Summary
Reason for performing study
Foals stand and walk immediately after birth, but insight into the subsequent longitudinal development of their gait kinetics in the early juvenile phase and the possible influence of osteochondrosis thereon is lacking.
Objectives
To quantify gait kinetics in foals during the first half year of life, taking into account their osteochondrosis status.
Study design
Prospective, cohort study performed at a single stud farm.
Methods
Pressure plate measurements at walk and trot from 11 Dutch Warmblood foals during the first 24 weeks of life were used to determine body mass normalised peak vertical force, normalised vertical impulse and stance duration. Coefficients of variation of peak vertical force and stance duration were used as measures for gait maturity. Radiographs of tarsocrural and femoropatellar joints were taken at age 4–6 weeks and after 6 months to check for osteochondrosis. A linear mixed model was used to determine the effects of age, limb, presence of osteochondrosis and speed on gait parameters.
Results
Mean walking and trotting velocity increased over time as did stance duration and normalised vertical impulse, normalised peak vertical force values however remained relatively constant. During the first weeks of their life only the coefficient of variation of stance duration decreased significantly, while the coefficient of variation of peak vertical force did not. None of the foals was visibly lame, but the presence of osteochondrosis resulted in a temporarily but significantly reduced normalised peak vertical force.
Main limitations
This study is a relatively small sample size of one breed from a single stud farm. A stand‐alone pressure plate was used and body mass was estimated rather than measured.
Conclusions
Despite being precocious, foals need time to mature their gait. During growth, velocity at walk and trot increases, but normalised peak vertical force remains relatively constant. Although not visibly lame, a temporary reduction in normalised peak vertical force was detected in osteochondrosis positive foals using a pressure plate.
Keywords: horse, foal, gait development, kinetics, osteochondrosis
Introduction
Research into equine gait spans centuries 1, but investigations into the development of equine gait are rare. It is stated by some that foals are born with mature horse‐like postural abilities and coordination 2, 3. However, recent work has indicated that foals show relatively poor postural control after birth, which gradually improves during early life 4. Denham et al.5 showed that foals have not yet achieved an even, 4‐beat rhythm at walk when aged 21 weeks, suggesting that there is a period of gait and balance maturation, as has been demonstrated in other animals 3, 6, 7.
The few studies focusing on the development of gait in foals have been kinematic in nature 5, 8, 9 and kinetic aspects of the development of equine gait have never been studied. Stabilographic analysis of young foals has already shown that suboptimal postural balance is characterised by larger swaying amplitudes and velocities 4. Similar incoordination can be expected during locomotion and would lead to inconsistent weightbearing per limb, hence to increased variation of vertical forces between different steps.
The prevalence of osteochondrosis in young foals can be very high 10, although the majority of the lesions present at an early age will heal 11. Osteochondrosis lesions might influence gait and hence locomotor parameters, as has been reported in pigs 12, 13. It is therefore imperative that studies of the longitudinal development of equine gait simultaneously monitor osteochondrosis status in the most commonly affected joints (i.e. the tarsocrural and femoropatellar joints) to exclude any influence from osteochondrosis lesions on conclusions drawn about normal gait development.
We hypothesised that foals initially exhibit an immature gait, characterised by a larger coefficient of variation of peak vertical forces, which would improve over time, in line with the development of their postural balance 4. We also hypothesised that the presence of osteochondrosis lesions would affect kinetic locomotor variables.
Materials and methods
Foals
Eleven privately owned Royal Dutch Sport Horse foals (5 female, 6 male), bred for showjumping were used in this study. They were all born and housed at the same stud farm and raised following usual standards in the Dutch horse breeding industry. Foals were kept together with their dams in a stable bedded with straw and had daily access to a pasture. After weaning at age between 20 and 24 weeks the foals were housed in a group in a large, half‐open stable with straw bedding.
Before each measurement session, foals were examined and only included if they were considered clinically sound. Limbs and joints were inspected and palpated for the presence of swelling and/or joint effusion and soundness was visually checked at walk and trot on a straight line on a hard surface. Height at the withers was regularly measured and body mass was estimated according to the method of Staniar et al.14.
Data collection
A pressure plate with a measuring surface of 1.95 × 0.32 m (Footscan 3D, 2 m system1), connected to a laptop computer with appropriate software (Gait Scientific1, version 7.99–27.05.2014) was used. These pressure plates are equipped with 16,384 sensors (sensitivity 0.27–127 N/cm2; 2.06 sensors/cm2), measuring at 126 Hz. Before every session, the system was calibrated according to the manufacturer's instructions and offset was manually adjusted to avoid saturation of the sensors. The pressure plate was embedded in a custom made, wooden frame to create a 1.5 m wide and 2.3 m long level measuring area with a small ramp in front and behind to prevent stumbling. To protect the pressure mat, the runway including the plate was covered with a 10 m long, 1.5 m wide and 5 mm thick rubber mat2 (natural rubber/styrene‐butadiene rubber, shore hardness 65 ± 5).
All measurements took place in an empty stable building at the stud farm. After a 5 min warm‐up period, foals were led over the pressure plate at their preferred speed by an experienced handler. Before weaning the foals followed their dams, led by another person next to the pressure plate; after weaning foals were handled alone. A trial was considered valid if the foal moved over the pressure plate in a consistent manner, looking straight ahead and with the hooves making full ground contact within the measuring area. When the foals were small, all limbs could be measured during one run, but with increasing size left and right limb data needed to be recorded in separate trials. For each limb, 5 valid measurements were collected.
On Day 2 or 3 and on Day 7 after birth, data were recorded at walk. From age 2–12 weeks, foals were measured every 2 weeks at both walk and trot and subsequently foals were evaluated at age 16, 20 and 24 weeks. Velocity was estimated by measuring the “limb velocity” of the first forelimb contacting the pressure plate (distance covered by 2 successive hoof strikes divided by the time between them). Relative velocity (Froude number) was calculated using the formula v2/gh (v: velocity, h: height at withers and g: gravitational acceleration). All trials were recorded with a small digital video camera (Philips full HD 1080p camcorder3).
Kinetic data
Collected footprints were manually assigned to left fore (LF), right fore (RF), left hind (LH) or right hind (RH) based on the video images. Peak vertical force and vertical impulse were normalised to body mass. The results of each set of 5 measurements per limb were averaged and considered representative for that limb at that measuring moment. Intra‐individual variability of peak vertical force and stance duration for each limb was assessed by calculating “within session” coefficients of variation (CV = s.d./mean × 100%) 15. Symmetry of hindlimb loading at trot was assessed by calculating asymmetry indexes of the peak vertical force (contralateral hindlimbs [CHL]: CHL = (LH–RH)/(0.5 × (LH+RH)) × 100) 13. The resulting value is a figure with no dimensions between −200 and 200, with 0 indicating perfect symmetry. Ground reaction force curves of 5 osteochondrosis negative foals at 2, 12 and 24 weeks were made with a custom‐made script (Matlab r2015b4) after extracting the raw force data. Vertical force data were normalised to body mass and stance time using linear interpolation.
Radiographic examinations
For logistic reasons, radiographic examinations of the foals were clustered and took place on location using a Gierth 400 X‐ray machine5 and FDR D‐EVO plus C24i panels6. The first examination was performed at age 4–6 weeks; the second examination at an age of ≥6 months (age range 6–9 months). Foals were sedated with detomidine (Domosedan7). Three radiographs (dorsoplantar, lateromedial and dorsomedial–palmarolateral oblique) were taken from the tarsocrural joints, and 2 (latero‐medial and craniolateral–caudomedial oblique) from the femoropatellar and femorotibial joints. The radiographs were evaluated by 2 board‐certified veterinary radiologists (A.J.M. van den Belt and M. Beukers).
Data analysis
Statistical analysis was performed using SPSS Statistics 228 with statistical significance set at P≤0.05. Correction for multiple comparisons was done with the false discovery rate method of Benjamini–Hochberg 16. Both for walk and trot, a linear mixed effect model with foal identity added as random intercept was used to evaluate the effects of age and limb (fore or hind) as fixed factors and velocity as a covariate on kinetic locomotor parameters and the coefficients of variation. The interaction between age and osteochondrosis status was added to the model to identify temporal effects of osteochondrosis within gait. Normality and homoscedasticity assumptions were met by log transformation of the kinetic parameters and square root transformation of the coefficients of variation. Data are reported as mean ± s.d.
Results
Average body mass increased from 58.1 ± 4.4 kg at age 2–3 days to 225.4 ± 18.7 kg) at age 24 weeks. In the same period average height at withers increased from 1.02 ± 0.02 to 1.39 ± 0.04 m (Fig 1). No abnormalities were found on clinical evaluation except in 2 individuals at 24 weeks. In both cases foals were lame, with mild effusion and painful passive flexion of a fetlock joint. One week later, they were considered sound and pressure plate measurements were taken, and were included in the dataset as being representative for Week 24.
Figure 1.
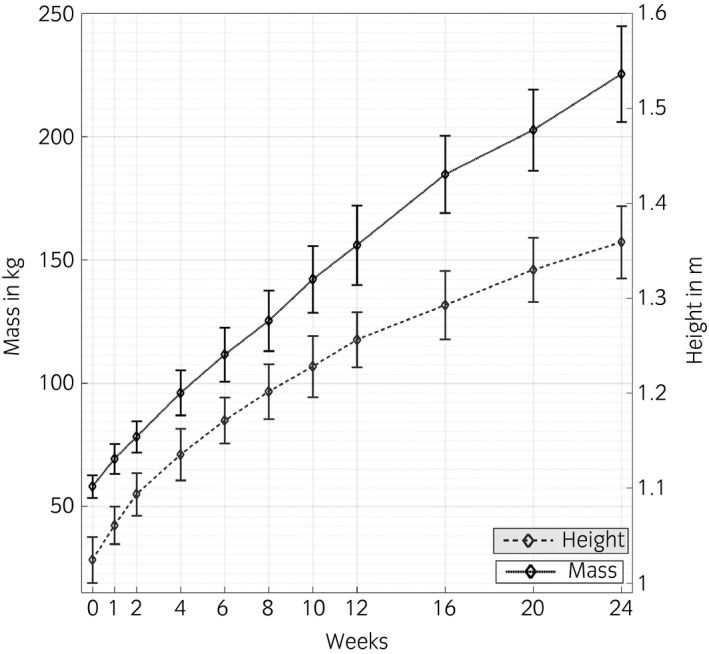
Mean (±s.d.) body mass (kg) and height at the withers (m) of all foals at the different evaluation periods.
At the first radiographic screening (age 4–6 weeks), the tarsal joints of 5 foals were osteochondrosis negative; 3 foals showed unilateral and 3 others had bilateral signs of osteochondrosis (Table 1). At 6 months, all but 3 of these 9 tarsal osteochondrosis lesions had resolved. Accurate evaluation of the stifle joints was not possible during the first radiographic examination due to the physiological irregular contour and granular subchondral bone opacity of the femoral trochlear ridges. At the second examination, no radiographic signs of osteochondrosis in the stifles were found.
Table 1.
Sex and osteochondrosis status (at 6 and 24 weeks) of the foals, 1 = unilateral, 2 = bilateral
| Foal number | Sex | OC status 6 weeks | OC status 6 months |
|---|---|---|---|
| 1 | Male | 2 | 0 |
| 2 | Male | 1 | 0 |
| 3 | Male | 0 | 0 |
| 4 | Male | 1 | 0 |
| 5 | Female | 2 | 2 |
| 6 | Female | 0 | 0 |
| 7 | Female | 1 | 0 |
| 8 | Female | 0 | 0 |
| 9 | Male | 2 | 1 |
| 10 | Female | 0 | 0 |
| 11 | Male | 0 | 0 |
All osteochondrosis lesions were detected in the tarsal joints, none in the stifle joints.
Walk
Gait parameters obtained at walk are presented in Table 2; results of the linear mixed model, 95% confidence intervals and P values can be found in Supplementary Item 1. The increase and variation in limb velocity at walk was limited, with average values increasing from 1.0 ± 0.1 to 1.2 ± 0.1 m/s during the study period. Both normalised peak vertical force and normalised vertical impulse were significantly higher in the front compared to the hindlimbs. Over time normalised peak vertical force values stayed relatively constant and, compared to Week 24, only average normalised peak vertical force of Week 1 was significantly lower. Over the complete study period, walking speed did not have a significant effect on normalised peak vertical force. At Week 8, average normalised peak vertical force was significantly lower in the osteochondrosis affected limbs, at Week 10 the opposite was the case. Mean coefficient of variation of normalised peak vertical force was significantly higher in the hindlimbs compared to the forelimbs and values fluctuated during the study; the highest variation was seen at Week 24. The coefficient of variation of front limb normalised peak vertical force was about 10–20%, whereas hindlimbs had coefficients of variation about 20–30%, except for Week 24 in which values of over 40% were found. Differences between osteochondrosis positive and negative foals were only significant at Weeks 2, 6 and 12; in all cases osteochondrosis positive animals had lower coefficient of variation values than osteochondrosis negative foals. Speed had no significant effect on coefficient of variation of normalised peak vertical force. Compared to Week 24, normalised vertical impulse was significantly lower in Weeks zero and one. Speed had a significant effect on normalised vertical impulse, which decreased with increasing speed. Osteochondrosis affected animals had significantly lower normalised vertical impulse in Week one whereas in Week 10 normalised vertical impulse was significantly higher in osteochondrosis positive animals. Stance duration increased significantly over time. When taking Week 24 as a reference, stance duration was significantly lower during the first 12 weeks. Limb (fore or hind) and osteochondrosis status did not have a significant effect on stance duration, whereas speed did; stance duration decreased with increasing speed. Variability of stance duration reduced over time, compared to Week 24; coefficient of variation of stance duration was significantly higher during the first 6 weeks of life. No significant effect of limb or osteochondrosis status was found.
Table 2.
Mean and s.d. of velocity, Froude numbers and kinetic gait parameters at walk for the different measuring moments
| Age (weeks) | 0.5 | 1 | 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | s.d. | Mean | s.d. | Mean | s.d. | |||||||
| Velocity (m/s) | 1.0 | 0.1 | 1.1 | 0.2 | 1.1 | 0.2 | ||||||
| Froude number | 0.10 | 0.03 | 0.11 | 0.03 | 0.12 | 0.03 | ||||||
| OC status 6 weeks | OC− | OC+ | OC− | OC+ | OC− | OC+ | ||||||
| Forelimbs | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. |
| nPVF (N/kg) | 7.7 | 1.4 | 6.8 | 1.6 | 7.1 | 2.0 | 5.9 | 1.0 | 7.6 | 2.0 | 6.6 | 1.5 |
| nVI (NS/kg) | 3.2 | 0.5 | 3.0 | 0.6 | 2.8 | 0.4 | 2.1 | 0.7 | 3.4 | 0.9 | 3.3 | 0.9 |
| StD (ms) | 619.4 | 89.4 | 642.8 | 75.8 | 588.3 | 64.1 | 698.1 | 113.8 | 661.4 | 35.2 | 714.0 | 92.6 |
| CV nPVF (%) | 18.6 | 8.0 | 11.3 | 6.6 | 15.7 | 9.8 | 13.9 | 5.1 | 16.3 | 6.2 | 12.2 | 4.8 |
| CV StD (%) | 13.7 | 7.0 | 14.9 | 5.7 | 13.4 | 5.9 | 11.2 | 5.3 | 14.1 | 7.8 | 15.3 | 3.8 |
| Hindlimbs | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. |
| nPVF (N/kg) | 5.4 | 1.4 | 4.8 | 1.0 | 5.3 | 1.2 | 4.6 | 0.7 | 5.5 | 1.2 | 5.1 | 0.8 |
| nVI (NS/kg) | 1.9 | 0.3 | 2.0 | 0.4 | 2.2 | 0.7 | 1.8 | 0.7 | 2.5 | 0.5 | 2.4 | 0.4 |
| StD (ms) | 600.7 | 81.5 | 640.2 | 92.5 | 615.1 | 58.3 | 708.0 | 67.6 | 667.1 | 29.9 | 714.7 | 83.8 |
| CV nPVF (%) | 22.3 | 16.6 | 14.8 | 8.5 | 18.9 | 7.5 | 25.2 | 11.5 | 19.5 | 10.0 | 12.3 | 6.2 |
| CV StD (%) | 15.9 | 7.2 | 15.4 | 10.2 | 11.3 | 4.4 | 12.0 | 6.5 | 12.8 | 6.4 | 11.8 | 5.7 |
| Age (weeks) | 4 | 6 | 8 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | s.d. | Mean | s.d. | Mean | s.d. | |||||||
| Velocity (m/s) | 1.1 | 0.1 | 1.1 | 0.1 | 1.2 | 0.2 | ||||||
| Froude number | 0.11 | 0.02 | 0.11 | 0.02 | 0.12 | 0.15 | ||||||
| OC status 6 weeks | OC− | OC+ | OC− | OC+ | OC− | OC+ | ||||||
| Forelimbs | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. |
| nPVF (N/kg) | 7.9 | 1.3 | 6.5 | 1.3 | 9.0 | 1.6 | 7.2 | 1.1 | 8.6 | 2.2 | 6.9 | 0.6 |
| nVI (NS/kg) | 3.7 | 0.6 | 3.0 | 0.7 | 4.2 | 0.9 | 3.6 | 0.8 | 4.0 | 1.0 | 3.4 | 0.6 |
| StD (ms) | 701.0 | 77.9 | 689.9 | 58.5 | 707.8 | 49.9 | 749.5 | 48.1 | 705.8 | 63.1 | 725.5 | 95.8 |
| CV nPVF (%) | 10.9 | 3.5 | 14.9 | 6.9 | 11.4 | 8.3 | 11.4 | 4.7 | 11.9 | 5.2 | 14.2 | 5.7 |
| CV StD (%) | 8.2 | 3.7 | 10.8 | 5.4 | 8.4 | 3.0 | 10.4 | 3.4 | 6.5 | 1.7 | 10.2 | 4.3 |
| Hindlimbs | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. |
| nPVF (N/kg) | 5.2 | 0.8 | 5.0 | 0.7 | 6.3 | 1.2 | 5.2 | 0.9 | 6.3 | 1.5 | 4.7 | 0.7 |
| nVI (NS/kg) | 2.5 | 0.5 | 2.4 | 0.4 | 3.1 | 0.6 | 2.8 | 0.6 | 2.9 | 0.9 | 2.4 | 0.4 |
| StD (ms) | 694.9 | 79.7 | 677.5 | 44.0 | 696.6 | 61.8 | 734.4 | 60.1 | 691.7 | 57.3 | 731.4 | 89.1 |
| CV nPVF (%) | 16.3 | 8.6 | 17.8 | 9.8 | 22.9 | 13.2 | 17.0 | 6.9 | 22.4 | 15.3 | 23.1 | 14.4 |
| CV StD (%) | 8.2 | 2.7 | 8.7 | 4.2 | 9.3 | 4.6 | 9.0 | 4.4 | 6.1 | 1.5 | 7.1 | 4.1 |
| Age (weeks) | 10 | 12 | 16 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | s.d. | Mean | s.d. | Mean | s.d. | |||||||
| Velocity (m/s) | 1.2 | 0.1 | 1.3 | 0.1 | 1.2 | 0.1 | ||||||
| Froude number | 0.11 | 0.01 | 0.13 | 0.02 | 0.12 | 0.02 | ||||||
| OC status 6 weeks | OC− | OC+ | OC− | OC+ | OC− | OC+ | ||||||
| Forelimbs | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. |
| nPVF (N/kg) | 7.3 | 1.2 | 7.8 | 1.4 | 8.1 | 1.4 | 7.7 | 1.8 | 9.1 | 1.4 | 8.4 | 1.7 |
| nVI (NS/kg) | 3.5 | 0.9 | 4.0 | 0.7 | 3.8 | 0.6 | 3.7 | 1.0 | 4.6 | 1.2 | 4.3 | 1.0 |
| StD (ms) | 707.1 | 81.1 | 750.5 | 49.3 | 714.5 | 91.5 | 717.0 | 54.6 | 753.1 | 97.5 | 761.6 | 95.3 |
| CV nPVF (%) | 15.6 | 7.0 | 13.1 | 7.4 | 15.3 | 6.1 | 12.3 | 5.2 | 16.0 | 7.5 | 12.7 | 5.1 |
| CV StD (%) | 6.8 | 3.7 | 7.2 | 2.5 | 7.1 | 3.6 | 6.4 | 2.1 | 5.6 | 2.4 | 6.6 | 3.6 |
| Hindlimbs | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. |
| nPVF (N/kg) | 5.1 | 1.3 | 6.0 | 1.3 | 5.5 | 1.0 | 5.1 | 1.0 | 5.4 | 2.0 | 5.6 | 1.4 |
| nVI (NS/kg) | 2.6 | 0.7 | 3.1 | 0.5 | 2.7 | 0.7 | 2.6 | 0.5 | 2.8 | 0.7 | 3.1 | 0.7 |
| StD (ms) | 705.2 | 72.1 | 739.4 | 65.3 | 704.8 | 64.9 | 710.7 | 78.0 | 744.1 | 80.4 | 775.1 | 82.7 |
| CV nPVF (%) | 24.1 | 13.2 | 23.4 | 9.9 | 18.2 | 12.7 | 18.7 | 8.2 | 31.3 | 11.6 | 24.0 | 10.7 |
| CV StD (%) | 7.0 | 3.2 | 6.5 | 3.9 | 5.6 | 3.8 | 8.9 | 4.6 | 6.9 | 4.2 | 7.2 | 4.1 |
| Age (weeks) | 20 | 24 | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | s.d. | Mean | s.d. | |||||
| Velocity (m/s) | 1.3 | 0.2 | 1.2 | 0.2 | ||||
| Froude number | 0.12 | 0.03 | 0.10 | 0.05 | ||||
| OC status 6 weeks | OC− | OC+ | OC− | OC+ | ||||
| Forelimbs | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. |
| nPVF (N/kg) | 9.3 | 2.2 | 9.4 | 2.0 | 9.1 | 2.3 | 8.0 | 2.3 |
| nVI (NS/kg) | 4.2 | 0.7 | 4.9 | 1.5 | 4.2 | 1.2 | 4.0 | 1.4 |
| StD (ms) | 723.6 | 68.4 | 764.8 | 73.6 | 721.3 | 98.3 | 754.7 | 54.8 |
| CV nPVF (%) | 12.7 | 4.5 | 14.3 | 8.3 | 19.8 | 8.8 | 16.1 | 9.2 |
| CV StD (%) | 10.7 | 5.5 | 8.5 | 4.7 | 7.8 | 4.8 | 6.9 | 2.6 |
| Hindlimbs | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. |
| nPVF (N/kg) | 5.3 | 1.5 | 5.8 | 1.6 | 5.2 | 1.1 | 4.9 | 1.5 |
| nVI (NS/kg) | 2.5 | 0.6 | 3.1 | 1.2 | 2.4 | 0.8 | 2.5 | 1.0 |
| StD (ms) | 717.3 | 98.7 | 760.1 | 71.0 | 720.9 | 72.4 | 758.3 | 47.5 |
| CV nPVF (%) | 23.2 | 18.3 | 24.9 | 11.9 | 41.7 | 13.0 | 29.4 | 17.3 |
| CV StD (%) | 8.1 | 3.6 | 10.1 | 4.9 | 6.6 | 2.6 | 6.3 | 5.1 |
Per limb (front or hind) and osteochondrosis (OC) status at Week 6, average body mass normalised peak vertical force (nPVF), normalised vertical impulse (nVI), stance duration (StD) and coefficients of variation (CV) of nPVF and StD are presented
At walk the ground reaction force curves of the osteochondrosis negative foals (Supplementary Items 2, 3 and 4) were quite constant. In the hindlimbs, the heights of the 2 peaks that constitute the time‐force curve were almost identical. For the front limbs, some variation in height between the peaks existed, but at Week 24, a consistently higher second peak was found.
Trot
The results for the trot are presented in Table 3. The results of the linear mixed model, 95% confidence intervals and P values can be found in Supplementary Item 1. At trot, average speed increased more compared to the walk (from 2.4 ± 0.4 to 3.2 ± 0.3 m/s). Just as at walk, normalised peak vertical force and normalised vertical impulse were significantly higher in the front limbs. Also at trot, average normalised peak vertical force stayed relatively constant and only the results found in Weeks 2 and 4 were significantly lower compared to Week 24. At Week 4 and 6, average normalised peak vertical force was significantly lower in the osteochondrosis positive foals. Again, when analysed for the complete study period, also at trot, velocity did not have a significant effect on normalised peak vertical force.
Table 3.
Mean and s.d. of velocity, Froude number and kinetic gait parameters at trot for the different measuring moments
| Age (weeks) | 2 | 4 | 6 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | s.d. | Mean | s.d. | Mean | s.d. | |||||||
| Velocity | 2.4 | 0.4 | 2.6 | 0.4 | 2.8 | 0.2 | ||||||
| Froude number | 0.55 | 0.16 | 0.6 | 0.16 | 0.67 | 0.12 | ||||||
| OC status 6 weeks | OC− | OC+ | OC− | OC+ | OC− | OC+ | ||||||
| Forelimbs | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. |
| nPVF (N/kg) | 11.6 | 2.5 | 10.0 | 2.8 | 12.9 | 3.8 | 9.6 | 2.4 | 14.4 | 3.3 | 11.1 | 2.4 |
| nVI (NS/kg) | 1.6 | 0.3 | 1.5 | 0.3 | 1.8 | 0.6 | 1.5 | 0.4 | 2.1 | 0.5 | 1.7 | 0.4 |
| StD (ms) | 253.9 | 27.1 | 267.7 | 30.6 | 253.1 | 17.5 | 274.0 | 18.1 | 269.4 | 13.6 | 268.3 | 24.5 |
| CV nPVF (%) | 11.2 | 7.8 | 9.3 | 5.9 | 12.6 | 7.0 | 10.1 | 5.7 | 14.9 | 7.2 | 12.1 | 4.7 |
| CV StD (%) | 6.6 | 3.6 | 12.3 | 5.7 | 8.8 | 3.6 | 9.7 | 4.8 | 8.6 | 3.5 | 8.4 | 2.8 |
| Hindlimbs | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. |
| nPVF (N/kg) | 9.5 | 2.3 | 8.9 | 1.6 | 10.3 | 1.9 | 8.2 | 1.2 | 11.8 | 1.9 | 9.5 | 2.0 |
| nVI (NS/kg) | 1.4 | 0.4 | 1.3 | 0.2 | 1.4 | 0.3 | 1.2 | 0.2 | 1.7 | 0.3 | 1.3 | 0.3 |
| StD (ms) | 256.3 | 18.8 | 269.9 | 31.6 | 260.0 | 23.9 | 281.1 | 23.3 | 263.5 | 32.6 | 262.9 | 28.5 |
| CV nPVF (%) | 15.7 | 12.4 | 15.1 | 9.4 | 12.1 | 4.1 | 14.5 | 6.8 | 16.8 | 8.0 | 14.0 | 7.4 |
| CV StD (%) | 6.8 | 4.8 | 13.8 | 11.0 | 11.4 | 7.6 | 14.9 | 9.7 | 8.5 | 4.6 | 11.3 | 8.5 |
| Age (weeks) | 8 | 10 | 12 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | s.d. | Mean | s.d. | Mean | s.d. | |||||||
| Velocity | 3.1 | 0.4 | 3.3 | 0.2 | 3.3 | 0.4 | ||||||
| Froude number | 0.81 | 0.18 | 0.92 | 0.1 | 0.88 | 0.21 | ||||||
| OC status 6 weeks | OC− | OC+ | OC− | OC+ | OC− | OC+ | ||||||
| Forelimbs | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. |
| nPVF (N/kg) | 14.2 | 4.1 | 11.4 | 2.0 | 13.1 | 3.1 | 13.1 | 3.3 | 14.5 | 2.0 | 12.0 | 2.4 |
| nVI (NS/kg) | 1.9 | 0.5 | 1.7 | 0.3 | 1.9 | 0.4 | 1.9 | 0.5 | 2.2 | 0.3 | 1.8 | 0.4 |
| StD (ms) | 247.6 | 9.4 | 264.2 | 21.0 | 254.8 | 14.2 | 264.7 | 16.7 | 269.9 | 17.3 | 270.6 | 17.4 |
| CV nPVF (%) | 13.7 | 7.5 | 12.5 | 5.9 | 17.8 | 5.9 | 9.8 | 5.4 | 15.1 | 8.7 | 12.0 | 3.1 |
| CV StD (%) | 9.2 | 2.3 | 9.2 | 5.0 | 8.1 | 3.0 | 8.2 | 2.4 | 7.5 | 2.7 | 7.2 | 3.3 |
| Hindlimbs | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. |
| nPVF (N/kg) | 11.5 | 3.7 | 9.2 | 1.4 | 10.1 | 2.4 | 11.0 | 2.5 | 11.3 | 2.4 | 10.1 | 1.9 |
| nVI (NS/kg) | 1.6 | 0.5 | 1.3 | 0.2 | 1.4 | 0.4 | 1.6 | 0.3 | 1.6 | 0.4 | 1.4 | 0.3 |
| StD (ms) | 248.9 | 8.8 | 263.5 | 32.5 | 263.0 | 13.4 | 266.9 | 23.2 | 264.9 | 21.8 | 267.1 | 17.2 |
| CV nPVF (%) | 18.9 | 7.3 | 15.9 | 6.0 | 19.3 | 9.9 | 13.3 | 7.2 | 16.2 | 9.2 | 13.0 | 5.7 |
| CV StD (%) | 9.2 | 3.4 | 8.1 | 5.2 | 7.7 | 3.3 | 9.4 | 6.0 | 8.3 | 4.7 | 8.4 | 4.2 |
| Age (weeks) | 16 | 20 | 24 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | s.d. | Mean | s.d. | Mean | s.d. | |||||||
| Velocity | 3.1 | 0.3 | 3.4 | 0.5 | 3.2 | 0.3 | ||||||
| Froude number | 0.77 | 0.15 | 0.92 | 0.25 | 0.78 | 0.16 | ||||||
| OC status 6 weeks | OC− | OC+ | OC− | OC+ | OC− | OC+ | ||||||
| Forelimbs | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. |
| nPVF (N/kg) | 15.1 | 1.7 | 13.4 | 2.3 | 15.5 | 3.4 | 15.1 | 1.5 | 15.1 | 3.7 | 13.5 | 3.5 |
| nVI (NS/kg) | 2.4 | 0.4 | 2.2 | 0.4 | 2.4 | 0.5 | 2.3 | 0.4 | 2.4 | 0.6 | 2.1 | 0.5 |
| StD (ms) | 284.4 | 12.9 | 285.2 | 18.2 | 276.0 | 18.9 | 276.3 | 32.7 | 287.2 | 14.5 | 289.3 | 12.4 |
| CV nPVF (%) | 14.9 | 6.0 | 18.1 | 11.3 | 11.7 | 5.4 | 15.3 | 7.4 | 19.2 | 10.3 | 13.8 | 11.8 |
| CV StD (%) | 7.1 | 2.9 | 7.6 | 3.0 | 7.3 | 1.6 | 6.7 | 3.6 | 5.7 | 3.3 | 6.9 | 2.6 |
| Hindlimbs | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. |
| nPVF (N/kg) | 11.8 | 2.0 | 11.0 | 1.6 | 12.1 | 1.7 | 12.1 | 1.8 | 11.3 | 2.8 | 10.8 | 3.8 |
| nVI (NS/kg) | 1.8 | 0.3 | 1.7 | 0.3 | 1.8 | 0.3 | 1.8 | 0.5 | 1.7 | 0.4 | 1.6 | 0.5 |
| StD (ms) | 282.0 | 21.1 | 294.5 | 24.3 | 275.7 | 16.1 | 288.8 | 44.0 | 287.8 | 15.5 | 280.9 | 24.3 |
| CV nPVF (%) | 13.5 | 6.2 | 17.2 | 8.8 | 14.4 | 6.2 | 19.0 | 10.8 | 24.5 | 10.7 | 17.4 | 11.7 |
| CV StD (%) | 10.3 | 6.9 | 10.7 | 5.3 | 7.4 | 6.4 | 9.5 | 6.1 | 7.3 | 5.2 | 8.2 | 5.3 |
Per limb (fore or hind) and osteochondrosis (OC) status at Week 6, average body mass normalised peak vertical force (nPVF), normalised vertical impulse (nVI), stance duration (StD) and coefficients of variation (CV) of nPVF and StD are presented.
At trot, coefficient of variation of normalised peak vertical force was lower than at walk and only at Week 10 osteochondrosis positive foals have significantly lower coefficient of variation of normalised peak vertical force. Speed had no significant effect on variability of peak vertical force. At trot, normalised vertical impulse increased over time, average values of Weeks 2–8 were significantly lower than in Week 24. With increasing speed, normalised vertical impulse decreased significantly. Osteochondrosis affected animals had only significantly lower normalised vertical impulse values in Week 6. Like at walk, stance duration increased over time, with stance duration being significantly lower during the first 12 weeks, compared to Week 24. Presence of osteochondrosis lesions did not affect stance duration significantly, whereas increasing speed was associated with a significant reduction of stance duration. At trot, coefficient of variation of stance duration at Week 2 was significantly higher compared to Week 24 and only at Week 2 was coefficient of variation of stance duration significantly higher in the osteochondrosis positive animals. There was no significant effect of speed on variability of stance duration. At trot, little variation in the shape of the ground reaction force curves was observed over time (Supplementary Items 2, 3 and 4). Presence of a persistent unilateral osteochondrosis lesion in the left limb led to a more asymmetric limb loading pattern compared to an osteochondrosis negative foal, shifting more weight to the contralateral hindlimb at trot (Supplementary Item 5).
Discussion
This study provides insight into the effects of growth and osteochondrosis on equine locomotor kinetics. Although limb velocity at walk and trot increased over time, normalised peak vertical force stayed relatively constant during the first half year of life. Variability of stance duration decreased over time, suggesting the presence of a period of gait maturation in foals. Although clinically sound, osteochondrosis positive foals in this study showed temporarily lower normalised peak vertical force values in the affected limb, indicative of subclinical lameness.
For logistic reasons, this study was limited to one stud farm, restricted to a cohort of 11 foals and a portable pressure plate system was used. Pressure plates are not as accurate and precise in determining absolute forces as force plates, which is a limitation of this study. Studies using the same pressure plate system simultaneously with a force plate reported that the maximal vertical forces recorded by the pressure plate are lower 17. Nonetheless, the accuracy of the system has proven to be acceptable 18, making it possible to use a pressure plate system to detect trends in gait kinetics over time. Furthermore, the measuring frequency (126 Hz) and with that temporal resolution of the pressure plate system used here is relatively low, especially compared to a force plate system. However, the impact of this lack of resolution is relatively low, as it will mainly affect the smoothness of the force–time curves, but only very minimally the height of the peak 17.
The method described by Staniar et al.14 is considered to be the most accurate way to estimate the mass of a foal, although this method has only been validated in Thoroughbred foals and not Dutch Warmblood foals. The birthweights of both breeds are similar 19, 20, 21 with similar weight range later in life 14, 20. We were not able to validate our results by comparing the estimated mass of the foals with actual measurements, the average foal mass we estimated was in line with values reported in Dutch Warmblood foals in studies in which mass was accurately measured 8, 20. Growth rate and velocity during walk and trot data were also in line with other studies on Warmblood foals 5, 8, 20.
Five foals were osteochondrosis‐negative throughout the study period and the remaining 6 were osteochondrosis‐positive at some stage. The number of foals is limited, however, other studies in the field of equine gait and balance analysis have used comparable numbers of animals (ranging from n = 5 18 to n = 24 8). The prevalence of osteochondrosis lesions at 6 weeks was lower than previously described 11, but foals in that study were bred from osteochondrosis‐positive sires and dams. At 6 months, prevalence data were similar to mature Warmblood populations 10, 11, 22, yet slightly lower than reported in yearlings 10, 23.
We collected 5 valid strides to calculate the average values for each parameter of each limb at each gait. When working with young animals, there is a delicate balance between the number of strides collected (increasing numbers increase the accuracy of “the average stride”) and the effects of the handling on the animals (inducing fatigue and thus increasing variation). A comparable study in young pigs 13 collected only 3 valid strides per animal. Studies in mature horses 17 and ponies 18 using the same system have also used 5 strides.
The observed normalised peak vertical force across the study period was about 9 N/kg (fore) and 6 N/kg (hind) at walk and 14 N/kg and 11 N/kg for the fore and hindlimbs at trot, almost the same as in mature ponies (fore: 8.9 N/kg, hind: 7.0 N/kg at walk; fore: 12.5 N/kg, hind: 11.2 N/kg at trot 24). Reported values in mature Warmbloods are lower with 6.5 N/kg 25 and 10.4 N/kg 26 measured in the forelimbs at walk and trot respectively. Locomotion parameters of differently sized animals can only be compared at the same relative velocity, which is indicated by the Froude number, correcting velocity for height. Calculated Froude numbers for these foals were comparable with values reported in ponies 24. Froude numbers reported in mature Dutch Warmbloods are similar for walk but higher for trot 25, 26. Animals differing in size but comparable in proportion, display the same locomotor pattern when moving at velocities corresponding to equal values of Froude numbers 27. However, conformation and proportion of foals change during growth 28, 29, leading to variability of inertial properties and centre of mass motion 30, 31. Consequently, the requirements of the dynamic similarity hypothesis are not met, precluding drawing of valid conclusions on the comparison of limb loading of foals with mature horses or ponies.
Speed influences most of the kinetic and kinematic gait parameters 32, 33, 34, 35, 36, 37, 38, 39. In mature horses, an increase in normalised peak vertical force and a reduction in stance duration and normalised vertical impulse is seen when the animal moves faster 35, 36, 37. In these foals, however, stance duration and normalised vertical impulse increased over time, whereas normalised peak vertical force values, as in young goats 40, remained relatively constant. This is seemingly contradictory with the observed increase in walking and trotting speed. However, in growing foals the increase in stride length due to their growing limbs is responsible for the increase in speed, whereas mature horses increase their stride frequency 36 when moving at higher speeds, explaining the differences described above. Furthermore, vertical forces are influenced by limb stiffness, which changes during growth 41.
Given that stabilographic analysis of young foals has shown that suboptimal postural balance is characterised by larger swaying amplitudes and velocities 4, comparable balance incoordination could be expected during locomotion. This imbalance would potentially lead to irregular weightbearing over the limbs over subsequent strides and hence to increased variation when comparing peak vertical forces between different steps, as seen with spinal ataxia in mature horses 42. Surprisingly, no significant reduction in variability of peak vertical force was observed during the study period. Nevertheless, average variability of peak vertical force, measured with the same pressure plate system in mature ponies was about 10–15% 24, which is somewhat lower than in our study and possibly indicating that reduction of peak vertical force variability takes place at a later age. Variability was highest at Week 24, and the foals were weaned at this age. The increased coefficient of variation was not due to more variation in velocity during the measurements. It may be that weaning and consequently handling the foals alone had an increasing effect on variability. There could also be an effect of the need to increase the number of trials, as at the end of the study left and right limb data were collected separately, possibly leading to more intertrial variability.
In contrast, a reduction in variability of stance duration was observed across the study period. This was most prominent during the first 2 weeks after birth when foals experience most progress in developing static balance 4 due to maturation of the neuromusculoskeletal system. Nauwelaerts et al.4 suggested that foals initially rely on fast, but imprecise and ballistic motor control (open loop), later switching to the more precise, closed loop control 43. More precise control leads to lower variability; therefore, this parameter can be used to quantify gait maturity in toddlers 44 and Warmblood foals 8. Variability of stance duration provides information about timing, whereas coefficient of variation of peak vertical force is an indicator of how wobbly foals are. As foals need to stand and walk almost immediately after birth, the “pacemaker” in locomotor pattern development may well develop earlier in time than stability of the centre of mass.
Vertical limb loading at trot, measured at Weeks 4 and 6 was significantly lower in osteochondrosis affected animals and more asymmetry was seen in unilaterally osteochondrosis‐positive animals. At walk, peak vertical force observed in the osteochondrosis positive foals was also significantly lower at Week 8; however, it was higher in these animals at Week 10. Reduction of peak vertical force was previously reported to be a gait adaptation mechanism in an induced lameness model 45, 46. Although the foals in the current study were not visibly lame, this finding strongly suggests that around Weeks 4 and 6 subclinical lameness was present in the osteochondrosis‐positive foals. The variable effect of osteochondrosis on gait parameters with age may possibly be explained by the highly dynamic character of osteochondrosis lesions in the early juvenile phase 11, which is likely to affect pain perception and hence manifestation of lameness. For ethical and economic reasons this was not confirmed with diagnostic anaesthesia.
Conclusions
At birth, a foal's gait is different from that of the mature horse. Similar to postural balance 4, gait maturation occurs after birth and this is reflected by a decrease in stance duration variability. During growth, preferred speed at walk and trot increases. Limb length and stance duration increase and increasing stride length rather than stride frequency is responsible for the increase in speed. Meanwhile, normalised peak vertical force and hence maximal loading of the internal structures of the distal limb remains relatively constant. Even when subjectively considered sound, osteochondrosis lesions may influence peak vertical force. Therefore, pressure plate systems might have the potential to serve as an early identification tool for detection of foals that might need further examination.
Authors' declaration of interests
No competing interests have been declared.
Ethical animal research
This study was reviewed and approved by the ethical committee of Utrecht University (DEC No. 2014.III.04.038). For all procedures carried out, written consent was obtained from the animal owner.
Sources of funding
None.
Authorship
All authors contributed to the conception and design of the study and interpretation of the results. B.M.C. Gorissen and F.M. Serra Bragança contributed to the acquisition and analysis of the pressure plate data. B.M.C. Gorissen drafted the manuscript and all authors revised it critically and approved the final version.
Supporting information
Supplementary Item 1: Results of the linear mixed model presenting the effect sizes, P values, 95% confidence intervals and Benjamini–Hochberg (B‐H) corrected P values of the parameters investigated.
Supplementary Item 2: Mean force (body mass normalised) time (normalised by linear interpolation) curves at Week 2 of the osteochondrosis‐negative foals for each limb (LF: left fore, RF: right fore, LH: left hind, RH: right hind) at walk and trot. The central line of the force time curves represents the mean and the shaded area represents the median absolute deviation (MAD).
Supplementary Item 3: Mean force (body mass normalised) time (normalised by linear interpolation) curves at Week 12 of the osteochondrosis‐negative foals for each limb (LF: left fore, RF: right fore, LH: left hind, RH: right hind) at walk and trot. The central line of the force time curves represents the mean and the shaded area represents the median absolute deviation (MAD).
Supplementary Item 4: Mean force (body mass normalised) time (normalised by linear interpolation) curves at Week 24 of the osteochondrosis‐negative foals for each limb (LF: left fore, RF: right fore, LH: left hind, RH: right hind) at walk and trot. The central line of the force time curves represents the mean and the shaded area represents the median absolute deviation (MAD).
Supplementary Item 5: Asymmetry indexes (ASI) of the contralateral hindlimbs (CHL) of a representative osteochondrosis‐negative and unilateral osteochondrosis‐positive foal at trot.
Acknowledgements
The authors are very grateful to the owner and staff of the stud farm, A.J.M. van den Belt and M. Beukers for evaluating the radiographs, M. Gillesen, D.M.E. de Hair, E.J. van Zoest and K. Goedknegt for assisting with the data collection, J.J.M Gorissen for the construction of the wooden frame and J.C.M. Vernooij for his assistance with the statistical analysis.
Manufacturers' addresses
RsScan International N.V., Paal, Belgium.
De Mulder Rubber and Plastics, Gent, Belgium.
Koninklijke Philips N.V., Eindhoven, the Netherlands.
MathWorks, Natick, Massachusetts, USA.
Gierth X‐Ray International GmbH, Riesa, Germany.
Fujifilm Holdings Corporation, Tokyo, Japan.
Orion Pharma Animal Health, Espoo, Finland.
IBM Corporation, Armonk, New York, USA.
References
- 1. Van Weeren, P.R. (2013) History In: Equine Locomotion, 2nd edn., Eds: Back W. and Clayton H.M., Saunders, London: pp 1–30. [Google Scholar]
- 2. Fox, M.W. (1964) Phylogenetic analysis of behavioral neuro‐ontogeny in precocial and non‐precocial mammals. Can. J. Comp. Med. Vet. Sci. 28, 197–202. [PMC free article] [PubMed] [Google Scholar]
- 3. Lelard, T. , Jamon, M. , Gasc, J.P. and Vidal, P.P. (2006) Postural development in rats. Exp. Neurol. 202, 112–124. [DOI] [PubMed] [Google Scholar]
- 4. Nauwelaerts, S. , Malone, S.R. and Clayton, H.M. (2013) Development of postural balance in foals. Vet. J. 198, 70–74. [DOI] [PubMed] [Google Scholar]
- 5. Denham, S.F. , Staniar, W.B. , Dascanio, J.J. , Phillips, A.B. and Splan, R.K. (2012) Linear and temporal kinematics of the walk in Warmblood foals. J. Equine. Vet. Sci. 32, 112–115. [Google Scholar]
- 6. Muir, G.D. , Gosline, J.M. and Steeves, J.D. (1996) Ontogeny of bipedal locomotion: walking and running in the chick. J. Physiol. 493, 589–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Westerga, J. and Gramsbergen, A. (1990) The development of locomotion in the rat. Brain Res. Dev. Brain Res. 57, 163–174. [DOI] [PubMed] [Google Scholar]
- 8. Back, W. , Barneveld, A. , Schamhardt, H.C. and Hartman, W. (1994) Longitudinal development of the kinematics of 4‐, 10‐, 18‐ and 26‐month‐old Dutch Warmblood horses. Equine Vet. J. 26, Suppl 17, 3–6. [Google Scholar]
- 9. Drevemo, S. , Fredricson, I. , Hjertén, G. and McMiken, D. (1987) Early development of gait asymmetries in trotting standardbred colts. Equine Vet. J. 19, 189–191. [DOI] [PubMed] [Google Scholar]
- 10. Van Grevenhof, E.M. , Ducro, B.J. , Van Weeren, P.R. , Van Tartwijk, J.M.F.M. , Van den Belt, A.J.M. and Bijma, P. (2009) Prevalence of various radiographic manifestations of osteochondrosis and their correlations between and within joints in Dutch warmblood horses. Equine Vet. J. 41, 11–16. [DOI] [PubMed] [Google Scholar]
- 11. Dik, K.J. , Enzerink, E. and Van Weeren, P.R. (1999) Radiographic development of osteochondral abnormalities, in the hock and stifle of Dutch Warmblood foals, from age 1 to 11 months. Equine Vet. J. 31, Suppl 31, 9–15. [DOI] [PubMed] [Google Scholar]
- 12. De Koning, D.B. , van Grevenhof, E.M. , Laurenssen, B.F.A. , Ducro, B.J. , Heuven, H.C.M. , de Groot, P.N. , Hazeleger, W. and Kemp, B. (2012) Associations between osteochondrosis and conformation and locomotive characteristics in pigs. J. Anim. Sci. 90, 4752–4763. [DOI] [PubMed] [Google Scholar]
- 13. Meijer, E. , Oosterlinck, M. , van Nes, A. , Back, W. and van der Staay, F.J. (2014) Pressure mat analysis of naturally occurring lameness in young pigs after weaning. BMC Vet. Res. 10, 193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Staniar, W.B. , Kronfeld, D.S. , Hoffman, R.M. , Wilson, J.A. and Harris, P.A. (2004) Weight prediction from linear measures of growing Thoroughbreds. Equine Vet. J. 36, 149–154. [DOI] [PubMed] [Google Scholar]
- 15. Petrie, A. and Watson, P. (2013) Descriptive statistics In: Statistics for Veterinary and Animal Science, 3rd edn., Wiley‐Blackwell, Hoboken: pp 12–27. [Google Scholar]
- 16. Benjamini, Y. and Hochberg, Y. (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Series B Stat. Methodol. 57, 289–300. [Google Scholar]
- 17. Oosterlinck, M. , Pille, F. , Huppes, T. , Gasthuys, F. and Back, W. (2010) Comparison of pressure plate and force plate gait kinetics in sound Warmbloods at walk and trot. Vet. J. 186, 347–351. [DOI] [PubMed] [Google Scholar]
- 18. Oosterlinck, M. , Pille, F. , Back, W. , Dewulf, J. and Gasthuys, F. (2010) Use of a stand‐alone pressure plate for the objective evaluation of forelimb symmetry in sound ponies at walk and trot. Vet. J. 183, 305–309. [DOI] [PubMed] [Google Scholar]
- 19. Hendriks, W.K. , Colenbrander, B. , van der Weijden, G.C. and Stout, T.A. (2009) Maternal age and parity influence ultrasonographic measurements of fetal growth in Dutch Warmblood mares. Anim. Reprod. Sci. 115, 110–123. [DOI] [PubMed] [Google Scholar]
- 20. Barneveld, A. and van Weeren, P.R. (1999) Conclusions regarding the influence of exercise on the development of the equine musculoskeletal system with special reference to osteochondrosis. Equine Vet. J. 31, 112–119. [DOI] [PubMed] [Google Scholar]
- 21. Whittaker, S. , Sullivan, S. , Auen, S. , Parkin, T.D. and Marr, C.M. (2012) The impact of birthweight on mare health and reproductive efficiency, and foal health and subsequent racing performance. Equine Vet. J. 44, Suppl 41, 26–29. [DOI] [PubMed] [Google Scholar]
- 22. Jönsson, L. , Dalin, G. , Egenvall, A. , Näsholm, A. , Roepstorff, L. and Philipsson, J. (2011) Equine hospital data as a source for study of prevalence and heritability of osteochondrosis and palmar/plantar osseous fragments of Swedish Warmblood horses. Equine Vet. J. 43, 695–700. [DOI] [PubMed] [Google Scholar]
- 23. Jacquet, S. , Robert, C. , Valette, J.P. and Denoix, J.M. (2013) Evolution of radiological findings detected in the limbs of 321 young horses between the ages of 6 and 18 months. Vet. J. 197, 58–64. [DOI] [PubMed] [Google Scholar]
- 24. Oosterlinck, M. , Pille, F. , Back, W. , Dewulf, J. and Gasthuys, F. (2011) A pressure plate study on fore and hindlimb loading and the association with hoof contact area in sound ponies at the walk and trot. Vet. J. 190, 71–76. [DOI] [PubMed] [Google Scholar]
- 25. Merkens, H.W. , Schamhardt, H.C. , Hartman, W. and Kersjes, A.W. (1986) Ground reaction force patterns of Dutch Warmblood horses at normal walk. Equine Vet. J. 18, 207–214. [DOI] [PubMed] [Google Scholar]
- 26. Merkens, H.W. , Schamhardt, H.C. , van Osch, G.J.V.M. and van den Bogert, A.J. (1993) Ground reaction force patterns of Dutch Warmblood horses at normal trot. Equine Vet. J. 25, 134–137. [DOI] [PubMed] [Google Scholar]
- 27. Alexander, R.M.N. and Jayes, A.S. (1983) A dynamic similarity hypothesis for the gaits of quadrupedal mammals. J. Zool. 201, 135–152. [Google Scholar]
- 28. Anderson, T.M. and McIlwraith, C.W. (2004) Longitudinal development of equine conformation from weanling to age 3 years in the Thoroughbred. Equine Vet. J. 36, 563–570. [DOI] [PubMed] [Google Scholar]
- 29. Thompson, K.N. (1995) Skeletal growth rates of weanling and yearling thoroughbred horses. J. Anim. Sci. 73, 2513–2517. [DOI] [PubMed] [Google Scholar]
- 30. Buchner, H.H. , Savelberg, H.H. , Schamhardt, H.C. and Barneveld, A. (1997) Inertial properties of Dutch Warmblood horses. J. Biomech. 30, 653–658. [DOI] [PubMed] [Google Scholar]
- 31. Buchner, H.H. , Obermüller, S. and Scheidl, M. (2000) Body centre of mass movement in the sound horse. Vet. J. 160, 225–234. [DOI] [PubMed] [Google Scholar]
- 32. Khumsap, S. , Clayton, H.M. , Lanovaz, J.L. and Bouchey, M. (2002) Effect of walking velocity on forelimb kinematics and kinetics. Equine Vet. J. 34, Suppl 34, 325–329. [DOI] [PubMed] [Google Scholar]
- 33. Khumsap, S. , Clayton, H.M. and Lanovaz, J.L. (2001) Effect of walking velocity on hindlimb kinetics during stance in normal horses. Equine Vet. J. 33, Suppl 33, 21–26. [DOI] [PubMed] [Google Scholar]
- 34. Khumsap, S. , Clayton, H.M. and Lanovaz, J.L. (2001) Effect of walking velocity on ground reaction force variables in the hind limb of clinically normal horses. Am. J. Vet. Res. 62, 901–906. [DOI] [PubMed] [Google Scholar]
- 35. McLaughlin, R.M.J. , Gaughan, E.M. , Roush, J.K. and Skaggs, C.L. (1996) Effects of subject velocity on ground reaction force measurements and stance times in clinically normal horses at the walk and trot. Am. J. Vet. Res. 57, 7–11. [PubMed] [Google Scholar]
- 36. Weishaupt, M.A. , Hogg, H.P. , Auer, J.A. and Wiestner, T. (2010) Velocity‐dependent changes of time, force and spatial parameters in Warmblood horses walking and trotting on a treadmill. Equine Vet. J. 42, 530–537. [DOI] [PubMed] [Google Scholar]
- 37. Robert, C. , Valette, J.P. , Pourcelot, P. , Audigié, F. and Denoix, J.M. (2002) Effects of trotting speed on muscle activity and kinematics in saddlehorses. Equine Vet. J. 34, Suppl 34, 295–301. [DOI] [PubMed] [Google Scholar]
- 38. Hoyt, D.F. , Molinari, M. , Wickler, S.J. and Cogger, E.A. (2002) Effect of trotting speed, load and incline on hindlimb stance‐phase kinematics. Equine Vet. J. 34, 330–336. [DOI] [PubMed] [Google Scholar]
- 39. Leach, D. and Cymbaluk, N.F. (1986) Relationships between stride length, stride frequency, velocity, and morphometrics of foals. Am. J. Vet. Res. 47, 2090–2097. [PubMed] [Google Scholar]
- 40. Main, R.P. and Biewener, A.A.J. (2004) Ontogenetic patterns of limb loading, in vivo bone strains and growth in the goat radius. Exp. Biol. 207, 2577–2588. [DOI] [PubMed] [Google Scholar]
- 41. Robilliard, J.J. and Wilson, A.M. (2005) Prediction of kinetics and kinematics of running animals using an analytical approximation to the planar spring‐mass system. J. Exp. Biol. 208, 4377–4389. [DOI] [PubMed] [Google Scholar]
- 42. Ishihara, A. , Reed, S.M. , Rajala‐Schultz, P.J. , Robertson, J.T. and Bertone, A.L. (2009) Use of kinetic gait analysis for detection, quantification, and differentiation of hind limb lameness and spinal ataxia in horses. J. Am. Vet. Med. Assoc. 234, 644–651. [DOI] [PubMed] [Google Scholar]
- 43. Collins, J.J. and De Luca, C.J. (1993) Open‐loop and closed‐loop control of posture: a random‐walk analysis of center‐of‐pressure trajectories. Exp. Brain Res. 95, 308–318. [DOI] [PubMed] [Google Scholar]
- 44. Clark, J.E. , Whitall, J. and Phillips, S.J. (1988) Human interlimb coordination: the first 6 months of independent walking. Dev. Psychobiol. 21, 445–456. [DOI] [PubMed] [Google Scholar]
- 45. Weishaupt, M.A. , Wiestner, T. , Hogg, H.P. , Jordan, P. and Auer, J.A. (2006) Compensatory load redistribution of horses with induced weight‐bearing forelimb lameness trotting on a treadmill. Vet. J. 171, 135‐–146. [DOI] [PubMed] [Google Scholar]
- 46. Weishaupt, M.A. (2008) Adaptation strategies of horses with lameness. Vet. Clin. North Am. Equine Pract. 24, 79–100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Item 1: Results of the linear mixed model presenting the effect sizes, P values, 95% confidence intervals and Benjamini–Hochberg (B‐H) corrected P values of the parameters investigated.
Supplementary Item 2: Mean force (body mass normalised) time (normalised by linear interpolation) curves at Week 2 of the osteochondrosis‐negative foals for each limb (LF: left fore, RF: right fore, LH: left hind, RH: right hind) at walk and trot. The central line of the force time curves represents the mean and the shaded area represents the median absolute deviation (MAD).
Supplementary Item 3: Mean force (body mass normalised) time (normalised by linear interpolation) curves at Week 12 of the osteochondrosis‐negative foals for each limb (LF: left fore, RF: right fore, LH: left hind, RH: right hind) at walk and trot. The central line of the force time curves represents the mean and the shaded area represents the median absolute deviation (MAD).
Supplementary Item 4: Mean force (body mass normalised) time (normalised by linear interpolation) curves at Week 24 of the osteochondrosis‐negative foals for each limb (LF: left fore, RF: right fore, LH: left hind, RH: right hind) at walk and trot. The central line of the force time curves represents the mean and the shaded area represents the median absolute deviation (MAD).
Supplementary Item 5: Asymmetry indexes (ASI) of the contralateral hindlimbs (CHL) of a representative osteochondrosis‐negative and unilateral osteochondrosis‐positive foal at trot.


