Abstract
Background
Chronic musculoskeletal pain conditions are a prevalent and disabling problem. Preventing chronic musculoskeletal pain requires multifactorial treatment approaches that address its complex etiology. Prior cohort studies identified a high risk subgroup comprised of variation in COMT genotype and pain catastrophizing. This subgroup had increased chance of heightened pain responses (in a pre-clinical model) and higher 12 month post-operatives pain intensity ratings (in a clinical model). This pre-clinical trial will test mechanisms and efficacy of personalized pain interventions matched to the genetic and psychological characteristics of the high-risk subgroup.
Methods
Potential participants will be screened for high risk subgroup membership, appropriateness for exercise-induced muscle injury protocol, and appropriateness for propranolol administration. Eligible participants that consent to the study will then be randomized into one of four treatment groups; 1) personalized pharmaceutical and psychological education; 2) personalized pharmaceutical and general education; 3) placebo pharmaceutical and psychological education; 4) placebo pharmaceutical and psychological education. Over the 5-day study period participants will complete an exercise-induced muscle injury protocol and receive study interventions. Pain and disability assessments will be completed daily, with primary outcomes being duration of shoulder pain (number of days until recovery), peak shoulder pain intensity, and peak shoulder disability. Secondary outcomes include inflammatory markers, psychological mediators, and measures of pain sensitivity regulation.
Conclusion
This pre-clinical trial builds on prior cohort studies and its completion will provide foundational data supporting efficacy and mechanisms of personalized interventions for individuals that may be at increased risk for developing chronic shoulder pain.
Trial Registration
ClinicalTrials.gov registry, NCT02620579 (Registered on November 13, 2015)
Introduction
Chronic musculoskeletal pain conditions are among the most prevalent and disabling medical problems experienced by individuals in the United States. Chronic pain affects 100 million people in the United States (U.S.) and produces annual costs up to $635 billion, exceeding the prevalence and costs of heart disease, cancer, and diabetes [1,2]. These costs are largely driven by musculoskeletal pain conditions. The burden of chronic pain is a global concern; in 2012 the Global Burden of Disease Study identified musculoskeletal pain as a primary contributor to years lived with disability worldwide [3]. The Institute of Medicine (IOM) has identified pain relief tailored to specific characteristics as a high priority for future research and practice initiatives, but very few accepted treatment models exist [1].
Preventing the development of chronic pain conditions is a high priority initiative for improving patient care. Unfortunately, current knowledge of mechanisms involved in the transition to chronic pain is limited, which decreases options for effective treatment of pain. Studies targeting validated risk factors that confer increased risk of experiencing chronic pain provide a unique opportunity to vertically advance the field. Indeed, interventions tailored to specific risk factor characteristics (i.e. personalized or precision medicine) hold great promise in reducing the impact of chronic pain [4,5]. Personalized medicine via identification of genetic risk factors has been successfully implemented for select areas of cardiac medicine [6–9] and oncology [10–12]. However, similar successes have not been achieved for pain treatment when focusing on genetic risk factors alone [5]. Because of their complex biopsychosocial etiologies, personalized interventions for chronic pain conditions will require identification of genetic factors in combination with psychological, environmental, and/or social risk factors [4]. We recently implemented this multiple risk factor approach in validating a high-risk subgroup comprised of psychological and genetic factors [13].
One component of this high risk subgroup, the catechol-O-methyltransferase (COMT) gene, encodes the COMT enzyme, which metabolizes catecholamines. COMT polymorphisms and haplotypes associated with low COMT activity have been linked to pain sensitivity and increased risk of multiple musculoskeletal pain conditions [14–16]. The impact of COMT on pain modulation occurs via multiple pathways, including endogenous μ-opioid function [17,18] and the beta-adrenergic system [19–22]. Pain catastrophizing, the psychological component of the high risk subgroup, is a negative cognitive style, comprised of pain-related rumination, magnification, and helplessness/pessimism, that leads to the perception that the experienced pain is beyond the control of the individual and will result in the worst possible outcome [23]. Pain catastrophizing has a well-established link to pain perception and disability in multiple pain populations [24–26], including shoulder pain as evidenced by our earlier studies [27,28]. In our pilot studies [29,30] we demonstrated an interaction between COMT genotype and pain catastrophizing as a stronger predictor of shoulder pain and disability than either factor alone [31]. In a pre-clinical cohort in whom shoulder pain was induced by eccentric exercise, we identified a subgroup comprised of COMT genotype associated with low enzyme activity plus elevated pain catastrophizing that was at higher risk for increased pain intensity and delayed recovery from the induced injury. This high risk subgroup was then validated by demonstrating that the subgroup experienced significantly poorer 12-month postsurgical outcomes in a separate clinical shoulder pain cohort [13].
These predictive findings provided the impetus to transition our biopsychosocial influence on shoulder pain (BISP) project to an intervention phase, which will advance scientific understanding of personalized or precision treatment options for musculoskeletal pain. The intervention phase will consist of using the pre-clinical model to determine the mechanisms and efficacy of pain interventions matched to the genetic and psychological characteristics of the high-risk subgroup. The purpose of this protocol paper is to describe the rationale, methods, and data analysis for the BISP pre-clinical proof of concept trial (NCT02620579).
Methods
Overview
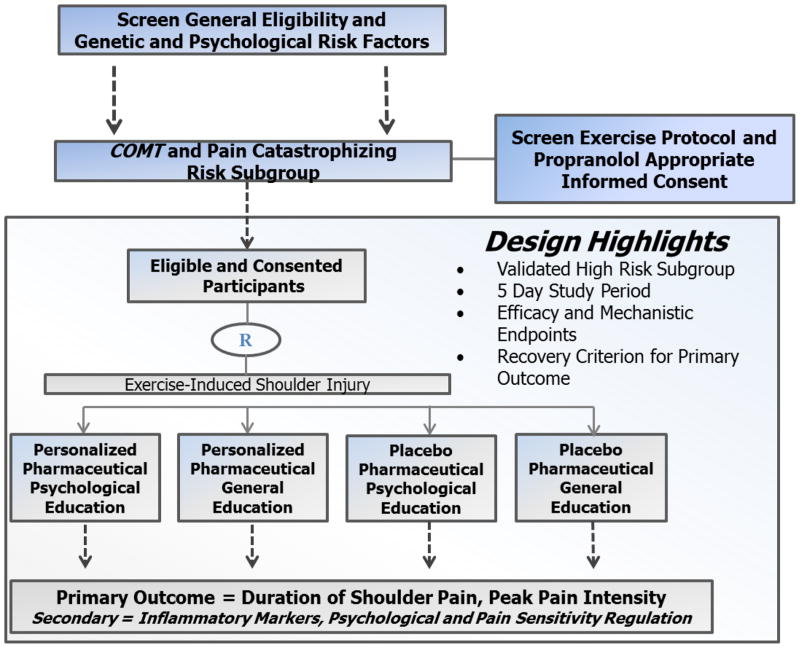
Figure 1 provides an overview of the study design following CONSORT recommendations [32] and Table 1 provides the enrollment, intervention, and assessment schedule following SPIRIT recommendations [33]. This study has been approved by the University of Florida Institutional Review Board and all participants will provide informed consent before being enrolled. Potential participants will be screened and those meeting the high-risk criteria will be randomized into one of four intervention groups created by crossing two pharmacologic conditions (propranolol vs. placebo) with two education conditions (psychological intervention vs. general education), with assigned treatments administered four consecutive days. Participants will then have shoulder pain induced via exercise-induced muscle injury. This pre-clinical model was selected because it controls the injury mechanism, allows for high treatment fidelity, and has an established translational link to a postoperative clinical model [13]. The pre-clinical model also offers logistical advantages and allows us to monitor inflammatory processes, psychological factors, and pain sensitivity regulation. The primary statistical analysis will determine whether the combined personalized intervention group experienced shorter shoulder pain duration, lower peak pain intensity, or lower upper-extremity disability. The combined personalized intervention versus the combined placebo condition is the primary comparison of interest for this study, but we also will evaluate the individual effects of both pharmaceutical and education interventions. Such comparisons will provide important information on whether the efficacy of the combined personalized intervention requires both components, or whether one component is sufficient for effective pain relief to occur. Secondary and exploratory analyses will determine which molecular, psychological, and pain sensitivity regulation mechanisms are associated with pain relief.
Figure 1.
Overview of Biopsychosocial Influence on Shoulder Pain Design
Table 1.
Schedule of Enrollment, Interventions, and Assessments for Biopsychosocial Influence on Shoulder Pain Pre-Clinical Trial
| STUDY PERIOD | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Enrollment | Allocation | Post-allocation | Close-out** | ||||||
|
| |||||||||
| TIME POINT | -7d+ | -3d | 0d | 0d | 1d | 2d | 3d | 4d | 5d+ |
|
| |||||||||
| ENROLLMENT: | |||||||||
|
|
|||||||||
| High risk screen | X | ||||||||
|
|
|||||||||
| Eligibility screen | X | ||||||||
|
|
|||||||||
| Informed consent | X | ||||||||
|
|
|||||||||
| Allocation | X | ||||||||
|
| |||||||||
| INTERVENTIONS: | |||||||||
|
|
|||||||||
| Propranolol | X | X | X | X | |||||
|
|
|||||||||
| Psychological Education | X | X | X | ||||||
|
|
|||||||||
| Placebo | X | X | X | X | |||||
|
|
|||||||||
| General Education | X | X | X | ||||||
|
| |||||||||
| ASSESSMENTS: | |||||||||
|
|
|||||||||
| Demographic | X | ||||||||
|
|
|||||||||
| Pain Intensity and Disability | X | X | X | X | X | X | |||
|
|
|||||||||
| Physical Impairment | X | X | X | X | X | ||||
|
|
|||||||||
| Psychological and Pain Sensitivity | X | X | X | X | X | ||||
|
|
|||||||||
| Inflammatory Markers | X | X | X | X | |||||
|
|
|||||||||
| Global Ratings and Blinding | X | ||||||||
Time point units are days (d)
Participants continued to be followed after last post-allocation day if they do not meet pain recovery criterion for pain intensity and disability measures only.
Participants
Healthy participants will be considered for study participation. Eligibility criteria were determined from established inclusion and exclusion criteria that determine appropriateness for the exercise-induced injury protocol, now modified to account for the risks of administering propranolol. Inclusion criteria are: a) ages ≥ 18 years to 65 years and b) English speaking. Exclusion criteria are reported in Table 2.
Table 2.
Exclusion Criteria for Biopsychosocial Influence on Shoulder Pain Pre-Clinical Trial
| Exclusion Criteria for Exercise-Induced Shoulder Injury Protocol | ||
| Chronic pain (> 3 months) in any area | Currently experiencing neck or shoulder pain | Previous history of upper extremity surgery |
| Neurologic impairment of the upper-extremity (determined by loss of sensation, muscle weakness, and reflex change) | Previous history of neck or shoulder pain (operationally defined as experiencing pain longer than 48 hours or seeking medical treatment) | Currently or regularly use pain medication |
| Regular participation in upper-extremity weight training | ||
| Exclusion Criteria for Propranolol | ||
| Clinically significant abnormal 12 lead ECG | Sinus bradycardia (resting heart rate below 55 beats per minute) | Uncontrolled hypertension (resting systolic blood pressure below 90 mm Hg) |
| Cardiac failure | Coronary heart disease | Wolff-Parkinson-White Syndrome |
| Greater than first degree heart block | Known hypersensitivity to propranolol | |
| General Exclusion Criteria for Study Participation | ||
| Bronchial asthma | Nonallergic bronchospasm | History of recent surgery requiring general anesthesia |
| Diabetes | Pregnancy | Major depression |
| Chronic obstructive pulmonary disease | Dementia | Breast feeding |
Screening Procedures
Because the personalized intervention is designed to match genetic and psychological makeup, screening will be required in order to identify and enroll high-risk participants. Therefore quarterly screenings will be completed on campus and in the local community. At each quarterly session, we anticipate screening at least 50 people and expect approximately 30% of those screened will meet the previously established high risk criteria. If eligible, each participant will provide a saliva sample and complete the Pain Catastrophizing Scale (PCS), a 13-item, 4-point rating scale [34,35]. A small monetary incentive will help to encourage participation. High-risk subgroup status will be based on PCS scores of 5 or greater and COMT genotype indicative of high pain sensitivity by rs6269 (i.e. “AA”) [13]. Those familiar with the PCS will notice that a score of 5 is not elevated for general or clinical populations. However, this cut-off is based on a healthy population and is specific for those with the COMT high pain sensitivity variation, so it is lower than if a general cut-off score independent of the genotype was used. Participants in the low risk subgroup are not further eligible, and their data will be destroyed.
Pre-Clinical Trial
Participants identified as high risk candidates are eligible for participation in the full intervention study. Key approach details for this trial are described in the subsequent sections.
Randomization
There are reported sex differences in pain conditions [36,37], and we have observed that females report higher pain sensitivity in our studies of shoulder pain [38]. Sex differences in how COMT impacts pain sensitivity have also been described, with females having a stronger association with pain sensitivity for certain genetic variants [39]. Therefore randomization will be stratified by sex to ensure equal allocation to different intervention groups within males and females, respectively. The randomization scheme will be prepared by computer and completed prior to the start of the study. After the randomization list is generated, treatment assignments will be accessed through a secured website that provides independent assignment for pharmaceutical and psychological interventions. Random assignment will be determined in sequential order as each participant enters the study. Research staff will be blinded to intervention assignment they are not involved with, for example, the pharmacy staff will not know the assignment for the psychological intervention. Randomization will be completed prior to the muscle injury protocol. The first 300 subjects will be equally randomized to the four groups; however, the allocation ratio may change for the remaining 148 subjects depending on interim analysis results. Specifically, if the combined personalized intervention is shown to be better than the combined placebo intervention at 0.04 significance level, then the combined placebo group will be dropped and the subjects will be equally allocated to the remaining three groups. On the other hand, if the conditional power to detect difference between the combined personalized intervention and combined placebo group is less than 70%, the two groups with one personalized intervention will be dropped and subjects will be equally allocated to the combined groups. Otherwise, the allocation ratio will remain the same for the four groups. Such an adaptive design will protect us from minor under or over estimation of response to the interventions.
Exercise-Induced Shoulder Injury
Research personnel performing the muscle injury protocol will be blinded to randomization results to prevent bias. Subjects will undergo exercise-induced shoulder injury to the dominant arm. The specific eccentric exercise fatigue protocol uses isokinetic equipment [40–44] and is an established protocol similar to that used in our prior studies [28,31,45]. Briefly, shoulder fatigue will be induced using a Biodex (Shirley, NY) isokinetic dynamometer. Subjects will be placed in a seated position, with shoulder straps applied to support the torso. Then, the dominant shoulder will be placed in the scapular plane because this position has been associated with high test-retest reliability and has decreased impingement of the greater tuberosity under the acromion [40,46]. Maximum voluntary isometric contraction (MVIC) will be determined by having the subjects perform 5 repetitions of isometric shoulder external rotation. Subjects will be asked to perform the contractions with maximal effort and given verbal encouragement during the contractions. The MVIC will be determined by averaging peak force from the 3 repetitions with the highest force [47,48].
After MVIC is determined, subjects will complete eccentric/concentric external rotation repetitions to induce muscle fatigue and microtrauma. All repetitions that constitute the fatigue protocol will be completed at 60 degrees/second in blocks of 3 sets of 10 repetitions. After completing those repetitions, subjects will be retested to determine if they can generate more than 50% of their respective MVIC. Previous research has indicated the inability to achieve 50% of initial peak MVIC is an indicator of muscle fatigue [41–44]. If they are unable to achieve at least 50% of their MVIC, the fatigue protocol is terminated. If they are able to generate more than 50% of their MVIC, subjects will perform additional sets of 10 repetitions at speeds of 60 degrees/second. This will be repeated until their peak force is less than 50% of the initial MVIC. Subjects will be allowed to rest 30 seconds between sets and the total amount of work performed to reach muscle fatigue will be recorded. The goal of the injury protocol is to induce delayed onset muscle soreness (DOMS) in the rotator cuff musculature. Shoulder DOMS is a clinically relevant model because subjects experience increased pain intensity, loss of range of motion, inflammatory responses, altered proprioception, and reduced self-care behaviors [41,42,44,49–53].
Personalized Pharmaceutical Intervention
The first pharmaceutical administration occurs before injury which allows for any immediate pre-emptive effects on the inflammatory or pain sensitivity regulation measures to be detected during the baseline session. This administration also matches when propranolol would be administered in a clinical model (i.e. pre-operatively), which maintains the translational component. Increasing evidence implicates β-adrenergic drive in the pathophysiology of chronic pain conditions. Indeed, musculoskeletal pain conditions are associated with heightened catecholamine levels and increased sympathetic responses to stressors [54–56]. Also, in rodents epinephrine produced a β-adrenergic receptor-mediated mechanical hyperalgesia [57]. Additional evidence suggests that these pro-nociceptive effects of catecholamines can be reversed by blocking beta-adrenergic receptors. For example, a single infusion of propranolol temporarily reduced clinical pain among individuals with temporomandibular disorder and fibromyalgia.[58] Another study showed that pindolol, a medication that blocks both β-adrenergic and serotonin 1A receptors, reduced pain and tenderness in patients with fibromyalgia [59]. Moreover, in rodents, propranolol has been found to decrease inflammation-evoked hyperalgesia in joint and muscle [60,61]. Catechol-O-methyltransferase (COMT), the enzyme encoded by COMT, metabolizes catecholamines, including epinephrine and norepinephrine [62]. COMT genotypes associated with lower COMT activity have been associated with increased risk of musculoskeletal pain[15,63] and greater pain sensitivity [14,64]. Preclinical work showed that COMT inhibition produced robust thermal and mechanical hyperalgesia, which was blocked by propranolol [21]. Furthermore, the analgesic effect of propranolol in people with orofacial pain was dependent on the subject’s COMT genotype – with greater analgesia observed in patients with a haplotype conferring low COMT activity [20]. Our personalized pharmaceutical intervention is designed to be consistent with that finding.
The University of Florida Investigational Drug Service will prepare long-acting propranolol (Propranolol LA) 60 mg to be administered orally in the Pain Clinical Research Unit once daily for the five days of the protocol. This dose will provide a bioequivalent dose to that recently reported in a clinical study examining responses to propranolol among patients with TMD pain [20]. The first dose will be administered prior to the exercise-induced shoulder injury to mimic pre-operative settings and to allow for immediate effects to be observed during the baseline session. The exercise-induced injury protocol occurs only on the first day and subsequent pharmacological doses will be applied at the beginning of each research session for the next 3 days. Cardiovascular response will be monitored 60 minutes after drug administration by a research nurse. The purpose of this monitoring is for safety (early identification of potential adverse events) and efficacy (demonstrate medication absorbed). These measures will be recorded by the research nurse in a blinded manner. These data will be used by the investigator team as a manipulation check to assure that propranolol absorption is occurring, and allow for adjustments to be made early in the protocol if not.
Placebo Pharmaceutical Intervention
Placebo capsules will be prepared by the UF Investigational Drug Service to be visually indistinguishable from the active medication. Placebo administration will be done in the same fashion as was described in the personalized pharmaceutical section to maintain blinding. This includes the same timing for each session and monitoring of cardiovascular responses.
Personalized Psychological Education Intervention
The Personalized Psychological Education interventions follow what has been used in low back pain clinical trials [65–68] and key principles in psychologically informed interventions [69]. These principles are not specific to the anatomical region of pain, for example there is a clinical trial of cognitive behavioral treatment for reducing catastrophizing in individuals with chronic headache [70]. These same principles were used to design an intervention for shoulder pain. Consistent evidence demonstrates that pain-related fear, kinesiophobia, and pain catastrophizing produce strong influences on exercise-induced shoulder pain [27,28,71], as has been reported for individuals with pain in other body regions [25,26,72,73]. Therefore, our personalized psychological education intervention will address these factors with special emphasis on pain catastrophizing, since that was the factor in the high risk subgroup and it has been established as an important therapeutic target for cognitive based interventions [74]. Additional justification for a separate psychological intervention comes from an indication that propranolol alone did not improve psychological status for subjects with orofacial pain [20]. The personalized psychological and education intervention will be administered on Days 2–4 of the exercise induced muscle injury protocol since the intervention is predicated on the individual experiencing pain. The duration of the personalized intervention will be 10–15 minutes. The overall goal of the intervention is to provide better understanding of pain processing and how psychological factors influence the pain experience. This information will encourage shoulder activation by: a) reducing the threat of muscle injury; b) encouraging normal use of the shoulder and arm; and c) addressing specific concerns expressed by the subject (e.g. pain with shoulder motion is a sign of re-injury). This intervention will be devoid of detailed information on shoulder anatomy, movement, and injury that characterizes the General Education modules. The psychological education modules will be scripted and structured for delivery during each research clinic visit to ensure consistent allocation. The participants view the module for content and then interact with research staff in scripted areas (i.e. demonstration of key principle and questions on module content). After completion of psychological modules participants will rate how useful and/or helpful the education module was for understanding their shoulder pain.
General Education Intervention
The General Education intervention will match the structure and administration of the personalized intervention with the participant remaining blinded to what is received. The general education module will serve as the comparator group and consist of content that did not change psychological measures in our low back clinical trials [66,67]. Since this content is not specific to anatomical region, it is likely appropriate for use in this study for shoulder pain. The general education intervention modules will be administered Days 2–4 following exercise enhance injury with the goal of participant understanding shoulder anatomy and injury while reviewing: a) structure and arthrokinematics of the shoulder joint; b) muscle anatomy of the shoulder with emphasis on the rotator cuff; and c) potential shoulder pain generators from the exercise-induced injury. The general education intervention modules will be devoid of information related to pain processing and psychological influences that characterizes the personalized intervention and will also last for 10–15 minutes. The general education modules will be scripted and structured for delivery during each research clinic visit to ensure consistent allocation. The participants view the module for content and then interact with research staff in scripted areas (i.e. demonstration of key principle and questions on module content). After completion of general education modules participants will rate how useful and/or helpful the education module was for understanding their shoulder pain.
Primary Outcome Measures
The primary outcome measures (listed below) were selected based on relevance to clinical outcomes and successful use in our prior studies. Another advantage of these measures is they are widely accepted as primary outcome measures in clinical studies, and will allow for specific effect size estimates for a subsequent randomized clinical trial in post-operative shoulder pain. Pain intensity ratings and self-report of upper-extremity disability will be used as primary outcome measures to determine efficacy for shoulder pain duration, peak shoulder pain intensity, and peak upper-extremity disability. These constructs have a conceptual link to chronic pain [1] and therefore can be used in a pre-clinical model to characterize the presence of persistent or continued pain following exercise-induced injury.
The Brief Pain Inventory (BPI) will be used to measure pain intensity as it has been found to have good test-rest reliability over short intervals [75]. Using an 11-point numerical rating scale ranging from 0 (no pain) to 10 (worst pain intensity imaginable), the BPI asks subjects to rate the intensity of their current pain and pain at its worst, best and average over the past 24 hours. To determine recovery, subjects will complete the BPI daily until they rated their current pain at 0/10 and their worst pain was rated less than 2/10. The number of days it takes to reach this recovery criterion will be recorded as duration of shoulder pain. The highest worst pain intensity recorded during recovery will be recorded as peak shoulder pain intensity.
The Disabilities of the Arm, Shoulder, and Hand Questionnaire (DASH) will be used to assess upper-extremity disability and we will continue to use a validated abridged version of the DASH (the QuickDASH) which consists of 11 functional items, with total scores ranging from 0 (no disability) to 100 (complete disability) [76]. We will use the QuickDASH because shoulder pain can also affect distal function of the arm and hand, and we wanted to obtain a global upper-extremity assessment. Similar to the BPI ratings, QuickDASH scores will be recorded daily until recovery and the highest score during this period will be recorded as peak upper extremity disability.
Secondary Outcome Measures
The measures listed below represent underlying mechanisms and/or processes that we hypothesize to be related to pain relief. These measures will be obtained at the same time each day (relative to the time of the initial shoulder injury) to avoid unwarranted variation.
Inflammatory Markers
These measures will capture relevant inflammatory biomarkers including IL-1β, IL-6, IL-8, and TNFα. The low COMT activity of our high-risk group results in increased catecholamine activity, which augments release of proinflammatory cytokines under conditions of stress [77–79]. This catecholamine-evoked cytokine release can be attenuated by propranolol [77–80]. Moreover, catastrophizing, which also characterizes our high-risk group, has been associated with greater increases in circulating proinflammatory cytokines following both acute pain [81] and induction of pain-related negative emotions [82]. Thus, we hypothesize that personalized pharmaceutical or psychological intervention will significantly attenuate cytokine levels, with the greatest reduction observed in the combined personalized intervention condition. Moreover, because the increased pain evoked by COMT inhibition has been found to be mediated by increased circulating cytokines and reversed by blockade of beta-adrenergic (β2 and β3) receptors [19], we further expect that the attenuation of cytokine release will be associated with the efficacy of our combined personalized intervention for reducing pain and disability. Thus, we plan to perform assays for several inflammatory cytokines, including IL1β, IL6, IL8, and TNFα at baseline, immediately after the exercise-induced injury, and at regular intervals.
Psychological
In addition to the aforementioned PCS used in screening, the Tampa Scale of Kinesiophobia which is an 11-item (TSK-11), 4 point rating scale to quantify avoidance and re-injury beliefs [83,84] and the Fear of Pain Questionnaire (FPQ-III) which is a 30-item, 5-point rating scale to quantify fear about specific situations that normally produce pain [85–87] will be used to capture psychological processes. Consistent with a fear-avoidance model of musculoskeletal pain [88], we hypothesize that the personalized psychological intervention will reduce these levels significantly via cognitive restructuring providing subsequent decreases in disability and pain.
Pain Sensitivity Regulation
These measures include suprathreshold heat pain responses, pressure pain threshold, and conditioned pain modulation which characterize nervous system processing of standard stimuli so that central or peripheral sensitization states indicative of pain amplification can be detected [89–91]. It is important to account for pain amplification separately, because it is hypothesized as a precursor to chronic musculoskeletal pain conditions that can occur with or independent of the molecular and psychological measures [4]. The potential contributors to pain amplification are multifactorial, therefore we hypothesize that the combined personalized intervention group will show the largest reduction in measures indicative of pain amplification. All pain sensitivity measures will be obtained by psychophysical sensory testing per established protocols established from the initial funding period [91–94]. All stimuli will be delivered to bilateral upper extremities to allow for side to side comparisons. Stimulation sites will be varied to prevent carryover effects due to local sensitization. The stimuli are to be applied by a research assistant blinded to intervention status who ensures proper application and the range of stimulus intensities will be presented beforehand to each subject. All subjects will undergo a brief training with the stimuli to be tested. We have found this procedure to be useful because it familiarizes subjects with the stimulus range, tends to obviate range effects in psychophysical scaling, and helps alleviate subject anxiety about the upper limit of stimulus intensities to be used. The research assistant will record patient visual analogue scale numeric pain rating response to each stimulus used. In order to standardize the scaling instructions, standard instructions[95] will be used for all subjects. Exact parameters for these QST measures are explained in more detail in publications from the initial funding period [91–94]. These measures were selected because we expected them to be responsive to the combined personalized intervention and associated with pain relief. For example, elevated suprathreshold heat pain responses normalized post-operatively in the clinical cohort [93] and its changes were associated with improvements in post-operative shoulder pain and disability [96].
Statistical Analysis Plan
All statistical analyses will be performed using the SAS software, version 9 (SAS Institute Inc, 1996). Summary statistics will be provided for baseline measures by intervention groups to determine if randomization produced balanced groups. Any group imbalance will be investigated further to determine if covariates should be considered. An interim analysis will be conducted when the first 300 subjects complete the follow-up. If the combined personalized intervention group is shown to be better than the combined placebo intervention at the 0.04 significance level, then the combined placebo and general education group will be dropped and the subjects will be equally allocated to the remaining three groups. On the other hand, if the conditional power to detect difference between combined personalized intervention group and combined placebo and general education group is less than 70%, the two groups with one personalized intervention will be dropped and subjects will be equally allocated to only the combined groups. Otherwise, the allocation ratio will remain the same for the four groups. For the latter two scenarios, one p-value (p1) will be derived from the interim analysis and another p-value (p2) will be derived from the logistic regressions based on only the second stage data. The primary comparison will then be tested using weighted normally inversed combination of the two p-values, i.e., the test statistics will be [√2 ??−1(1−p1)+ ??−1(1−p2)]/√3, where ?? is the normal cumulative distribution function.
A primary analysis will compare shoulder pain duration across the four randomly assigned groups with the use of logistic regression. The primary outcome variable will be dichotomized based on duration of at least 6 days or not. There is an a priori plan to include age, sex, and race as covariates in this analysis, additional variables will be added as covariates only if imbalanced across groups and correlated with outcome measures. We anticipate very little missing data because this is a pre-clinical study, but any missing outcomes will be predicted by subject pain intensity trajectory plus baseline demographic factors. The primary comparison between the combined personalized intervention and the combined placebo and general education condition will be tested at the 0.05 significance level, with early stopping boundary chosen to be p<0.04 in the interim. On the other hand, the other five between group contrasts will be tested using Holm’s step-down procedure so that family-wise error rate is controlled at 0.05 [97]. For the other primary outcomes (peak pain intensity and upper-extremity disability as continuous measures) we will perform a similar analysis process (i.e. same considerations for post randomization imbalance, covariates, and missing data) with linear regression analysis to compare the four intervention groups.
In secondary analyses we will fit path models with predetermined orders to investigate the direct (intervention group) and indirect effects (through the mediating inflammatory, psychological, and pain sensitivity paths) of the randomly assigned condition for predicting pain relief. The combined placebo intervention will serve as reference group for these analyses. The total, direct and indirect mechanistic effects on pain duration (dichotomous) and peak pain intensity and upper-extremity disability (continuous) will be estimated and tested at the 0.05 significance level for the comparison of assigned conditions. The total effect will be estimated and tested through a logistic regression for pain duration and a general linear model for peak pain intensity, both of which include the assigned intervention conditions as independent variables while controlling for participant age, sex, and race. The direct effect will be estimated and tested with the use of the same models including the same independent variables, but controlling for mediating molecular, psychological, and pain sensitivity variables in addition to age, sex and race. The two regression models in the second step will reveal the contributions of the mediating psychological, molecular, and pain sensitivity variables on pain relief. On the other hand, the indirect effect will be calculated based on the difference between the total and direct effects. In addition, we will conduct a third set of regression analyses to evaluate the effect of the assigned condition on the mediating variables which will determine which indirect paths are statistically significant.
Exploratory Analyses
We intentionally presented a focused plan for primary and secondary analyses. We do acknowledge that response to these interventions may not be as predictable as we have hypothesized. In the event that these analyses indicate no group differences we will perform additional analyses to inform future research in this area. For example as already mentioned, there is potential for sex differences in pain sensitivity and COMT variant influence on pain sensitivity to impact study results. We have accounted for this with stratified randomization based on sex and by a priori including sex as a covariate in primary analyses. In the case of null findings, however, a specific exploratory analysis will determine if sex-specific intervention effects occurred. Other potential exploratory analyses could look at post-hoc treatment responder characteristics and will be enhanced by the storage of excess DNA, plasma, and RNA so additional genetic predictors and molecular mechanisms can be considered.
Sample Size Estimate
A total of 448 high-risk subjects will be recruited. In our earlier study we found that 40.5% of high risk subjects and 21.4% of low risk subjects had pain duration ≥ 7 days [13]. In our power analysis, we assumed that 40% of the combined placebo and general education group will have long pain duration (rate for high risk subgroup), while the rate for the combined personalized intervention group will be 20% (rate for low risk group). Those with one personalized intervention were assumed to have 30% chance of having duration ≥ 7 days. The planned sample size will provide 80% power to detect the assumed differences across the four intervention groups and 91% power for the primary comparison between the combined personalized and the combined placebo and general education interventions at a type-I error level of 0.05.
Discussion
Shoulder pain is a common musculoskeletal pain condition. In the aforementioned IOM report, the shoulder was the 5th most frequently reported pain site (estimated at 9.0% of all US adults over age 18).[1] Other estimates of shoulder pain prevalence document high rates, including 1-year prevalence rates from 5 – 47% [98,99] and point prevalence rates from 14 – 21% [100,101]. Shoulder pain is characterized by poor outcomes and resulting disability. For example, in one cohort 40% of the individuals did not report full recovery at 1 year after new onset of shoulder pain [102]. Among those with shoulder pain, 17.7% had difficulty with basic daily activities while 21.4% had difficulty with complex daily activities [1].
There remains a clear need for a pre-clinical study to establish proof of principle for efficacy and identify mechanisms of pain relief in this high risk subgroup before a highly resource-intensive clinical trial could be justified. Very few treatment models for personalized or precision pain interventions exist [4,5]; therefore, this pre-clinical trial represents a meaningful advance towards the reality of providing personalized or targeted treatments for musculoskeletal pain. Our approach was based on established genetic and psychological risk factors and the interventions were specifically designed with those factors in mind, so we felt the term “personalized” was more appropriate. These findings could strongly impact the field if individuals in the high risk subgroup respond favorably to the interventions included in this study. Moreover, we will determine the relevant psychological, physiological, and pain sensitivity regulation mechanisms involved in pain relief.
Chronic musculoskeletal pain is a significant public health problem. Our ultimate goal is to conduct a randomized clinical trial in patients with post-operative shoulder pain. This pre-clinical trial builds on our predictive cohort studies and its completion will provide foundational data by demonstrating the efficacy of personalized interventions for those individuals that are at increased risk for developing chronic shoulder pain. Furthermore, completion of this pre-clinical trial will provide important proof of principle support for a subsequent clinical trial in post-operative shoulder pain that could potentially improve standard of care for pain management.
Acknowledgments
Funding
This study was completed with funding from the National Institutes of Health; NIAMS (AR055899). All authors were independent from this funding source and the funding source played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review or approval of the manuscript.
Luda Diatchenko provided feedback on study design and selection of inflammatory markers.
Mark Bishop, Geoffrey Dover, and Will Hedderson assisted with the exercise-induced muscle injury protocol. Kristy Shimp developed content and assisted with the psychological education module.
Footnotes
Ethics Approval and Consent to Participate
This study was approved the University of Florida Institutional Review Board – 01 (Human Subjects Review). All participants will provide informed consent before participating in this study.
Availability of Data and Materials
Not appropriate. This paper is a protocol description and does not contain any data.
Competing Interest
The authors have no conflict of interest, financial or otherwise, to declare with submission of this manuscript.
Authors’ Contributions
SZG, RS, SSW, PAB, MRW, RBF all provided input on study design, writing, and reviewed final version of this manuscript. WHG and LNM provided input on protocol details, study implementation, and reviewed final version of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.IOM (Institute of Medicine) Relieving pain in America: A blueprint for transforming prevention, care, education, and research. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 2.Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012;13:715–724. doi: 10.1016/j.jpain.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 3.Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basanez MG, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabe E, Bhalla K, Bhandari B, Bikbov B, Bin AA, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, Brooks P, Brugha TS, Bryan-Hancock C, Bucello C, Buchbinder R, Buckle G, Budke CM, Burch M, Burney P, Burstein R, Calabria B, Campbell B, Canter CE, Carabin H, Carapetis J, Carmona L, Cella C, Charlson F, Chen H, Cheng AT, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahiya M, Dahodwala N, Damsere-Derry J, Danaei G, Davis A, De LD, Degenhardt L, Dellavalle R, Delossantos A, Denenberg J, Derrett S, Des Jarlais DC, Dharmaratne SD, Dherani M, Diaz-Torne C, Dolk H, Dorsey ER, Driscoll T, Duber H, Ebel B, Edmond K, Elbaz A, Ali SE, Erskine H, Erwin PJ, Espindola P, Ewoigbokhan SE, Farzadfar F, Feigin V, Felson DT, Ferrari A, Ferri CP, Fevre EM, Finucane MM, Flaxman S, Flood L, Foreman K, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabbe BJ, Gabriel SE, Gakidou E, Ganatra HA, Garcia B, Gaspari F, Gillum RF, Gmel G, Gosselin R, Grainger R, Groeger J, Guillemin F, Gunnell D, Gupta R, Haagsma J, Hagan H, Halasa YA, Hall W, Haring D, Haro JM, Harrison JE, Havmoeller R, Hay RJ, Higashi H, Hill C, Hoen B, Hoffman H, Hotez PJ, Hoy D, Huang JJ, Ibeanusi SE, Jacobsen KH, James SL, Jarvis D, Jasrasaria R, Jayaraman S, Johns N, Jonas JB, Karthikeyan G, Kassebaum N, Kawakami N, Keren A, Khoo JP, King CH, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lalloo R, Laslett LL, Lathlean T, Leasher JL, Lee YY, Leigh J, Lim SS, Limb E, Lin JK, Lipnick M, Lipshultz SE, Liu W, Loane M, Ohno SL, Lyons R, Ma J, Mabweijano J, MacIntyre MF, Malekzadeh R, Mallinger L, Manivannan S, Marcenes W, March L, Margolis DJ, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGill N, McGrath J, Medina-Mora ME, Meltzer M, Mensah GA, Merriman TR, Meyer AC, Miglioli V, Miller M, Miller TR, Mitchell PB, Mocumbi AO, Moffitt TE, Mokdad AA, Monasta L, Montico M, Moradi-Lakeh M, Moran A, Morawska L, Mori R, Murdoch ME, Mwaniki MK, Naidoo K, Nair MN, Naldi L, Narayan KM, Nelson PK, Nelson RG, Nevitt MC, Newton CR, Nolte S, Norman P, Norman R, O’Donnell M, O’Hanlon S, Olives C, Omer SB, Ortblad K, Osborne R, Ozgediz D, Page A, Pahari B, Pandian JD, Rivero AP, Patten SB, Pearce N, Padilla RP, Perez-Ruiz F. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diatchenko L, Fillingim RB, Smith SB, Maixner W. The phenotypic and genetic signatures of common musculoskeletal pain conditions. Nat Rev Rheumatol. 2013;9:340–350. doi: 10.1038/nrrheum.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mogil JS. Pain genetics: past, present and future. Trends Genet. 2012;28:258–266. doi: 10.1016/j.tig.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Rader DJ. New Therapies for Coronary Artery Disease: Genetics Provides a Blueprint. Sci Transl Med. 2014;6:239ps4. doi: 10.1126/scitranslmed.3008535. [DOI] [PubMed] [Google Scholar]
- 7.Scott SA, Sangkuhl K, Gardner EE, Stein CM, Hulot JS, Johnson JA, Roden DM, Klein TE, Shuldiner AR. Clinical Pharmacogenetics Implementation Consortium guidelines for cytochrome P450-2C19 (CYP2C19) genotype and clopidogrel therapy. Clin Pharmacol Ther. 2011;90:328–332. doi: 10.1038/clpt.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson JA, Liggett SB. Cardiovascular pharmacogenomics of adrenergic receptor signaling: clinical implications and future directions. Clin Pharmacol Ther. 2011;89:366–378. doi: 10.1038/clpt.2010.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roden DM, Johnson JA, Kimmel SE, Krauss RM, Medina MW, Shuldiner A, Wilke RA. Cardiovascular pharmacogenomics. Circ Res. 2011;109:807–820. doi: 10.1161/CIRCRESAHA.110.230995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Couch FJ, Nathanson KL, Offit K. Two decades after BRCA: setting paradigms in personalized cancer care and prevention. Science. 2014;343:1466–1470. doi: 10.1126/science.1251827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alanee SR, Glogowski EA, Schrader KA, Eastham JA, Offit K. Clinical features and management of BRCA1 and BRCA2-associated prostate cancer. Front Biosci (Elite Ed) 2014;6:15–30. doi: 10.2741/e686. [DOI] [PubMed] [Google Scholar]
- 12.Bombard Y, Bach PB, Offit K. Translating genomics in cancer care. J Natl Compr Canc Netw. 2013;11:1343–1353. doi: 10.6004/jnccn.2013.0158. [DOI] [PubMed] [Google Scholar]
- 13.George SZ, Wallace MR, Wu SS, Moser MW, Wright TW, Farmer KW, Borsa PA, Parr JJ, Greenfield WH, III, Dai Y, Li H, Fillingim RB. Biopsychosocial influence on shoulder pain: risk subgroups translated across preclinical and clinical prospective cohorts. Pain. 2015;156:148–156. doi: 10.1016/j.pain.0000000000000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diatchenko L, Slade GD, Nackley AG, Bhalang K, Sigurdsson A, Belfer I, Goldman D, Xu K, Shabalina SA, Shagin D, Max MB, Makarov SS, Maixner W. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum Mol Genet. 2005;14:135–143. doi: 10.1093/hmg/ddi013. [DOI] [PubMed] [Google Scholar]
- 15.Belfer I, Segall S. COMT genetic variants and pain. Drugs Today (Barc) 2011;47:457–467. doi: 10.1358/dot.2011.47.6.1611895. [DOI] [PubMed] [Google Scholar]
- 16.Tammimaki A, Mannisto PT. Catechol-O-methyltransferase gene polymorphism and chronic human pain: a systematic review and meta-analysis. Pharmacogenet Genomics. 2012;22:673–691. doi: 10.1097/FPC.0b013e3283560c46. [DOI] [PubMed] [Google Scholar]
- 17.Li PP, Warsh JJ, Godse DD. Formation and clearance of norepinephrine glycol metabolites in mouse brain. J Neurochem. 1984;43:1425–1433. doi: 10.1111/j.1471-4159.1984.tb05404.x. [DOI] [PubMed] [Google Scholar]
- 18.Cumming P, Brown E, Damsma G, Fibiger H. Formation and clearance of interstitial metabolites of dopamine and serotonin in the rat striatum: an in vivo microdialysis study. J Neurochem. 1992;59:1905–1914. doi: 10.1111/j.1471-4159.1992.tb11026.x. [DOI] [PubMed] [Google Scholar]
- 19.Hartung JE, Ciszek BP, Nackley AG. beta2- and beta3-adrenergic receptors drive COMT-dependent pain by increasing production of nitric oxide and cytokines. Pain. 2014;155:1346–1355. doi: 10.1016/j.pain.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tchivileva IE, Lim PF, Smith SB, Slade GD, Diatchenko L, McLean SA, Maixner W. Effect of catechol-O-methyltransferase polymorphism on response to propranolol therapy in chronic musculoskeletal pain: a randomized, double-blind, placebo-controlled, crossover pilot study. Pharmacogenet Genomics. 2010;20:239–248. doi: 10.1097/FPC.0b013e328337f9ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nackley AG, Tan KS, Fecho K, Flood P, Diatchenko L, Maixner W. Catechol-O-methyltransferase inhibition increases pain sensitivity through activation of both beta(2)- and beta(3)-adrenergic receptors. Pain. 2007;128:199–208. doi: 10.1016/j.pain.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Segall SK, Maixner W, Belfer I, Wiltshire T, Seltzer Z, Diatchenko L. Janus molecule I: dichotomous effects of COMT in neuropathic vs nociceptive pain modalities. CNS Neurol Diso rd Drug Targets. 2012;11:222–235. doi: 10.2174/187152712800672490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sullivan MJ, Thorn B, Haythornthwaite JA, Keefe F, Martin M, Bradley LA, Lefebvre JC. Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain. 2001;17:52–64. doi: 10.1097/00002508-200103000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Edwards RR, Cahalan C, Mensing G, Smith M, Haythornthwaite JA. Pain, catastrophizing, and depression in the rheumatic diseases. Nat Rev Rheumatol. 2011;7:216–224. doi: 10.1038/nrrheum.2011.2. [DOI] [PubMed] [Google Scholar]
- 25.Wideman TH, Finan PH, Edwards RR, Quartana PJ, Buenaver LF, Haythornthwaite JA, Smith MT. Increased sensitivity to physical activity among individuals with knee osteoarthritis: relation to pain outcomes, psychological factors, and responses to quantitative sensory testing. Pain. 2014;155:703–711. doi: 10.1016/j.pain.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 26.Day MA, Thorn BE. The relationship of demographic and ps ychosocial variables to pain-related outcomes in a rural chronic pain population. Pain. 2010;151:467–474. doi: 10.1016/j.pain.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.George SZ, Hirsh AT. Psychologic influence on experimental pain sensitivity and clinical pain intensity for patients with shoulder pain. J Pain. 2009;10:293–299. doi: 10.1016/j.jpain.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parr JJ, Borsa PA, Fillingim RB, Tillman MD, Manini TM, Gregory CM, George SZ. Pain-related fear and catastrophizing predict pain intensity and disability independently using an induced muscle injury model. J Pain. 2012;13:370–378. doi: 10.1016/j.jpain.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.George SZ, Wallace MR, Wright TW, Moser MW, Greenfield WH, III, Sack BK, Herbstman DM, Fillingim RB. Evidence for a biopsychosocial influence on shoulder pain: Pain catastrophizing and catechol-O-methyltransferase (COMT) diplotype predict clinical pain ratings. Pain. 2007;136:53–61. doi: 10.1016/j.pain.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.George SZ, Dover GC, Wallace MR, Sack BK, Herbstman DM, Aydog E, Fillingim RB. Biopsychosocial influence on exercise-induced delayed onset muscle soreness at the shoulder: pain catastrophizing and catechol-o-methyltransferase (COMT) diplotype predict pain ratings. Clin J Pain. 2008;24:793–801. doi: 10.1097/AJP.0b013e31817bcb65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.George SZ, Parr JJ, Wallace MR, Wu SS, Borsa PA, Dai Y, Fillingim RB. Biopsychosocial influence on exercise-induced injury: genetic and psychological combinations are predictive of shoulder pain phenotypes. J Pain. 2014;15:68–80. doi: 10.1016/j.jpain.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. J Am Podiatr Med Assoc. 2001;91:437–442. doi: 10.7547/87507315-91-8-437. [DOI] [PubMed] [Google Scholar]
- 33.Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gotzsche PC, Krleza-Jeric K, Hrobjartsson A, Mann H, Dickersin K, Berlin JA, Dore CJ, Parulekar WR, Summerskill WS, Groves T, Schulz KF, Sox HC, Rockhold FW, Rennie D, Moher D. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158:200–207. doi: 10.7326/0003-4819-158-3-201302050-00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychological Assessment. 1995;7:524–532. [Google Scholar]
- 35.Van Damme S, Crombez G, Bijttebier P, Goubert L, Van Houdenhove B. A confirmatory factor analysis of the Pain Catastrophizing Scale: invariant factor structure across clinical and non -clinical populations. Pain. 2002;96:319–324. doi: 10.1016/S0304-3959(01)00463-8. [DOI] [PubMed] [Google Scholar]
- 36.Bartley EJ, Fillingim RB. Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth. 2013;111:52–58. doi: 10.1093/bja/aet127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greenspan JD, Craft RM, LeResche L, Arendt-Nielsen L, Berkley KJ, Fillingim RB, Gold MS, Holdcroft A, Lautenbacher S, Mayer EA, Mogil JS, Murphy AZ, Traub RJ. Studying sex and gender differences in pain and analgesia: a consensus report. Pain. 2007;132(Suppl 1):S26–S45. doi: 10.1016/j.pain.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kindler LL, Valencia C, Fillingim RB, George SZ. Sex differences in experimental and clinical pain sensitivity for patients with shoulder pain. Eur J Pain. 2011;15:118–123. doi: 10.1016/j.ejpain.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Belfer I, Segall SK, Lariviere WR, Smith SB, Dai F, Slade GD, Rashid NU, Mogil JS, Campbell CM, Edwards RR, Liu Q, Bair E, Maixner W, Diatchenko L. Pain modality- and sex-specific effects of COMT genetic functional variants. Pain. 2013;154:1368–1376. doi: 10.1016/j.pain.2013.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Plotnikoff NA, MacIntyre DL. Test-retest reliability of glenohumeral internal and external rotator strength. Clin J Sport Med. 2002;12:367–372. doi: 10.1097/00042752-200211000-00008. [DOI] [PubMed] [Google Scholar]
- 41.Borsa PA, Sauers EL. The importance of gender on myokinetic deficits before and after microinjury. Med Sci Sports Exerc. 2000;32:891–896. doi: 10.1097/00005768-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 42.Carpenter JE, Blasier RB, Pellizzon GG. The effects of muscle fatigue on shoulder joint position sense. Am J Sports Med. 1998;26:262–265. doi: 10.1177/03635465980260021701. [DOI] [PubMed] [Google Scholar]
- 43.Myers JB, Guskiewicz KM, Schneider RA, Prentice WE. Proprioception and Neuromuscular Control of the Shoulder After Muscle Fatigue. J Athl Train. 1999;34:362–367. [PMC free article] [PubMed] [Google Scholar]
- 44.Voight ML, Hardin JA, Blackburn TA, Tippett S, Canner GC. The effects of muscle fatigue on and the relationship of arm dominance to shoulder proprioception. J Orthop Sports Phys Ther. 1996;23:348–352. doi: 10.2519/jospt.1996.23.6.348. [DOI] [PubMed] [Google Scholar]
- 45.George SZ, Parr JJ, Wallace MR, Wu SS, Borsa PA, Dai Y, Fillingim RB. Inflammatory Genes and Psychological Factors Predict Induced Shoulder Pain Phenotype. Med Sci Sports Exerc. 2014 doi: 10.1249/MSS.0000000000000328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tis LL, Maxwell T. The effect of positioning on shoulder isokinetic measures in females. Med Sci Sports Exerc. 1996;28:1188–1192. doi: 10.1097/00005768-199609000-00016. [DOI] [PubMed] [Google Scholar]
- 47.Dauty M, Delbrouck C, Hugue D, Rousseau B. Reproducibility of concentric an d eccentric isokinetic strength of the shoulder rotators in normal subjects 40 to 55 years old. Isokinetics and Exercise Science. 2003:95–100. [Google Scholar]
- 48.Mandalidis DG, Donne B. Reliability of isokinetic internal and external rotation of the shoulder in the sca pular plane. Isokinetics and Exercise Science. 2001:65–72. [Google Scholar]
- 49.Dannecker EA, Hausenblas HA, Kaminski TW, Robinson ME. Sex differences in delayed onset muscle pain. Clin J Pain. 2005;21:120–126. doi: 10.1097/00002508-200503000-00002. [DOI] [PubMed] [Google Scholar]
- 50.Dannecker EA, Gagnon CM, Jump RL, Brown JL, Robinson ME. Self-care behaviors for muscle pain. J Pain. 2004;5:521–527. doi: 10.1016/j.jpain.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 51.Dannecker EA, Koltyn KF, Riley JL, III, Robinson ME. Sex differences in delayed onset muscle soreness. J Sports Med Phys Fitness. 2003;43:78–84. [PubMed] [Google Scholar]
- 52.Poudevigne MS, O’Connor PJ, Pasley JD. Lack of both sex differences and influence of resting blood pressure on muscle pain intensity. Clin J Pain. 2002;18:386–393. doi: 10.1097/00002508-200211000-00007. [DOI] [PubMed] [Google Scholar]
- 53.Clarkson PM, Hubal MJ. Exercise-induced muscle damage in humans. Am J Phys Med Rehabil. 2002;81:S52–S69. doi: 10.1097/00002060-200211001-00007. [DOI] [PubMed] [Google Scholar]
- 54.Evaskus DS, Laskin DM. A biochemical measure of stress in patients with myofascial pain -dysfunction syndrome. J Dent Res. 1972;51:1464–1466. doi: 10.1177/00220345720510053501. [DOI] [PubMed] [Google Scholar]
- 55.Perry F, Heller PH, Kamiya J, Levine JD. Altered autonomic function in patients with arthritis or with chronic myofascial pain. Pain. 1989;39:77–84. doi: 10.1016/0304-3959(89)90177-2. [DOI] [PubMed] [Google Scholar]
- 56.Torpy DJ, Papanicolaou DA, Lotsikas AJ, Wilder RL, Chrousos GP, Pillemer SR. Responses of the sympathetic nervous system and the hypothalamic-pituitary-adrenal axis to interleukin-6: a pilot study in fibromyalgia. Arthritis Rheum. 2000;43:872–880. doi: 10.1002/1529-0131(200004)43:4<872::AID-ANR19>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 57.Khasar SG, McCarter G, Levine JD. Epinephrine produces a beta-adrenergic receptor-mediated mechanical hyperalgesia and in vitro sensitization of rat nociceptors. J Neurophysiol. 1999;81:1104–1112. doi: 10.1152/jn.1999.81.3.1104. [DOI] [PubMed] [Google Scholar]
- 58.Light KC, Bragdon EE, Grewen KM, Brownley KA, Girdler SS, Maixner W. Adrenergic dysregulation and pain with and without acute beta-blockade in women with fibromyalgia and temporomandibular disorder. J Pain. 2009;10:542–552. doi: 10.1016/j.jpain.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wood PB, Kablinger AS, Caldito GS. Open trial of pindolol in the treatment of fibromyalgia. Ann Pharmacother. 2005;39:1812–1816. doi: 10.1345/aph.1G014. [DOI] [PubMed] [Google Scholar]
- 60.Rodrigues LL, Oliveira MC, Pelegrini-da-Silva A, de Arruda Veiga MC, Parada CA, Tambeli CH. Peripheral sympathetic component of the temporomandibular joint inflammatory pain in rats. J Pain. 2006;7:929–936. doi: 10.1016/j.jpain.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 61.Tonussi CR, Ferreira SH. Rat knee-joint carrageenin incapacitation test: an objective screen for central and peripheral analgesics. Pain. 1992;48:421–427. doi: 10.1016/0304-3959(92)90095-S. [DOI] [PubMed] [Google Scholar]
- 62.Mannisto PT, Kaakkola S. Catechol-O-methyltransferase (COMT): biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacol Rev. 1999;51:593–628. [PubMed] [Google Scholar]
- 63.Gursoy S, Erdal E, Herken H, Madenci E, Alasehirli B, Erdal N. Significance of catechol-O-methyltransferase gene polymorphism in fibromyalgia syndrome. Rheumatol Int. 2003;23:104–107. doi: 10.1007/s00296-002-0260-5. [DOI] [PubMed] [Google Scholar]
- 64.Zubieta JK, Heitzeg MM, Smith YR, Bueller JA, Xu K, Xu Y, Koeppe RA, Stohler CS, Goldman D. COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science. 2003;299:1240–1243. doi: 10.1126/science.1078546. [DOI] [PubMed] [Google Scholar]
- 65.George SZ, Zeppieri G, Jr, Cere AL, Cere MR, Borut MS, Hodges MJ, Reed DM, Valencia C, Robinson ME. A randomized trial of behavioral physical therapy interventions for acute and sub -acute low back pain ( NCT00373867) Pain. 2008;140:145–157. doi: 10.1016/j.pain.2008.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.George SZ, Fritz JM, Bialosky JE, Donald DA. The effect of a fear-avoidance-based physical therapy intervention for patients with acute low back pain: results of a randomized clinical trial. Spine. 2003;28:2551–2560. doi: 10.1097/01.BRS.0000096677.84605.A2. [DOI] [PubMed] [Google Scholar]
- 67.George SZ, Teyhen DS, Wu SS, Wright AC, Dugan JL, Yang G, Robinson ME, Childs JD. Psychosocial education improves low back pain beliefs: results from a cluster randomized clinical trial ( NCT00373009) in a primary prevention setting. Eur Spine J. 2009;18:1050–1058. doi: 10.1007/s00586-009-1016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.George SZ, Childs JD, Teyhen DS, Wu SS, Wright AC, Dugan JL, Robinson ME. Briefy psychosocial education, not core stabilization, reduced incidence of low back pain: results from the Prevention of Low Back Pain in the Military (POLM) cluster randomized trial. BMC Medicine. 2011;9:128. doi: 10.1186/1741-7015-9-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nicholas MK, George SZ. Psychologically informed interventions for low back pain: an update for physical therapists. Phys Ther. 2011;91:765–776. doi: 10.2522/ptj.20100278. [DOI] [PubMed] [Google Scholar]
- 70.Thorn BE, Pence LB, Ward LC, Kilgo G, Clements KL, Cross TH, Davis AM, Tsui PW. A randomized clinical trial of targeted cognitive behavioral treatment to reduce catastrophizing in chronic headache sufferers. J Pain. 2007;8:938–949. doi: 10.1016/j.jpain.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 71.George SZ, Dover GC, Fillingim RB. Fear of Pain Influences Outcomes After Exercise-induced Delayed Onset Muscle Soreness at the Shoulder. Clin J Pain. 2007;23:76–84. doi: 10.1097/01.ajp.0000210949.19429.34. [DOI] [PubMed] [Google Scholar]
- 72.Trost Z, France CR, Sullivan MJ, Thomas JS. Pain-related fear predicts reduced spinal motion following experimental back injury. Pain. 2012;153:1015–1021. doi: 10.1016/j.pain.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 73.Trost Z, France CR, Thomas JS. Pain-related fear and avoidance of physical exertion following delayed -onset muscle soreness. Pain. 2011;152:1540–1547. doi: 10.1016/j.pain.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 74.Burns JW, Day MA, Thorn BE. Is reduction in pain catastrop hizing a therapeutic mechanism specific to cognitive-behavioral therapy for chronic pain? Transl Behav Med. 2012;2:22–29. doi: 10.1007/s13142-011-0086-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, Cleeland CS. Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain. 2004;20:309–318. doi: 10.1097/00002508-200409000-00005. [DOI] [PubMed] [Google Scholar]
- 76.Gummesson C, Ward MM, Atroshi I. The shortened disabilities of the arm, shoulder and hand questionnaire (QuickDASH): validity and reliability based on responses within the full-length DASH. BMC Musculoskelet Disord. 2006;7:44. doi: 10.1186/1471-2474-7-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Johnson JD, Campisi J, Sharkey CM, Kennedy SL, Nickerson M, Greenwood BN, Fleshner M. Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience. 2005;135:1295–1307. doi: 10.1016/j.neuroscience.2005.06.090. [DOI] [PubMed] [Google Scholar]
- 78.Soszynski D, Kozak W, Conn CA, Rudolph K, Kluger MJ. Beta-adrenoceptor antagonists suppress elevation in body temperature and increase in plasma IL-6 in rats exposed to open field. Neuroendocrinology. 1996;63:459–467. doi: 10.1159/000127072. [DOI] [PubMed] [Google Scholar]
- 79.Hanke ML, Powell ND, Stiner LM, Bailey MT, Sheridan JF. Beta adrenergic blockade decreases the immunomodulatory effects of social disruption stress. Brain Behav Immun. 2012;26:1150–1159. doi: 10.1016/j.bbi.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huang QH, Takaki A, Arimura A. Central noradrenergic system modulat es plasma interleukin-6 production by peripheral interleukin-1. Am J Physiol. 1997;273:R731–R738. doi: 10.1152/ajpregu.1997.273.2.R731. [DOI] [PubMed] [Google Scholar]
- 81.Edwards RR, Kronfli T, Haythornthwaite JA, Smith MT, McGuire L, Page GG. Association of catastrophizing with interleukin-6 responses to acute pain. Pain. 2008;140:135–144. doi: 10.1016/j.pain.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Darnall BD, Aickin M, Zwickey H. Pilot study of inflammatory responses following a negative imaginal focus in persons with chronic pain: analysis by sex/gender. Gend Med. 2010;7:247–260. doi: 10.1016/j.genm.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 83.Goubert L, Crombez G, Van Damme S, Vlaeyen JW, Bijttebier P, Roelofs J. Confirmatory factor analysis of the Tampa Scale for Kinesiophobia: invariant two-factor model across low back pain patients and fibromyalgia patients. Clin J Pain. 2004;20:103–110. doi: 10.1097/00002508-200403000-00007. [DOI] [PubMed] [Google Scholar]
- 84.Woby SR, Roach NK, Urmston M, Watson PJ. Psychometric properties of the TSK-11: a shortened version of the Tampa Scale for Kinesiophobia. Pain. 2005;117:137–144. doi: 10.1016/j.pain.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 85.McNeil DW, Rainwater AJ. Development of the Fear of Pain Questionnaire--III. J Behav Med. 1998;21:389–410. doi: 10.1023/a:1018782831217. [DOI] [PubMed] [Google Scholar]
- 86.Albaret MC, Munoz Sastre MT, Cottencin A, Mullet E. The Fear of Pain questionnaire: factor structure in samples of young, middle-aged and elderly European people. Eur J Pain. 2004;8:273–281. doi: 10.1016/j.ejpain.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 87.Osman A, Breitenstein JL, Barrios FX, Gutierrez PM, Kopper BA. The Fear of Pain Questionnaire-III: further reliability and validity with nonclinical samples. J Behav Med. 2002;25:155–173. doi: 10.1023/a:1014884704974. [DOI] [PubMed] [Google Scholar]
- 88.Leeuw M, Goossens ME, Linton SJ, Crombez G, Boersma K, Vlaeyen JW. The fear-avoidance model of musculoskeletal pain: current state of scientific evidence. J Behav Med. 2007;30:77–94. doi: 10.1007/s10865-006-9085-0. [DOI] [PubMed] [Google Scholar]
- 89.Hubscher M, Moloney N, Leaver A, Rebbeck T, McAuley JH, Refshauge KM. Relationship between quantitative sensory testing and pain or disability in people with spinal pain -a systematic review and meta-analysis. Pain. 2013;154:1497–1504. doi: 10.1016/j.pain.2013.05.031. [DOI] [PubMed] [Google Scholar]
- 90.Cruz-Almeida Y, Fillingim RB. Can quantitative sensory testing move us closer to mechanism-based pain management? Pain Med. 2014;15:61–72. doi: 10.1111/pme.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Coronado RA, Kindler LL, Valencia C, George SZ. Thermal and pressure pain sensitivity in patients with unilateral shoulder pain: comparison of involved and uninvolved sides. J Orthop Sports Phys Ther. 2011;41:165–173. doi: 10.2519/jospt.2011.3416. [DOI] [PubMed] [Google Scholar]
- 92.Valencia C, Fillingim RB, George SZ. Suprathreshold heat pain response is associated with clinical pain intensity for patients with shoulder pain. J Pain. 2011;12:133–140. doi: 10.1016/j.jpain.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Valencia C, Kindler LL, Fillingim RB, George SZ. Investigation of central pain processing in shoulder pain: converging results from 2 musculoskeletal pain models. J Pain. 2012;13:81–89. doi: 10.1016/j.jpain.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Valencia C, Kindler LL, Fillingim RB, George SZ. Stability of conditioned pain modulation in two musculoskeletal pain models: investigating the influence of shoulder pain intensity and gender. BMC Musculoskelet Disord. 2013;14:182. doi: 10.1186/1471-2474-14-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983;17:45–56. doi: 10.1016/0304-3959(83)90126-4. [DOI] [PubMed] [Google Scholar]
- 96.Valencia C, Fillingim RB, Bishop M, Wu SS, Wright TW, Moser M, Farmer K, George SZ. Investigation of Central Pain Processing in Post-Operative Shoulder Pain and Disability. Clin J Pain. 2013:775–786. doi: 10.1097/AJP.0000000000000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Holm S. A simple sequentially rejective multiple test procedure. Scand J Statistics. 1979;6:65–70. [Google Scholar]
- 98.Kuijpers T, van der Windt DA, van der Heijden GJ, Bouter LM. Systematic review of prognostic cohort studies on shoulder disorders. Pain. 2004;109:420–431. doi: 10.1016/j.pain.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 99.van der Heijden GJ. Shoulder disorders: a state-of-the-art review. Baillieres Clin Rheumatol. 1999;13:287–309. [PubMed] [Google Scholar]
- 100.Picavet HS, Schouten JS. Musculoskeletal pain in the Netherlands: prevalences, consequences and risk groups, the DMC(3)-study. Pain. 2003;102:167–178. doi: 10.1016/s0304-3959(02)00372-x. [DOI] [PubMed] [Google Scholar]
- 101.Urwin M, Symmons D, Allison T, Brammah T, Busby H, Roxby M, Simmons A, Williams G. Est imating the burden of musculoskeletal disorders in the community: the comparative prevalence of symptoms at different anatomical sites, and the relation to social deprivation. Ann Rheum Dis. 1998;57:649–655. doi: 10.1136/ard.57.11.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.van der Windt DA, Koes BW, Boeke AJ, Deville W, De Jong BA, Bouter LM. Shoulder disorders in general practice: prognostic indicators of outcome. Br J Gen Pract. 1996;46:519–523. [PMC free article] [PubMed] [Google Scholar]