | 43 (25.6) | .765 1 |
| HbA1c, mmol/mol | 70.8 ± 9.5 | 71.2 ± 8.4 | .723 2 |
| HbA1c, % | 8.62 ± 0.86 | 8.67 ± 0.77 | .640 2 |
| FPG, mmol/L | 8.91 ± 2.41 | 8.87 ± 2.53 | .921 2 |
| FPG mg/dL | 160.5 ± 43.4 | 159.9 ± 45.7 | .921 2 |
| C‐peptide, nmol/L | 0.36 ± 0.23 | 0.33 ± 0.21 | .327 2 |
| eGFR, mL/min/1.73 m2 | 80.11 ± 21.94 | 83.98 ± 20.27 | .162 2 |
| eGFR ≥ 90 mL/min/1.73 m2 | 20 (23.0) | 59 (35.1) | .063 1 |
| Insulin type | .953 1 | ||
| Mixed | 24 (27.6) | 50 (29.8) | |
| Intermediate‐acting | 6 (6.9) | 11 (6.5) | |
| Long‐acting | 57 (65.5) | 107 (63.7) | |
| Total insulin dose (units/day) | .693 1 | ||
| <15 | 30 (34.5) | 59 (35.1) | |
| ≥15 to <30 | 41 (47.1) | 71 (42.3) | |
| ≥30 | 16 (18.4) | 38 (22.6) |
Values are presented as the n (%) or mean ± standard deviation.
BMI, body mass index; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; SBP, systolic blood pressure; DBP, diastolic blood pressure.
Fisher's exact test.
Two‐sample t test.
3.2. Efficacy
Small changes in HbA1c levels were observed in the placebo group: from 8.62% (baseline) to 8.90% (end of treatment) (70.8 to 73.7 mmol/mol), whereas HbA1c levels in the ipragliflozin group decreased from 8.67% to 7.88% (71.2 to 62.5 mmol/mol), resulting in an intergroup adjusted mean difference of −1.07% [95% confidence interval (CI) −1.24, −0.91] or −11.7 mmol/mol (−13.5, −9.9), p < .001 (Table 2). Figure 2A shows that the mean HbA1c level decreased from baseline until week 12, and remained stable until week 16.
Table 2.
Efficacy variables
| Placebo (N = 87) |
Ipragliflozin (N = 168) |
||
|---|---|---|---|
| HbA1c, mmol/mol [%] | Baseline / EOT | 70.8 ± 9.5 / 73.7 ± 11.7 | 71.2 ± 8.4 / 62.5 ± 8.9 |
| [8.62 ± 0.86 / 8.90 ± 1.07] | [8.67 ± 0.77 / 7.88 ± 0.81] | ||
| Change | 2.9 ± 7.0 | −8.7 ± 7.2 | |
| [0.27 ± 0.65] | [−0.79 ± 0.66] | ||
| AMD (95% CI) | −11.7 (−13.5, −9.9) | ||
| [−1.07 (−1.24, −0.91)], p < .001 | |||
| FPG, mmol/L [mg/dL] | Baseline / EOT | 8.91 ± 2.41 / 9.48 ± 2.88 | 8.87 ± 2.53 / 7.26 ± 1.79 (167) |
| [160.5 ± 43.4 / 170.9 ± 52.0] | [159.9 ± 45.7 / 130.7 ± 32.3] | ||
| Change | 0.58 ± 2.19 | −1.62 ± 2.19 (167) | |
| [10.4 ± 39.6] | [−29.3 ± 39.4] | ||
| AMD (95% CI) | −2.23 (−2.70, −1.75) | ||
| [−40.3 (−48.9, −31.7)], p < .001 | |||
| Glycoalbumin, % | Baseline / EOT | 23.43 ± 3.92 / 23.63 ± 4.26 | 23.48 ± 3.58 / 19.88 ± 3.41 |
| Change | 0.20 ± 2.08 | −3.60 ± 2.33 | |
| AMD (95% CI) | −3.84 (−4.40, −3.29), p < .001 | ||
| SMBG, mmol/L [mg/dL] | |||
| Fasting | Baseline / EOT | 8.739 ± 2.353 (86) / 7.761 ± 1.831 (58) | 8.349 ± 2.355 (161) / 6.935 ± 1.492 (123) |
| [157.44 ± 42.40] / [139.82 ± 32.99] | [150.41 ± 42.44]/ [124.94 ± 26.87] | ||
| Change | −0.101 ± 1.517 (58) | −1.643 ± 1.674 (119) | |
| [−1.83 ± 27.33] | [−29.60 ± 30.16] | ||
| AMD (95% CI) | −1.185 (−1.591, −0.779), p < .001 | ||
| [−21.36 (−28.67, −14.04)], p < .001 | |||
| 1 h after breakfast | Baseline / EOT | 13.458 ± 3.131 (83) / 12.797 ± 3.229 (58) | 12.817 ± 3.029 (159) / 11.349 ± 2.374 (123) |
| [242.46 ± 56.40] / [230.53 ± 58.18] | [230.91 ± 54.57] / [204.46 ± 42.78] | ||
| Change | 0.438 ± 3.114 (57) | −1.464 ± 2.830 (117) | |
| [7.89 ± 56.08] | [−26.37 ± 50.99] | ||
| AMD (95% CI) | −1.639 (−2.431, −0.847), p < .001 | ||
| [−29.51 (−43.78, −15.24)], p < .001 | |||
| Before lunch | Baseline / EOT | 9.359 ± 3.240 (83) / 8.686 ± 2.427 (58) | 9.181 ± 2.983 (161) / 7.507 ± 2.355 (120) |
| [168.61 ± 58.38] / [156.48 ± 43.71] | [165.39 ± 53.75] / [135.24 ± 42.42] | ||
| Change | 0.247 ± 2.410 (57) | −1.852 ± 3.113 (116) | |
| [4.45 ± 43.41] | [−33.37 ± 56.07] | ||
| AMD (95% CI) | −1.464 (−2.194, −0.733), p < .001 | ||
| [−26.37 (−39.52, −13.22)], p < .001 | |||
| 1 h after lunch | Baseline / EOT | 13.340 ± 2.729 (84) / 13.271 ± 2.521 (59) | 13.201 ± 2.937 (161) / 11.866 ± 2.361 (122) |
| [240.31 ± 49.16] / [239.08 ± 45.42] | [237.81 ± 52.91] / [213.78 ± 42.53] | ||
| Change | 0.636 ± 2.523 (59) | −1.378 ± 2.955 (116) | |
| [11.45 ± 45.46] | [−24.81 ± 53.24] | ||
| AMD (95% CI) | −1.591 (−2.311, −0.871), p < .001 | ||
| [−28.65 (−41.62, −15.69)], p < .001 | |||
| Before dinner | Baseline / EOT | 9.715 ± 3.203 (84) / 9.655 ± 2.853 (59) | 9.658 ± 2.813 (161) / 7.966 ± 2.381 (121) |
| [175.02 ± 57.69] / [173.93 ± 51.41] | [173.99 ± 50.67] / [143.51 ± 42.89] | ||
| Change | 0.621 ± 2.633 (58) | −1.647 ± 2.613 (116) | |
| [11.17 ± 47.44] | [−29.67 ± 47.07] | ||
| AMD (95% CI) | −1.968 (−2.680, −1.256), p < .001 | ||
| [−35.44 (−48.26, −22.62)], p < .001 | |||
| 1 h after dinner | Baseline / EOT | 12.854 ± 3.385 (83) / 12.546 ± 3.354 (59) | 12.711 ± 3.281 (160) / 11.378 ± 2.763 (121) |
| [231.57 ± 60.99] / [226.01 ± 60.44] | [228.99 ± 59.11] / [204.98 ± 49.79] | ||
| Change | 0.602 ± 2.713 (57) | −1.404 ± 2.944 (115) | |
| [10.85 ± 48.89] | [−25.28 ± 53.04] | ||
| AMD (95% CI) | −1.489 (−2.287, −0.692), p < .001 | ||
| [−26.82 (−41.19, −12.45)], p < .001 | |||
| Before bedtime | Baseline / EOT | 12.359 ± 3.213 (84) / 11.750 ± 3.529 (59) | 11.998 ± 3.299 (156) / 10.236 ± 3.031 (120) |
| [222.65 ± 57.88] / [211.68 ± 63.56] | [216.14 ± 59.45] / [184.39 ± 54.60] | ||
| Change | 0.397 ± 3.379 (58) | −1.684 ± 2.922 (111) | |
| [7.16 ± 60.86] | [−30.34 ± 52.65] | ||
| AMD (95% CI) | −1.838 (−2.722, −0.954), p < .001 | ||
| [−33.12 (−49.05, −17.19)], p < .001 | |||
| Body weight, kg | Baseline / EOT | 70.26 ± 12.16 / 70.21 ± 11.94 | 69.03 ± 11.67 / 67.94 ± 11.58 |
| Change | −0.05 ± 1.42 | −1.09 ± 1.27 | |
| AMD (95% CI) | −1.07 (−1.41, −0.73), p < .001 | ||
| Waist circumference, cm | Baseline / EOT | 92.80 ± 9.33 / 92.00 ± 8.69 (68) | 90.79 ± 9.59 / 90.41 ± 9.37 (127) |
| Change | −0.84 ± 3.62 (68) | −1.36 ± 3.36 (127) | |
| AMD (95% CI) | −0.63 (−1.63, 0.37), p = .215 | ||
| C‐peptide, nmol/L | Baseline / EOT | 0.36 ± 0.23 / 0.37 ± 0.22 (68) | 0.33 ± 0.21 / 0.34 ± 0.21 (127) |
| Change | 0.05 ± 0.13 (68) | −0.03 ± 0.14 (127) | |
| AMD (95% CI) | −0.07 (−0.11, −0.03), p < .001 | ||
| Glucagon, ng/L | Baseline / EOT | 145.5 ± 148.5 1 / 122.4 ± 26.4 | 124.4 ± 21.9 (167) / 120.5 ± 22.3 |
| Change | −23.2 ± 150.8 | −3.9 ± 20.3 (167) | |
| AMD (95% CI) | −1.5 (−7.8, 4.8), p = .637 | ||
| Glucagon, ng/L (per‐protocol analysis set) | Baseline / EOT | 129.4 ± 25.7 / 118.6 ± 19.9 (65) | 124.6 ± 23.0 / 117.7 ± 22.2 (123) |
| Change | −10.8 ± 20.1 (65) | −6.9 ± 19.6 (123) | |
| AMD (95% CI) | 1.7 (−3.4, 6.8), p = .510 | ||
| Leptin, nmol/L | Baseline / EOT | 0.763 ± 0.511 / 0.742 ± 0.523 | 0.750 ± 0523 (167) / 0.691 ± 0.508 |
| Change | −0.021 ± 0.243 | −0.057 ± 0.219 (167) | |
| AMD (95% CI) | −0.040 (−0.097, 0.018), p = .172 | ||
| Adiponectin, µg/mL | Baseline / EOT | 7.17 ± 3.62 / 7.60 ± 3.94 | 7.24 ± 3.65 (167) / 7.99 ± 3.71 |
| Change | 0.43 ± 1.10 | 0.76 ± 1.07 (167) | |
| AMD (95% CI) | 0.33 (0.05, 0.62), p = .022 | ||
Values are presented as the mean ± standard deviation or adjusted mean difference (95% confidence interval). Where the number of patients differed from the number of patients in the full analysis set, the number is given in parentheses.
AMD, adjusted mean difference (ipragliflozin − placebo); EOT, end of the randomized treatment period; FPG, fasting plasma glucose; SMBG, self‐monitored blood glucose.
One patient had an outlier of 1490 ng/L, which resulted in the large standard deviation. This patient was administered glucagon prior to endoscopy on the day of the blood test at baseline. Therefore, glucagon concentrations were also analysed in the per‐protocol analysis set after excluding this outlier.
Figure 2.
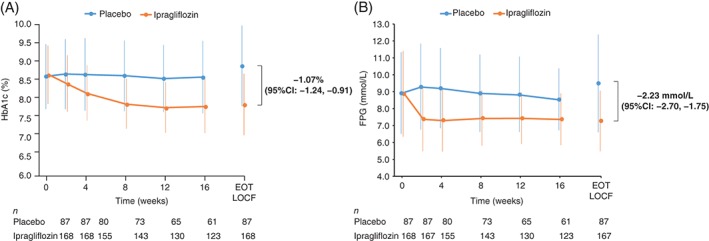
Time‐course of A, HbA1c and B, FPG. Data are shown as the mean ± s.d. (full analysis set). The number of patients at each time point is shown below the x‐axis. EOT, end of the randomized treatment period.
The FPG levels increased from 8.91 mmol/L (160.5 mg/dL) at baseline to 9.48 mmol/L (170.9 mg/dL) at the end of treatment (+0.58 mmol/L or +10.4 mg/dL) in the placebo group and decreased from 8.87 to 7.26 mmol/L (−1.62 mmol/L) or 159.9 to 130.7 mg/dL (−29.3 mg/dL) in the ipragliflozin group (Table 2). The adjusted mean difference for the change in FPG from baseline to the end of treatment was −2.23 mmol/L (95% CI −2.70, −1.75), or −40.3 mg/dL (95% CI −48.9, −31.7; p < .001) (Table 2). Figure 2B shows a decrease in FPG in the ipragliflozin group by week 2, which remained constant until week 16. Consistent with the changes in HbA1c and FPG, the mean change in glycoalbumin from baseline to end of treatment was greater in the ipragliflozin (−3.60%) than in the placebo (+0.20%) group [adjusted mean difference: −3.84%; 95% CI −4.40, −3.29; p < .001 (Table 2)]. SMBG was performed at seven time points (before and after each meal and at bedtime) at baseline and at the end of the treatment period. Table 2 shows that ipragliflozin, but not placebo, was associated with significant reductions in SMBG at all time points (p = .001).
The adjusted mean difference (ipragliflozin − placebo) for changes in HbA1c and FPG (Figure 3A,B) was numerically greater in patients who used a DPP‐4 inhibitor (−1.20% [−13.0 mmol/mol] and −2.47 mmol/L [−44.5 mg/dL]) than in patients who did not (−0.84% [−9.3 mmol/mol] and −1.84 mmol/L [−33.4 mg/dL]). The mean ± s.d. baseline HbA1c levels were 8.63% ± 0.91% (70.9 ± 10.1 mmol/mol) and 8.70% ± 0.77% (71.6 ± 8.5 mmol/mol) for placebo and ipragliflozin, respectively, in patients with concomitant use of a DPP‐4 inhibitor, and 8.60% ± 0.76% (70.7 ± 8.3 mmol/mol) and 8.62% ± 0.76% (70.7 ± 8.3 mmol/mol) for placebo and ipragliflozin, respectively, in patients without concomitant use of a DPP‐4 inhibitor. To assess the influence of DPP‐4 inhibitors on the between‐group difference in HbA1c, an interaction analysis was performed for HbA1c changes. Interaction analyses for changes in HbA1c showed a statistically significant interaction for use/non‐use of a DPP‐4 inhibitor × treatment group (p = .042).
Figure 3.
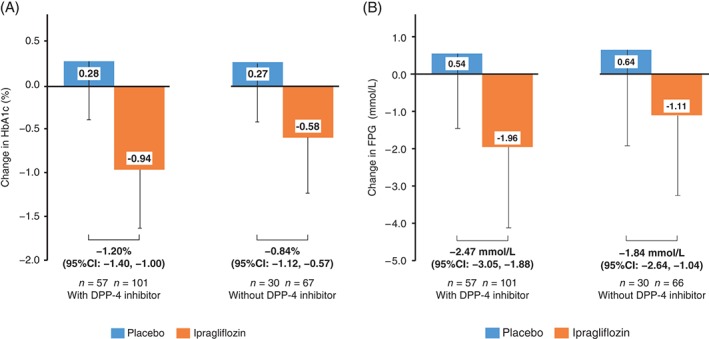
Change in A, HbA1c and B, FPG from baseline to the end of treatment (with last observation carried forward) in patients treated with or without a concomitant DPP‐4 inhibitor. Values are shown as the mean change minus the s.d.
3.3. Metabolic parameters
The reduction in body weight was significantly greater in the ipragliflozin (−1.09 kg) than in the placebo (−0.05 kg) group (adjusted mean difference −1.07 kg; p < .001; Table 2). By contrast, the change in waist circumference was not significantly different between the placebo (−0.84 cm) and ipragliflozin (−1.36 cm) groups [adjusted mean difference −0.63 cm; p = .215 (Table 2)]. The mean change in adiponectin from baseline to the end of treatment was slightly but significantly greater in the ipragliflozin (0.76 µg/mL) than in the placebo (0.43 µg/mL) group [adjusted mean difference +0.33 µg/mL; p = .022 (Table 2)]. The change in C‐peptide level from baseline to the end of treatment was significantly different between the placebo (0.05 nmol/L) and the ipragliflozin (−0.03 nmol/L) group [adjusted mean difference −0.07 nmol/L; p < .001 (Table 2)]. We also analysed the effects of DPP‐4 inhibitors on C‐peptide levels. The adjusted mean difference (ipragliflozin − placebo) in the change in C‐peptide from baseline to the end of treatment was statistically significant in patients who used a DPP‐4 inhibitor (−0.10 nmol/L; 95% CI −0.15, −0.05; p < .001), but not in patients who did not use a DPP‐4 inhibitor (−0.03 nmol/L; 95% CI −0.09, 0.04; p = .403). Regarding glucagon concentrations, we found one outlier in the ipragliflozin group, which markedly affected the values. Accordingly, we excluded this patient and report the glucagon concentrations in the per‐protocol analysis set. The mean changes in glucagon concentrations from baseline to the end of treatment were not significantly different between the placebo and ipragliflozin groups (Table 2). In addition, the glucagon concentrations were not significantly different between baseline and the end of treatment in either group (Table 2). Glucagon concentrations remained unchanged in the ipragliflozin group in patients with and without the use of a concomitant DPP‐4 inhibitor (data not shown). The mean changes in leptin concentrations from baseline to the end of treatment were not significantly different between the placebo and ipragliflozin groups (Table 2).
3.4. Safety
We found that TEAEs (Table 3) occurred in 56.3% (49/87) and 74.3% (130/175) of patients, and drug‐related TEAEs occurred in 21.8% (19/87) and 42.3% (74/175) of patients in the placebo and ipragliflozin groups, respectively. No deaths were reported during the treatment period.
Table 3.
Treatment‐emergent adverse events
| Placebo (N = 87) | Ipragliflozin (N = 175) | |||
|---|---|---|---|---|
| n (%) | Events | n (%) | Events | |
| Total TEAEs | 49 (56.3) | 112 | 130 (74.3) | 349 |
| Serious TEAEs | 2 (2.3) | 2 | 2 (1.1) | 3 |
| TEAEs leading to permanent discontinuation | 2 (2.3) | 2 | 2 (1.1) | 2 |
| Total drug‐related TEAEs | 19 (21.8) | 44 | 74 (42.3) | 205 |
| Drug‐related serious TEAEs | 1 (1.1) | 1 | 1 (0.6) | 2 |
| Drug‐related TEAEs leading to permanent discontinuation | 1 (1.1) | 1 | 2 (1.1) | 2 |
| TEAEs of special interest | ||||
| Hypoglycaemia‐related events | 13 (14.9) | 36 | 52 (29.7) | 168 |
| Urinary tract infection | 1 (1.1) | 1 | 4 (2.3) | 4 |
| Genital infection | 0 | 0 | 7 (4.0) | 7 |
| Body fluid volume and electrolytes | 1 (1.1) | 1 | 4 (2.3) | 4 |
| Drug‐related TEAEs by preferred term in ≥1% of patients in either group | ||||
| Hypoglycaemia | 13 (14.9) | 36 | 51 (29.1) | 164 |
| Pollakiuria | 1 (1.1) | 1 | 8 (4.6) | 8 |
| Blood ketone bodies increased | 1 (1.1) | 1 | 5 (2.9) | 5 |
| Thirst | 0 | 0 | 3 (1.7) | 3 |
| Vulvovaginal candidiasis | 0 | 0 | 3 (1.7) | 3 |
| Muscle spasms | 2 (2.3) | 2 | 1 (0.6) | 2 |
| Constipation | 1 (1.1) | 1 | 2 (1.1) | 2 |
| Rash | 0 | 0 | 2 (1.1) | 2 |
| Urine β2 microglobulin increased | 1 (1.1) | 1 | 1 (0.6) | 1 |
| Cerebral infarction | 1 (1.1) | 1 | 0 | 0 |
| Dizziness | 1 (1.1) | 1 | 0 | 0 |
During the treatment period, serious TEAEs were reported in two patients (2.3%) in the placebo group and two patients (1.1%) in the ipragliflozin group (Table 3). Two serious TEAEs in the placebo group were cerebral infarction and coronary arterial stent insertion, in one patient each. Both events led to permanent discontinuation of the study for those patients. Cerebral infarction was classified as severe, and was considered by the investigator to be probably related to the study drug; however, that patient was later shown to be in the placebo group, indicating the cerebral infarction was not related to the study drug. Coronary arterial stent insertion was classified as moderate in severity, but was not considered to be related to the study drug. Serious TEAEs in the ipragliflozin group included cough in one patient, and abnormal hepatic function and hypokalaemia in one patient. These events were classified as mild in severity. Abnormal hepatic function and hypokalaemia were considered to be possibly related to the study drug. Both events were resolved 29 days after onset, and the patient continued the study drug.
Hypoglycaemia‐related events were observed in 13 patients (14.9%) in the placebo group and 52 patients (29.7%) in the ipragliflozin group. In the placebo group, there were 36 events of hypoglycaemia, all of which were considered possibly or probably related to the study drug, but the events were mild in severity and were resolved on the day of onset. Hypoglycaemia‐related TEAEs in the ipragliflozin group were hypoglycaemia (167 events) and hunger (1 event). All of these events were classified as mild in severity. Except for three events of hypoglycaemia in two patients, all of these events were considered possibly or probably related to the study drug. All of the hypoglycaemia‐related TEAEs were resolved without any treatment or by the ingestion of sugary foods. None of these patients discontinued the study.
We also analysed whether occurrence of hypoglycaemia was influenced by concomitant use of DPP‐4 inhibitors. Of those not receiving DPP‐4 inhibitor, seven patients (23.3%) treated with placebo and 22 (32.4%) treated with ipragliflozin experienced hypoglycaemia. In contrast, for those with concomitant use of a DPP‐4 inhibitor, hypoglycaemia occurred in six patients (10.5%) treated with placebo and 30 (28.0%) treated with ipragliflozin. Although concomitant use of DPP‐4 inhibitor led to a greater reduction in HbA1c, it did not increase hypoglycaemia frequency.
Drug‐related TEAEs linked to urinary tract infection in the ipragliflozin group were cystitis (one patient), asymptomatic bacteriuria (one patient) and urethritis (one patient). Two episodes of cystitis (one patient in each group) were considered unrelated to the study drug. TEAEs related to genital infection in the ipragliflozin group included vulvovaginal candidiasis in three patients, and eczema, pruritus genital, epididymitis and genital erosion in one patient each. These events were considered related to the study drug, except for the epididymitis.
Ipragliflozin caused slight decreases in estimated glomerular filtration rate (eGFR; −1.19 ± 9.53 mL/min/1.73 m2) compared with placebo (+1.21 ± 8.19). Systolic and diastolic blood pressures were unaltered from baseline in placebo (1.5 ± 8.19 and 0.3 ± 9.2 mm Hg for systolic and diastolic changes, respectively) and ipragliflozin groups (−1.4 ± 14.5 and −0.3 ± 9.9). No change in pulse rate was observed in the placebo (−0.5 ± 10.0 per min) or ipragliflozin groups (−0.1 ± 0.0 per min). There were no changes in total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides or free fatty acid levels from baseline to the end of treatment in either group.
4. DISCUSSION
In this study, the efficacy and safety of ipragliflozin were examined in patients with type 2 diabetes and inadequate glycaemic control in the early stage of insulin therapy. Ipragliflozin was superior to placebo in terms of improving glycaemic control, as shown by the significant reductions in HbA1c, FPG, glycoalbumin and SMBG values. Notably, the change in HbA1c from baseline was consistent with the changes reported in earlier studies of ipragliflozin administered as monotherapy or in combination with other oral antidiabetic drugs.5, 6, 7 The results of SMBG performed seven times per day at baseline and at the end of treatment confirmed that ipragliflozin was superior to placebo in terms of the reduction in glucose levels at both fasting and postprandial periods.
Intriguingly, when we compared the changes in glycaemic control between patients treated with or without a concomitant DPP‐4 inhibitor, the reductions in HbA1c from baseline to the end of treatment were numerically greater in patients treated with concomitant DPP‐4 inhibitors. An interaction analysis showed a statistically significant correlation (p = .042) between ipragliflozin treatment and the use of DPP‐4 inhibitors, indicating better efficacy in patients using a DPP‐4 inhibitor. This might be explained by the combined improvement of insulin resistance by ipragliflozin and the improved β‐cell responsiveness in patients using a DPP‐4 inhibitor. Alternatively, the glucagon‐suppressing effects of DPP‐4 inhibitors may be beneficial when ipragliflozin is added, because other SGLT2 inhibitors reportedly increase serum glucagon concentrations10, 11; however, the present study did not identify changes in glucagon levels in patients treated with ipragliflozin. Notably, the changes in glucagon concentrations were similar between the placebo and ipragliflozin groups in patients who did or did not use a concomitant DPP‐4 inhibitor (data not shown). It is unclear why our results differ from those reported for empagliflozin10 and dapagliflozin,11 which were associated with increases in glucagon concentrations. It is possible that this lack of effect on glucagon could be a unique feature of ipragliflozin, or may be attributable to the combination of drugs used in each study, or other differences in study design. In the present study, we measured glucagon concentrations after 16 weeks of treatment. In a study of empagliflozin,10 glucagon concentrations increased after the initial dose and later diminished after 28 days of treatment, although they did not return to baseline values. We also found that the better efficacy of ipragliflozin in patients treated with a DPP‐4 inhibitor was not accompanied by an increase in hypoglycaemia. This observation strengthens the clinical benefits of combined ipragliflozin and DPP‐4 inhibitor therapy in patients treated with insulin.
A number of studies using animal models showed that SGLT2 inhibition reduces insulin resistance.12, 13, 14 We found that serum adiponectin levels increased with ipragliflozin treatment, suggesting that SGLT2 inhibition also ameliorates insulin resistance in humans. Further studies, including direct evaluation of insulin sensitivity, are needed to establish the possible effects of ipragliflozin on insulin sensitivity in humans.
We observed a significant reduction in C‐peptide concentrations in ipragliflozin‐treated patients. This was, however, observed in patients with concomitant use of a DPP‐4 inhibitor. Preserving endogenous insulin secretion by adding ipragliflozin to ongoing therapy might be beneficial in terms of β‐cell protection. We would expect to see a considerable reduction in the number of β cells in our cohort of patients with a mean HbA1c of ~8.6% ∼ (71 mmol/mol) at baseline because it was previously reported that β‐cell area was reduced by ~45% in Japanese patients with a mean HbA1c of 7.8% (62 mmol/mol).15
Although the incidence of hypoglycaemia was greater in the ipragliflozin group than in the placebo group, none of these events was classified as serious. It was reported that 10 and 20 mg dapagliflozin treatment for 12 weeks in insulin‐treated patients caused hypoglycaemia in 29.2% and 25.0% of patients, respectively.16 Empagliflozin 10 or 25 mg for 18 weeks caused hypoglycaemia in 20% and 28% of patients, respectively.17 Thus, hypoglycaemic frequency by ipragliflozin (29.1%) was similar to that of other SGLT2 inhibitors.
In the present study, urinary tract infection and genital infection occurred in four (2.3%) and seven (4.0%) patients, respectively, in the ipragliflozin group, and in one (1.1%) and no patients, respectively, in the placebo group. These rates are similar to or lower than those reported in other global clinical trials in which patients were treated with an SGLT2 inhibitor in combination with insulin.16, 17, 18
There is concern about the risk of ketoacidosis with SGLT2 inhibitors.19 In the present study, an increase in blood ketone bodies was reported as a TEAE in five patients (2.9%) in the ipragliflozin group and one patient (1.1%) in the placebo group, but none of the patients reported any symptoms of ketoacidosis during the study. We observed slight decreases in eGFR in patients treated with ipragliflozin for 16 weeks. An initial decline in eGFR at 18 weeks by canagliflozin was reported to be partially reversed after 52 weeks.18 Future studies should carefully follow the long‐term changes in renal function in patients treated with SGLT2 inhibitors such as ipragliflozin.
The present study had some limitations. First, none of the patients were using intensive insulin regimens; therefore, we cannot generalize the present results to regimens such as basal‐bolus injections. Second, other oral antidiabetic drugs, such as sulphonylureas that could cause hypoglycaemia, were washed out before the initial observation period; therefore, caution is necessary when ipragliflozin is used in patients treated with insulin and sulphonylureas.
In conclusion, ipragliflozin add‐on therapy to insulin for 16 weeks significantly decreased HbA1c, FPG and SMBG values. Amelioration of glycaemic control was greater in patients concomitantly treated with a DPP‐4 inhibitor. Incidences of other TEAEs were within expected ranges according to previous studies of ipragliflozin as monotherapy or in combination with other oral antidiabetic drugs.
Supporting information
File S1. Exclusion criteria, additional details of sample size calculation, and list of participating sites.
ACKNOWLEDGMENTS
The authors wish to thank all of the investigators involved in this trial. This study was sponsored by Astellas Pharma Inc., Japan. Medical writing and editorial support was funded by Astellas and provided by Dr. Nicholas D. Smith (Edanz Group Ltd.) and Elsevier/ELMCOM.
Conflict of interest
H. I. has served on the scientific advisory board of Astellas Pharma Inc.; received lecture or consulting fees from Astellas Pharma Inc., MSD, Sanofi, Mitsubishi Tanabe Pharma, Boehringer Ingelheim Japan and Novartis Pharma; received grants/research support from Astellas Pharma Inc., Ono Pharmaceutical, Boehringer Ingelheim Japan, AstraZeneca, Sanofi, Mitsubishi Tanabe Pharma, Eli Lilly Japan, Daiichi‐Sankyo, Novo Nordisk Pharma, Kyowa Hakko Kirin, and MSD. S. Y., I. N., A. O. and S. A. are employees of Astellas Pharma Inc., Japan.
Author contributions
H. I. and S. A. contributed to the study design, data analysis and writing of the manuscript; S. Y., I. N. and A. O. contributed to the study design, study conduct, data collection and analysis, and writing of the manuscript.
Ishihara H, Yamaguchi S, Nakao I, Okitsu A and Asahina S. Efficacy and safety of ipragliflozin as add‐on therapy to insulin in Japanese patients with type 2 diabetes mellitus (IOLITE): a multi‐centre, randomized, placebo‐controlled, double‐blind study, Diabetes Obes Metab 2016, 18, 1207–1216. DOI:10.1111/dom.12745
Funding Information This study was sponsored by Astellas Pharma Inc., Japan. Medical writing and editorial support was funded by Astellas and provided by Dr. Nicholas D. Smith (Edanz Group Ltd.) and Elsevier/ELMCOM.
The copyright line for this article was changed on 1 June after original online publication.
REFERENCES
- 1. Pettus J, Santos Cavaiola T, Tamborlane WV, Edelman S. The past, present, and future of basal insulins. Diabetes Metab Res Rev. 2015 Oct 28. doi:10.1002/dmrr.2763. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 2. Lovre D, Fonseca V. Benefits of timely basal insulin control in patients with type 2 diabetes. J Diabetes Complications. 2015;29:295–301. [DOI] [PubMed] [Google Scholar]
- 3. Wu T, Betty B, Downie M, et al. Practical Guidance on the use of premix insulin analogs in initiating, intensifying, or switching insulin regimens in type 2 diabetes. Diabetes Ther. 2015;6:273–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ross SA, Tildesley HD, Ashkenas J. Barriers to effective insulin treatment: the persistence of poor glycemic control in type 2 diabetes. Curr Med Res Opin. 2011;27(suppl 3):13–20. [DOI] [PubMed] [Google Scholar]
- 5. Kashiwagi A, Kazuta K, Yoshida S, Nagase I. Randomized, placebo‐controlled, double‐blind glycemic control trial of novel sodium‐dependent glucose cotransporter 2 inhibitor ipragliflozin in Japanese patients with type 2 diabetes mellitus. J Diabetes Investig. 2014;5:382–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kashiwagi A, Kazuta K, Goto K, Yoshida S, Ueyama E, Utsuno A. Ipragliflozin in combination with metformin for the treatment of Japanese patients with type 2 diabetes: ILLUMINATE, a randomized, double‐blind, placebo‐controlled study. Diabetes Obes Metab. 2015;17:304–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kashiwagi A, Takahashi H, Ishikawa H, et al. A randomized, double‐blind, placebo‐controlled study on long‐term efficacy and safety of ipragliflozin treatment in patients with type 2 diabetes mellitus and renal impairment: results of the long‐term ASP1941 safety evaluation in patients with type 2 diabetes with renal impairment (LANTERN) study. Diabetes Obes Metab. 2015;17:152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Katsuno T, Ikeda H, Ida K, Miyagawa J, Namba M. Add‐on therapy with the DPP‐4 inhibitor sitagliptin improves glycemic control in insulin‐treated Japanese patients with type 2 diabetes mellitus. Endocr J. 2013;60:733–742. [DOI] [PubMed] [Google Scholar]
- 9. Otsuka Y, Yamaguchi S, Furukawa A, Kosuda M, Nakazaki M, Ishihara H. Addition of sitagliptin or metformin to insulin monotherapy improves blood glucose control via different effects on insulin and glucagon secretion in hyperglycemic Japanese patients with type 2 diabetes. Endocr J. 2015;62:133–143. [DOI] [PubMed] [Google Scholar]
- 10. Ferrannini E, Muscelli E, Frascerra S, et al. Metabolic response to sodium‐glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest. 2014;124:499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Merovci A, Solis‐Herrera C, Daniele G, et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest. 2014;124:509–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rossetti L, Smith D, Shulman GI, Papachristou D, DeFronzo RA. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest. 1987;79:1510–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen L, Klein T, Leung PS. Effects of combining linagliptin treatment with BI‐38335, a novel SGLT2 inhibitor, on pancreatic islet function and inflammation in db/db mice. Curr Mol Med. 2012;12:995–1004. [DOI] [PubMed] [Google Scholar]
- 14. Jurczak MJ, Lee HY, Birkenfeld AL, et al. SGLT2 deletion improves glucose homeostasis and preserves pancreatic beta‐cell function. Diabetes. 2011;60:890–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Inaishi J, Saisho Y, Saito S, et al. Effect of obesity and diabetes on alpha and beta cell mass in surgically resected human pancreas. J Clin Endocrinol Metab. 2016;101:2874–2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wilding JP, Norwood P, T'joen C, et al. A study of dapagliflozin in patients with type 2 diabetes receiving high doses of insulin plus insulin sensitizers: applicability of a novel insulin‐independent treatment. Diabetes Care. 2009;32:1656–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rosenstock J, Jelaska A, Zeller C, et al. Impact of empagliflozin added on to basal insulin in type 2 diabetes inadequately controlled on basal insulin: a 78‐week randomized, double‐blind, placebo‐controlled trial. Diabetes Obes Metab. 2015;17:936–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Neal B, Perkovic V, de Zeeuw D, et al. Efficacy and safety of canagliflozin, an inhibitor of sodium‐glucose cotransporter 2, when used in conjunction with insulin therapy in patients with type 2 diabetes. Diabetes Care. 2015;38:403–411. [DOI] [PubMed] [Google Scholar]
- 19. Taylor SI, Blau JE, Rother KI. SGLT2 inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metab. 2015;100:2849–2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
File S1. Exclusion criteria, additional details of sample size calculation, and list of participating sites.


