Abstract
The ability to regenerate damaged or lost tissues has remained the lofty goal of regenerative medicine. Unfortunately, humans, like most mammals, suffer from very minimal natural regenerative capabilities. Certain non-mammalian animal species, however, are not so limited in their healing capabilities, and several have attracted the attention of researchers hoping to recreate enhanced healing responses in humans. This review focuses on one such animal group with remarkable regenerative abilities, the lizards. As the closest relatives of mammals that exhibit enhanced regenerative abilities as adults, lizards potentially represent the most relevant model for direct comparison and subsequent improvement of mammalian healing. Lizards are able to regenerate amputated tails, and exhibit adaptations that both limit tissue damage in response to injury and initiate coordinated regenerative responses. This review summarizes the salient aspects of lizard tail regeneration as they relate to the overall regenerative process, and also presents the relevant information pertaining to regrowth of specific tissues, including skeletal, muscular, nervous, and vascular tissues. The goal of this review is to introduce the topic of lizard tail regeneration to new audiences with the hope of expanding the knowledge base of this under-utilized but potentially powerful model organism.
Keywords: lizard, salamander, cartilage, muscle, spinal cord, peripheral nerve, regeneration
Introduction
The quest to improve human wound healing has benefited from the use of a variety of mammalian animal models in all areas of research, from elucidating the mechanisms of healing processes to testing therapies. However, comparatively little work has been extended to non-mammalian animals, some of which exhibit the most amazing wound healing abilities among vertebrate animals. The goal of this review is to introduce these organisms and their regenerative capabilities to an audience who otherwise might not consider these non-traditional, but potentially instructive, animals for research aimed at augmenting mammalian regeneration.
Reptiles and amphibians spontaneously regenerate cartilaginous skeletons in response to skeletal injury
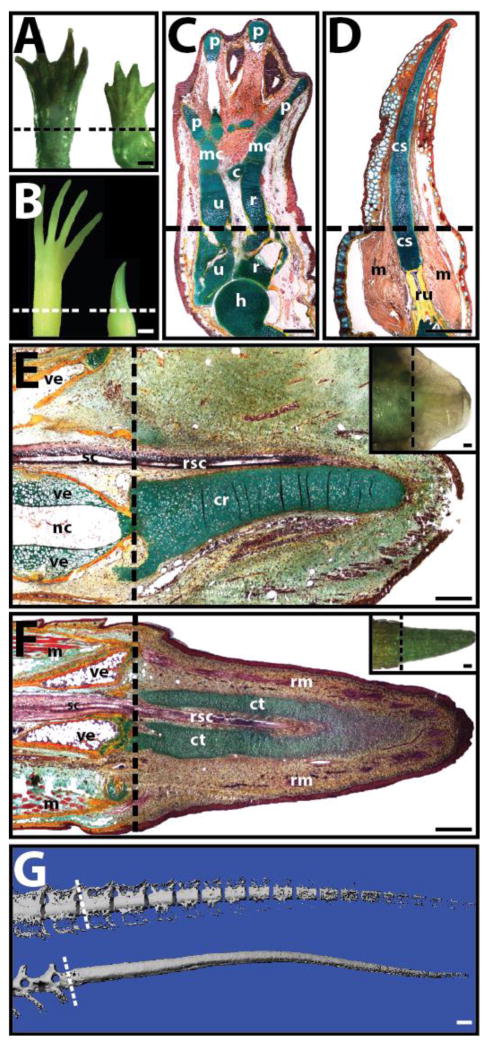
The ability to regenerate whole appendages (i.e. limbs and tails) is a rarity among adult vertebrates. The most impressive examples of appendage regeneration are exhibited by the amphibians, including the urodeles (salamanders and newts) and anurans (frogs and toads). Salamanders and frogs are able to regenerate limbs (Fig. 1A–D). Neotenic salamanders, which never fully develop and retain non-ossified, cartilaginous skeletons into adulthood, are able to regenerate fully formed limbs (Fig. 1A), with all the original cartilaginous skeletal elements of the originals (Fig. 1C). Regenerated salamander limbs also recreate the musculature of the amputated arms/legs. Frogs, which do fully develop and exhibit ossified skeleton as adults, regenerate cartilage spikes rather than limbs following amputation (Fig. 1B). Cartilage spikes are continuous with the radio-ulna bone of the original limb, and no other skeletal elements are formed, and very little muscle is regenerated (Fig. 1D).
Fig. 1.
Examples of limb and tail regeneration in amphibians and lizards. (A, B) Morphological comparison of (A) salamander (Ambystoma mexicanum) and (B) frog (Xenopus laevis) forelimbs before (left) and 8 weeks after (right) amputation. Salamanders regenerate new limbs, while frogs regenerate cartilage spikes. (C, D) Histological analysis (pentachrome) of regenerated (C) salamander and (D) frog limbs. Salamanders regenerate all the skeletal elements of the upper arm and hand, while frogs regenerate a single cartilage spike. (E, F) Histological (pentachrome) and (E, F Insets) morphological analysis of (E) salamander tail 5-weeks post amputation and (F) lizard (Anolis carolinensis) tail 2 weeks post-amputation. (G) Salamander (top) and lizard (bottom) tails 10 weeks after amputation analyzed by micro computed tomography. The regenerated salamander, but not lizard, skeleton segments. Pentachrome stains cartilage green, bone orange, muscle red, and spinal cord and epidermis purple. Dashed lines denote amputation planes. c, carpal; cr, cartilage rod; cs, cartilage spike; ct, cartilage tube; h, humerus; m, muscle; mc, metacarpal; nc, notochord; p, phalanges; r, radius; rm, regenerated muscle; rsc, regenerated spinal cord; ru, radio-ulna; sc, spinal cord; u, ulna; ve, vertebra. Bar = 1 mm.
This inverse relationship between skeletal development/maturity and regeneration fidelity, as well as the preference for producing cartilage, are also observed in tail regeneration. Lizards are the only group of amniotes capable of tail regeneration as adults, and, unlike the anamniotic salamanders, adult lizard axial skeletons are fully ossified. Both salamanders and lizards regenerate tails, and both regenerated tail skeletons are almost completely cartilaginous (Fig. 1E, F). Salamanders regenerate cartilage rods ventral to regenerated spinal cords (Fig. 1E), while lizards regenerate cartilage tubes that enclose regenerated spinal cords (Fig. 1F). However, regenerated tails of the less skeletally developed salamander segment and develop neural and hemal arches, and mature regenerated salamander tails are almost perfect copies of originals (Fig. 1G). The more skeletally matured lizards, on the other hand, regrow imperfect regenerated tails, and lizard cartilage tubes never segment and are easily distinguishable from original tail skeletons (Fig. 1G).
In summation, non-mammalian vertebrate skeletal regeneration favors cartilage regeneration over bone. This is particularly interesting given that cartilage is a tissue that most mammals, and humans, are completely unable to heal, let alone regenerate. Given that, among the regenerative vertebrates, only lizards are grouped with mammals as amniotes, and that many of the regenerative properties and processes exemplified in lizards is shared with amphibians, the bulk of this review will focus on the lizard in its discussion of enhanced wound healing capabilities.
Lizard tails exhibit modifications that facilitate tail loss and minimize tissue damage
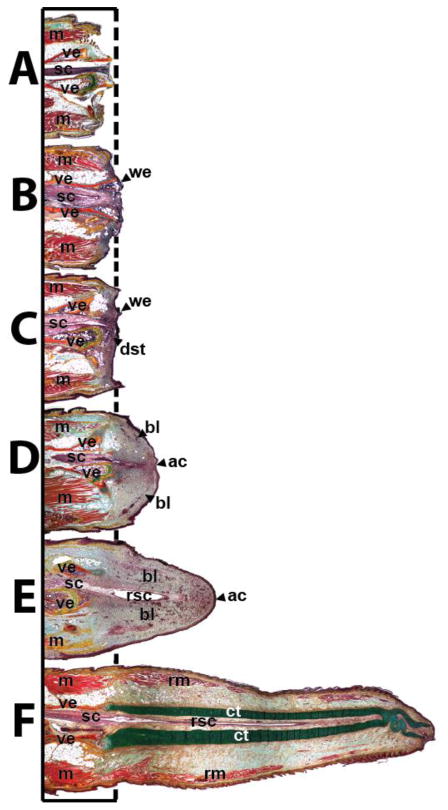
As a general rule, all lizard species capable of tail regeneration exhibit the ability to shed or readily lose their tails as a method of escape from potential predators. This ability of “self-amputation”, known as autotomy, is facilitated by several modifications to lizard tail tissues that work to minimize tissue damage during tail loss. For example, lizard tail vertebrae contain structures known as fracture planes, pre-formed breaks in the bone along which tails readily separate (1) (Fig. 2). Each fracture plane is continuous with autotomy septa that pass through fat, muscle, and connective tissue. Furthermore, fracture planes and autotomy septa are positioned just distal to sphincters and valves in caudal arteries and veins, respectively. Autotomy planes/septa do not bisect the tail spinal cord, however, which is severed during tail loss. Thus, following tail loss by autotomy, tail tissues separate along fracture planes and autotomy septa, minimizing bone and muscle tissue damage, and blood vessel sphincters/valves remaining in tail stumps contract to limit blood loss. The resultant tail stump ends at the vertebrae fracture plane and includes a severed spinal cord (Fig. 3A).
Fig. 2.
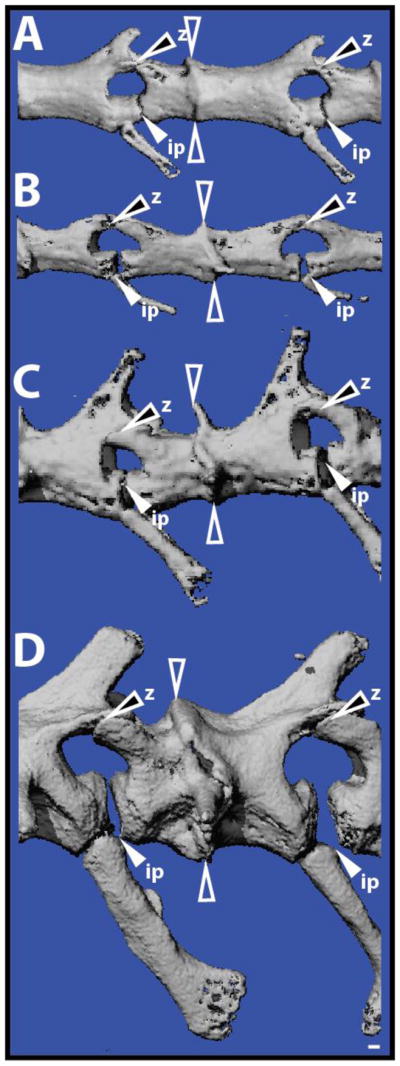
Autotomous lizard tail vertebrae contain fracture planes. Original tails of (A) Anolis carolinensis, (B) Hemidactylus frenatus, (C) Heteronotia binoei, and (D) Hoplodactylus duvaucelii analyzed by micro computed tomography and highlighting the diversity of fracture plane position and structure. Open arrowheads denote intravertebral fracture plane. White-filled arrowheads mark intervertebral pads (ip). Black-filled arrowheads identify Z-joints (z). Bar = 100 μm.
Fig. 3.
Representative lizard tails (A) 0, (B) 3, (C) 6, (D) 9, (E) 12, (F) 15, (G) and 28 days following tail loss highlighting the important structures involved with tail regrowth. ac, apical cap; bl, blastema; ct, cartilage tube; dst, degenerated stump tissue; m, muscle; rm, regenerated muscle; rsc, regenerated spinal cord; sc, spinal cord; we, wound epithelium; ve, vertebra.
While fracture planes and autotomy septa greatly reduce tissue damage following autotomy, they are apparently not required for regeneration. For example, amputation of gecko tails by scalpel blades outside of fracture planes does not appear to affect tail regeneration (2), with both autotomized and amputated tails regenerating similarly. However, other studies involving Anolis lizards have indicated that amputated tails significantly regenerate shorter compared to autotomized tails (3). Perhaps the most telling evidence supporting the hypothesis that tail regeneration does not require autotomy planes is the fact that amputations made to regenerated tail regions are able to re-regenerate (Fig. 4). As previously mentioned, regenerated lizard tail skeletons consist of unsegmented cartilage tubes (Fig. 4A,B). While lizard cartilage tubes partially calcify, they do not develop fracture planes (Fig. 4B). Interestingly, amputations to either proximal or distal regenerated regions resulted in re-regenerated tails of similar lengths to those regrown from original tail amputation sites (Fig. 4C) (Lozito, unpublished data). Regeneration efficiency was influenced by both position and lizard species. For example, in Anolis lizards, proximal regenerated tail regions re-regenerated in response to 98% of amputations, while more distal regions re-regenerated in response to only 9.57% of amputations (Lozito, unpublished data). In geckos, however, distal regenerated tails regions re-regenerated in response to 100% of amputations (Lozito, unpublished data). In this light, it appears that fracture planes and regeneration are separate, and the inability of other reptilian tails to regenerate (snakes, turtles, rarely in crocodilians) is probably not due to a lack of fracture planes. Instead, it is probable that regenerative lizards possess some cell-type and/or signal that is not found in non-regenerative reptiles and that is responsible for initiating regeneration.
Fig. 4.
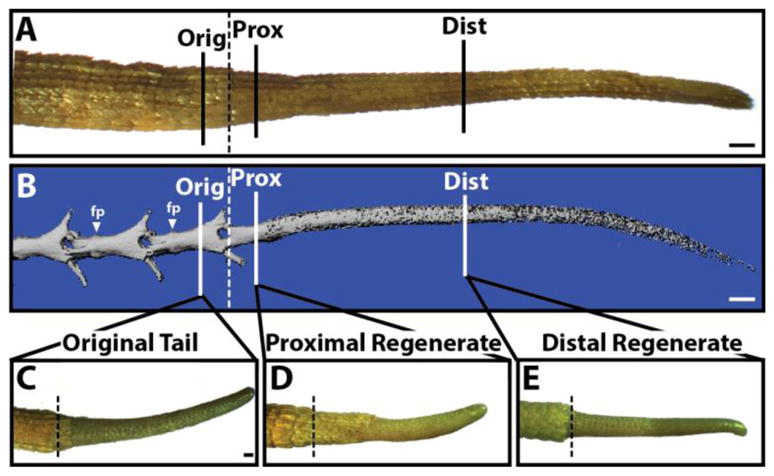
Regenerated lizard tails are able to re-regenerate following amputation. (A,B) Mature lizard tail regenerates with CTs were amputated in (1) the original tail vertebra (Orig), (2) the proximal regenerated tail (Prox), or (3) the distal regenerated tail (Dist). (A) Morphological and (B) microCT analyses of an intact mature lizard regenerate showing relative position of amputation sites. (B) The skeletons of original, but not regenerated, tail regions. Fracture planes (fp) are present in (C–E) Following 4 weeks of regeneration, tails amputated in the (C) original tail, (D) proximal regenerate, or (E) distal regenerates were analyzed for overall regenerate elongation. fp, fracture plane. Bar = 0.5 cm.
Lizard tail regeneration follows waves of degeneration, proliferation, and differentiation
Regardless of whether the lizard tail is amputated or autotomized, the first stage of regeneration is actually characterized by tail stump tissue degeneration and breakdown. Within days of tail loss, macrophages and osteoclasts home to stump tissues, where they proliferate and secrete proteases such as matrix metallopeptidase 9 (MMP-9) (1, 4). These proteases breakdown stump tissues, including the terminal tail vertebra, which is effectively cut in half by osteoclasts (1) (Fig. 5). The exception to this tissue degeneration is the epidermis, which proliferates around the wound surface and migrates through the break in the terminal tail vertebra created by the osteoclasts (Fig. 3B). Several days later, when the most distal portion of the tail stump is shed, a process known as ablation, the stump is completely covered by new epidermal tissues, which is referred to as wound epidermis (Fig. 3C). Macrophages, osteoclasts, and wound epidermis secrete proteases that degenerate stump bone, muscle, and connective tissue, releasing a variety of cell types directly under the wound epidermis (Fig. 3C). Cells derived from degenerated stump tissues secrete signals, such as insulin-like growth factor-2 (IGF-2) (Fig. 6A,B) (Lozito, unpublished data), which have been shown to potentiate wound epidermis development in other models of regeneration (5). Wound epidermis thickens and stratifies as it forms the structure known as the apical cap (Fig. 3D) (Note: varied terminology has been used to refer to the apical cap, including apical epidermal cap (AEC) and apical epidermal peg (AEP).) In turn, the apical cap produces another set of signals, including Wnt5a and FGF-2 (Fig. 6B,C) (6, 7). FGF-2 signals induce proliferation and chemotaxis in tail stump ependymal cells, which line the central canal of the spinal cord (Fig. 6D–F). As they proliferate and migrate towards the apical cap, ependymal cells self-organize into a structure known as the ependymal tube, which forms the bulk of the regenerated spinal cord. Implantation of FGF-soaked beads attracts ependymal cells toward implantation sites, resulting in ependymal tube branching (Fig. 6E) (Lozito, unpublished data). Alternatively, implantation of beads soaked in the drug SU5402, a specific inhibitor of FGF receptors, blocks ependymal tube extension (Fig. 6F) (Lozito, unpublished data).
Fig. 5.
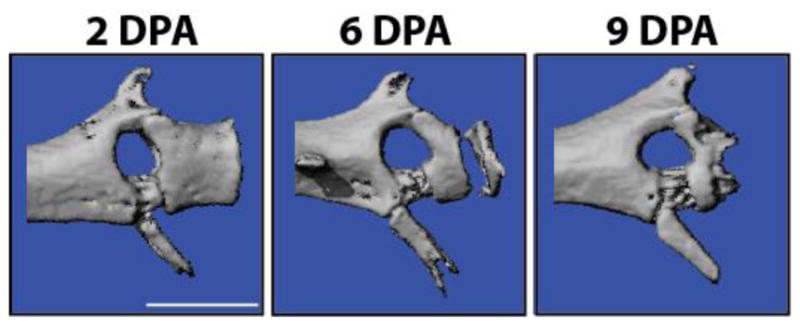
Micro computed tomography scans of original lizard tail terminal vertebrae 2, and 6, and 9 days post-autotomy (DPA) analyzed by micro computed tomography. The distal portions of terminal vertebrae are completely degraded by 9 DPA. Bar = 100 μm.
Fig. 6.
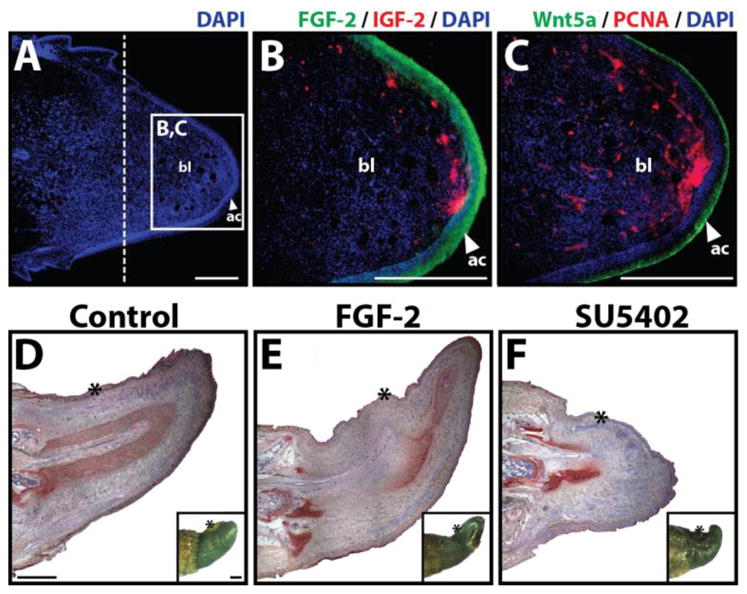
(A–C) Analysis of signaling molecules and cell proliferation markers within the regenerating lizard tail. Lizard tail blastemas (9 days following tail loss) were immunostained for IGF-2, FGF-2, Wnt5a, and PCNA. Panels B and C depict higher magnification views of region identified in Panel A. Dashed lines denote amputation planes. (D–F) Effects of FGF-2 and the FGF inhibitor SU5402 on lizard tail regeneration. Beads soaked in (D) vehicle control, (E) 100 μg/ml FGF-2, (F) or 2 mg/ml SU5402 were implanted in lizard tail blastemas. Following 7 days of growth, the tails were collected and analyzed by collagen type II immunohistochemistry. Insets of each panel depict gross tail morphology. Stars denote bead implantation sites. (D) Control tails exhibit normal ependymal tube extension. (E) Tails treated with FGF-2-soaked beads exhibit ependymal tube branches that invade toward implantation sites. (F) Tails treated with SU5402-soaked beads exhibit stunted ependymal tube invasion. ac, apical cap; bl, blastema; et, ependymal tube; ct, cartilage tube. Bar = 2 mm.
As the ependymal tube infiltrates the mass of cells released from degenerated stump tissues, stump cell populations proliferate and swell beneath the apical cap, forming the lizard tail blastema (Fig. 3D). This ends the degeneration phase of tail regeneration, also known as the latent period due to the lack of tail elongation. Blastema cell proliferation now drives rapid tail growth (Fig. 3E), which can reach 4–5 mm per day in some species (3). At this point, blastema cells begins differentiating into regenerated tissues, including muscle and skeletal tissues (Fig. 3F).
The regenerated lizard skeleton develops two distinct cartilage regions
The regenerated lizard tail skeleton takes the form of an unsegmented cartilage tube (Fig. 3F) (1, 8). At its extreme proximal end, the cartilage tube contacts the original tail vertebrae, while the distal cartilage tube extends toward the apical cap. The development and properties of the proximal and distal cartilage tube regions are different enough that they can be thought of as separate cartilaginous tissues (1). The proximal cartilage tube develops a growth-plate like region that undergoes endochondral ossification (Fig. 7). Proximal cartilage tube chondrocytes undergo hypertrophy and express Indian hedgehog (Ihh), and ossification centers from in between the terminal vertebra and proximal cartilage tube (1). Osteoclasts are recruited, and eventually the entire proximal cartilage area is replaced by bone tissue. Later, the perichondrium of the distal cartilage tube also mineralizes in most lizard species, but without undergoing endochondral ossification (Fig. 8A). The sub-perichondrium of the distal cartilage tube expresses Ihh, but the growth plate-like structure that characterizes the proximal cartilage tube never develops (1). Instead, the cartilage tube perichondrium directly calcifies, initially beginning at distinct regions along the cartilage tube edges, which grow and merge until the entire perichondrium is calcified. Distal cartilage tube calcification is restricted to the perichondrium, and the entire cartilage tube interior resists mineralization for the lifetime of the regenerate. Interestingly, while both proximal and distal cartilage tube mineralization events are spatially and temporally distinct, both are dependent upon hedgehog signaling and are inhibited by the hedgehog inhibitor cyclopamine (1).
Fig. 7.
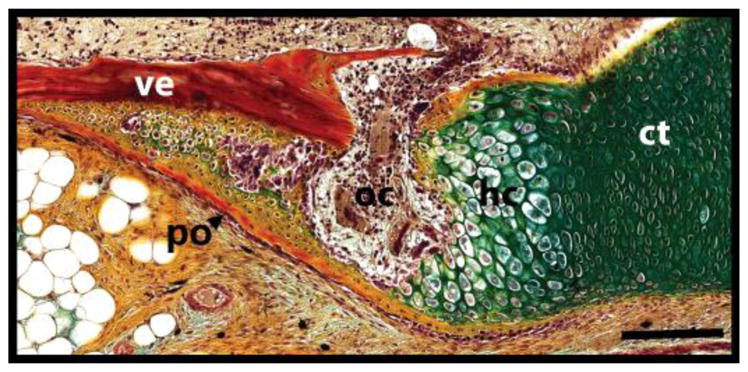
Close-up of extreme proximal cartilage tube highlighting its resemblance to a fracture callus. ct, cartilage tube; hc, hypertrophic chondrocytes; oc, ossification center; po, periosteum; ve, vertebra. Bar = 100 μm.
Fig. 8.
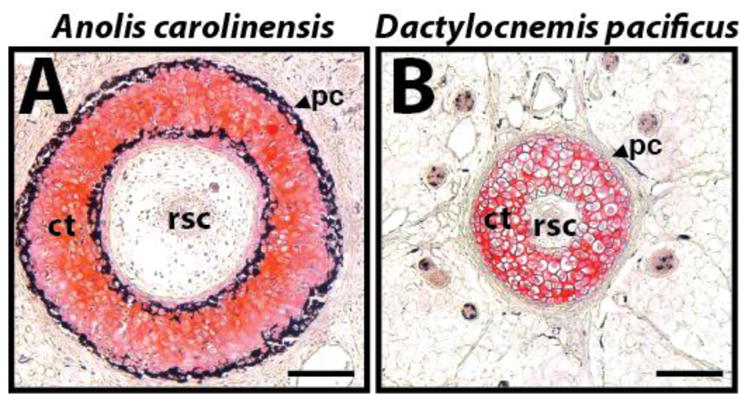
Comparison of cartilage tube perichondrium calcification between two species of lizards. Cross-sections of mature regenerated tails of (A) Anolis carolinensis and (B) the gecko Dactylocnemis pacificus analyzed by histology (von Kossa / Safranin O). Cartilage stains red, and calcified tissues stain black. The perichondrium of the Anolis cartilage tube calcifies, while the gecko cartilage tube resists calcification. ct, cartilage tube; pc, perichondrium; rsc, regenerated spinal cord. Bar = 50 μm.
Outwardly, the mature lizard cartilage tube of most lizard species appears inert and functionally limited as it is encased in a calcified perichondrium. Indeed, regenerates with calcified cartilage tunes are completely rigid, incapable of the wide degrees of mobility of the original tail. The regenerated tails of some geckonid lizards, however, remain flexible, without any appreciable decrease in functionality compared to original tails. The cartilage tube perichondria of these lizards resist calcification (Fig. 8B), and the cartilage tube matrices produced by these species contain significant amounts of elastin, resulting in much more versatile regenerated tails (9). Many of these “calcification-resistant” species are arboreal, and their tails are semi-prehensile for use as a fifth limb in climbing. In these cases, one can imagine evolutionary pressures to keep regenerated tails flexible enough to allow these lizards to survive in their niche habitats. An interesting direction for future research is determining how the cartilage tube perichondria of these arboreal geckos resist mineralization and whether the elastin found in the cartilage matrix plays a role.
Recent evidence has also indicated that even the mature cartilage tube is quite dynamic thanks to a population of cartilage stem/progenitor cells found within the perichondrium (1). These perichondrial cells respond to TGF-β signals by forming new cartilage, causing the cartilage tube to expand in circumference. The mature cartilage tube is also capable of regeneration, as amputations made within regenerated tail regions “re-regenerate” and form new cartilage tubes continuous with old ones. Thus, lizards not only regenerate robust cartilage, a rarity among higher vertebrates, but regenerated lizard tail cartilage is also able to self-renew.
Lizard tail muscle regeneration begins at distinct segments despite the absence of somites in the regenerated tail
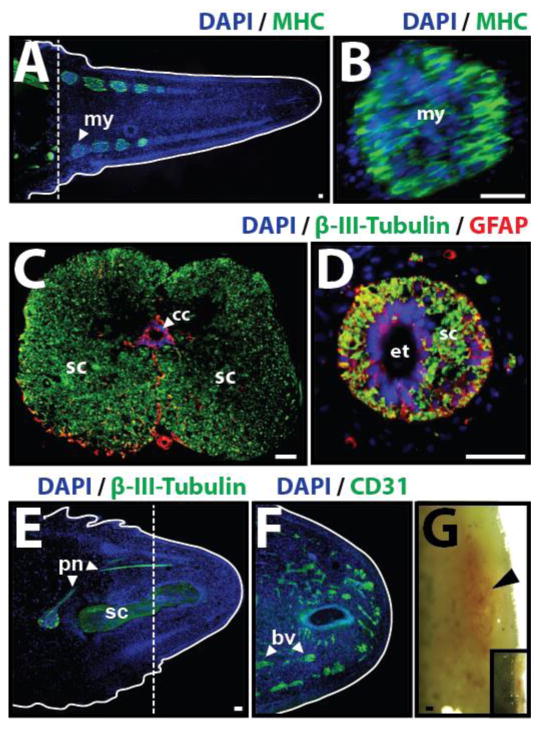
Lizard tail skeletal muscle regeneration provides an interesting and poignant contrast to lizard axial skeleton regeneration. Unlike the unsegmented cartilage tube, which develops proximally to distally as a continuous structure around the ependymal tube, regenerated lizard tail muscles begin at distinct regions in between the cartilage tube and epidermis (4, 10) (Fig. 9A). Blastema cells aggregate into defined condensations and differentiate into mononuclear myoblasts to form myomeres (Fig. 9B). Muscle differentiation proceeds proximodistally, with new myoblast aggregations forming at regular spatial and temporal intervals along the regenerating tail (Fig. 9A). As new distal myomeres form, myoblasts of older proximal myotomes elongate and align, eventually fusing to form multinucleate myofibers. Each myotome grows in size as new myoblasts differentiate and fuse with existing myofibers until successive myomeres touch, forming myomeric segments. The connective tissue between segments forms myosepta. Eventually, myomeres develop processes that interdigitate with the muscle of adjoining segments, similar to the musculature of the original tail. The first, most proximal regenerated muscle segment attaches to the last segment of the original tail stump. While regenerated muscle segments, important differences compared to the original tail musculature exist (3). Original muscle is divided into quadrants separated by horizontal and median longitudinal septa. Regenerated muscles are separated by radial septa into smaller, more numerous, bundles arranged in a ring around the cartilage tube. Also, regenerated myotubes are comparatively small in diameter and exhibit centrally located nuclei. Still, compared to lizard tail skeletal regeneration, muscle regeneration more closely resembles the processes that take place during embryonic development. How regenerated lizard tail muscle manages to segment without the formation of somites and in a tail environment in which all other tissues do not segment remains an interesting, yet unexplored, area of study.
Fig. 9.
Lizard tail muscle, spinal cord, peripheral nerve, and blood vessel regeneration. (A) Lizard tail 21 days post amputation (DPA) immunostained for muscle marker myosin heavy chain (MHC). Regenerated muscles begin as distinct myomeres. (B) Close-up of a regenerated muscle myomere. (C) Original and (D) regenerated tail spinal cords analyzed immunostained for neural marker β-III-tubulin and glial cell marker glial fibrillary acidic protein (GFAP). (E, F) Lizard tail 9 DPA immunostained for (E) β-III-tubulin and (F) the blood vessel marker CD31. (E) Peripheral nerves regenerate from dorsal root ganglia in tail stumps into tail blastemas. (F) Blood vessels form at the distal lizard tail blastema tip (G) Blood vessels also form at the distal salamander tail blastema tip. Black arrowhead marks prominent vascular bed. Inset depicts gross morphology of entire salamander tail blastema. Dashed lines denote amputation planes, and solid lines trace tail outlines. bl, blastema; drg, dorsal root ganglion; pn, peripheral nerve; sc, spinal cord. Bar = 25 μm.
Regenerated lizard tails lack true tendons but provide important resources for fat storage
Lizard tail tendon regeneration has received little attention compared to other musculoskeletal tissues, but it would appear that lizard tails do not regenerate true tendons. Unlike the originals, regenerated muscles are unattached to the skeleton, represented by the cartilage tube. Tracts of connective tissue, which has been referred to as “tendon-like”, do link radial septa with fibrous tissue surrounding the cartilage tube perichondrium (8), but the roles of these tissues in tail movement, if any, remains unclear. For example, regenerated lizard tails are able move until the cartilage tube perichondrium becomes calcified, but whether the “tendon-like” structures play roles in this movement remains to be seen. Once the perichondrium calcifies, adipose tissue develops in between muscles and cartilage in regenerated lizard tails, and obvious musculoskeletal attachments are absent. In fact, fat regeneration appears to be extremely efficient in lizard tails. Adipocytes, presumably differentiated from blastema cells, mature in a proximodistal fashion during tail regrowth, and, during later stages of regeneration, tail fat content increases as adipocytes cells hypertrophy, causing the regenerated tail to increase in circumference (11). Like the cartilage tube, the regenerated adipose layer is unsegmented as it is undivided by septa. This fat layer acts as an extremely important energy reserve critical for lizards (12), so much so that fat storage is theorized to be the most important function of most regenerated lizard tails (3).
The regenerated lizard tail nervous system consists of functional peripheral nerves and a largely nonfunctional spinal cord
Both the central and peripheral nervous systems are regenerated in the new lizard tail. The regenerated lizard tail central nervous system takes the form of a regenerated spinal cord that sprouts from the original. The central canal of the original spinal cord is lined by radial glial/ependymal cells and is surrounded by the descending and ascending axons that make up the spinal cord proper (Fig. 9C). Upon regeneration, the radial glia of the central canal proliferate and self-organize into the ependymal tube. In turn, the ependymal tube acts as a tract for the outgrowth of descending nerve fibers that sprout from the original cord (13–15). Ascending nerves are not regenerated, no new nerve cell bodies are formed, with the exception of cerebrospinal-fluid-contacting-neurons (CSFCNs) that contribute to the ependymal tube (16). Furthermore, the majority of descending nerve fibers in new tails are unmyelinated (15). Indeed, the regenerated lizard tail spinal cord can accurately be described as an ependymal tube surrounded by a disorganized mass of descending neurons and glial cells (Fig. 9D). Interestingly, the vast majority of the descending neurons of the regenerated spinal cord are confined to the interior of the cartilage tube, the functional nerve supply of the regenerate is probably derived entirely from the peripheral nerves of the stump (3).
The regenerated lizard tail peripheral nerves sprout from the last several dorsal root ganglia of the original tail stump (Fig 9E). New peripheral nerves grow distally through the early blastema and continue to extend as the regenerating tail lengthens. The regenerating motor fibers of peripheral nerves branch at regular intervals and innervate the newly formed muscle bundles, and new motor end-plates are developed. No new dorsal root ganglia are regenerated, but the last 1–3 ganglia of the original tail stump increase in size and cellularity in proportion with their expanded innervated sensory fields. Further work is needed to elucidate the exact details of lizard peripheral nerve regeneration, such as the homing and guidance mechanisms that allow new nerves to find regenerated muscle fibers, but it remains one of the most promising areas of study.
Lizard tail blood vessel regeneration is largely uncharacterized
Blood vessels form within the early blastema shortly after wound epithelium closure, and the regenerated lizard tail blastema exhibits a prominent vascular bed (Fig. 9F) (7, 17). Interestingly, the lizard tail vascular bed has been used in the past to argue against classifying the early regenerated lizard tail as a true blastema. These views are included in reports from the 1960s published by P. G. Cox highlighting differences between the early regenerated lizard tail and the classical blastema described from amphibian limb regeneration (10). More recently, however, we and others have observed that the salamander tail blastema exhibits a prominent vascular bed, just like the lizard tail blastema (Fig. 9G). These observations support the hypothesis that differences in vasculature are actually due to differences in limb versus tail regeneration rather than differences between salamanders and lizards. Thus, the true distinction to be made is between limb and tail blastemas rather than excluding early lizard tail regenerates from classification as blastemas.
Presumably regenerated tail blood vessels are directly derived from outgrowths of original tail vasculature (3). Blood vessels near the apical cap form sinusoids and associate with the apical ependymal tube (18). However, the specific cellular origins of regenerated blood vessel cells, and the signals regulating their proliferation and migration, remain unknown. Interestingly, VEGF has been detected in regenerated lizard tails, were it may play roles in blood and lymphatic vessel regeneration (19). Outgrowths derived from the two largest tail blood vessels, the caudal artery and vein, continue to grow in size and complexity during regenerate maturation. However, the regenerated caudal arteries and veins lack sphincters and valves, respectively, which echoes the lack of segmentation seen in other regenerated tail tissue.
Lizard tail regeneration is dependent upon the spinal cord
One of the first questions that arises when studying regeneration in reptiles and amphibians is “Why can these organisms regenerate, while mammals cannot?”. Comparisons between non-regenerative and regenerative organisms have identified two tissues/structures both unique to regenerative species and required for regeneration: (1) the apical cap and (2) the tail spinal cord (3). For example, wound epidermis that forms over the stumps of amputate mouse tails never develops into the thickened apical caps observed in early lizard tail regenerates. Furthermore, removal of the lizard apical cap, or replacement of the apical cap with mature skin grafts, inhibits tail regeneration (20).
Perhaps the most important tissue to regeneration is the tail spinal cord, specifically spinal cord ependyma (3). Organisms that are not capable of tail regeneration, such as mice, do not possess tail spinal cords as adults (21), while both lizards and salamanders retain tail spinal cords into adult stages. We and others have observed that destruction of spinal cords/ependyma at lizard tail amputation sites results in regeneration failure (4, 22, 23) (Fig. 10A, B). Furthermore, spinal cord/ependyma autograft implants produce small but normally structured ectopic tails at implantation sites (Fig. 10C, D).
Fig. 10.

The lizard spinal cord is necessary and sufficient for inducing regenerated tails in lizards. (A, B) Morphological comparison of lizard tails (A) with and (B) without intact spinal cords two weeks after tail loss. (A) Lizard tails with intact spinal cords regenerate new tails, but (B) tails with disrupted spinal cords fail to regenerate. Dashed line marks amputation plane. (C, D) Morphologies of lizard tails (C) with or (D) without spinal cord implants. After two weeks, (C) tails treated with exogenous spinal cord implants develop ectopic tails at implantation sites, while (D) control tails that did not receive exogenous spinal cord implants did not exhibit any growth. ect, ectopic tail. Bar = 1 mm.
Finally, it seems that interactions between the apical cap and tail spinal cord is as important to regeneration as the presence of these two tissues. The interactions are blocked by dermal tissue implants, and interposition of dermal tissue between tail spinal cords and apical caps inhibits regeneration (3, 4). Furthermore, spinal cord regeneration and extension is confined to the tail and is dependent on interactions with the apical cap. Spinal cord transection in the thoracic regions results in effectively no regeneration of the ependymal and nerves (15).
Conclusions
Lizards are amniotes with the remarkable ability to regenerate amputated tails, making them the closest relatives of mammals to exhibit enhanced healing capabilities as adults. This is important because lizard tail regeneration may offer perhaps the best opportunity for translating principles of enhanced healing towards the improvement of mammalian regeneration. Despite this promise, the study of lizard tail regeneration has lagged far behind the study of regenerative amphibians. Indeed, only basic knowledge exists for many of the regenerated tissues, and very few of the regenerative processes have been evaluated with modern techniques. Indeed, there exists a tremendous opportunity, therefore, for important contributions to the field in major subjects of study. For example, two recent transcriptomic analyses of regenerating lizard tails have yielded new insights into the cell and molecular aspects of tail regrowth (7, 24). In this review, we have highlighted the most relevant information gleaned from previous studies in the hopes of introducing the area of lizard tail regeneration to a new audience with command of the tools and expertise to take this topic in new and revealing directions. It is our hope that more researchers consider lizard tail regeneration in their work to elucidate the mechanisms regulating wound healing in amniotes, including mammals.
Acknowledgments
We thank Dr. Gary Gibson, PhD, for the invitation to write this review. We also thank Dr. José Padial, PhD, Stephen P. Rogers, MS, and the Carnegie Museum of Natural History Department of Herpetology for allowing us to perform micro computed topography and histological analyses on herpetology specimens. This manuscript presents results collected from specimens 55522, 49954, 64476, P-1683. This project was supported by the Commonwealth of Pennsylvania Department of Health (SAP 4100050913) and the NIH (R01 GM115444).
References
- 1.Lozito TP, Tuan RS. Lizard tail regeneration: regulation of two distinct cartilage regions by Indian hedgehog. Developmental Biology. 2015;399(2):249–62. doi: 10.1016/j.ydbio.2014.12.036. [DOI] [PubMed] [Google Scholar]
- 2.Delorme SL, Lungu IM, Vickaryous MK. Scar-free wound healing and regeneration following tail loss in the leopard gecko, Eublepharis macularius. Anatomical record. 2012;295(10):1575–95. doi: 10.1002/ar.22490. [DOI] [PubMed] [Google Scholar]
- 3.Bellairs AD, Bryant SV. Autotomy and Regeneration in Reptiles. In: Billet CGaF., editor. The Biology Of The Reptilia. Vol. 15. New York: John Wiley & Sons, Inc; 1985. pp. 303–410. [Google Scholar]
- 4.Alibardi L. In: Morphological and Cellular Aspects of Tail and Limb Regeneration in Lizards: A Model System With Implications for Tissue Regeneration in Mammals. Korf H-W, editor. Springer; 2010. [PubMed] [Google Scholar]
- 5.Chablais F, Jazwinska A. IGF signaling between blastema and wound epidermis is required for fin regeneration. Development. 2010;137(6):871–9. doi: 10.1242/dev.043885. [DOI] [PubMed] [Google Scholar]
- 6.Alibardi L, Lovicu FJ. Immunolocalization of FGF1 and FGF2 in the regenerating tail of the lizard Lampropholis guichenoti: implications for FGFs as trophic factors in lizard tail regeneration. Acta histochemica. 2010;112(5):459–73. doi: 10.1016/j.acthis.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Hutchins ED, Markov GJ, Eckalbar WL, George RM, King JM, Tokuyama MA, Geiger LA, Emmert N, Ammar MJ, Allen AN, Siniard AL, Corneveaux JJ, Fisher RE, Wade J, DeNardo DF, Rawls JA, Huentelman MJ, Wilson-Rawls J, Kusumi K. Transcriptomic Analysis of Tail Regeneration in the Lizard Anolis carolinensis Reveals Activation of Conserved Vertebrate Developmental and Repair Mechanisms. PloS one. 2014;9(8):e105004. doi: 10.1371/journal.pone.0105004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisher RE, Geiger LA, Stroik LK, Hutchins ED, George RM, Denardo DF, Kusumi K, Rawls JA, Wilson-Rawls J. A histological comparison of the original and regenerated tail in the green anole, Anolis carolinensis. Anatomical record. 2012;295(10):1609–19. doi: 10.1002/ar.22537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alibardi L, Meyer-Rochow VB. Comparative fine structure of the axial skeleton inside the regenerated tail of some lizard species and the tuatara (Sphenodon punctatus) Gegenbaurs morphologisches Jahrbuch. 1989;135(5):705–16. [PubMed] [Google Scholar]
- 10.Cox PG. Some Aspects of Tail Regeneration in Lizard, Anolis Carolinensis .I. A Description Based on Histology and Autoradiography. Journal of Experimental Zoology. 1969;171(2):127. [Google Scholar]
- 11.Alibardi L. Histogenesis of Fat Tissue in the Regenerating Tail of the Lizard (Lampropholis Spp) Can J Zool. 1995;73(6):1077–84. [Google Scholar]
- 12.Cencetti T, Poli P, Mele M, Zuffi MAL. Preliminary results on tail energetics in the Moorish gecko, Tarentola mauritanica. Acta Herpetol. 2011;6(1):101–3. [Google Scholar]
- 13.Egar M, Simpson SB, Singer M. The growth and differentiation of the regenerating spinal cord of the lizard, Anolis carolinensis. Journal of morphology. 1970;131(2):131–51. doi: 10.1002/jmor.1051310202. [DOI] [PubMed] [Google Scholar]
- 14.Simpson SB., Jr Morphology of the regenerated spinal cord in the lizard, Anolis carolinensis. The Journal of comparative neurology. 1968;134(2):193–210. doi: 10.1002/cne.901340207. [DOI] [PubMed] [Google Scholar]
- 15.Simpson SB., Jr Studies on regeneration of the lizard’s tail. Am Zool. 1970;10(2):157–65. doi: 10.1093/icb/10.2.157. [DOI] [PubMed] [Google Scholar]
- 16.Alibardi L, Gibbons J, Simpson SB., Jr 3H-GABA administration during tail regeneration of lizards and autoradiographical localization. Journal fur Hirnforschung. 1993;34(1):67–77. [PubMed] [Google Scholar]
- 17.McLean KE, Vickaryous MK. A novel amniote model of epimorphic regeneration: the leopard gecko, Eublepharis macularius. BMC Dev Biol. 2011;11:50. doi: 10.1186/1471-213X-11-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alibardi L. Observations of the ultrastructure of blood capillaries in the regenerating blastema of lizard in relation to the blood brain barrier. European Archives Of Biology. 1993;104(1):21–7. [Google Scholar]
- 19.Daniels CB, Lewis BC, Tsopelas C, Munns SL, Orgeig S, Baldwin ME, Stacker SA, Achen MG, Chatterton BE, Cooter RD. Regenerating lizard tails: A new model for investigating lymphangiogenesis. Faseb J. 2003;17(1):479. doi: 10.1096/fj.02-0579fje. [DOI] [PubMed] [Google Scholar]
- 20.Whimster IW. Nerve Supply as a Stimulator of Growth of Tissues Including Skin .2. Animal Evidence. Clin Exp Dermatol. 1978;3(4):389–410. doi: 10.1111/j.1365-2230.1978.tb01518.x. [DOI] [PubMed] [Google Scholar]
- 21.Economides KD, Zeltser L, Capecchi MR. Hoxb13 mutations cause overgrowth of caudal spinal cord and tail vertebrae. Dev Biol. 2003;256(2):317–30. doi: 10.1016/s0012-1606(02)00137-9. [DOI] [PubMed] [Google Scholar]
- 22.Kamrin RP, Singer M. The Influence of the Spinal Cord in Regeneration of the Tail of the Lizard, Anolis-Carolinensis. Journal of Experimental Zoology. 1955;128(3):611–27. [Google Scholar]
- 23.Simpson SB., Jr Analysis of Tail Regeneration in the Lizard Lygosoma Laterale. I. Initiation of Regeneration and Cartilage Differentiation: The Role of Ependyma. Journal of morphology. 1964;114:425–35. doi: 10.1002/jmor.1051140305. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Zhou Q, Wang Y, Luo L, Yang J, Yang L, Liu M, Li Y, Qian T, Zheng Y, Li M, Li J, Gu Y, Han Z, Xu M, Wang Y, Zhu C, Yu B, Yang Y, Ding F, Jiang J, Yang H, Gu X. Gekko japonicus genome reveals evolution of adhesive toe pads and tail regeneration. Nature communications. 2015;6:10033. doi: 10.1038/ncomms10033. [DOI] [PMC free article] [PubMed] [Google Scholar]