Abstract
To compare patterns of gene expression following preconditioning cyclic light rearing versus preconditioning aerobic exercise. BALB/C mice were preconditioned either by rearing in 800 lx 12:12 h cyclic light for 8 days or by running on treadmills for 9 days, exposed to toxic levels of light to cause light-induced retinal degeneration (LIRD), then sacrificed and retinal tissue harvested. Subsets of mice were maintained for an additional 2 weeks and for assessment of retinal function by electroretinogram (ERG). Both preconditioning protocols partially but significantly preserved retinal function and morphology and induced similar leukemia inhibitory factor (LIF) gene expression pattern. The data demonstrate that exercise preconditioning and cyclic light preconditioning protect photoreceptors against LIRD and evoke a similar pattern of retinal LIF gene expression. It may be that similar stress response pathways mediate the protection provided by the two preconditioning modalities.
Keywords: Aerobic exercise, Retinal degeneration, Preconditioning, Light-induced retinal degeneration, Cyclic light rearing
59.1 Introduction
Preconditioning of neural tissue produces local and systemic responses that protect the tissue from toxic levels of stress. For instance mild hypoxia/ischemia (Roth et al. 1998; Grimm et al. 2005, 2006; Gidday 2006; Li et al. 2006; Zhu et al. 2007; Thiersch et al. 2009; Grimm and Willmann 2012; Wacker et al. 2012), moderate-intensity cyclic light (Li et al. 2001, 2003; Chollangi et al. 2009; Ueki et al. 2009), and even whole body exercise (Zhang et al. 2011) are preconditioning stressors that protect several neuronal structures from the effects of exposure to toxic levels of stress. These reports and others further suggest that the mechanisms of preconditioning stressors may be common across preconditioning and toxic modalities. We recently demonstrated that modest treadmill exercise protects photoreceptor morphology and function against light-induced retinal degeneration (LIRD; reported elsewhere at this meeting and in (Lawson et al. 2014)). We hypothesized that preconditioning cyclic light rearing and preconditioning exercise will be protective against LIRD and that the two forms of preconditioning elicit similar patterns of gene expression.
59.2 Methods
Adult male albino BALB/C mice were used in all experiments; n = 3–6 per experimental condition. For cyclic light preconditioning, mice were reared on 12:12 h light:dark cycles. Half the mice were exposed to light at ~ 50 lx (i.e., normal maintenance level) and for the other half to 800 lx. After 8 days, half of each light-rearing group was exposed to 5000 lx white light for 4 h (i.e., toxic light) and the other half were exposed to 50 lx of light. Immediately following light exposure, subsets of mice were sacrificed and retinas harvested for RNA extraction for use in real-time reverse-transcriptase PCR. In some cases, retinal extracts were pooled from individual eyes prior to PCR. To confirm the putative protective effect, another subset of mice was returned to maintenance housing and after 2 weeks were assessed for visual function by electroretinogram (ERG), after which they were sacrificed and ocular paraffin sections prepared for morphological assessment (data not shown).
For exercise preconditioning, mice were exercised on a treadmill running at 10 m/min for 1 h for 9 consecutive days. Controls were mice placed on a stationary treadmill at the same time. Immediately following the last exercise period, mice were exposed to either maintenance levels or toxic levels of light as above. At the end of exposure, mice were sacrificed and retinas harvested for RNA extraction. To confirm the putative protective effect, in other experiments, mice were exercised for 2 weeks, exposed to maintenance or toxic light, then exercised 2 more weeks, after which their ERGs were obtained, they were sacrificed, and ocular paraffin sections prepared for morphological assessment.
59.3 Results
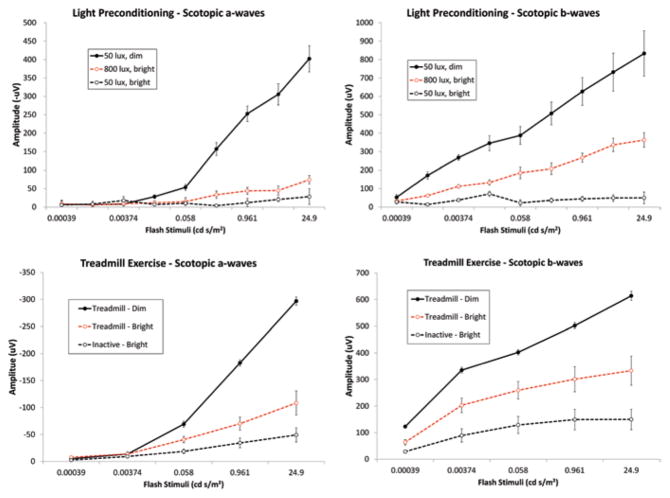
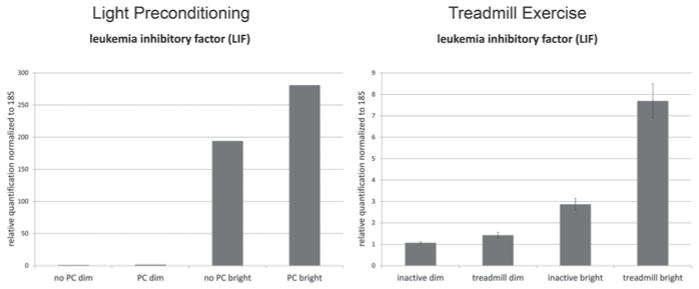
Both forms of preconditioning protected against LIRD to remarkably similar extents, with cyclic light and exercise preconditioned mice showing significantly preserved retinal function (Fig. 59.1) and photoreceptor nuclei (2x greater total counts) and thicker outer nuclear layers than non-preconditioned mice exposed to toxic light (data not shown). Real-time polymerase chain reaction assays using retina RNA revealed that preconditioning by cyclic light rearing and aerobic exercise similarly increased the expression of LIF (Fig. 59.2). Increases were also seen in expression of other preconditioning or stress response genes (e.g., HMOX1, IL-6, PPARgamma, STAT3, HIF1alpha, etc.), but not in expression of CLU and citrate synthetase (data not shown).
Fig. 59.1.
Preconditioning protects retinal function. Panels show ERG stimulus response curves 2 weeks after light exposure for dark-adapted a-wave ( left panels) and b-wave ( right panels) amplitudes. Exposure to “bright” light (5000 lx for 4 h; dashed black lines) suppressed ERG amplitudes compared to exposure to “dim” (50 lx) light ( solid black lines). Rearing in 800 lx cyclic light ( top panels) or treadmill running ( bottom panels) preserved ERG amplitudes ( dashed red lines)
Fig. 59.2.
Preconditioning by cyclic light rearing or by treadmill running increases expression of LIF. Rearing in 800 lx cyclic light (“PC”) or treadmill running (“treadmill”) similarly increased expression of the preconditioning gene LIF immediately following exposure toxic light (5000 lx for 4 h)
59.4 Discussion
The data suggest that exercise preconditioning and cyclic light preconditioning protect photoreceptors against LIRD and evoke similar patterns of retinal gene expression. Models of protective preconditioning have been used well to increase our understanding of innate protective stress responses in retina (Roth et al. 1998; Li et al. 2001; Grimm et al. 2002, 2004, 2005, 2006; Li et al. 2003; Zhu et al. 2006, 2007, 2008; Chollangi et al. 2009; Thiersch et al. 2009; Ueki et al. 2009; Gidday 2010; Grimm and Willmann 2012; Zhu et al. 2012; McLaughlin and Gidday 2013); such approaches are revealing several exciting potential therapeutic targets. In the case of exercise, though, it may be that this form of preconditioning, which is accessible to the majority of the population, is itself a therapeutic intervention. To that end, additional studies on the mechanisms underlying this neuroprotection, the optimal exercise regimen, and effects in humans are being pursued.
Acknowledgments
Supported by NIH P30 EY006360, NIH R01 EY014026 and a grant from the Abraham J. & Phyllis Katz Foundation (to J.H.B.), Rehabilitation Research and Development Service Veterans Affairs Research Career Scientist Award (to M.T.P.), Atlanta VA Center of Excellence in Vision and Neurocognitive Rehabilitation, and Departmental Award from Research to Prevent Blindness.
Contributor Information
Micah A. Chrenek, Department of Ophthalmology, Emory University School of Medicine, Room B5500, Emory Eye Center, 1365B, Clifton Road, N.E., Atlanta, GA 30322, USA
Jana T. Sellers, Department of Ophthalmology, Emory University School of Medicine, Room B5500, Emory Eye Center, 1365B, Clifton Road, N.E., Atlanta, GA 30322, USA
Eric C. Lawson, Department of Ophthalmology, Emory University School of Medicine, Room B5500, Emory Eye Center, 1365B, Clifton Road, N.E., Atlanta, GA 30322, USA. Atlanta VA Center of Excellence for Visual and Neurocognitive Rehabilitation, Atlanta VA Medical Center, Decatur, GA 30033, USA
Priscila P. Cunha, Department of Ophthalmology, Emory University School of Medicine, Room B5500, Emory Eye Center, 1365B, Clifton Road, N.E., Atlanta, GA 30322, USA
Jessica L. Johnson, Department of Ophthalmology, Emory University School of Medicine, Room B5500, Emory Eye Center, 1365B, Clifton Road, N.E., Atlanta, GA 30322, USA
Preston E. Girardot, Department of Ophthalmology, Emory University School of Medicine, Room B5500, Emory Eye Center, 1365B, Clifton Road, N.E., Atlanta, GA 30322, USA
Cristina Kendall, Department of Ophthalmology, Emory University School of Medicine, Room B5500, Emory Eye Center, 1365B, Clifton Road, N.E., Atlanta, GA 30322, USA.
Moon K. Han, Department of Ophthalmology, Emory University School of Medicine, Room B5500, Emory Eye Center, 1365B, Clifton Road, N.E., Atlanta, GA 30322, USA. Atlanta VA Center of Excellence for Visual and Neurocognitive Rehabilitation, Atlanta VA Medical Center, Decatur, GA 30033, USA
Adam Hanif, Department of Ophthalmology, Emory University School of Medicine, Room B5500, Emory Eye Center, 1365B, Clifton Road, N.E., Atlanta, GA 30322, USA. Atlanta VA Center of Excellence for Visual and Neurocognitive Rehabilitation, Atlanta VA Medical Center, Decatur, GA 30033, USA.
Vincent T. Ciavatta, Department of Ophthalmology, Emory University School of Medicine, Room B5500, Emory Eye Center, 1365B, Clifton Road, N.E., Atlanta, GA 30322, USA. Atlanta VA Center of Excellence for Visual and Neurocognitive Rehabilitation, Atlanta VA Medical Center, Decatur, GA 30033, USA
Marissa A. Gogniat, Department of Ophthalmology, Emory University School of Medicine, Room B5500, Emory Eye Center, 1365B, Clifton Road, N.E., Atlanta, GA 30322, USA. Atlanta VA Center of Excellence for Visual and Neurocognitive Rehabilitation, Atlanta VA Medical Center, Decatur, GA 30033, USA
John M. Nickerson, Department of Ophthalmology, Emory University School of Medicine, Room B5500, Emory Eye Center, 1365B, Clifton Road, N.E., Atlanta, GA 30322, USA
Machelle T. Pardue, Department of Ophthalmology, Emory University School of Medicine, Room B5500, Emory Eye Center, 1365B, Clifton Road, N.E., Atlanta, GA 30322, USA. Atlanta VA Center of Excellence for Visual and Neurocognitive Rehabilitation, Atlanta VA Medical Center, Decatur, GA 30033, USA
Jeffrey H. Boatright, Department of Ophthalmology, Emory University School of Medicine, Room B5500, Emory Eye Center, 1365B, Clifton Road, N.E., Atlanta, GA 30322, USA. Atlanta VA Center of Excellence for Visual and Neurocognitive Rehabilitation, Atlanta VA Medical Center, Decatur, GA 30033, USA
References
- Chollangi S, Wang J, Martin A, et al. Preconditioning-induced protection from oxidative injury is mediated by leukemia inhibitory factor receptor (LIFR) and its ligands in the retina. Neurobiol Dis. 2009;34:535–544. doi: 10.1016/j.nbd.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci. 2006;7:437–448. doi: 10.1038/nrn1927. [DOI] [PubMed] [Google Scholar]
- Gidday JM. Pharmacologic preconditioning: translating the promise. Transl Stroke Res. 2010;1:19–30. doi: 10.1007/s12975-010-0011-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm C, Willmann G. Hypoxia in the eye: a two-sided coin. High Alt Med Biol. 2012;13:169–175. doi: 10.1089/ham.2012.1031. [DOI] [PubMed] [Google Scholar]
- Grimm C, Wenzel A, Groszer M, et al. HIF-1-induced erythropoietin in the hypoxic retina protects against light-induced retinal degeneration. Nat Med. 2002;8:718–724. doi: 10.1038/nm723. [DOI] [PubMed] [Google Scholar]
- Grimm C, Wenzel A, Stanescu D, et al. Constitutive overexpression of human erythropoietin protects the mouse retina against induced but not inherited retinal degeneration. J Neurosci. 2004;24:5651–5658. doi: 10.1523/JNEUROSCI.1288-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm C, Hermann DM, Bogdanova A, et al. Neuroprotection by hypoxic preconditioning: HIF-1 and erythropoietin protect from retinal degeneration. Semin Cell Dev Biol. 2005;16:531–538. doi: 10.1016/j.semcdb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Grimm C, Wenzel A, Acar N, et al. Hypoxic preconditioning and erythropoietin protect retinal neurons from degeneration. Adv Exp Med Biol. 2006;588:119–131. doi: 10.1007/978-0-387-34817-9_11. [DOI] [PubMed] [Google Scholar]
- Lawson EC, Han MK, Sellers JT, et al. Aerobic exercise protects retinal function and structure from light-induced retinal degeneration. J Neurosci. 2014;34:2406–2412. doi: 10.1523/JNEUROSCI.2062-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Cao W, Anderson RE. Protection of photoreceptor cells in adult rats from light-induced degeneration by adaptation to bright cyclic light. Exp Eye Res. 2001;73:569–577. doi: 10.1006/exer.2001.1068. [DOI] [PubMed] [Google Scholar]
- Li F, Cao W, Anderson RE. Alleviation of constant-light-induced photoreceptor degeneration by adaptation of adult albino rat to bright cyclic light. Invest Ophthalmol Vis Sci. 2003;44:4968–4975. doi: 10.1167/iovs.03-0140. [DOI] [PubMed] [Google Scholar]
- Li W, Luo Y, Zhang F, et al. Ischemic preconditioning in the rat brain enhances the repair of endogenous oxidative DNA damage by activating the base-excision repair pathway. J Cereb Blood Flow Metab. 2006;26:181–198. doi: 10.1038/sj.jcbfm.9600180. [DOI] [PubMed] [Google Scholar]
- McLaughlin B, Gidday JM. Poised for success: implementation of sound conditioning strategies to promote endogenous protective responses to stroke in patients. Transl Stroke Res. 2013;4:104–113. doi: 10.1007/s12975-012-0240-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth S, Li B, Rosenbaum PS, et al. Preconditioning provides complete protection against retinal ischemic injury in rats. Invest Ophthalmol Vis Sci. 1998;39:777–785. [PubMed] [Google Scholar]
- Thiersch M, Lange C, Joly S, et al. Retinal neuroprotection by hypoxic preconditioning is independent of hypoxia-inducible factor-1 alpha expression in photoreceptors. Eur J Neurosci. 2009;29:2291–2302. doi: 10.1111/j.1460-9568.2009.06781.x. [DOI] [PubMed] [Google Scholar]
- Ueki Y, Le YZ, Chollangi S, et al. Preconditioning-induced protection of photoreceptors requires activation of the signal-transducing receptor gp130 in photoreceptors. Proc Natl Acad Sci U S A. 2009;106:21389–21394. doi: 10.1073/pnas.0906156106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker BK, Perfater JL, Gidday JM. Hypoxic preconditioning induces stroke tolerance in mice via a cascading HIF, sphingosine kinase, and CCL2 signaling pathway. J Neurochem. 2012;123:954–962. doi: 10.1111/jnc.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Wu Y, Jia J. Exercise preconditioning and brain ischemic tolerance. Neuroscience. 2011;177:170–176. doi: 10.1016/j.neuroscience.2011.01.018. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Ohlemiller KK, McMahan BK, et al. Constitutive nitric oxide synthase activity is required to trigger ischemic tolerance in mouse retina. Exp Eye Res. 2006;82:153–163. doi: 10.1016/j.exer.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Zhang Y, Ojwang BA, et al. Long-term tolerance to retinal ischemia by repetitive hypoxic preconditioning: role of HIF1-alpha and heme oxygenase-1. Invest Ophthalmol Vis Sci. 2007;48:1735–1743. doi: 10.1167/iovs.06-1037. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Zhang L, Gidday JM. Deferroxamine preconditioning promotes long-lasting retinal ischemic tolerance. J Ocul Pharmacol Ther. 2008;24:527–535. doi: 10.1089/jop.2008.0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Zhang L, Schmidt JF, et al. Glaucoma-induced degeneration of retinal ganglion cells prevented by hypoxic preconditioning: a model of glaucoma tolerance. Mol Med. 2012;18:697–706. doi: 10.2119/molmed.2012.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]