Abstract
Context
Methadone is an important drug in the management of both cancer-related and non-cancer-related pain and is the main pharmacologic agent used in the treatment of opioid addiction. Unexpected hypoglycemia has been observed in patients receiving methadone, prompting a more detailed investigation.
Objectives
To evaluate the incidence of hypoglycemia in a cohort of inpatients receiving methadone versus other opioids including fentanyl, hydromorphone and morphine.
Methods
Retrospective observational cohort of inpatients in a tertiary cancer center admitted for more than 48 hours from November 1, 2011 to October 30, 2013. The main outcomes were lowest measured daily blood glucose (in mg/dl) and incidence of hypoglycemia (defined as blood glucose < 70 mg/dl, equivalent to 3.9 mmol/l) with variable methadone doses compared with other non-methadone opioids.
Results
Of the 641 eligible patients admitted during the study period who received at least one dose of methadone during admission, multivariable logistic regression showed significant associations between methadone and hypoglycemia at doses greater than 40 mg oral equivalents per day or with patient-controlled analgesia (PCA) use. A dose-response relationship was observed, with an odds ratio of 3.1 (95% confidence interval 2.5, 3.6) when doses greater than 80 mg/day were used. No evidence of increased risk of hypoglycemia or of a dose-response curve was seen for the other opioids.
Conclusion
The risk of hypoglycemia is increased for patients taking more than 40 mg oral methadone equivalents per day. When starting methadone at or above 40 mg a day, we recommend routine blood glucose monitoring.
Keywords: methadone, hypoglycemia
Introduction
Methadone, an opioid agonist, can be an effective medication for severe pain when administered by experienced prescribers. For patients with liver and renal impairment, severe neuropathic pain or pain refractory to other opioids, methadone may be the preferred opioids.1 Its use in the United States has increased in the past decade, with approximately 4.4 million prescriptions written annually.2 Methadone is the main pharmacological intervention in the treatment of opioid dependence.3 Potential advantages over other opioids include low cost, high bioavailability, long half-life and lack of active metabolites. Methadone also interacts with the N-methyl-D-aspartate (NMDA) receptor, which is thought to mediate peripheral sensitization and visceral pain.4 Safety, however, has been a recurring theme in the expansion of methadone use, with problems related both to overdose and QT-prolongation with high doses.5,6,7,8
Methadone shares a similar side-effect profile with other opioids including morphine, hydromorphone and fentanyl, with serious reactions that include respiratory depression, apnea, and risk of dependency and common reactions including dizziness, sedation, nausea/vomiting and constipation. Uniquely, methadone has been shown to increase QTC in selected cases, and dosing of the medication at over 100 milligrams per day warrants periodic electrocardiogram screening.9
Previous literature has suggested an association between methadone and hypoglycemia, although no human studies have compared the effect of methadone versus other opioids on blood glucose. A case report and subsequent case series by Moryl et al. showed hypoglycemia in 19% of 59 patients during rapid escalation of methadone dose in the inpatient setting.10 Follow-up research in a mouse model showed that intravenous methadone lowered blood glucose in a dose-dependent manner, with only doses greater than 10 mg/kg significantly lowering blood glucose levels.11 Other opioids in that preclinical study included morphine, fentanyl, and oxycodone, none of which affected blood glucose levels. A similar effect has been described in the literature with tramadol and dextropropoxyphene.12, 13, 14, 15,16 The evidence to date raises a potentially serious safety concern, as even mild hypoglycemia can significantly impact patients' quality of life, and severe hypoglycemia can lead to morbidity and death through mechanisms ranging from neurological damage to cardiac arrhythmias.17, 18
We undertook a retrospective cohort study in order to test the hypothesis that methadone causes hypoglycemia in humans, quantify such risk and its relation to measured confounders, and assess for a dose-response effect. We designed the present study to evaluate the incidence of hypoglycemia in a cohort of inpatients receiving methadone versus other commonly prescribed opioids.
Methods
Design
This was a retrospective cohort study designed to test the a priori hypothesis that methadone administration is acutely associated with lowering of blood glucose and episodes of hypoglycemia defined as blood glucose < 70 mg/dl, equivalent to 3.9 mmol/l. The cohort comprised patients 18 years of age or older admitted for more than 48 hours at a tertiary cancer center between November 1, 2011 and October 30, 2013 who received at least one dose of methadone during their inpatient stay. No further exclusion criteria were applied.
The study was designed as a retrospective cohort with repeated measures of glucose levels over time, hierarchical at the patient level. The primary unit of analysis was person-days, with analysis focused on changes in blood glucose and risk of hypoglycemia over time. Patients receiving methadone commonly received other opioids during the same admission, before, during, and after methadone exposure. These exposures to other opioids were treated as covariates and included in the analysis. The major advantage of this design was to allow patients to serve as their own controls, reducing the effect of non-time-varying confounders. 19 This approach was chosen over a more conventional propensity-score matching design (in which users of methadone would have been matched to other patients with no methadone exposure) because methadone may be used in refractory pain for patients who have failed other opioids and may intrinsically be “sicker,” with higher baseline risk for hypoglycemia because of advanced disease, poor oral intake, and other factors than matched controls.
In a secondary analysis, separate cohorts of patients who were admitted and treated with monotherapy of hydromorphone, morphine, or fentanyl were examined for evidence of hypoglycemic effect. Patients were only eligible for this cohort if they received at least one dose of the opioid of interest and no doses of any other opioid. Inclusion criteria were otherwise identical to the primary cohort.
Data Collection
Exposure to methadone was defined based on electronic health records (EHR) of opioid administration and it included oral (PO), intravenous (IV) and IV delivered via patient-controlled analgesia (PCA). As conversion ratios from methadone to other opioids vary greatly, we converted morphine, hydromorphone, and fentanyl to methadone based on the well-established opioid ratios recommended by the National Comprehensive Cancer Network.20 All opioids are reported as their equivalent in mg of oral methadone throughout the manuscript.21 Opioid doses were divided into quartiles corresponding to the observed doses of daily oral methadone in this population so that dose categories were directly comparable across classes. Each person-day when the patient was on or off methadone was included in the study, so single patients may be included in multiple categories. In the primary analysis, all non-methadone opioids were analyzed as a single group. In the secondary analysis, each opioid was treated as a distinct exposure category.
Exposure to PCA was represented as a dichotomous variable as total dose administered through PCA was not recorded in the EHR. Clinician administered boluses via PCA were captured in the EHR and quantified as normal IV exposure; in such cases, patients would be classified as exposed to both a known clinician-administered IV dose and the unquantified basal and patient-administered opioid.
Outcomes
The study has two primary outcomes: lowest recorded blood glucose on a given day, and presence or absence of hypoglycemia on a given day.22 On days where glucose was not measured, hypoglycemia was assumed not to occur. In sensitivity analysis, the cohort was restricted to patients with glucose measured on over 90% of days. The values were extracted from the EHR and included both glucose reported on serum chemistry analyses and those measured by finger stick. Methadone and other opioid exposures on a given day (t) were assumed to affect blood glucose on the next day (t+1).
Statistical Analysis
Baseline covariates were: sex, age, body mass index (BMI) calculated as kg/m2, estimated glomerular filtration rate (eGFR) calculated using a Modification of Diet in Renal Disease study equation, serum albumin and admitting service. 23 Time-varying covariates were dose and route of administration of other opioids, and factors affecting blood glucose including nil per os (NPO) status, use of antidiabetic drugs, and use of steroids. In the primary multivariable analysis the three other commonly used opioids (morphine, hydromorphone, and fentanyl) were combined into one exposure category (non-methadone).
Initial descriptive analysis using admission and person-days as the units of analysis was conducted. For adjusted estimates of the effect of methadone exposure on lowest blood glucose, mixed linear modeling, hierarchical at the level of admission, was used. For adjusted estimates of methadone's effect on risk of hypoglycemia, a mixed logistic modeling, hierarchical at the level of admission, was used. For both models, for each admission, sex, age, BMI, renal function, type of admission (surgical, medical, or other) and serum albumin were forced into the model as baseline covariates. In addition, dose of non-methadone opioids, exposure to steroids, and exposure to hypoglycemic drugs were forced into the model as time-varying covariates. Additional post-hoc analysis was undertaken using random slopes models to examine how the risk of hypoglycemia changed over time before, during, and after methadone exposure. R v. 3.0.1 was used for all analyses, with the Hmisc and lme4 libraries (The R foundation, Vienna, Austria).
This retrospective study was reviewed and approved by the Memorial Sloan Kettering Cancer Center Institutional Review Board.
Results
During the two-year study period, 641 unique patients were admitted who met the study criteria of admission greater than 48 hours and who received at least one dose of methadone during admission, with a total of 1085 eligible admissions and 11,980 person-days. Of admissions, 47.0% were female; 21.7% were older than 65 years, 85.9% had a BMI greater than 20 on admission, and 86.9% had an eGFR of greater than 60 ml/min.
Of the patients with methadone exposure (7682 patient days [64%]), a majority (86.3%) also received opioids other than methadone. The interquartile range of oral methadone equivalent dosing was 15 to 80 mg/day. For over half of admissions (51.2%), glucose was measured on over 90% of days (Table 1).
Table 1. Patient characteristics by admission.
| N | ||
|---|---|---|
| Patients | 641 | |
| Number of Admissions | 1085 | |
| Patient-Days | 11980 | |
|
| ||
| Number of Admissions | (%) | |
| Male | 575 | 53.0% |
| Female | 510 | 47.0% |
| Age < 40 | 242 | 22.3% |
| Age 40-65 | 608 | 56.0% |
| Age > 65 | 235 | 21.7% |
| Medical Admission | 820 | 75.6% |
| Surgical Admission | 179 | 16.5% |
| Other Admission | 54 | 5.0% |
| BMI < 20 kg/m2 | 153 | 14.1% |
| BMI 20-25 kg/m2 | 336 | 31.0% |
| BMI 25-30 kg/m2 | 282 | 26.0% |
| BMI > 30 kg/m2 | 186 | 17.1% |
| GFR < 30 ml/min | 31 | 2.9% |
| GFR 30-60 ml/min | 101 | 9.3% |
| GFR > 60 ml/min | 943 | 86.9% |
| Albumin < 3 g/dl | 1907 | 15.9% |
| Albumin >= 3 g/dl | 8518 | 71.1% |
| Glucose Measured < 50% of Days | 115 | 10.6% |
| Glucose Measured 50-99% of Days | 494 | 45.5% |
| Glucose Measured 100% of Days | 476 | 43.9% |
| % Using Methadone | 1085 | 100.0% |
| % Using Other Opioids | 936 | 86.3% |
| Metastatic Cancer | 878 | 75.0% |
| Hematologic | 93 | 7.9% |
| Connective Tissue | 109 | 9.3% |
| Breast | 107 | 9.1% |
| Bowel | 96 | 8.2% |
| Prostate | 76 | 6.5% |
| Lung | 98 | 8.4% |
| Nonovarian Gyn | 68 | 5.8% |
| Liver | 28 | 2.4% |
| Other | 58 | 5.0% |
| Renal Disease | 87 | 7.4% |
| Major Liver Disease | 15 | 1.3% |
| Diabetes | 31 | 2.6% |
| Cardiovascular Disease | 72 | 6.2% |
Methadone PCA was used on 4.5% of patient days. Other opioids were administered by both PO and non-PCA IV routes. Non-methadone PCA was used on 42.2% of patient-days; 5.2% of patient-days were exposed to diabetes drugs, and 9.9% of person-days had documented NPO status. Also, 38.8% of patient days involved steroid exposure (Table 2).
Table 2.
Patient days on methadone versus other opioids. All opioid doses are adjusted and reported as their equivalent in oral methadone.
| Number of Patient-Days | (%) | |
|---|---|---|
| Oral Methadone 0-15 mg | 1033 | 8.6% |
| Oral Methadone 15-40 mg | 1883 | 15.7% |
| Oral Methadone 40-80 mg | 1827 | 15.3% |
| Oral Methadone > 80 mg | 1761 | 14.7% |
| IV Methadone 0-15 mg equivalent | 314 | 2.6% |
| IV Methadone 15-40 mg equivalent | 498 | 4.2% |
| IV Methadone 40-80 mg equivalent | 349 | 2.9% |
| IV Methadone > 80 mg equivalent | 345 | 2.9% |
| PCA Methadone | 542 | 4.5% |
| Oral Non-methadone Opioid 0-15 mg equivalent | 1372 | 11.5% |
| Oral Non-methadone Opioid 15-40 mg equivalent | 130 | 1.1% |
| Oral Non-methadone Opioid 40-80 mg equivalent | 43 | 0.4% |
| Oral Non-methadone Opioid > 80 mg equivalent | 71 | 0.6% |
| IV Non-methadone Opioid 0-15 mg equivalent | 2597 | 21.7% |
| IV Non-methadone Opioid 15-40 mg equivalent | 791 | 6.6% |
| IV Non-methadone Opioid 40-80 mg equivalent | 530 | 4.4% |
| IV Non-methadone Opioid > 80 mg equivalent | 1056 | 8.8% |
| PCA Non-methadone Opioid | 5060 | 42.2% |
| Transdermal Fentanyl | 1236 | 10.3% |
| Days With Diabetes Drug Exposure | 628 | 5.2% |
| Days With Steroid Exposure | 4653 | 38.8% |
| Days With NPO Order | 1181 | 9.9% |
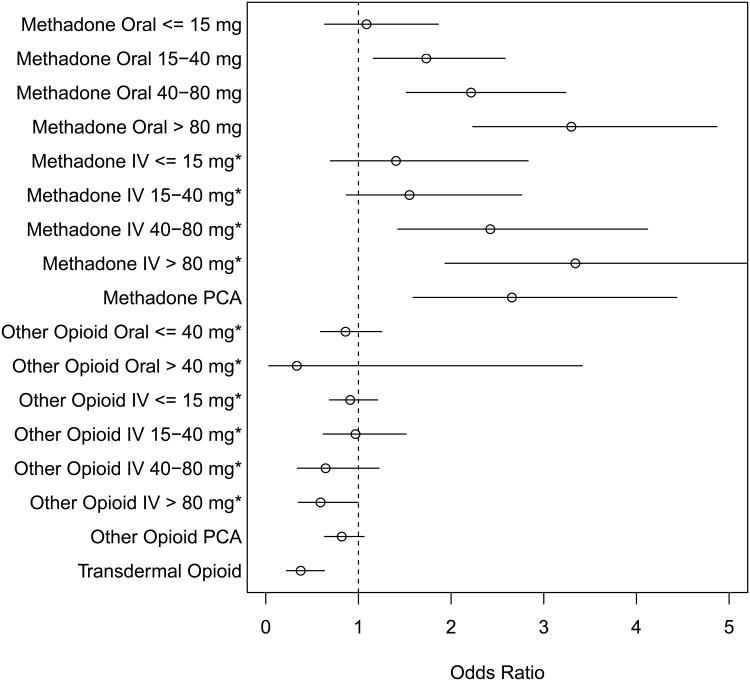
Of days without methadone exposure, 188 person-days had hypoglycemia (3.7%). Of days with methadone exposure, there were 532 patient-days (6.9%) with hypoglycemia. Of these events occurring while on methadone, 35% was to a glucose < 60 mg/dl (3.3 mmol/l), 10% was to a glucose < 50 mg/dl (2.8 mmol/l), and 3% was to a glucose < 40 mg/dl (2.2 mmol/l). Logistic multivariable regression showed a significant association between methadone and hypoglycemia, with an odds ratio (OR) of 2.2 (95% confidence interval [CI] 1.6, 2.9). An approximately linear association between hypoglycemia and methadone dose was present, with ORs of 2.9-3.1 at the highest dosing quartile and an OR of 2.9 (95% CI 1.8, 4.6) when a PCA was used. No evidence of increased risk of hypoglycemia or of a dose-response curve was seen with these same patients while exposed to the other opioids, including at daily doses exceeding the equivalent of 80 mg/day of oral methadone (Fig. 1).
Fig. 1.
Adjusted odds ratio for hypoglycemia. * denotes adjusted dose, reported as its equivalent in oral methadone. Reference category is days with no methadone or other opioid exposure.
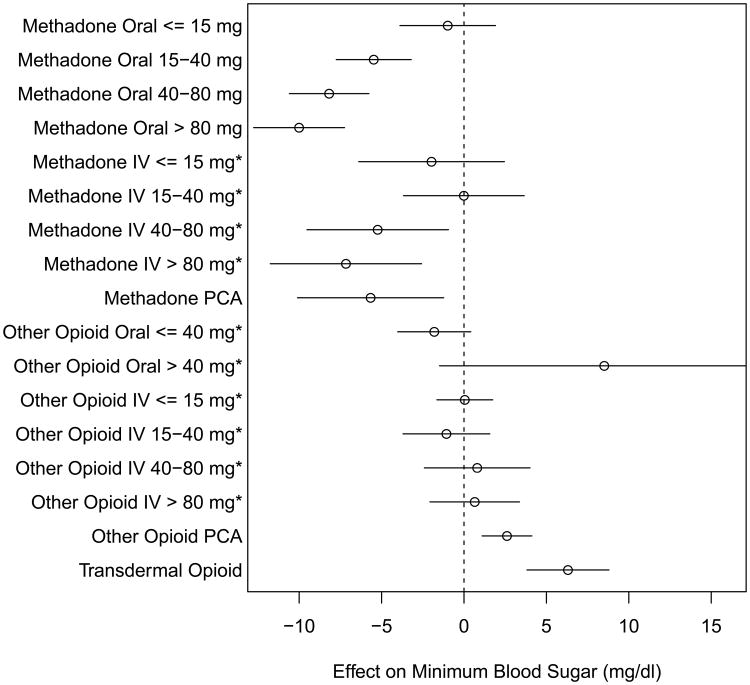
Linear multivariable regression showed methadone to be significantly associated with reduced average minimum daily blood sugar by -5.7 mg/dl (95% CI -7.3, -4.1, equivalent to mmol/l 0.31), with increasing doses associated with greater negative effect (Fig. 2).
Fig. 2.
Changes in blood glucose with time-varying exposures. * denotes adjusted dose, reported as its equivalent in oral methadone. Reference category is days with no methadone or other opioid exposure.
On secondary analysis, three separate cohorts were identified consisting of patients admitted and exposed to only one opioid: morphine (3557 admissions with 10,347 exposed patient-days), hydromorphone (4076 with 11,639 exposed patient-days), or fentanyl (8199 admissions with 2748 exposed patient-days). Consistent with the primary analysis, no association between opioid exposure and either decreased blood glucose or increased incidence of hypoglycemia was seen in any of these cohorts.
Glucose data were missing on 16.8% of patient-days overall, although 43.9% of the cohort had no missing blood glucose values. An additional 52% of patient-days had a single blood glucose measured, and the remainder had multiple measurements per day. Blood glucose in the overall cohort was missing more often on days when methadone was being administered, with an OR of missing data of 1.04 (95% CI 1.03, 1.06), while other opioids were not associated with more missing data. On sensitivity analysis, the cohort was restricted to admissions on which glucose was measured on 100% of patient days, and results were not materially affected (Supplementary Data, available at jpsmjournal.com). Of note, this restriction eliminated any association between rate of missingness and methadone exposure.
Additional sensitivity analyses were undertaken. The assumption that opioids given on one day would affect glucose levels the next day was varied, from modeling that assumed the effect would be seen on the same day as opioid administration to models that assumed the effect would be seen two days later. In additional sensitivity analysis, other opioids were treated as distinct exposure categories. Results were not materially altered in any of these analyses, except that assuming the effect of methadone would be seen on days other than t+1 attenuated the strength of the association.
On post-hoc analysis, the cohort was restricted to patients who were admitted for at least 24 hours with no methadone administration and then started on methadone. The association of methadone with reduced serum blood glucose was time-dependent and progressive when the dose of methadone exceeded 40 mg oral equivalents per day (P <0.01, linear test for trend) (Fig. 3, left upper panel). For methadone doses below 40 mg oral equivalents per day, no such relationship was present (Fig. 3, left lower panel). The increased risk of hypoglycemia was seen within 24 hours of methadone use at higher doses (P < 0.01) (Fig. 3, right upper panel). Patients who experienced hypoglycemia on methadone were at elevated risk for additional episodes while on methadone; an episode of hypoglycemia conferred an OR of 4.0 (95% CI 2.3, 7.0) for hypoglycemia the next day.
Fig. 3. Time trends with higher dose (> 40 mg/day equivalent dose, or PCA) versus lower dose (≤ 40 mg/day equivalent dose) methadone.
Discussion
This retrospective study found a strong association between methadone exposure and reduction in blood glucose and a significantly increased rate of hypoglycemia. For any methadone exposure, the per-day rate of hypoglycemia was 6.9%. The effect appears to be comparable for both IV and PO administration of methadone (Table 3). These risks exceed those commonly seen with patients receiving insulin in the inpatient setting (approximately 3.2% per day).24 A clear dose-response curve is evident, and is robust to multivariable adjustment. The association is time-dependent, occurring approximately 24 hours after initiation of methadone. Similar effects are not seen for other opioids in the same cohort of patients, suggesting that this effect may be specific to methadone.
Table 3.
Event rates in drug exposure categories including methadone, hydromorphone, morphine and fentanyl. All opioid doses are adjusted and reported as their equivalent in oral methadone.
| Methadone | Hydromorphone | |||||
|---|---|---|---|---|---|---|
| Days Exposed | % Days With Hypoglycemia | 95% CI | Days Exposed | % Days With Hypoglycemia | 95% CI | |
|
| ||||||
| Oral 0-15 mg equivalent | 1033 | 3.9 | (2.9-5.2) | 1103 | 3.8 | (2.8-5.1) |
| Oral 15-40 mg equivalent | 1883 | 4.2 | (3.4-5.2) | 72 | 4.2 | (1.4-11.5) |
| Oral 40-80 mg equivalent | 1827 | 7.4 | (6.3-8.7) | 0 | ||
| Oral > 80 equivalent | 1761 | 8.0 | (6.8-9.3) | 1 | 0.0 | (0.0-94.9) |
|
| ||||||
| IV 0-15 mg equivalent | 314 | 5.7 | (3.7-8.9) | 1995 | 5.0 | (4.1-6.1) |
| IV 15-40 mg equivalent | 498 | 5.6 | (3.9-8.0) | 562 | 5.2 | (3.6-7.3) |
| IV 40-80 mg equivalent | 349 | 12.6 | (9.5-16.5) | 188 | 6.4 | (3.7-10.8) |
| IV > 80 mg equivalent | 345 | 13.0 | (9.9-17.0) | 106 | 2.8 | (1.0-8.0) |
|
| ||||||
| PCA | 542 | 16.4 | (13.5-19.8) | 2375 | 4.7 | (3.9-5.6) |
|
| ||||||
| Morphine | Fentanyl | |||||
| Days Exposed | % Days With Hypoglycemia | 95% CI | Days Exposed | % Days With Hypoglycemia | 95% CI | |
|
| ||||||
| Oral 0-15 mg equivalent | 297 | 5.1 | (3.1-8.2) | 0 | ||
| Oral 15-40 mg equivalent | 56 | 1.8 | (0.1-9.4) | 1 | 0.0 | (0.0-94.9) |
| Oral 40-80 mg equivalent | 37 | 0.0 | (0.0-9.4) | 6 | 0.0 | (0.0-39.0) |
| Oral > 80 equivalent | 43 | 0.0 | (0.0-8.2) | 27 | 3.7 | (0.2-18.3) |
|
| ||||||
| IV 0-15 mg equivalent | 710 | 5.1 | (3.7-6.9) | 102 | 7.8 | (4.0-14.7) |
| IV 15-40 mg equivalent | 33 | 18.2 | (8.6-34.4) | 227 | 4.8 | (2.7-8.5) |
| IV 40-80 mg equivalent | 2 | 0.0 | (0.0-65.8) | 345 | 2.0 | (1.0-4.1) |
| IV > 80 mg equivalent | 0 | 948 | 2.8 | (2.0-4.1) | ||
|
| ||||||
| PCA | 575 | 5.2 | (3.7-7.4) | 2241 | 3.1 | (2.4-3.9) |
|
| ||||||
| Transdermal | 1236 | 2.3 | (1.6-3.3) | |||
These findings support the a priori hypothesis that methadone can cause hypoglycemia and shows a clinically relevant increase in rates of hypoglycemia, particularly at doses greater than 40 mg orally daily and with methadone PCA. While observational, these data suggest that serum glucose should be monitored in patients taking higher doses of methadone, and methadone use should be included in the differential diagnosis for unexplained hypoglycemia.
Opioids have previously been noted to affect endocrine systems in a wide variety of ways, including modulation of pancreatic exocrine secretion and suppression of the hypothalamic-pituitary-adrenal (HPA) axis. 25,26,27 Methadone's uniquely variable and relatively long half-life in comparison to other opioids may suggest a mechanism related to its constant effect on the HPA axis. Methadone displays antagonistic activity at the NMDA receptor and inhibits the reuptake of serotonin and norepinephrine in the central nervous system. 28,29 Possible etiologies of hypoglycemia may include suppression of counter-regulatory mechanisms or promotion of pancreatic insulin release, although additional investigation is required. Other more general possibilities include suppression of counter-regulatory mechanisms such as glucagon, epinephrine and sympathoadrenal responses to hypoglycemia.
This study has limitations. As an observational study based on data automatically extracted from a large number of EHR, we did not have access to all relevant covariates, such as functional status. The outcomes are based on glucose as measured in routine clinical care, which means that glucose was measured at a variety of times, with a variety of methods (both bedside glucometers and serum glucose measurements), without a record of whether samples represent fasting or non-fasting glucose. All of these sources of variation could potentially introduce bias. Also, glucose was not measured on all patient days, creating a potential for ascertainment bias in the primary analysis. The pattern of missing data was non-random; glucose was less often measured when methadone was used, which would bias the analysis against showing an increased risk of hypoglycemia with methadone. This suggests that the risk of hypoglycemia from methadone may be greater than reported here. Results were robust to sensitivity analysis, including restriction to admissions with no missing data.
An additional limitation introduced by the random nature of the glucose measurements and the complex real-world dosing regimens observed is that it was difficult to assess how the effect of exposure to a single dose of methadone evolved over time. The crude assumption that effects of methadone on blood sugar on a given day would be most strongly seen the next day is a limitation of this study that would be best addressed by prospective research.
In addition, while the design minimizes problems with baseline confounders, it is still vulnerable to problems with time-varying confounding. If methadone, particularly at high doses, tends be used in clinical scenarios where hypoglycemia is particularly likely, the pattern of results found here could occur in the absence of a true pharmacologic effect. The fact that these results are robust to multivariable adjustment and are not seen even at high doses of other opioids is reassuring. Finally, the retrospective design of the study does not provide evidence that the hypoglycemic events were clinically significant. However, a third of the incidents of hypoglycemia associated with methadone exposure were to a glucose of < 60 mg/dl (3.3 mmol/l), and 10% were to a glucose < 50 mg/dl (2.8 mmol/l), levels at which both autonomic nervous system activation and impairment of cognitive function are typically seen. 30
In conclusion, this observational study raises a significant and easily remedied safety concern about the use of methadone. Clinicians who prescribe methadone should be well-informed of the risk and symptoms of hypoglycemia and monitor their patients appropriately. Particularly when higher doses of methadone are used, periodic blood glucose checks may be prudent, and methadone use should be considered on the differential for unexplained hypoglycemia. Further research is warranted to verify these findings using randomized data, exploration of their implications in the outpatient setting and elucidation of possible mechanisms.
Supplementary Material
Supplementary Data: Primary analysis repeated after restriction to population of patients with no missing data. Panel 1 is odds ratio for hypoglycemia; panel 2 is change in lowest recorded serum glucose in mg/dl.
Acknowledgments
No funding was received for this study
Footnotes
Disclosures: The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brown R, Kraus C, Fleming M, Reddy S. Methadone: applied pharmacology and use as adjunctive treatment in chronic pain. Postgrad Med J. 2004;80:654–659. doi: 10.1136/pgmj.2004.022988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) Vital signs: Risk for overdose from methadone used for pain relief – United States, 1999 – 2010. MMWR Morb Mortal Wkly Rep. 2012;61:493–497. [PubMed] [Google Scholar]
- 3.Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2008;2:CD002207. doi: 10.1002/14651858.CD002207.pub3. [DOI] [PubMed] [Google Scholar]
- 4.Petrenko AB, Yamakura T, Baba H, Shimoji K. The role of N-methyl-D-aspartate (NMDA) receptors in pain: a review. Anesth Analg. 2003;97:1108–1116. doi: 10.1213/01.ANE.0000081061.12235.55. [DOI] [PubMed] [Google Scholar]
- 5.Strain EC, Stitzer ML, Liebson IA, Bigelow GE. Dose-response effects of methadone in the treatment of opioid dependence. Ann Intern Med. 1993;119:23–27. doi: 10.7326/0003-4819-119-1-199307010-00004. [DOI] [PubMed] [Google Scholar]
- 6.Pearson EC, Woosley RL. QT prolongation and torsades de pointes among methadone users: reports to the FDA spontaneous reporting system. Pharmacoepidemiol Drug Saf. 2005;14:747–753. doi: 10.1002/pds.1112. [DOI] [PubMed] [Google Scholar]
- 7.Maingi S, Moryl N, Faskowitz A. Symptomatic hypoglycemia due to escalating doses of intravenous methadone. J Pain. 2008;9(Suppl 2):37. [Google Scholar]
- 8.Ray WA, Chung CP, Murray KT, et al. Out-of-hospital mortality among patients receiving methadone for noncancer pain. JAMA Intern Med. 2015;175:420–427. doi: 10.1001/jamainternmed.2014.6294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chou R, Cruciani RA, Fiellin DA, et al. Methadone safety: a clinical practice guideline from the American Pain Society and College on Problems of Drug Dependence, in collaboration with the Heart Rhythm Society. J Pain. 2014;15:321–337. doi: 10.1016/j.jpain.2014.01.494. [DOI] [PubMed] [Google Scholar]
- 10.Moryl N, Pope J, Obbens E. Hypoglycemia during rapid methadone dose escalation. J Opioid Manag. 2013;9:29–34. doi: 10.5055/jom.2013.0144. [DOI] [PubMed] [Google Scholar]
- 11.Faskowitz AJ, Kramskiy VN, Pasternak GW. Methadone-induced hypoglycemia Cell Mol Neurobiol. 2013;33:537–542. doi: 10.1007/s10571-013-9919-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abadie D, Durrieu G, Roussin A, Montastruc JL. “Serious” adverse drug reactions with tramadol: a 2010-2011 pharmacovigilance survey in France. Therapie. 2013;68:77–84. doi: 10.2515/therapie/2013021. [DOI] [PubMed] [Google Scholar]
- 13.Mugunthan N, Davoren P. Danger of hypoglycemia due to acute tramadol poisoning. Endocr Pract. 2012;18:e151–e152. doi: 10.4158/EP12070.CR. [DOI] [PubMed] [Google Scholar]
- 14.Bourne C, Gouraud A, Daveluy A, et al. French Association of Regional Pharmacovigilance Centres. Tramadol and hypoglycaemia: comparison with other step 2 analgesic drugs. Br J Clin Pharmacol. 2013;75:1063–1067. doi: 10.1111/j.1365-2125.2012.04451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lowenstein W, Fadlallah JP, Haas C, Durand H. Hypoglycemia induced by dextropropoxyphene: an emergency in drug addicts. Presse Medicale. 1993;22:133. In French. [PubMed] [Google Scholar]
- 16.Laurent M, Gallinari C, Bonnin M, Soubrie C. Hypoglycemia induced by dextropropoxyphene in very old subjects. 7 cases. Presse Medicale. 1991;20:1628. In French. [PubMed] [Google Scholar]
- 17.Fulcher G, Singer J, Castañeda R, et al. The psychosocial and financial impact of non-severe hypoglycemic events on people with diabetes: two international surveys. J Med Econ. 2014;5:1–11. doi: 10.3111/13696998.2014.946992. [DOI] [PubMed] [Google Scholar]
- 18.Cryer PE. Mechanisms of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med. 2013;369:362–372. doi: 10.1056/NEJMra1215228. [DOI] [PubMed] [Google Scholar]
- 19.Louis TA, Lavori PW, Bailar JC, III, et al. Crossover and self-controlled designs in clinical research. N Engl J Med. 1984;310:24–31. doi: 10.1056/NEJM198401053100106. [DOI] [PubMed] [Google Scholar]
- 20.National Comprehensive Cancer Network. Practice guidelines in oncology. [Accessed November 4, 2014]; Available at: Palliative care. http://www.nccn.org/professionals/physicians_gls/PDP/palliative.pdf.
- 21.Bruera E, Pereira J, Watanabe S, et al. Opioid rotation in patients with cancer pain. A retrospective comparison of dose ratios between methadone, hydromorphone, and morphine. J Cancer. 1996;78:852–857. doi: 10.1002/(SICI)1097-0142(19960815)78:4<852::AID-CNCR23>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 22.Workgroup on Hypoglycemia, American Diabetes Association. Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care. 2005;28:1245–1249. doi: 10.2337/diacare.28.5.1245. [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group? Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 24.Flory JH, Aleman JO, Furst J, Seley JJ. Basal insulin use in the non-critical care setting: is fasting hypoglycemia inevitable or preventable? J Diabetes Sci Technol. 2014;8:427–428. doi: 10.1177/1932296813520367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chariot J, Appia F, Vaille C, Rozé C. Etorphine inhibition of pancreatic exocrine secretion in rats: comparison with methadone. Eur J Pharmacol. 1986;121:73–81. doi: 10.1016/0014-2999(86)90394-8. [DOI] [PubMed] [Google Scholar]
- 26.Aloisi AM, Buonocore M, Merlo L, et al. Chronic pain therapy and hypothalamic-pituitary-adrenal axis impairment. Psychoneuroendocrinology. 2011;36:1032–1039. doi: 10.1016/j.psyneuen.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 27.Brase DA, Singha AK, Estrada U, Lux F, Dewey WL. Hypoglycemia induced by intrathecal opioids in mice: stereospecificity, drug specificity and effect of fasting. J Pharmacol Exp Ther. 1990;253:899–904. [PubMed] [Google Scholar]
- 28.Gorman AL, Elliott KJ, Inturrisi CE. The d- and l-isomers of methadone bind to the non-competitive site on the N-methyl-D-aspartate (NMDA) receptor in rat forebrain and spinal cord. Neurosci Lett. 1997;223:5–8. doi: 10.1016/s0304-3940(97)13391-2. [DOI] [PubMed] [Google Scholar]
- 29.Codd EE, Shank RP, Schupsky JJ, Raffa RB. Serotonin and norepinephrine uptake inhibiting activity of centrally acting analgesics: structural determinants and role in antinociception. J Pharmacol Exp Ther. 1995;274:1263–1270. [PubMed] [Google Scholar]
- 30.Cryer PE. Hypoglycemia, functional brain failure, and brain death. J Clin Invest. 2007;117:868–870. doi: 10.1172/JCI31669. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data: Primary analysis repeated after restriction to population of patients with no missing data. Panel 1 is odds ratio for hypoglycemia; panel 2 is change in lowest recorded serum glucose in mg/dl.