Abstract
During embryonic development, tissues deform by a succession and combination of morphogenetic processes. Tissue compaction is the morphogenetic process by which a tissue adopts a tighter structure. Recent studies characterized the respective roles of cells’ adhesive and contractile properties in tissue compaction. In this review, we formalize the mechanical and molecular principles of tissue compaction and we analyze through the prism of this framework several morphogenetic events: the compaction of the early mouse embryo, the formation of the fly retina, the segmentation of somites and the separation of germ layers during gastrulation.
Keywords: Mechanics, Compaction, Cell adhesion, Contractility, Theory
During embryonic development or pathologies, the cohesion of cells within tissues can evolve significantly. Tissue compaction is a process by which cells increase their cohesion. During tissue compaction, cells get in closer contact with their neighbors, a process associated to the spreading of cells onto one another. Failure in compaction can result in severe pathologies, such as isolated left ventricular non-compaction cardiomyopathy [1], or developmental arrest, in particular during compaction of the mammalian embryo [2,3]. Since adhesion molecules are essential to tissue compaction [4,5], this morphogenetic process is generally described as an adhesion process that is driven by adhesive forces [6–9]. However, recent measurements challenge the idea that adhesion molecules would be able to generate sufficient forces to deform tissues [10,11]. As any tissue shape change, tissue compaction results from the combined action of intra- and inter-cellular forces, which are not solely of adhesive nature. We will describe in this review how compaction relies in fact on the adhesive and tensile properties at cells’ surface, which are controlled by the adhesion and contractile machineries of the cell. Understanding the forces involved in cell–cell interactions is therefore essential to apprehend tissue compaction beyond its molecular aspect. In this review, we initially formalize the process of compaction and then use this framework to interpret several morphogenetic processes during embryonic development that involve some degree of tissue compaction.
1. Mechanics of tissue compaction
Tissue compaction is a fundamental morphogenetic process that relies on cells mechanical interactions. This mechanical coupling between cells is primarily governed by their surface properties. The spreading of a cellular interface is hence controlled by two main properties: adhesion, which fosters interface spreading, and surface tension, which, on the contrary, promotes interface shrinkage. In fact, adhesion and tension, despite being of distinct nature, can both be counted in units of tensions. In physics, a surface tension is described as the cost of energy per unit surface (joule per square meter) or equivalently as a force per unit length (newton per meter). Adhesion is generally described as a negative surface tension, because it plays exactly the opposite role to a regular surface tension. In other words, adhesion and tension can be described in the same quantity and therefore can be added up.
In principle, the spreading of a specific interface will result from the balance of its tensile and adhesive forces. In particular, the contact angle θ characterizing the shape of a contacting interface results from the balance of the surface tensions at the contact and contact-free interfaces (Fig. 1). Along the contact, both adhesive (negative) and tensile (positive) contributions add up. This process was formalized 200 years ago by Young [12] and Dupré [13] for the spreading of a liquid droplet on a surface. The analogy of cells and tissues with liquids was proposed 100 years ago by Thompson [14]. Since then, the mathematical description of tissues using the physics of wetting, and more generally the mechanics of fluids at small scales, has been very successful in describing a variety of morphogenetic events [6,7,15–21]. This description remains valid for any homogeneous fluid interface, alive or inert [22–24]. However, contrary to their inanimate counterparts, living materials can actively control those interfacial tensions in space and time.
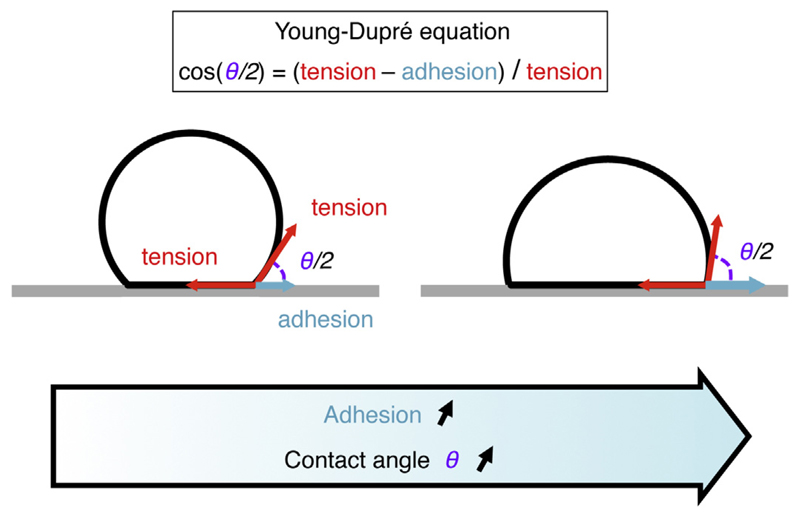
Fig. 1.
Adhesion of a droplet, vesicle or bubble to a surface. Schematic of an inert droplet, vesicle or a bubble adhering onto a surface. The adhesion can be counted in terms of tension, but it acts in the direction opposite as the surface tension along the contact. The spreading is described by the contact angle θ/2 and is governed by the Young–Dupré tension balance at the contact: cos(θ/2) = (tension – adhesion)/tension.
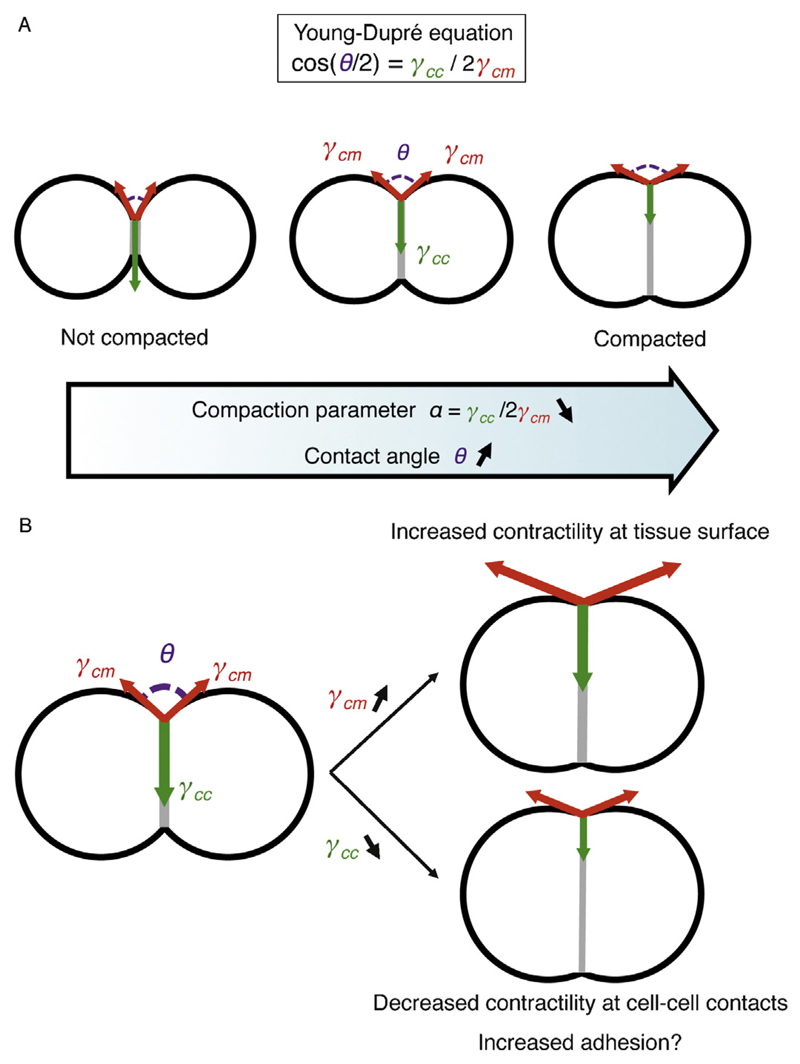
For a minimal tissue composed of two identical cells (Fig. 2), the spreading of the contacting interface depends on the surface tension γcm at the cell–medium interface of each cell and on the cell–cell interfacial tension γcc at the cell contact. In all generality, the medium surrounding the cells can be an extracellular medium or another tissue with different mechanical properties. For a given cellular interface, the Young–Dupré tension balance relates the contact angle to the cell–medium and cell–cell tensions: cos(θ/2) = γcc/2γcm (Fig. 2A). As a result, it is convenient to describe the shape of tissues by considering the geometry of contact points and contact edges within the tissue.
Fig. 2.
Mechanical control of compaction. A – Schematic of a minimal tissue from low (left) to high (right) compaction. The degree of compaction is given by the angles of contact θ (magenta), which results from the ratio of tension within the tissue γcc (green) and between the outside of the tissue and its surrounding γcm (red). Therefore, the compaction parameter α = γcc/2γcm = cos(θ/2), as given by the Young–Dupré equation, reflects both the degree of compaction and the balance of tensions in the tissue. Compaction corresponds to a decrease of the compaction parameter α. B – Compaction occurs when the tension γcm increases (top right) and/or the tension γcc decreases (bottom right). Contractility controls both γcm and γcc. Adhesion may theoretically control γcc directly, but its signaling to decrease contractility at cell–cell contacts constitutes the primary influence of adhesion to tissue compaction.
During tissue compaction, cells spread their contact onto each other while minimizing the interface of the tissue with its surrounding. Changing either of the cell–medium or cell–cell surface tensions will modify the compactness of the tissue (Fig. 2B). Reducing the interfacial tension γcc promotes contact spreading and therefore compaction. Alternatively, increasing the surface tension γcm reduces the surface of the tissue and compacts it. In other words, the tug of war between the contact and interfaces outside of this contact is what shapes the contact. We define a dimensionless parameter α = γcc/2γcm = cos(θ/2), which varies between zero and one. This parameter characterizes directly and uniquely the shape of contacts and hence the state of tissue compaction. The compaction parameter α is close to one when the tissue is not compacted: the surface tension is half the interfacial tension (γcc ∼ 2γcm). The compaction parameter is close to zero when the tissue is well compacted: the tension within the tissue is small compared to the tension at the surface of the tissue (γcc ≪ γcm). In other words, the compaction parameter α can be used as a quantitative measure of the compactness of a tissue. However, this parameter cannot tell if compaction is driven by an increase of γcm or a decrease of γcc, or both. To determine the relative contribution of those two mechanisms, it is possible to evaluate γcm, γcc or both by using laser ablation, laser manipulation, micropipette aspiration or atomic force microscopy [25–28]. Finally, to identify the actual motor of compaction, one has to characterize the molecular machinery that controls the surface tensions γcc and γcm at the cell–cell and cell–medium interfaces
2. Molecular control of tissue compaction
Several cellular components are implicated in the control of tissue interfacial tensions in space and time: the plasma membrane, the acto-myosin cortex and adhesion molecules. However, they differ in their relative contribution and surface of action.
Within the cell, the plasma membrane and associated actomyosin cortex generate and control cells interfacial tension. The plasma membrane can itself be under tension, which is generally of the order of a few to tens of piconewton per micrometer (pN/μm) [29–32]. The magnitude of the membrane tension is regulated by reservoirs of membrane, which can unfold when solicited [33,34], for example by contractility [35,36]. Although membrane tension is large enough to control some cellular behaviors, such as blebbing or polarity [32,37,38], the acto-myosin cortex associated to the plasma membrane can generate tensions up to a hundred times higher than that of the plasma membrane [39,40]. The cortex tension, which is typically on the order of tens to thousands of pN/μm [27,28,38,39,41,42], is therefore considered to govern primarily the tension of cellular interfaces [43]. In other words, the forces of the membrane can generally be ignored compared to those of the cortex, as far as tissue compaction is concerned. The cortex is composed of cross-linked actin filaments bound to the plasma membrane [44,45]. The membrane–cortex attachment can be mediated by ERM (Ezrin–Radixin–Moesin) proteins [32,46], Myo1 [31,32] and/or, as recently proposed, the cadherin adhesion complex [47,48]. Myosin motors, and most generally non-muscle myosin 2, pull on this network and thereby generate a tension that is transmitted to the cell surface. The magnitude of the tension generated by the acto-myosin cortex is expected to depend on the density and turnover of the network as well as on the number and activity of motor proteins [43,49]. These molecular properties are regulated by signaling pathways, that often converge to Rho-GTPases [38,44,50] and the phosphorylation of the myosin regulatory light-chain [51–53] and heavy chain [54]. In summary, the acto-myosin cortex generates large forces that can pull on cell contacts and deform them. In this way, a tissue would compact because cells pull themselves into a tighter structure.
Adhesion molecules contribute negatively to interfacial tensions between cells and thus could promote interface spreading. Adhesion molecules bind to extra-cellular matrices or to adhesion molecules on the surface of neighboring cells [55]. Therefore, unlike contractility, adhesion molecules may only exert tensions at adhesive contacts. The magnitude of the adhesion tension that could be generated by adhesion molecules remains poorly characterized. However, it was estimated that the contribution of adhesion to cells interfacial tension is minor compared to the tension generated by the acto-myosin cortex [10,11]. It is difficult, however, to specifically manipulate adhesion tension because changing the number, or the activity of adhesion molecules has additional indirect effects. Indeed, adhesion molecules, such as cadherins or integrins, anchor the acto-myosin cytoskeleton of the cell to its environment [10,56,57] and act as surface receptors that signal to the cytoskeleton [58–60]. Therefore, adhesion molecules can indirectly control interfacial tension via the acto-myosin cytoskeleton by modulating force transmission to the cells’ surroundings (adhesion coupling) and/or by changing its activity (adhesion signaling) [61]. In summary, to deform cell contacts, adhesion molecules can have a more significant effect by acting indirectly than by directly generating adhesive forces [61,62].
By modulating acto-myosin contractility and adhesion molecule activity, tissues will modify their compaction state. Acto-myosin directly generates large tensions that can be controlled in a cell-autonomous and interface-specific fashion. Adhesion molecules, among other signaling conduits, can modulate contractility and can indirectly control tension in a non-cell autonomous way.
Using the mechanical framework presented above, we present an analysis of tissue compaction processes observed during embryonic development: from the simple observation of the geometry of cell–cell interfaces, one can conjecture, based on our framework, the relative value of tensions between interfaces within the tissue. This type of inverse problem approach, called force inference methods, was recently developed on a rigorous mathematical basis to deduce the relative interfacial tensions between cells from the sole analysis of their 2-dimensional shape in epithelial tissues [65–69]. Here we develop a more naïve approach and argue that, together with the localization of adhesive and contractile molecules, the simple analysis framework above is often sufficient to develop a good understanding of how a morphogenetic event is driven. Functional biological tests can then be accurately designed to evaluate the working hypothesis. Using this mechanical framework, in the following sections, we will describe examples of morphogenetic events occurring during embryonic development for which some geometrical, mechanical and/or molecular data are available. We will start with the simplest case: the compaction of the 8-cell stage mouse embryo during which the whole embryo increases its compactness. We will then discuss the formation of the fly ommatidium in which a cluster of four epithelial cells compacts within its surrounding tissue, before moving to somitogenesis during which thousands of cells separate into a compact block of heterogeneous tissue. Finally, we will extend our analysis to the more complex case of gastrulation, when the germ layers change their compactness while they segregate from one another and interact with extra-embryonic tissues and the extra-cellular matrix.
3. Compaction of the mouse embryo
The most emblematic example of tissue compaction during embryonic development takes place at the beginning of mammalian development [70]. Formally denominated “compaction”, this developmental process consists in the spreading of the blastomeres onto each other. During the 8-cell stage of the mouse embryo, the compaction parameter α drops from ∼0.7 to ∼0.2 transforming a grape-like cluster of cells into a mulberry-like ball (Figure 2A) [27,71]. This gave the name “uvomorulin” (from the latin uvo meaning “grape” and morula for “mulberry”) to the cell–cell adhesion molecule now called Cdh1 (for Cadherin1, also known as E-cadherin) that is required for compaction to occur [4,72,73]. It was therefore generally assumed that increased expression or post-translational modifications of Cdh1 increases cell–cell adhesion and drives compaction [2,3,7,9,74]. Interestingly, it was shown that an intact actin cytoskeleton is also required for compaction to occur [75]. So far, the role of actin in the early mouse embryo was linked to its binding to adhesion molecules and to the establishment of apico-basal polarity [76]. Cell polarization also occurs during the 8-cell stage but it is not required for compaction [77] or vice versa [78]. In this historical view, compaction is driven by the adhesion complex increasing cells’ “stickiness” (Fig. 2B).
Recently, two new studies have proposed alternative mechanisms for compaction involving both actin and cadherin. In a first study [79], Fierro-González, White et al. observe thick adhesive filopodia growing at the surface of a subset of the blastomeres of the 8-cell stage mouse embryo. After qualitatively probing cells tension using laser ablation of cells surface, they propose that adhesive filopodia exert pulling forces on neighboring cells, and that these pulling forces compact the embryo. In a second study [27], Maître et al. (authors of this review) use non-invasive micropipette aspiration to quantitatively measure all blastomeres’ interfacial tensions in space and time. The authors find a two-fold increase of the cell–medium surface tension γcm and a 1/3rd decrease of the cell–cell interfacial tension γcc during compaction. Using the mechanical framework presented above, the authors predict that 3/4th of compaction is explained by the increase in cell–medium surface tension, while 1/4th only can be attributed to the decrease of tension at cell–cell contacts.
Both studies report that tension at the surface of the embryo is larger than at cell–cell contacts. Therefore, both studies conclude that, contrary to previous hypotheses, adhesive forces are not the only or the main driver of compaction. However, while contractility is a cell-autonomous force generator, adhesive filopodia are a non-autonomous process as they operate by pulling on neighboring cells. When measuring the tension of cells genetically devoid of Cdh1 or mechanically isolated from the embryo, both being unable to grow filopodia to pull on their neighbors, Maître et al. find intact surface tensions, indicating that tension generation is cell-autonomous. Moreover, the knock-down of Myosin 10, which is essential for filopodia formation [80], in one half of the embryo presented in Fierro-González, White et al. shows that the healthy half of the embryo does not seem to rescue the injected half, which supports a cell-autonomous mechanism, in contrast with the authors conclusions. Therefore, cell-autonomous acto-myosin contractility is the most realistic force generator for embryo compaction. A putative role of adhesive filopodia might be to provide adhesion signaling, which is essential for mouse embryo blastomeres to survive [81].
The mechanism by which contractility decreases the compaction parameter α is by modulating its localization and/or activity between different interfaces. During compaction, actomyosin accumulates at the surface of the embryo while it clears from cell–cell contacts [27]. In this view, the embryo compacts by forming an effective contractile shell at its surface (Fig. 2B). If the increase in contractility is cell-autonomous, the decrease of contractility requires Cdh1 to signal at cell–cell contacts, as indicated by ectopic accumulation of acto-myosin at cell–cell contacts of maternal zygotic Cdh1 knock-out embryos [3,27]. Therefore, Cdh1 is required for compaction to occur, in agreement with previous studies [2–5,73], but, contrary to previous conclusions, its role is not to directly generate forces but to clear acto-myosin away from contacts to facilitate the work of contractile forces generated at the cell–medium interface [27,58,59]. Another function of adhesion molecules, which was not investigated in the compacting embryo may be to transmit the contractile forces across the cell–cell contact [10,56,82]. The cell-autonomous signal that triggers the increase in contractility in each blastomere remains unknown and will surely be the focus of future studies.
4. Ommatidium
During morphogenesis of the fly retina, cells group together to form hundreds of ommatidia, the photoreceptive units of the compound eye. Each ommatidium is composed of eight epithelial cells. At the level of their apical adherens junction belt, the cells within an ommatidium compact distinctly, which is essential for retinal function (Fig. 3B). The cone cells in the center of the ommatidium strongly compact (α ∼ 0.3) and minimize their contact to the surrounding pigment cells, which, in a way, act like the surrounding medium in the compacting mouse embryo. Careful analysis of cell shape in combination with powerful genetics described how differences in cadherin expressions control the geometry of the ommatidium [83]. The quantitative data allowed for modeling and simulation of ommatidium morphogenesis with great accuracy [17]. The model predicted the tensions of each interface, which, however, remain to be experimentally measured. Interestingly, to faithfully simulate ommatidium compaction, the model must consider not only the differences in cadherin expression but also cells’ contractility. Although the role of myosin was investigated during the initial formation of the cell cluster that eventually constitutes the ommatidium [84], little is known about contractility within the maturing ommatidium. Therefore, it is unclear whether differences in cadherin expression directly translate into different adhesive forces sufficient to drive compaction of the cone cells or, alternatively, if the role of cadherins is to control interfacial tension indirectly via signaling to contractile elements, like during mouse embryo compaction.
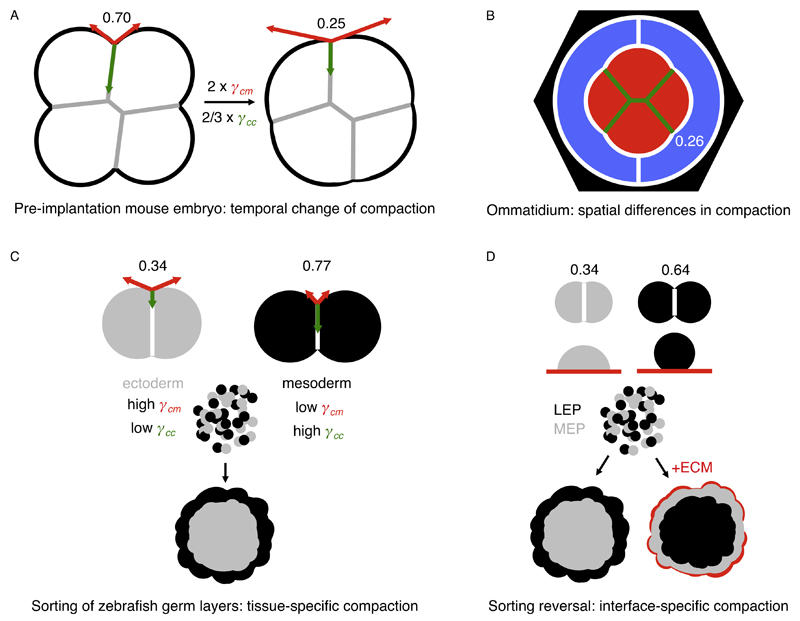
Fig. 3.
Examples of homogeneous and heterogeneous tissue compaction processes. In these examples, the compaction parameter could be calculated from published quantitative data. The compaction parameter is written next to the corresponding contacting interface. A – Schematic of a compacting mouse embryo. During the 8-cell stage, the compaction parameter decreases homogeneously as the tension at the cell–medium interface (γcm, red) doubles and the tension at the cell–cell contact (γcc, green) decreases by 1/3. These temporal changes are primarily controlled by contractility, not adhesion [27]. B – Schematic of an ommatidium of a drosophila retina. During ommatidium morphogenesis, the primary pigment cells (blue), expressing Cdh1 only, surround the cone cells (red), which express both Cdh1 and Cdh2. Interfaces containing Cdh2 (green) have a lower tension than those with Cdh1 only (white), as suggested by the compaction parameters [17]. Additional pigments cells are in black. C – Schematic of ectoderm and mesoderm homotypic doublets (top) and of a sorting experiment of ectoderm and mesoderm germ layers (bottom). During gastrulation, ectoderm and mesoderm compact differently due to their differences in tension both at the cell–medium and cell–cell interfaces [10,28]. This is primarily controlled by contractility, not adhesion [10]. When mixed, ectoderm and mesoderm progenitors sort out so that the tissue of highest compaction (ectoderm) is enveloped by the tissue of lowest compaction (mesoderm) [28]. D – Schematic of luminal (LEP, black) and myoepithelial (MEP, gray) mammary gland homotypic doublets (top), of individual LEP and MEP cells on extracellular matrix (ECM, red) and of a sorting experiment with or without external ECM. MEP cells are more compact than LEP cells, causing MEP cells to sort to the inside when mixed with LEP cells. This is the opposite configuration to the one observed in vivo and modifying cell–cell adhesion molecules expression does not explain this sorting reversal [111,112]. Unlike LEP cells, MEP cells adhere to ECM. In the presence of ECM around the cell aggregate during the sorting experiment, MEP cells stick to the ECM and remain on the outside while LEP cells now sort to the inside [107].
5. Somitogenesis
During gastrulation, the lateral mesoderm segments itself into blocks of tissue called somites. Somites form one after the other during body elongation when groups of cells separate into compact blocks of tissue. With the development of live-reporters [85,86], major advances were made in our understanding of the periodic specification of the pre-somitic mesoderm. This has led to comprehensive quantitative models of somitogenesis [87,88]. However, little is understood, in comparison, about the final step of somitogenesis: the segmentation of a new somite block, since the cellular processes controlling the segmentation need further characterization.
During somitogenesis, the cells at the nascent boundary of somites change their surface proteins composition, notably their adhesion molecules [89]. In chicken embryos, N-CAM (Neural Cell Adhesion Molecule), an adhesion molecule of the immunoglobulin super-family [90,91], becomes restricted to cells present at the surface of the segmenting somite, whereas cells within the somite express Cdh2 (also known as N-Cadherin) [92]. At the same time, EphA4, of the ephrin receptor family of receptor tyrosine kinase, becomes restricted to the anterior part of the somite where it could interact with the ligand ephrinB2 present at the posterior part of the somite [93]. This molecular redefinition of the tissue has been proposed to modify the tensions of the forming somite with surrounding tissues, in particular with the pre-somitic mesoderm [94]. Surface energy minimization, which is, to some conditions, equivalent to considering the balance of surface tensions at cell edges, is sufficient to generate a new compact somite block in silico [88,94]. From a mechanical point of view it is uncertain that N-CAM or ephrin localization at the surface of the forming somite may directly generate forces rounding up the somite. Instead, signaling to the acto-myosin cytoskeleton may once again be a better candidate for regulating the forces compacting new somites. For example, the presence of ephrin receptors at the anterior and posterior sides of the forming somites may regulate the local contractility [95], like for germ layer separation in amphibian embryos. This could reduce the contacting surface between the forming somite and the pre-somitic mesoderm. However, myosin localization has not been investigated enough during somite formation. At this stage, too little is known to distinguish among the initial mechanical models of somite formation and contractility-dependent rounding [88,94,96]. Quantitative measurements of tissues mechanical properties, together with careful temporal analysis of tissue remodeling and contractile elements localization, may help us understand how somites form.
6. Gastrulation
Gastrulation is the process by which the three germ layers acquire their distinct molecular signatures and position themselves within the body to form all somatic tissues. During fly, amphibian, fish or mouse gastrulation, the mesoderm becomes less compact than the ectoderm (whether it is an epithelium or not). In vitro, mixing these cells results in self-organized sorting into distinct layers with the most compact tissue positioned at the center of the cell mass [28,97]. The understanding of cell sorting and of its hierarchy greatly benefited from the original hypotheses from Holtfreter [98] and from the pioneering work and idea of Steinberg, who formulated 50 years ago the differential adhesion hypothesis [15]. During his career, Steinberg showed and measured that tissues have an effective tissue-scale surface tension that controls their relative positioning when they are mixed [20]. For example, as measured both in zebrafish and frog embryos, the more compact ectoderm shows a higher tissue surface tension than mesoderm [99,100]. This causes the ectoderm to be surrounded by mesoderm when the two different cell types or tissues are mixed. However, the tissue surface tension and compactness does not necessarily correlates with the number of adhesion molecules, as earlier proposed by Steinberg [7,101]. On the contrary, in the zebrafish embryo, for instance, ectoderm, the most compact tissue, expresses less cadherin adhesion molecules than the comparably less compact mesoderm tissue (Fig. 3C) [28]. Instead, the higher contractility of ectoderm compared to mesoderm cells is directly responsible for the difference in tissue surface tension, compaction and sorting [10,28]. Regardless of the levels of expression of cadherins, the contribution of adhesion forces to the compaction of ectoderm or mesoderm tissues was found negligible when compared to that of contractility [10]. How adhesion molecules modulate contractility remains to be elucidated in zebrafish embryos. In amphibians, adhesion signaling is mediated by the paraxial proto-cadherin (PAPC or protocadherin-8) and ephrin receptors, which regulate contractility at cell–cell contacts [95] and tissue surface tension [102]. Throughout decades of quantitative experimental characterization of cell sorting, theoretical modeling supported and tested the different mechanisms that were proposed. Numerical simulations validated the idea that either an increase of cells adhesiveness [103] or more generally a differential of interfacial tensions involving cells contractility [18,28] could, in theory, control tissue compaction and sorting. Only by measuring the relative contribution of adhesive and contractile forces can we distinguish between the two theoretically plausible mechanisms.
Although germ layer progenitors robustly sort from one another when mixed, it remains unclear whether this is the mechanism by which germ layers actually separate during normal development [104]. In fact, the configuration obtained after cell sorting in vitro, with ectoderm enveloped by mesoderm, is opposite to the situation in vivo. The presence of additional interfaces could control the sorting direction. In zebrafish for example, additional interfaces may be extra-embryonic tissues such as the yolk syncytium to which mesoderm cells adhere [105] or the enveloping layer [106]. Recently, extra-cellular matrix (ECM) has been used to invert the sorting configuration of luminal and myoepithelial mammary gland cells [107]. As mentioned earlier, when cells with different compaction parameters are mixed, the most compacted tissue (the myoepithelial cells here) consistently adopts the inner position, enveloped by less compacted tissues (the luminal cells here). However, adding ECM outside the aggregate can reverse this organization because only the myoepithelial cells adhere significantly to the ECM (Fig. 2D) [107]. Similar mechanisms by which additional interfaces control the direction of sorting could apply during gastrulation. In fact, adding such interfaces to isolated germ layers changes their surface tensions and shape [108]. Future studies will help understanding whether ECM and extra-embryonic tissues provide signaling and/or mechanical scaffolds to correctly arrange the germ layers.
7. Conclusion
Because tissue compaction is a developmentally regulated adhesion process, great efforts were made to characterize the adhesion molecules required for tissue compaction to occur. However, recent studies now emphasize the essential role of contractility in controlling the interfacial tensions driving tissue compaction. This conceptually adjusts our vision of tissue compaction, which is not necessarily a process of increased adhesiveness between cells but rather of differential interfacial contractility. In this case, adhesion molecules act as signaling and mechanical scaffold molecules, rather than force generators. How adhesion molecules instruct contractility to act at different interfaces remains to be elucidated in most systems. Several pathways, both in cell culture [59] and developing animals [19,58,109,110], may constitute good candidates for future studies. This will be key to understand how morphogenetic events are orchestrated during development.
Glossary.
Surface or interfacial tension: the energy required to decrease by a unit area a given surface (or interface). Similar as to liquids, surface tension gives cells and tissues an apparent stiffness, which resists mechanical stresses normal to the surface. Tensions are measured in newton per meter.
Cortex tension: contribution of the acto-myosin cortex to the interfacial tension. It is specific to one cell in a cell–cell contact.
Membrane tension: contribution of the plasma membrane to the interfacial tension. It is specific to one cell in a cell–cell contact.
Adhesion: process by which cells spread and stick to their surrounding.
Adhesion tension: negative tension arising from the binding of adhesion molecules (in principle, proportional to the binding energy times their surface density). Unlike cortex and membrane tension, it is associated to both contacting interfaces. Unlike adhesion coupling, adhesion tension acts only parallel to the contact surface.
Adhesion coupling: mechanical resistance of adhesion molecules (on the cell surface and cytoplasm) to forces that would detach cells from their adhesion site.
Adhesion signaling: biochemical changes resulting from the signaling of adhesion molecules upon binding.
Compaction parameter: dimensionless number describing both the shape of a tissue and the balance of forces shaping this tissue. When approaching 1, the compaction parameter describes cells with little cohesion and equivalent tensions on all their interfaces. When nearing 0, the compaction parameter reflects cohesive tissues with high surface tension with its surrounding.
Wetting: describes the extent of spreading of a liquid material on a surface. The wetting is partial when the contact angle θ is below 180° (like a mercury droplet on glass) and the wetting is complete when θ is above 180° (like a water droplet on glass). During tissue compaction, the contact angle θ remains below 180°. Complete wetting is typically reached when cells spread on a surface using protrusions such as lamellipodia [63,64], for which the framework presented here is not suited.
Acknowledgements
We are grateful to T. Hiiragi for reading earlier versions of this review. H. T. and J.-L. M. are supported by Marie Curie Actions Intra-European Fellowships. H. T. acknowledges support from the Young Researcher Prize of the Bettencourt-Schueller Foundation.
References
- [1].Chen H, Zhang W, Sun X, Yoshimoto M, Chen Z, Zhu W, et al. Fkbp1a controls ventricular myocardium trabeculation and compaction by regulating endocardial Notch1 activity. Development. 2013;140:1946–57. doi: 10.1242/dev.089920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Larue L, Ohsugi M, Hirchenhain J, Kemler R. E-cadherin null mutant embryos fail to form a trophectoderm epithelium. Proc Natl Acad Sci U S A. 1994;91:8263–7. doi: 10.1073/pnas.91.17.8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Stephenson RO, Yamanaka Y, Rossant J. Disorganized epithelial polarity and excess trophectoderm cell fate in preimplantation embryos lacking E-cadherin. Development. 2010;137:3383–91. doi: 10.1242/dev.050195. [DOI] [PubMed] [Google Scholar]
- [4].Shirayoshi Y, Okada TS, Takeichi M. The calcium-dependent cell–cell adhesion system regulates inner cell mass formation and cell surface polarization in early mouse development. Cell. 1983;35:631–8. doi: 10.1016/0092-8674(83)90095-8. [DOI] [PubMed] [Google Scholar]
- [5].Hyafil F, Morello D, Babinet C, Jacob F. A cell surface glycoprotein involved in the compaction of embryonal carcinoma cells and cleavage stage embryos. Cell. 1980;21:927–34. doi: 10.1016/0092-8674(80)90456-0. [DOI] [PubMed] [Google Scholar]
- [6].Steinberg M. Adhesion in development: an historical overview. Dev Biol. 1996;180:377–88. doi: 10.1006/dbio.1996.0312. [DOI] [PubMed] [Google Scholar]
- [7].Steinberg MS, Takeichi M. Experimental specification of cell sorting, tissue spreading, and specific spatial patterning by quantitative differences in cadherin expression. Proc Natl Acad Sci U S A. 1994;91:206–9. doi: 10.1073/pnas.91.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wolpert L. Principles of Development. Oxford University Press; 2011. [Google Scholar]
- [9].Fleming TP, Sheth B, Fesenko I. Cell adhesion in the preimplantation mammalian embryo and its role in trophectoderm differentiation and blastocyst morphogenesis. Front Biosci. 2001;6:D1000–7. doi: 10.2741/fleming. [DOI] [PubMed] [Google Scholar]
- [10].Maître J-L, Berthoumieux H, Krens SFG, Salbreux G, Jülicher F, Paluch E, et al. Adhesion functions in cell sorting by mechanically coupling the cortices of adhering cells. Science. 2012;338:253–6. doi: 10.1126/science.1225399. [DOI] [PubMed] [Google Scholar]
- [11].Stirbat TV, Mgharbel A, Bodennec S, Ferri K, Mertani HC, Rieu J-P, et al. Fine tuning of tissues’ viscosity and surface tension through contractility suggests a new role for α-catenin. PLOS ONE. 2013;8:e52554. doi: 10.1371/journal.pone.0052554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Young T. An essay on the cohesion of fluids. Philos Trans R Soc Lond. 1805:65–87. [Google Scholar]
- [13].Dupré A, Dupré P. In: Théorie mécanique de la chaleur. Dupré Athanase, Dupré Paul., editors. Google Livres; 1869. [Google Scholar]
- [14].Thompson DW. On Growth and Form. Cambridge University Press; 1917. [Google Scholar]
- [15].Steinberg MS. Reconstruction of tissues by dissociated cells. Some morphogenetic tissue movements and the sorting out of embryonic cells may have a common explanation. Science. 1963;141:401–8. doi: 10.1126/science.141.3579.401. [DOI] [PubMed] [Google Scholar]
- [16].Farhadifar R, Röper J-C, Aigouy B, Eaton S, Jülicher F. The influence of cell mechanics, cell–cell interactions, and proliferation on epithelial packing. Curr Biol. 2007;17:2095–104. doi: 10.1016/j.cub.2007.11.049. [DOI] [PubMed] [Google Scholar]
- [17].Kafer J, Hayashi T, Maree A, Carthew R, Graner F. Cell adhesion and cortex contractility determine cell patterning in the Drosophila retina. Proc Natl Acad Sci U S A. 2007;104:18549–54. doi: 10.1073/pnas.0704235104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Brodland G. The Differential Interfacial Tension Hypothesis (DITH): a comprehensive theory for the self-rearrangement of embryonic cells and tissues. J Biomech Eng. 2002;124:188–97. doi: 10.1115/1.1449491. [DOI] [PubMed] [Google Scholar]
- [19].Bosveld F, Bonnet I, Guirao B, Tlili S, Wang Z, Petitalot A, et al. Mechanical control of morphogenesis by Fat/Dachsous/Four-jointed planar cell polarity pathway. Science. 2012;336:724–7. doi: 10.1126/science.1221071. [DOI] [PubMed] [Google Scholar]
- [20].Foty RA, Pfleger CM, Forgacs G, Steinberg MS. Surface tensions of embryonic tissues predict their mutual envelopment behavior. Development. 1996;122:1611–20. doi: 10.1242/dev.122.5.1611. [DOI] [PubMed] [Google Scholar]
- [21].Behrndt M, Salbreux G, Campinho P, Hauschild R, Oswald F, Roensch J, et al. Forces driving epithelial spreading in zebrafish gastrulation. Science. 2012;338:257–60. doi: 10.1126/science.1224143. [DOI] [PubMed] [Google Scholar]
- [22].Saye RI, Sethian JA. Multiscale modeling of membrane rearrangement, drainage, and rupture in evolving foams. Science. 2013;340:720–4. doi: 10.1126/science.1230623. [DOI] [PubMed] [Google Scholar]
- [23].Cira NJ, Benusiglio A, Prakash M. Vapour-mediated sensing and motility in two-component droplets. Nature. 2015:1–15. doi: 10.1038/nature14272. [DOI] [PubMed] [Google Scholar]
- [24].de Gennes P-G, Brochard-Wyart F, Quéré D. Capillarity and Wetting Phenomena: Drops, Bubbles, Pearls, Waves. Pierre-Gilles de Gennes, Francoise Brochard-Wyart, David Quere. Google Books; 2004. [Google Scholar]
- [25].Bambardekar K, Clément R, Blanc O, Chardès C, Lenne P-F. Direct laser manipulation reveals the mechanics of cell contacts in vivo. Proc Natl Acad Sci U S A. 2015 doi: 10.1073/pnas.1418732112. 201418732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rauzi M, Verant P, Lecuit T, Lenne P-F. Nature and anisotropy of cortical forces orienting Drosophila tissue morphogenesis. Nat Cell Biol. 2008;10:1401–10. doi: 10.1038/ncb1798. [DOI] [PubMed] [Google Scholar]
- [27].Maître J-L, Niwayama R, Turlier H, Nédélec F, Hiiragi T. Pulsatile cell-autonomous contractility drives compaction in the mouse embryo. Nat Cell Biol. 2015;17:849–55. doi: 10.1038/ncb3185. [DOI] [PubMed] [Google Scholar]
- [28].Krieg M, Arboleda-Estudillo Y, Puech P, Kafer J, Graner F, Muller D, et al. Tensile forces govern germ-layer organization in zebrafish. Nat Cell Biol. 2008;10:429–36. doi: 10.1038/ncb1705. [DOI] [PubMed] [Google Scholar]
- [29].Dai J, Sheetz M. Membrane tether formation from blebbing cells. Biophys J. 1999;77:3363–70. doi: 10.1016/S0006-3495(99)77168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hochmuth R, Marcus W. Membrane tethers formed from blood cells with available area and determination of their adhesion energy. Biophys J. 2002;82:2964–9. doi: 10.1016/S0006-3495(02)75637-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Nambiar R, McConnell RE, Tyska MJ. Control of cell membrane tension by myosin-I. Proc Natl Acad Sci U S A. 2009;106:11972–7. doi: 10.1073/pnas.0901641106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Diz-Muñoz A, Krieg M, Bergert M, Ibarlucea-Benitez I, Muller DJ, Paluch E, et al. Control of directed cell migration in vivo by membrane-to-cortex attachment. PLoS Biol. 2010;8:e1000544. doi: 10.1371/journal.pbio.1000544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Raucher D, Sheetz M. Characteristics of a membrane reservoir buffering membrane tension. Biophys J. 1999;77:1992–2002. doi: 10.1016/S0006-3495(99)77040-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sinha B, Köster D, Ruez R, Gonnord P, Bastiani M, Abankwa D, et al. Cells respond to mechanical stress by rapid disassembly of caveolae. Cell. 2011;144:402–13. doi: 10.1016/j.cell.2010.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Gauthier NC, Fardin M-A, Roca-Cusachs P, Sheetz MP. Temporary increase in plasma membrane tension coordinates the activation of exocytosis and contraction during cell spreading. Proc Natl Acad Sci U S A. 2011;108:14467–72. doi: 10.1073/pnas.1105845108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lieber AD, Yehudai-Resheff S, Barnhart EL, Theriot JA, Keren K. Membrane tension in rapidly moving cells is determined by cytoskeletal forces. Curr Biol. 2013;23(15):1409–17. doi: 10.1016/j.cub.2013.05.063. [DOI] [PubMed] [Google Scholar]
- [37].Houk AR, Jilkine A, Mejean CO, Boltyanskiy R, Dufresne ER, Angenent SB, et al. Membrane tension maintains cell polarity by confining signals to the leading edge during neutrophil migration. Cell. 2012;148:175–88. doi: 10.1016/j.cell.2011.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Tinevez J-Y, Schulze U, Salbreux G, Roensch J, Joanny J-F, Paluch E. Role of cortical tension in bleb growth. Proc Natl Acad Sci U S A. 2009;106:18581–6. doi: 10.1073/pnas.0903353106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Dai J, Ting-Beall H, Hochmuth R, Sheetz M, Titus M. Myosin I contributes to the generation of resting cortical tension. Biophys J. 1999;77:1168–76. doi: 10.1016/s0006-3495(99)76968-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Larson SM, Lee HJ, Hung P-H, Matthews LM, Robinson DN, Evans JP. Cortical mechanics and meiosis II completion in mammalian oocytes are mediated by myosin-II and Ezrin-Radixin-Moesin (ERM) proteins. Mol Biol Cell. 2010;21:3182–92. doi: 10.1091/mbc.E10-01-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Evans JP, Robinson DN. The spatial and mechanical challenges of female meiosis. Mol Reprod Dev. 2011;78:769–77. doi: 10.1002/mrd.21358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Chaigne A, Campillo C, Gov NS, Voituriez R, Azoury J, Umaña-Diaz C, et al. A soft cortex is essential for asymmetric spindle positioning in mouse oocytes. Nat Cell Biol. 2013;15:958–66. doi: 10.1038/ncb2799. [DOI] [PubMed] [Google Scholar]
- [43].Salbreux G, Charras G, Paluch E. Actin cortex mechanics and cellular morphogenesis. Trends Cell Biol. 2012;22:536–45. doi: 10.1016/j.tcb.2012.07.001. [DOI] [PubMed] [Google Scholar]
- [44].Biro M, Romeo Y, Kroschwald S, Bovellan M, Boden A, Tcherkezian J, et al. Cell cortex composition and homeostasis resolved by integrating proteomics and quantitative imaging. Cytoskeleton. 2013;70:741–54. doi: 10.1002/cm.21142. [DOI] [PubMed] [Google Scholar]
- [45].Morone N, Fujiwara T, Murase K, Kasai RS, Ike H, Yuasa S, et al. Three-dimensional reconstruction of the membrane skeleton at the plasma membrane interface by electron tomography. J Cell Biol. 2006;174(6):851–62. doi: 10.1083/jcb.200606007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kunda P, Pelling A, Liu T, Baum B. Moesin controls cortical rigidity, cell rounding, and spindle morphogenesis during mitosis. Curr Biol. 2008;18:91–101. doi: 10.1016/j.cub.2007.12.051. [DOI] [PubMed] [Google Scholar]
- [47].Borghi N, Sorokina M, Shcherbakova OG, Weis WI, Pruitt BL, Nelson WJ, et al. E-cadherin is under constitutive actomyosin-generated tension that is increased at cell–cell contacts upon externally applied stretch. Proc Natl Acad Sci U S A. 2012;109:12568–73. doi: 10.1073/pnas.1204390109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Schepis A, Sepich D, Nelson WJ. αE-catenin regulates cell–cell adhesion and membrane blebbing during zebrafish epiboly. Development. 2012;139:537–46. doi: 10.1242/dev.073932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Clark AG, Wartlick O, Salbreux G, Paluch EK. Stresses at the cell surface during animal cell review morphogenesis. Curr Biol. 2014;24:R484–94. doi: 10.1016/j.cub.2014.03.059. [DOI] [PubMed] [Google Scholar]
- [50].Mayer M, Depken M, Bois JS, Jülicher F, Grill SW. Anisotropies in cortical tension reveal the physical basis of polarizing cortical flows. Nature. 2010;467:617–21. doi: 10.1038/nature09376. [DOI] [PubMed] [Google Scholar]
- [51].Sherrard K, Robin F, Lemaire P, Munro E. Sequential activation of apical and basolateral contractility drives ascidian endoderm invagination. Curr Biol. 2010;20:1499–510. doi: 10.1016/j.cub.2010.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Vasquez CG, Tworoger M, Martin AC. Dynamic myosin phosphorylation regulates contractile pulses and tissue integrity during epithelial morphogenesis. J Cell Biol. 2014;206:435–50. doi: 10.1083/jcb.201402004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Zhou J, Kim H, Davidson L. Actomyosin stiffens the vertebrate embryo during crucial stages of elongation and neural tube closure. Development. 2009;136:677–88. doi: 10.1242/dev.026211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Smutny M, Cox HL, Leerberg JM, Kovacs EM, Conti MA, Ferguson C, et al. Myosin II isoforms identify distinct functional modules that support integrity of the epithelial zonula adherens. Nat Cell Biol. 2010;12:696–702. doi: 10.1038/ncb2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Maartens AP, Brown NH. Anchors and signals: the diverse roles of integrins in development. 1st ed. Elsevier Inc; 2015. [DOI] [PubMed] [Google Scholar]
- [56].Engl W, Arasi B, Yap LL, Thiery JP, Viasnoff V. Actin dynamics modulate mechanosensitive immobilization of E-cadherin at adherens junctions. Nat Cell Biol. 2014;16:587–94. doi: 10.1038/ncb2973. [DOI] [PubMed] [Google Scholar]
- [57].Selhuber-Unkel C, Erdmann T, López-García M, Kessler H, Schwarz US, Spatz JP. Cell adhesion strength is controlled by intermolecular spacing of adhesion receptors. Biophys J. 2010;98:543–51. doi: 10.1016/j.bpj.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Klompstra D, Anderson DC, Yeh JY, Zilberman Y, Nance J. An instructive role for C. elegans E-cadherin in translating cell contact cues into cortical polarity. Nat Cell Biol. 2015;17:726–35. doi: 10.1038/ncb3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Yamada S, Nelson WJ. Localized zones of Rho and Rac activities drive initiation and expansion of epithelial cell–cell adhesion. J Cell Biol. 2007;178:517–27. doi: 10.1083/jcb.200701058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Ratheesh A, Gomez GA, Priya R, Verma S, Kovacs EM, Jiang K, et al. Centralspindlin and α-catenin regulate Rho signalling at the epithelial zonula adherens. Nat Cell Biol. 2012;14:818–28. doi: 10.1038/ncb2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Maître J-L, Heisenberg C-P. Three functions of cadherins in cell adhesion. Curr Biol. 2013;23:R626–33. doi: 10.1016/j.cub.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Lecuit T, Yap AS. E-cadherin junctions as active mechanical integrators in tissue dynamics. Nat Cell Biol. 2015;17:533–9. doi: 10.1038/ncb3136. [DOI] [PubMed] [Google Scholar]
- [63].Cai Y, Rossier O, Gauthier N, Biais N, Fardin M, Zhang X, et al. Cytoskeletal coherence requires myosin-IIA contractility. J Cell Sci. 2010;123:413–23. doi: 10.1242/jcs.058297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Douezan S, Guevorkian K, Naouar R, Dufour S, Cuvelier D, Brochard-Wyart F. Spreading dynamics and wetting transition of cellular aggregates. Proc Natl Acad Sci U S A. 2011;108:7315–20. doi: 10.1073/pnas.1018057108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Chiou KK, Hufnagel L, Shraiman BI. Mechanical stress inference for two dimensional cell arrays. PLoS Comput Biol. 2012;8:e1002512. doi: 10.1371/journal.pcbi.1002512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Sugimura K, Ishihara S. The mechanical anisotropy in a tissue promotes ordering in hexagonal cell packing. Development. 2013;140:4091–101. doi: 10.1242/dev.094060. [DOI] [PubMed] [Google Scholar]
- [67].Brodland GW, Conte V, Cranston PG, Veldhuis J, Narasimhan S, Hutson MS, et al. Video force microscopy reveals the mechanics of ventral furrow invagination in Drosophila. Proc Natl Acad Sci U S A. 2010;107:22111–6. doi: 10.1073/pnas.1006591107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Brodland GW, Veldhuis JH, Kim S, Perrone M, Mashburn D, Hutson MS. CellFIT:. A cellular force-inference toolkit using curvilinear cell boundaries. PLOS ONE. 2014;9:e99116. doi: 10.1371/journal.pone.0099116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Ishihara S, Sugimura K, Cox SJ, Bonnet I, Bellaiche Y, Graner F. Comparative study of non-invasive force and stress inference methods in tissue. Eur Phys J E Soft Matter. 2013;36:9859. doi: 10.1140/epje/i2013-13045-8. [DOI] [PubMed] [Google Scholar]
- [70].Ducibella T, Ukena T, Karnovsky M, Anderson E. Changes in cell surface and cortical cytoplasmic organization during early embryogenesis in the preimplantation mouse embryo. J Cell Biol. 1977;74:153–67. doi: 10.1083/jcb.74.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Goel NS, Doggenweiler CF, Thompson RL. Simulation of cellular compaction and internalization in mammalian embryo development as driven by minimization of surface energy. Bull Math Biol. 1986;48:167–87. doi: 10.1007/BF02460021. [DOI] [PubMed] [Google Scholar]
- [72].Peyriéras N, Hyafil F, Louvard D, Ploegh HL, Jacob F. Uvomorulin: a nonintegral membrane protein of early mouse embryo. Proc Natl Acad Sci U S A. 1983;80:6274–7. doi: 10.1073/pnas.80.20.6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kemler R, Babinet C, Eisen H, Jacob F. Surface antigen in early differentiation. Proc Natl Acad Sci U S A. 1977;74:4449–52. doi: 10.1073/pnas.74.10.4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Winkel GK, Ferguson JE, Takeichi M, Nuccitelli R. Activation of protein kinase C triggers premature compaction in the four-cell stage mouse embryo. Dev Biol. 1990;138:1–15. doi: 10.1016/0012-1606(90)90171-e. [DOI] [PubMed] [Google Scholar]
- [75].Pratt HP, Ziomek CA, Reeve WJ, Johnson MH. Compaction of the mouse embryo: an analysis of its components. J Embryol Exp Morphol. 1982;70:113–32. [PubMed] [Google Scholar]
- [76].Johnson MH, Maro B. The distribution of cytoplasmic actin in mouse 8-cell blastomeres. J Embryol Exp Morphol. 1984;82:97–117. [PubMed] [Google Scholar]
- [77].Dard N, Le T, Maro B, Louvet-Vallée S. Inactivation of aPKClambda reveals a context dependent allocation of cell lineages in preimplantation mouse embryos. PLoS ONE. 2009;4:e7117. doi: 10.1371/journal.pone.0007117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Ziomek CA, Johnson MH. Cell surface interaction induces polarization of mouse 8-cell blastomeres at compaction. Cell. 1980;21:935–42. doi: 10.1016/0092-8674(80)90457-2. [DOI] [PubMed] [Google Scholar]
- [79].Fierro-González JC, White MD, Silva JC, Plachta N. Cadherin-dependent filopodia control preimplantation embryo compaction. Nat Cell Biol. 2013;15:1–10. doi: 10.1038/ncb2875. [DOI] [PubMed] [Google Scholar]
- [80].Bohil AB, Robertson BW, Cheney RE. Myosin-X is a molecular motor that functions in filopodia formation. Proc Natl Acad Sci U S A. 2006;103:12411–6. doi: 10.1073/pnas.0602443103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Bedzhov I, Liszewska E, Kanzler B, Stemmler MP. Igf1r signaling is indispensable for preimplantation development and is activated via a novel function of E-cadherin. PLoS Genet. 2012;8:e1002609. doi: 10.1371/journal.pgen.1002609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Roh-Johnson M, Shemer G, Higgins CD, McClellan JH, Werts AD, Tulu US, et al. Triggering a cell shape change by exploiting preexisting actomyosin contractions. Science. 2012;335:1232–5. doi: 10.1126/science.1217869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Hayashi T, Carthew R. Surface mechanics mediate pattern formation in the developing retina. Nature. 2004;431:647–52. doi: 10.1038/nature02952. [DOI] [PubMed] [Google Scholar]
- [84].Robertson F, Pinal N, Fichelson P, Pichaud F. Atonal and EGFR signalling orchestrate rok- and Drak-dependent adherens junction remodelling during ommatidia morphogenesis. Development. 2012;139:3432–41. doi: 10.1242/dev.080762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Lauschke VM, Tsiairis CD, François P, Aulehla A. Scaling of embryonic patterning based on phase-gradient encoding. Nature. 2013;493:101–5. doi: 10.1038/nature11804. [DOI] [PubMed] [Google Scholar]
- [86].Soroldoni D, Jorg DJ, Morelli LG, Richmond DL, Schindelin J, Julicher F, et al. A Doppler effect in embryonic pattern formation. Science. 2014;345:222–5. doi: 10.1126/science.1253089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Ares S, Morelli LG, Jörg DJ, Oates AC, Jülicher F. Collective modes of coupled phase oscillators with delayed coupling. Phys Rev Lett. 2012;108:204101. doi: 10.1103/PhysRevLett.108.204101. [DOI] [PubMed] [Google Scholar]
- [88].Hester SD, Belmonte JM, Gens JS, Clendenon SG, Glazier JA. A multi-cell, multi-scale model of vertebrate segmentation and somite formation. PLoS Comput Biol. 2011;7:e1002155. doi: 10.1371/journal.pcbi.1002155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].McMillen P, Holley SA. The tissue mechanics of vertebrate body elongation and segmentation. Curr Opin Genet Dev. 2015;32:106–11. doi: 10.1016/j.gde.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Brackenbury R, Thiery JP, Rutishauser U, Edelman GM. Adhesion among neural cells of the chick embryo. I. An immunological assay for molecules involved in cell–cell binding. J Biol Chem. 1977;252:6835–40. [PubMed] [Google Scholar]
- [91].Thiery JP, Brackenbury R, Rutishauser U, Edelman GM. Adhesion among neural cells of the chick embryo. II. Purification and characterization of a cell adhesion molecule from neural retina. J Biol Chem. 1977;252:6841–5. [PubMed] [Google Scholar]
- [92].Linask KK, Ludwig C, Han MD, Liu X, Radice GL, Knudsen KA. N-cadherin/catenin-mediated morphoregulation of somite formation. Dev Biol. 1998;202:85–102. doi: 10.1006/dbio.1998.9025. [DOI] [PubMed] [Google Scholar]
- [93].Baker RK, Antin PB. Ephs and ephrins during early stages of chick embryogenesis. Dev Dyn. 2003;228:128–42. doi: 10.1002/dvdy.10354. [DOI] [PubMed] [Google Scholar]
- [94].Glazier JA, Zhang Y, Swat M, Zaitlen B, Schnell S. Coordinated action of N-CAM, N-cadherin, EphA4, and ephrinB2 translates genetic prepatterns into structure during somitogenesis in chick. Curr Top Dev Biol. 2008;81:205–47. doi: 10.1016/S0070-2153(07)81007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Rohani N, Canty L, Luu O, Fagotto F, Winklbauer R. EphrinB/ephb signaling controls embryonic germ layer separation by contact-induced cell detachment. PLoS Biol. 2011;9:e1000597. doi: 10.1371/journal.pbio.1000597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Grima R, Schnell S. Can tissue surface tension drive somite formation? Dev Biol. 2007;307:248–57. doi: 10.1016/j.ydbio.2007.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Davis G, Phillips H, Steinberg M. Germ-layer surface tensions and “tissue affinities” in Rana pipiens gastrulae: quantitative measurements. Dev Biol. 1997;192:630–44. doi: 10.1006/dbio.1997.8741. [DOI] [PubMed] [Google Scholar]
- [98].Townes PL, Holtfreter J. Directed movements and selective adhesion of embryonic amphibian cells. J Exp Zool. 1955;128(1):53–120. doi: 10.1002/jez.a.114. [DOI] [PubMed] [Google Scholar]
- [99].Schötz E-M, Burdine RD, Jülicher F, Steinberg MS, Heisenberg C-P, Foty RA. Quantitative differences in tissue surface tension influence zebrafish germ layer positioning. HFSP J. 2008;2:42–56. doi: 10.2976/1.2834817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].David R, Luu O, Damm EW, Wen JWH, Nagel M, Winklbauer R. Tissue cohesion and the mechanics of cell rearrangement. Development. 2014;141:3672–82. doi: 10.1242/dev.104315. [DOI] [PubMed] [Google Scholar]
- [101].Foty R, Steinberg M. The differential adhesion hypothesis: a direct evaluation. Dev Biol. 2005;278:255–63. doi: 10.1016/j.ydbio.2004.11.012. [DOI] [PubMed] [Google Scholar]
- [102].Luu O, Damm EW, Parent SE, Barua D, Smith THL, Wen JWH, et al. PAPC mediates self/non-self-distinction during Snail1-dependent tissue separation. J Cell Biol. 2015;208:839–56. doi: 10.1083/jcb.201409026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Graner F, Glazier J. Simulation of biological cell sorting using a two-dimensional extended Potts model. Phys Rev Lett. 1992;69:2013–6. doi: 10.1103/PhysRevLett.69.2013. [DOI] [PubMed] [Google Scholar]
- [104].Krens SFG, Heisenberg C-P. Cell sorting in development. Curr Top Dev Biol. 2011;95:189–213. doi: 10.1016/B978-0-12-385065-2.00006-2. [DOI] [PubMed] [Google Scholar]
- [105].Carvalho L, Stühmer J, Bois JS, Kalaidzidis Y, Lecaudey V, Heisenberg C-P. Control of convergent yolk syncytial layer nuclear movement in zebrafish. Development. 2009;136:1305–15. doi: 10.1242/dev.026922. [DOI] [PubMed] [Google Scholar]
- [106].Krens SFG, Möllmert S, Heisenberg C-P. Enveloping cell-layer differentiation at the surface of zebrafish germ-layer tissue explants. Proc Natl Acad Sci U S A. 2011;108:E9–E10. doi: 10.1073/pnas.1010767108. authorreplyE11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Cerchiari AE, Garbe JC, Jee NY, Todhunter ME, Broaders KE, Peehl DM, et al. A strategy for tissue self-organization that is robust to cellular heterogeneity and plasticity. Proc Natl Acad Sci U S A. 2015;112:2287–92. doi: 10.1073/pnas.1410776112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Ninomiya H, Winklbauer R. Epithelial coating controls mesenchymal shape change through tissue-positioning effects and reduction of surface-minimizing tension. Nat Cell Biol. 2008;10:61–9. doi: 10.1038/ncb1669. [DOI] [PubMed] [Google Scholar]
- [109].Wang Y-C, Khan Z, Wieschaus EF. Distinct Rap1 Activity States Control the Extent of Epithelial Invagination via α-Catenin. Dev Cell. 2013;25:299–309. doi: 10.1016/j.devcel.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Bardet P-L, Guirao B, Paoletti C, Serman F, Léopold V, Bosveld F, et al. PTEN controls junction lengthening and stability during cell rearrangement in epithelial tissue. Dev Cell. 2013;25(5):534–46. doi: 10.1016/j.devcel.2013.04.020. [DOI] [PubMed] [Google Scholar]
- [111].Boussadia O, Kutsch S, Hierholzer A, Delmas V, Kemler R. E-cadherin is a survival factor for the lactating mouse mammary gland. Mech Dev. 2002;115:53–62. doi: 10.1016/s0925-4773(02)00090-4. [DOI] [PubMed] [Google Scholar]
- [112].Radice GL, Ferreira-Cornwell MC, Robinson SD, Rayburn H, Chodosh LA, Takeichi M, et al. Precocious mammary gland development in P-cadherin-deficient mice. J Cell Biol. 1997;139:1025–32. doi: 10.1083/jcb.139.4.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]