ABSTRACT
Small intestinal muscularis externa macrophages have been associated with interstitial cells of Cajal. They have been proposed to play various roles in motility disorders and to take part in a microbiota‐driven regulation of gastrointestinal motility. Our objective was to understand the reaction of resident macrophages of the musculature to a pro‐inflammatory stimulator, lipopolysaccharide (LPS). Mice were injected with LPS or saline and sacrificed after 6 hr. Whole mounts were stained with antibodies toward CD169, ionized calcium‐binding adaptor molecule 1 (iba1) (microglial/macrophage marker) and heme oxygenase‐1 (HO‐1). Cell densities were measured using unbiased stereology. Results: iba1pos cells showed an overall higher density than CD169pos and HO‐1pos cells. Most HO‐1pos and iba1pos cells were positive for CD 169 in serosa and at Auerbach's plexus (AP). At the deep muscular plexus, mainly iba1pos cells were present, and were mostly CD169neg; a few HO‐1pos cells were present. Conclusions: A new subset of resident macrophages in the intestinal muscularis externa was discovered, identified as iba1pos CD169neg. HO‐1 is constitutively present in most macrophages in serosa and at AP, suggesting a M2 phenotype. LPS‐treatment results in an up‐regulation of HO‐1pos/CD169neg cells in serosa and at AP. Anat Rec, 300:1114–1122, 2017. © 2016 The Authors. The Anatomical Record published by Wiley Periodicals, Inc. on behalf of American Association of Anatomists
Keywords: macrophages, intestine, HO‐1, iba1, LPS, immunohistochemistry
Resident macrophages have several functions; they play a role in development, tissue homeostasis and repair, in addition to immune responses to pathogens (Phillips and Powley, 2012; Wynn et al., 2013).
Macrophages in the muscularis externa of the small intestine are evenly distributed and have a close spatial relationship to interstitial cells of Cajal (ICC) (Thuneberg, 1982; Mikkelsen et al., 2011) suggesting potential regulation of motility. Indeed, others have recently confirmed that macrophages may play a role in pathological conditions associated with motility disturbances; for example, inflammation, diabetic gastroparesis, and postoperative ileus (Zhao et al., 2008; Choi et al., 2010; Neshatian et al., 2015; Bauer, 2008). Particularly the macrophages at Auerbach's plexus (AP) have attracted attention. It has been suggested that they take part in a microbiota driven regulation of gastrointestinal motility (Muller et al., 2014), and in enteric neural regulation of macrophage function (Matteoli et al., 2014;Verheijden et al., 2015; Gabanyi et al., 2016).
It is therefore important to characterize the macrophage population in muscularis externa further with newer macrophage antibodies that may identify physiologically important subgroups.
In rodents, macrophages in muscularis externa of the small intestine are distributed in three layers; in the serosa, associated with AP and with the deep muscular plexus (DMP) (Mikkelsen et al.,2011; Mikkelsen, 1995; 2010). Osteopetrotic (op/op) mice lack the colony‐stimulating factor‐1 (M‐CSF‐1) (Yoshida et al., 1990) and subgroups of macrophages are absent (Wiktor‐Jedrzejczak et al., 1990). We have shown that macrophages in all three layers of the small intestine muscularis are absent in op/op mice (Mikkelsen and Thuneberg, 2008; Mikkelsen et al., 1985), indicating that this macrophage population is M‐CSF‐1‐dependent.
Ionized calcium‐binding adaptor molecule 1 (iba1) was first demonstrated in microglia (resident macrophages in the brain) (Imai et al., 1996) and has been shown to stain cells in both normal and pathological lesions in rodents (Ito et al., 1998; 2001). Outside the CNS, iba1/AIF‐1 has been shown to stain most macrophage subpopulations (except alveolar macrophages) and has therefore been suggested to be a “pan‐macrophage marker” (Kohler, 2007; DeFalco et al., 2015).
To further characterize the activation state of the macrophages during normal and inflammatory conditions we used an antibody against heme oxygenase‐1 (HO‐1). In preliminary studies of healthy rats and mice we had observed that many jejunal muscularis macrophages are HO‐1pos (Mikkelsen and Kirkeby, 2012), although HO‐1 immunoreactivity has previously been reported to be lacking in muscularis externa (Miller et al., 1998; Moore et al., 2003).
HO‐1 is a highly inducible antioxidant enzyme, which facilitates the degradation of heme and has immunomodulatory and anti‐inflammatory properties (Morse and Choi, 2005). The final products of heme catabolism (CO and biliverdin) are considered to have antioxidant effects (Paine et al., 2010). Under normal conditions, HO‐1 is considered to be undetectable in most tissues, but is highly inducible under conditions of stress or inflammation (Paine et al., 2010). HO‐1 is expressed in many cell types including some macrophage types, and is often described as being associated with both proinflammatory and alternative activation (Wang et al., 2009; Wang and Chau, 2010; Ryter and Choi, 2016).
Lipopolysaccharide (LPS) is an activator of Toll‐like receptors that induces the transcription factor NF‐ϰB, which among other things, controls the activity of the promoters of pro‐inflammatory cytokines (Ruetten et al., 1999; Jersmann et al., 2001). LPS‐administration has been shown to cause abnormal gastrointestinal motility with delayed gastric emptying, altered transit time and intussusceptions of the small and large intestines in mice (Nissan et al., 1997; De Winter et al., 2005). LPS was therefore used as a pro‐inflammatory activator.
The muscularis externa macrophages were originally demonstrated by light and electron microscopy (Mikkelsen et al., 2011). In later studies, we combined FITC‐dextran endocytosis with immunostaining with the macrophage markers F4/80, M1/70, MHCII (IE‐antigen), scavenger receptor (2F8), and CD169 (Mikkelsen et al., 2004; 1999; 1988).
In this study, we chose CD169 as the macrophage marker, as it allows double‐staining with iba1 and HO‐1. We compared the densities of CD169, iba1, and HO‐1 in the orad part of jejunum to map macrophage subtypes in the muscularis externa. As we have previously shown, that a well‐defined LPS injection scheme in mice gave the highest density of MHCII positive macrophages after 6 hr (Mikkelsen et al., 1985), we used the same dose and time to assess whether inflammation changes macrophage density (CD169, iba1, and HO‐1), phenotypes or HO‐1 up‐regulation.
MATERIALS AND METHODS
Animals
Eight to nine week‐old female C57Bl/6 (B6) mice (Taconic) were used. One group of mice (N = 10) was injected intraperitoneally with 20 µg/g LPS (Escherichia coli) (Sigma, Serotype 055:B5, L‐2880) dissolved in sterile saline. Another group of animals (N = 8) was sham‐injected with sterile saline. The animals were fed with standard laboratory chow, had free access to water and food, and were killed by cervical dislocation 6 hr after injection. The mice were housed in a high quality barrier with high hygienic standards and housed and experimentally manipulated in accordance with current national regulations issued by The Danish Council on Animal Care.
Antibodies
The antibodies used were rat anti‐CD169 (AbD Serotec, MCA‐884; 1:200), rabbit anti‐iba1 (Waco, 019‐19741; 1:200) and rabbit anti‐HO‐1 (Abcam, ab13243; 1:250). The secondary antibodies were biotin conj. goat anti‐rat (Amersham, RPN 1005; 1:1500), followed by Vectastain ABC reagent Vector (PK‐7100) and DAB (DakoCytomation), using the companies' recommendations. For rabbit antibodies, EnVision (DakoCytomation) was used. For double‐staining RH RedX‐conjugated goat anti‐rat (Jackson, 112‐295‐102) and Dylight 488‐conjugated goat anti‐rabbit (Jackson, 111‐485.144) were used. Specimens incubated with rat IgG2a (AbD Serotec), irrelevant rabbit antibodies or no primary antibodies served as controls.
Tissue Preparation and Immunohistochemistry
Whole mounts were prepared from the orad jejunum starting about 3½ cm after the pyloric sphincter. We have previously shown that the first part of jejunum seems to have the smallest inter‐animal variance in cell densities (Mikkelsen et al., 1988). The whole mounts were prepared by peeling off the whole muscularis externa from jejunum, which subsequently was cut open along the mesentery. For details see (Mikkelsen et al., 1985; 1988). The isolated muscle coat was placed in TBS with Nifedipine (1 µmol L−1) for 10 min to ensure relaxation and was then stretched and pinned onto a Sylgard plate. After fixation with 4% paraformaldehyde for 3 hr, the muscle coat was kept in TBS at 4°C until immunostaining the following day, and was divided into about 1½ cm whole mount pieces. To obtain the smallest inter‐animal variance in cell densities (Mikkelsen et al., 1988), we used the first 3 whole mounts for the stereological analyses; the following whole mounts were used for double staining and for negative controls, respectively. Some of the whole mounts for stereology analyses were of substandard quality (uneven staining, damage to serosa), and were therefore omitted from the stereological quantification. The discarded whole mounts were 7 from the iba1 groups, 4 from the HO‐1 groups, and 8 from the CD169 groups.
All washing solutions contained 0.5% triton‐X 100. The tissue was quenched in 1% H2O2 for 15 min. Dako Real Antibody Diluent (S2022) was used for preincubation (½ hr) and for diluting all primary and secondary antibodies. Incubations were carried out at 4°C, overnight for primary antibodies, 2 hr for Envision and biotin‐conjugated antibodies, and 1 hr for ABC‐complex at room temperature. The chromogene was 0.5% diaminobenzidine in 0.035% H2O2. Double‐staining with iba1/CD169 and HO‐1/CD169 were carried out with a mixture of primary rat and rabbit antibodies overnight, followed by secondary anti‐rat antibodies for 2 hr and finally secondary anti‐rabbit antibodies for 2 hr.
In addition, frozen specimens from the distal part of ileum from op/op and control mice from a previous study were added (Mikkelsen et al., 1985).
A Zeiss Axioplan 2 microscope was used for microscopy, and confocal stacks (15–18) were obtained with an LSM 700 through a Plan‐Apochromat 20/0,8 NA objective. A 488‐nm argon laser was used for the excitation of Dylight 488, and a 555‐nm laser was used for the excitation of RH RedX.
Stereological Analysis
The areal densities (the number of cells per surface area of the muscle layer) of CD169pos, iba1pos, and HO‐1pos cells were estimated in whole mounts from the proximal jejunum using unbiased stereological counting through the full‐thickness of the muscularis externa. For further details see (Alberti et al., 2005; Mikkelsen et al., 1985; 1988). An Olympus BX51 microscope was used for counting the cell populations at a final magnification of ×1022 using an ×20 UPanApo oil immersion objective (NA ¼ 0.8) to which the ×2 objective used for delineation was paracentered.
In the CD169 whole mounts, the mean number of counted cells was 67 in both treatment groups. The cells were counted in 123 fields of view (range: 68–166) in the saline group and in 109 (range: 44–138) in the LPS‐treated group. In the iba1 whole mounts, the mean numbers of counted cells were 60 and 61 in the saline‐ and LPS‐treated group, respectively. This number of cells were counted in 75 fields of view (range: 32–123) and 70 (range: 48–98), respectively. In the HO‐1 whole mounts, the mean numbers of counted cells were 35 and 51 in the saline‐ and LPS‐treated groups and the numbers of fields of view were 62 (range: 29–132) and 60 (range: 14–110).
Statistics
Group comparisons were carried out with the unpaired t‐test assuming unequal variance. When comparing the densities of the different cell populations within groups (i.e., within the sham group and the LPS group, respectively) we first used an ANOVA test to verify that there were indeed significant differences between the cell populations in both the sham‐group and the LPS group. Subsequently, an unpaired t‐test assuming unequal variances was applied to compare one cell population against another. We chose the unpaired test instead of the paired test since not all animals were represented by all antibody stains. A P‐value in the two‐tailed test below 0.05 was considered to be a statistical significant difference between groups.
A semi‐quantitative assessment of fluorescent double‐stains was performed to clarify if CD169 co‐localizes with iba1 and/or HO1. Cells were counted in both combinations of double‐stained whole mounts.
The counting of cells was performed directly at the microscope going from serosa to AP. Cells counted had processes, stained for iba1 or HO‐1 respectively, and showed double‐staining with CD169pos cells, no CD169 staining, or were negative when CD169 stained cells were present in the field of view.
Cells at the deep muscular layer were counted separately, because of the modest number of cells.
RESULTS
In sham‐treated mice, CD169pos macrophages were present in muscularis externa at three positions. In the serosa (SS), the cells were oblong or ramified with slender processes (Figs. 1A and 2A,G). At the level of AP, they were situated between the longitudinal and circular muscle layers, and were stellate‐shaped with thin slender, ramified processes (Figs. 1B and 2B,H). At the level of the DMP, between the inner and outer circular muscle layers, CD169pos cells were solitary, oblong, rare, and with very few processes (Fig. 1C).
Figure 1.
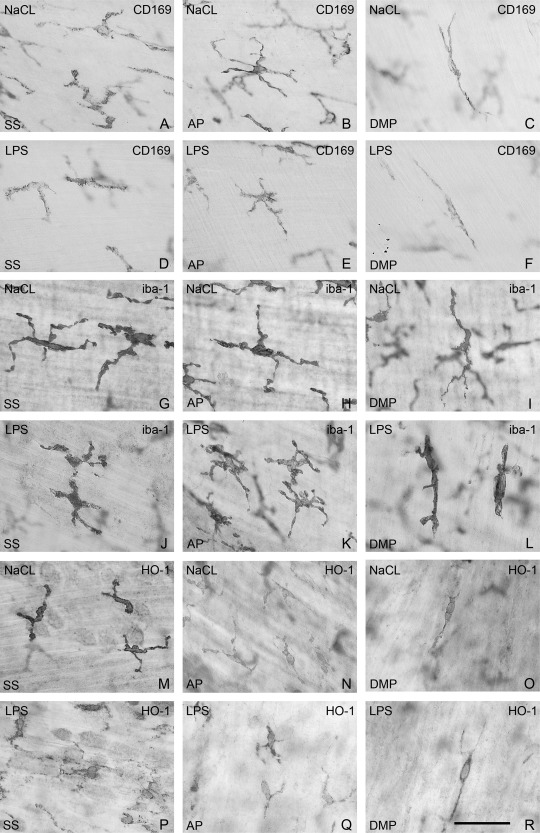
Muscularis externa whole mounts from the orad part of jejunum. Immunostaining of macrophages with antibodies towards CD169 (A–F), iba1 (G–L), and HO‐1(M–R). Sham‐treated mice (A–C, G–I, and M–O). Mice treated with LPS injection (D–F, J–L, and P–R). Cells in serosa (A, D, G, J, M, P), cells at AP (B, E, H, K, N, Q), cells at DMP (C, F, I, L, O, R). Bar: 50 μm.
Figure 2.
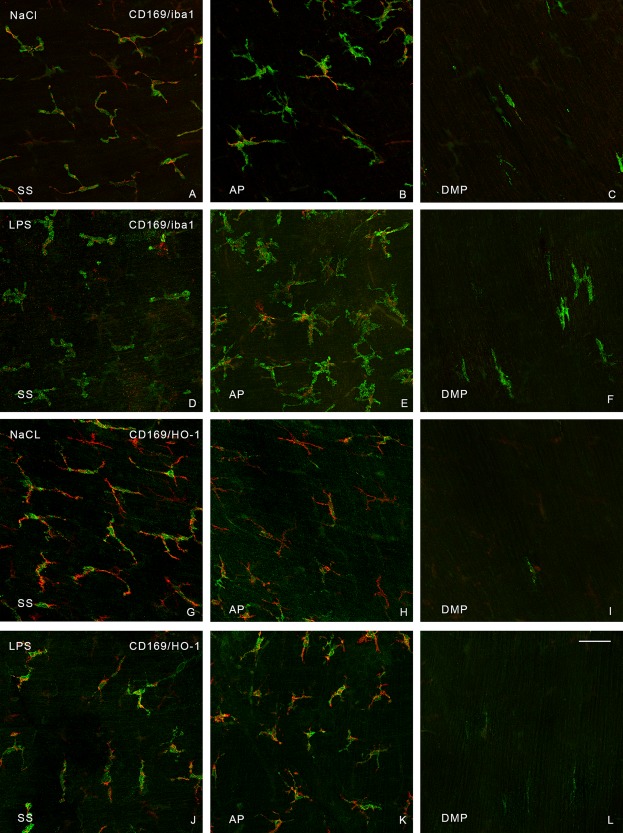
Muscularis externa whole mounts from the orad part of jejunum. The confocal micrographs were taken through the thickness of the jejunal muscularis. The images are single confocal images. Double‐staining was performed with antibodies toward CD169 (red) and iba1 (green) (A–F), and CD169 (red) and HO‐1(green) (G–L). A, D, G, and J are from the serosa (SS). B, E, H, and K are from the level of AP. C, F, I, and L are from the level of the DMP, very few CD169pos cells were present in this layer. A–C and G–I are from sham‐treated mice. D–F and J–L are from LPS‐treated mice. H‐shaped iba1pos cells can be observed in F. Bar: 50 μm.
Cell densities were quantified on sham‐ and LPS‐treated specimens (Fig. 3). Only HO‐1 cell density was altered by LPS‐treatment. In sham‐treated mice, the density of HO‐1pos cells was comparable to the density of CD169pos cells, whereas the density of HO‐1pos cells in LPS‐treated animals increased and was comparable to the density of lba1pos cells (Fig. 3). Moreover, we found in the within‐group comparisons, that in the sham group the density of HO‐1pos cells was statistically significantly different from the density of lba1pos cells, but did not differ significantly from the density of the CD169pos cells. Conversely, in the LPS‐group the density of HO‐1pos cells was statistically significantly different from the density of CD169pos cells, but was comparable to the density of lba‐1pos cells (P‐values given in Table 1).
Figure 3.
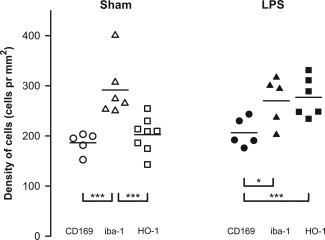
Densities of immunostained macrophages (cells/mm2). Open symbols represent the sham‐treated animals and solid symbols represent the LPS‐treated animals. CD169pos cells are shown by circles, iba1pos cells by triangles, and HO‐1pos cells by squares. The horizontal lines indicate the group mean. Three asterisks indicate that the two cell populations are significantly different with a Bonferroni corrected P‐value below 0.017. One asterisk indicates a P‐value below 0.05.
Table 1.
P‐values for all statistical tests performed in this study
| Sham group versus LPS group using unpaired t‐test | ||
|---|---|---|
| P‐value | ||
| HO‐1‐positive cells | 0.0041 | |
| CD169‐positive cells | Ns | |
| lba‐1‐positive cells | Ns | |
| Within group comparisons using ANOVA | ||
|---|---|---|
| P‐value | ||
| Sham group | 0.00078 | |
| LPS group | 0.024 | |
| Within group comparisons using unpaired t‐test | ||
|---|---|---|
| P‐value for sham group | P‐value for LPS group | |
| HO‐1‐positive cells vs. CD169‐positive cells | 0.31 (N = 8/N = 5) | 0.0074 (N = 6/N = 5) |
| HO‐1‐positive cells vs. lba‐1‐positive cells | 0.0096 (N = 8/N = 6) | 0.801 (N = 6/N = 5) |
| CD169‐positive cells vs. lba‐1‐positive cells | 0.0029 (N = 5/N = 6) | 0.039 (N = 5/N = 5) |
The uppermost part compares the LPS‐ and sham‐treated animals with regard to the three different cell populations. The lower part compares the cell densities of the individual cell populations within the group using an unpaired t‐test.
To try to evaluate in which layer the cells differed, we used doubled‐stained preparations. In serosa, iba1pos cells co‐stained with CD‐169 (Fig. 2A); at AP CD169pos cells were also iba1pos, but there were also iba1pos/CD169neg cells (Fig. 2B and Table 2) with the same morphology as both iba1pos and CD169pos cells (Fig. 1B,H). At the DMP, iba1pos cells were oblong with small cell processes (Fig. 1I), but they were seldom positive for CD169 (Fig. 2C and Table 2). Hence, iba1 staining showed a population of macrophages at DMP that were seldom recognized with CD169 staining.
Table 2.
The percentage of the double‐stained cell populations counted using the epifluorescence microscope
| Semi‐quantitative assessment of double‐stained cells | |||
|---|---|---|---|
| Sham‐treated | LPS‐treated | ||
| SS | CD169pos/iba1pos | 100% | 97% |
| CD169pos/HO‐1pos | 80% | 76% | |
| iba1pos/CD169pos | 100% | 93% | |
| HO‐1pos/CD169pos | 94% | 84% | |
| AP | CD169pos/iba1pos | 100% | 81% |
| CD169pos/HO‐1 pos | 92% | 67% | |
| iba1pos/CD169pos | 56% | 67% | |
| HO‐1 pos/CD169pos | 88% | 65% | |
| DMP | CD169pos/iba1pos | 80% | 86% |
| CD169pos/HO‐1pos | 64% | 82% | |
| iba1pos/CD169pos | 22% | 12% | |
| HO‐1pos/CD169pos | 35% | 34% | |
Cells that stained positive for the stain mentioned before the slash was sampled, and it was recorded how many of those cells that were stained positive for the stain mentioned after the slash.
To verify that these cells belong to the macrophage population, we used frozen sections of muscularis externa from control and op/op mice: the latter have previously been shown to lack macrophages (F4/80pos and MHCIIpos cells) (Mikkelsen and Thuneberg, 2008; Mikkelsen et al., 1985). In control mice, iba1pos cells were observed in serosa, at AP and DMP (Fig. 4A), but were absent in op/op mice (Fig. 4B).
Figure 4.
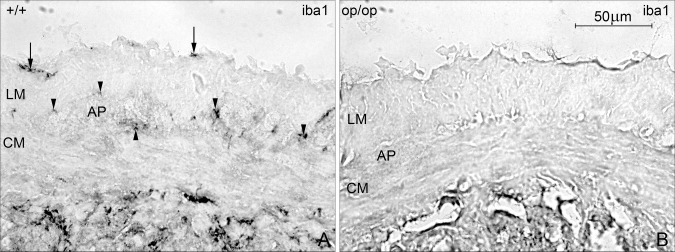
iba1‐staining in the muscularis externa in ileal frozen sections from control and op/op mice. A: iba1 positive cells in control mice were present in the serosa (arrow), at the level of AP, between the longitudinal (LM), and circular muscle layer (CM; arrow head). B: In op/op mice, iba1‐immunoreactivity was absent in muscularis externa. Bar 50 μm.
In serosa and at AP, HO‐1pos cells appeared to have the same morphology as iba1pos cells (Fig. 1M,N) and most CD169pos cells harbored HO‐1(Fig. 2G,H). At DMP, HO‐1pos oblong cells were rare (Fig. 1O) and about a third co‐stained with CD169 (Fig. 2I and Table 2).
LPS‐treatment resulted in HO‐1pos/CD169pos, HO‐1pos/CD169neg, and HO‐1neg/CD169pos cells in serosa and at AP (Fig. 2J,K). In LPS‐treated animals an up‐regulation of HO‐1 staining took place in cells in serosa and AP resulting in an increase in HO‐1pos/CD169neg cells. No change was apparent at the DMP.
DISCUSSION
In sham‐treated animals iba1pos cells had a significantly higher density than CD169pos and HO‐1pos cells. The densities of iba1pos and CD169pos cells were unaffected by LPS‐treatment. At DMP, neither iba1pos nor HO‐1pos cells showed any significant co‐staining with CD169, in either sham‐ or in LPS‐treated animals. In addition, both HO‐1pos and CD169pos cells were rare at DMP.
Traditionally, macrophages in mouse small intestine muscularis externa have immunophenotypically been considered to consist of an almost homogenous population (Mikkelsen et al., 2004; 1999; 1988), in general, F4/80 is considered to be a pan macrophage marker, although we previously have found that the density of F4/80pos cells is lower than the density of CD169pos and MHCIIpos cells in C57Bl/6 mice (Mikkelsen et al., 1985). This indicates the need to clarify the existence of subgroups of macrophages that may have different roles in physiology and/or pathophysiology. iba1 has mainly been used to stain microglia (resident macrophages in the brain) (Imai et al., 1996). Outside the CNS AIF1 (allograft inflammatory factor1), which is identical to iba1 with regard to gene and protein, stains most macrophage sub‐populations (except alveolar macrophages), and is expressed by all ordinary macrophages (Kohler, 2007). In small and large intestines, AIF stains macrophages in the lamina propria and between the muscle layers. In lymphoid and hemopoietic tissues, iba1/AIF1 stains more macrophages than the macrophage marker F4/80, for example, marginal zone macrophages in the spleen. In contrast to F4/80, it does not stain monocytes and has been suggested to be a “pan‐macrophage marker” (Kohler, 2007; DeFalco et al., 2015).
To determine if the iba1pos cells in muscularis externa of the small intestine are indeed macrophages and not other ramified cells (for example ICC or fibroblasts), we used osteopetrotic (op/op) mice. They lack M‐CSF‐1 and therefore lack subgroups of macrophages, especially those located in the tunica muscularis of the small intestine (Mikkelsen and Thuneberg, 2008). In ileal frozen sections from op/op mice, no iba1‐immunoreactivity was found in muscularis externa. This is in accord with the observation that brain iba1pos cells are M‐CSF‐1‐dependent (Imai and Kohsaka, 2002), and microglia are reduced in op/op mice (Wegiel et al.,1998; Kondo and Duncan, 2009). iba1 has been reported to have actin‐crosslinking activities and to be functionally involved in certain aspects of motility‐associated rearrangements of the actin cytoskeleton, such as in membrane ruffling, and in the building of phagocytic cups.
Little is known about the macrophage/microglia‐specific components that regulate the reorganization of actin filaments. However, in mouse microglia, LPS‐exposure resulted in swelling of the cell body, a thickening of the proximal processes, and a reduction in number of distal ramifications (Kloss et al., 2001). In a pilot study on sham‐ and LPS‐treated mice, we did not find any indications of changes in the morphology of iba1pos cells (number of ramifications and thickening of the processes) with the same dose of LPS.
HO‐1 is an inducible enzyme (Wang and Chau, 2010). It has been reported that HO‐1 may be protective towards inducible nitric oxide synthase (Ashino et al., 2008). The presence of HO‐1 in murine small intestine muscularis macrophages in control material has not been demonstrated previously (Miller et al., 1998; Moore et al., 2003). However, in this study we observed that HO‐1 is expressed in CD169pos cells in sham‐treated mice. This difference could be due to differences in antibodies and fixation techniques.
When we stained frozen sections from germ‐free mice with HO‐1 antibodies, the immunoreactivity was very low in mouse muscularis externa, and was only detectable in ganglion cells in AP and to a very limited degree in cells in serosa and at DMP (unpublished observations). We have also previously observed in embryonic and germ‐free mice, without any microbiota, that the muscularis macrophages are MHCIIneg, in contrast to conventional adult mice, which are constitutively MHCIIpos (Mikkelsen et al., 1999). This up‐regulation might be due to normal microbiota. Hence, the HO‐1 positivity of CD169pos macrophages may be induced by normal gut microbiota in sham‐treated mice.
HO‐1 is associated with alternative macrophage activation (M2) and recently it has been shown that muscularis macrophages express wound healing and tissue protective genes (M2), whereas lamina propria macrophages express pro‐inflammatory genes (M1) (Gabanyi et al., 2016).
Also intragastric inoculation with nonpathogenic or noninvasive bacteria strains resulted in a further local up‐regulation in M2 genes in muscularis externa macrophages, but changes in gene expressions were few in lamina propria macrophages (Gabanyi et al., 2016).
In this study, after sham‐treatment, confocal microscopy and conventional fluorescence microscopy demonstrated that many iba1pos and HO‐1pos cells were also positive for CD169 in serosa and at AP, whereas at DMP CD169pos cells seemed to be rare. A few oblong CD169neg/HO‐1pos cells were present and both oblong and H‐shaped CD169neg/iba1pos cells were observed in this layer. After LPS‐treatment more CD169neg/HO‐1pos cells were present in serosa and at AP, which suggests an HO‐1 up‐regulation in the Iba1pos/CD169neg cell population. Furthermore, at DMP, HO‐1pos/CD169neg cells as well as iba1pos/CD169neg cells were observed, but the proportion of iba1pos/CD169neg and HO‐1pos/CD169neg cells was unaltered compared to sham‐treated mice. We have been unsuccessful interpreting specimens that are double‐stained with two antibodies derived from the same species (rabbits); therefore, we did not attempt double‐staining with iba1 and HO‐1.
In the CNS of CX3CR1‐GFP mice, brain cells express the microglial marker iba1 and overlap precisely with the GFPpos population (Cardona et al., 2006). Since the ligand CXCL1 is localized on neurons, several hypotheses on interactions between microglia and nerves have been proposed (see review; Limatola and Ransohoff, 2014).
Intestinal macrophages, in both lamina propria and in muscularis externa, are characterized by very high levels of the chemokine receptor CX3CR1 (Zigmond and Jung, 2013). In a recent study, cross sections (colonic muscularis externa) from CX3CR1‐GFPpos/neg mice, revealed a strong fluorescence in cells in the muscularis externa at AP (Muller et al., 2014). It is not clear if CX3CR1 is also present on macrophages in the serosa.
Neuro‐immune interactions in muscularis externa have been proposed (Matteoli et al., 2014; Gabanyi et al., 2016). Extrinsic sympathetic nerves have been shown to be situated close to muscularis macrophages, which—when using gene expression profiling and qPCR—show high levels of Adrb2 (encoding β2 adrenergic receptors), but it is uncertain whether this is the case for all muscularis macrophages.
The cholinergic anti‐inflammatory pathway, which is associated with modulation of inflammation has been associated with muscularis macrophages, which express α7 nicotinic acetylcholine receptor (α7nAChR). The vagus nerve interacts with cholinergic nerves located near muscularis macrophages, but does not innervate them (Matteoli et al., 2014).
Ultrastructural studies of normal mouse muscularis externa showed that direct intimate contacts between macrophages and neurons/ganglion cells at AP and at DMP are absent (Thuneberg, 1982; Mikkelsen et al., 2011). Hence, communication between nerves and macrophages may be indirect, potentially via ICC that are close to macrophages and are heavily innervated by neurons at AP and DMP.
In diabetic gastroparesis, the close spatial contact between macrophages and ICC and up‐regulation of HO‐1 in M2 macrophages, seems to protect against the loss of ICC and nNOS in enteric nerves and to protect against gastroparesis (Choi et al., 2010).
With regard to function, it has been suggested recently that muscularis macrophages communicate with enteric nerves, and may be involved in the regulation of motility (Muller et al., 2014), although no direct measurements of peristalsis were carried out. Hence, a direct role in the genesis of this motor pattern has yet to be confirmed.
In conclusion, different macrophage immunophenotypes seem to exist in the muscularis externa, specifically iba1posCD169neg and iba1pos CD169pos cells. At the DMP macrophages, which are iba1pos seem rarely to costain with CD169.
Importantly, many CD169pos macrophages in sham‐treated mice express HO‐1, suggesting a M2 phenotype. LPS administration up‐regulates HO‐1 in the macrophages at AP and in serosa.
ACKNOWLEDGEMENT
The authors wish to thank Ha Nguyen, Pernille Froh, Hanne Hadberg, and Keld Ottesen for skilled technical assistance.
Literature Cited
- Alberti E, Mikkelsen HB, Larsen JO, Jimenez M. 2005. Motility patterns and distribution of interstitial cells of Cajal and nitrergic neurons in the proximal, mid‐ and distal‐colon of the rat. Neurogastroenterol Motil 17:133–147. [DOI] [PubMed] [Google Scholar]
- Ashino T, Yamanaka R, Yamamoto M, Shimokawa H, Sekikawa K, Iwakura Y, Shioda S, Numazawa S, Yoshida T. 2008. Negative feedback regulation of lipopolysaccharide‐induced inducible nitric oxide synthase gene expression by heme oxygenase‐1 induction in macrophages. Mol Immunol 45:2106–2115. [DOI] [PubMed] [Google Scholar]
- Bauer AJ. 2008. Mentation on the immunological modulation of gastrointestinal motility. Neurogastroenterol Motil 20 Suppl 1:81–90. [DOI] [PubMed] [Google Scholar]
- Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, et al. 2006. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci 9:917–924. [DOI] [PubMed] [Google Scholar]
- Choi KM, Kashyap PC, Dutta N, Stoltz GJ, Ordog T, Shea DT, Bauer AJ, Linden DR, Szurszewski JH, Gibbons SJ, et al. 2010. CD206‐positive M2 macrophages that express heme oxygenase‐1 protect against diabetic gastroparesis in mice. Gastroenterology 138:2399–2409, 2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Winter BY, Van Nassauw L, De Man JG, de Jonge F, Bredenoord AJ, Seerden TC, Herman AG, Timmermans JP, Pelckmans PA. 2005. Role of oxidative stress in the pathogenesis of septic ileus in mice. Neurogastroenterol Motil 17:251–261. [DOI] [PubMed] [Google Scholar]
- DeFalco T, Potter SJ, Williams AV, Waller B, Kan MJ, Capel B. 2015. Macrophages Contribute to the Spermatogonial Niche in the Adult Testis. Cell Rep 12:1107–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabanyi I, Muller PA, Feighery L, Oliveira TY, Costa‐Pinto FA, Mucida D. 2016. Neuro‐immune Interactions Drive Tissue Programming in Intestinal Macrophages. Cell 164:378–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y, Ibata I, Ito D, Ohsawa K, Kohsaka S. 1996. A novel gene iba1 in the major histocompatibility complex class III region encoding an EF hand protein expressed in a monocytic lineage. Biochem Biophys Res Commun 224:855–862. [DOI] [PubMed] [Google Scholar]
- Imai Y, Kohsaka S. 2002. Intracellular signaling in M‐CSF‐induced microglia activation: role of Iba1. Glia 40:164–174. [DOI] [PubMed] [Google Scholar]
- Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S. 1998. Microglia‐specific localisation of a novel calcium binding protein, Iba1. Brain Res Mol Brain Res 57:1–9. [DOI] [PubMed] [Google Scholar]
- Ito D, Tanaka K, Suzuki S, Dembo T, Fukuuchi Y. 2001. Enhanced expression of Iba1, ionized calcium‐binding adapter molecule 1, after transient focal cerebral ischemia in rat brain. Stroke 32:1208–1215. [DOI] [PubMed] [Google Scholar]
- Jersmann HP, Hii CS, Ferrante JV, Ferrante A. 2001. Bacterial lipopolysaccharide and tumor necrosis factor alpha synergistically increase expression of human endothelial adhesion molecules through activation of NF‐kappaB and p38 mitogen‐activated protein kinase signaling pathways. Infect Immun 69:1273–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloss CU, Bohatschek M, Kreutzberg GW, Raivich G. 2001. Effect of lipopolysaccharide on the morphology and integrin immunoreactivity of ramified microglia in the mouse brain and in cell culture. Exp Neurol 168:32–46. [DOI] [PubMed] [Google Scholar]
- Kohler C. 2007. Allograft inflammatory factor‐1/Ionized calcium‐binding adapter molecule 1 is specifically expressed by most subpopulations of macrophages and spermatids in testis. Cell Tissue Res 330:291–302. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Duncan ID. 2009. Selective reduction in microglia density and function in the white matter of colony‐stimulating factor‐1‐deficient mice. J Neurosci Res 87:2686–2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limatola C, Ransohoff RM. 2014. Modulating neurotoxicity through CX3CL1/CX3CR1 signaling. Front Cell Neurosci 8:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteoli G, Gomez‐Pinilla PJ, Nemethova A, Di GM, Cailotto C, van Bree SH, Michel K, Tracey KJ, Schemann M, Boesmans W, et al. 2014. A distinct vagal anti‐inflammatory pathway modulates intestinal muscularis resident macrophages independent of the spleen. Gut 63:938–948. [DOI] [PubMed] [Google Scholar]
- Mikkelsen HB. 1995. Macrophages in the external muscle layers of mammalian intestines. Histol Histopathol 10:719–736. [PubMed] [Google Scholar]
- Mikkelsen HB. 2010. Interstitial cells of Cajal, macrophages and mast cells in the gut musculature: morphology, distribution, spatial and possible functional interactions. J Cell Mol Med 14:818–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen HB, Thuneberg L. 1999. Op/op mice defective in production of functional colony‐stimulating factor‐1 lack macrophages in muscularis externa of the small intestine. Cell Tissue Res 295:485–493. [DOI] [PubMed] [Google Scholar]
- Mikkelsen HB, Kirkeby S. 2012. Do mice and rat differ regarding intestinal muscularis macrophage activation? Neurogastroenterol Motil 24[s.2]:64. [Google Scholar]
- Mikkelsen HB, Thuneberg L, Rumessen JJ, Thorball N. 1985. Macrophage‐like cells in the muscularis externa of mouse small intestine. Anat Rec 213:77–86. [DOI] [PubMed] [Google Scholar]
- Mikkelsen HB, Mirsky R, Jessen KR, Thuneberg L. 1988. Macrophage‐like cells in muscularis externa of mouse small intestine: Immunohistochemical localization of F4/80, M1/70, and Ia‐antigen. Cell Tissue Res 252:301–306. [DOI] [PubMed] [Google Scholar]
- Mikkelsen HB, Garbarsch C, Tranum‐Jensen J, Thuneberg L. 2004. Macrophages in the small intestinal muscularis externa of embryos, newborn and adult germ‐free mice. J Mol Histol 35:377–387. [DOI] [PubMed] [Google Scholar]
- Mikkelsen HB, Larsen JO, Hadberg H. 2008. The macrophage system in the intestinal muscularis externa during inflammation: an immunohistochemical and quantitative study of osteopetrotic mice. Histochem Cell Biol 130:363–373. [DOI] [PubMed] [Google Scholar]
- Mikkelsen HB, Larsen JO, Froh P, Nguyen TH. 2011. Quantitative Assessment of Macrophages in the Muscularis Externa of Mouse Intestines. Anat Rec (Hoboken) 294:1557–1565. [DOI] [PubMed] [Google Scholar]
- Miller SM, Farrugia G, Schmalz PF, Ermilov LG, Maines MD, Szurszewski JH. 1998. Heme oxygenase‐2 is present in interstitial networks of the mouse small‐intestine. Gastroenterology 114:239–244. [DOI] [PubMed] [Google Scholar]
- Moore BA, Otterbein LE, Turler A, Choi AM, Bauer AJ. 2003. Inhaled carbon monoxide suppresses the development of postoperative ileus in the murine small intestine. Gastroenterology 124:377–391. [DOI] [PubMed] [Google Scholar]
- Morse D, Choi AM. 2005. Heme oxygenase‐1: from bench to bedside. Am J Respir Crit Care Med 172:660–670. [DOI] [PubMed] [Google Scholar]
- Muller PA, Koscso B, Rajani GM, Stevanovic K, Berres ML, Hashimoto D, Mortha A, Leboeuf M, Li XM, Mucida D, et al. 2014. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell 158:300–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neshatian L, Gibbons SJ, Farrugia G. 2015. Macrophages in diabetic gastroparesis–the missing link? Neurogastroenterol Motil 27:7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissan A, Zhang JM, Lin Z, Haskel Y, Freund HR, Hanani M. 1997. The contribution of inflammatory mediators and nitric oxide to lipopolysaccharide‐induced intussusception in mice. J Surg Res 69:205–207. [DOI] [PubMed] [Google Scholar]
- Paine A, Eiz‐Vesper B, Blasczyk R, Immenschuh S. 2010. Signaling to heme oxygenase‐1 and its anti‐inflammatory therapeutic potential. Biochem Pharmacol 80:1895–1903. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Powley TL. 2012. Macrophages associated with the intrinsic and extrinsic autonomic innervation of the rat gastrointestinal tract. Auton Neurosci 169:12–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruetten H, Thiemermann C, Perretti M. 1999. Upregulation of ICAM‐1 expression on J774.2 macrophages by endotoxin involves activation of NF‐kappaB but not protein tyrosine kinase: Comparison to induction of iNOS. Mediators Inflamm 8:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter SW, Choi AM. 2016. Targeting heme oxygenase‐1 and carbon monoxide for therapeutic modulation of inflammation. Transl Res 167:7–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuneberg L. 1982. Interstitial cells of Cajal: Intestinal pacemaker cells? Adv Anat Embryol Cell Biol 71:1–130. [PubMed] [Google Scholar]
- Verheijden S, De SS, Boeckxstaens GE. 2015. Neuron‐macrophage crosstalk in the intestine: a “microglia” perspective. Front Cell Neurosci 9:403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CY, Chau LY. 2010. Heme oxygenase‐1 in cardiovascular diseases: molecular mechanisms and clinical perspectives. Chang Gung Med J 33:13–24. [PubMed] [Google Scholar]
- Wang XM, Kim HP, Nakahira K, Ryter SW, Choi AM. 2009. The heme oxygenase‐1/carbon monoxide pathway suppresses TLR4 signaling by regulating the interaction of TLR4 with caveolin‐1. J Immunol 182:3809–3818. [DOI] [PubMed] [Google Scholar]
- Wegiel J, Wisniewski HM, Dziewiatkowski J, Tarnawski M, Kozielski R, Trekner E, Wiktor‐Jedrzejczak W. 1998. Reduced number and altered morphology of microglial cells in colony stimulating‐1‐deficient osteopetrotic op/op mice. Brain Res 803:135–139. [DOI] [PubMed] [Google Scholar]
- Wiktor‐Jedrzejczak W, Bartocci A, Ferrante AW, Ansari AA, Sell KW, Pollard JW, Stanley ER. 1990. Total absence of colony‐stimulating factor 1 in the macrophage‐deficient osteopetrotic (op/op) mouse. Proc Natl Acad Sci USA 87:4828–4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA, Chawla A, Pollard JW. 2013. Macrophage biology in development, homeostasis and disease. Nature 496:445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H, Hayashi S, Kunisada T, Ogawa M, Nishikawa S, Okamura H, Sudo T, Schultz LD. 1990. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature 345:442–444. [DOI] [PubMed] [Google Scholar]
- Zhao A, Urban JF, Jr. , Anthony RM, Sun R, Stiltz J, Van RN, Wynn TA, Gause WC, Shea‐Donohue T. 2008. Th2 cytokine‐induced alterations in intestinal smooth muscle function depend on alternatively activated macrophages. Gastroenterology 135:217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond E, Jung S. 2013. Intestinal macrophages: Well educated exceptions from the rule. Trends Immunol 34:162–168. [DOI] [PubMed] [Google Scholar]


