Abstract
Background
Deficiencies of the monoamine neurotransmitters, such as dopamine synthesized from Tyr and serotonin synthesized from Trp, are of concern in PKU. Our objective was to utilize metabolomics analysis to assess monoamine metabolites in subjects with PKU consuming amino acid medical foods (AA-MF) and glycomacropeptide medical foods (GMP-MF).
Methods
Subjects with PKU consumed a low-Phe diet combined with AA-MF and GMP-MF for 3 weeks each in a randomized, controlled, crossover study. Metabolomic analysis was conducted by Metabolon, Inc. on plasma (n=18) and urine (n=9) samples. Catecholamines and 6-sulfatoxymelatonin were measured in 24-hr urine samples.
Results
Intake of Tyr and Trp was ~50% higher with AA-MF, and AA-MF were consumed in larger quantities, less frequently during the day compared with GMP-MF. Performance on neuropsychological tests and concentrations of neurotransmitters derived from Tyr and Trp were not significantly different with AA- MF or GMP-MF. Plasma serotonin levels of gut origin were higher in subjects with variant compared with classical PKU, and with GMP-MF compared with AA-MF in subjects with variant PKU. Metabolomics analysis identified higher levels of microbiome-derived compounds synthesized from Tyr, such as phenol sulfate, and higher levels of compounds synthesized from Trp in the kynurenine pathway, such as quinolinic acid, with ingestion of AA-MF compared with GMP-MF.
Conclusions
The Tyr from AA-MF is less bioavailable due, in part, to greater degradation by intestinal microbes compared with the Tyr from prebiotic GMP-MF. Research is needed to understand how metabolism of Trp via the kynurenine pathway and changes in the intestinal microbiota affect health for individuals with PKU.
Keywords: catecholamines, serotonin, intestinal microbiota, glycomacropeptide, 6-sulfatoxymelatonin, phenol sulfate
1. Introduction
Phenylketonuria (PKU; OMIM 261600) is an autosomal recessive disorder caused by deficiency of hepatic phenylalanine hydroxylase (PAH; EC 1.14.16), which catalyzes the conversion of Phe to Tyr using tetrahydrobiopterin as a cofactor (1). In untreated PKU, consumption of a normal diet causes Phe to accumulate in blood and brain resulting in cognitive impairment. The mainstay of PKU treatment is a low-Phe diet restricted in protein from natural foods and supplemented with amino acid medical foods (AA-MF) or glycomacropeptide medical foods (GMP-MF) to provide the majority of dietary nitrogen and micronutrients (2, 3). Glycomacropeptide (GMP) is a 64-amino acid glycophosphopeptide derived from κ-casein in bovine milk that is produced during the manufacture of cheese and shows prebiotic properties (4, 5). The absence of aromatic amino acids in GMP (Phe, Tyr and Trp) enables the formulation of a variety of palatable GMP-MF for the management of PKU and tyrosinemia. Because GMP is not a complete protein, it requires supplementation with the following indispensable amino acids for PKU: Arg, His, Leu, Trp and Tyr (6, 7). Individuals who are diagnosed in infancy and maintain control of blood Phe levels within 120–360 μM/L show IQs within the normal range, although neurocognitive symptoms, such as decreased executive function, and neuropsychological symptoms, such as anxiety and depression, persist (8, 9).
Abnormal metabolism of the monoamine neurotransmitters, particularly deficiencies of dopamine synthesized from Tyr and serotonin synthesized from Trp in the central nervous system (CNS), may relate to the occurrence of neurocognitive and neuropsychological symptoms in individuals with PKU (9, 10). High blood Phe levels are presumed to cause deficiencies of the precursor amino acids Tyr and Trp for synthesis of the monoamine neurotransmitters by competitive inhibition for the large neutral amino acid (LNAA) transporter 1 (LAT1) which transports the LNAA from blood into the brain (11). Supplementation with LNAA, including Tyr and Trp, in individuals with PKU has been reported to increase cerebrospinal concentrations of metabolites of dopamine and serotonin (10–12). Thus, the concentration of Tyr and Trp in medical foods for the management of PKU may impact neurotransmitter synthesis; however, the bioavailability of these amino acids for absorption from the gastrointestinal tract has not been studied. This is an important consideration, given evidence that the intestinal microbiome is altered in human PKU with ingestion of AA-MF (13) and improved with ingestion of a diet containing GMP. GMP alters microbial phyla and increases production of short chain fatty acids compared with a diet containing amino acids (4, 5). Moreover, genes for metabolism of amino acids, especially Tyr and Trp, are overrepresented in bacteria comprising the microbiome in the distal gut (14).
In our recent randomized, controlled, crossover trial, we observed a disparity between intake and plasma concentrations of Tyr and Trp (6). Despite significantly higher intakes of Tyr and Trp in subjects consuming a low-Phe diet in combination with AA-MF compared with GMP-MF, fasting plasma concentrations of Tyr and Trp were not significantly different. This led us to the hypothesis that changes in the intestinal microbiota with ingestion of AA-MF result in degradation of Tyr and Trp, thus reducing their bioavailability for synthesis of neurotransmitters. Our objective was to utilize metabolomics analysis to assess metabolites and neurotransmitters derived from Tyr and Trp in plasma and urine samples from subjects with PKU consuming both AA-MF and GMP-MF.
2. Materials and Methods
2.1. Experimental Design
We conducted metabolomics analysis of a subset of plasma (n=18) and 24-hr urine (n=9) samples obtained from PKU subjects who completed our randomized, controlled, crossover trial where 30 early-treated PKU subjects consumed, for 3-wk each, their usual low-Phe diet combined with AA-MF or GMP-MF (6). The University of Wisconsin-Madison Health Sciences Institutional Review Board approved the study protocol. All subjects provided written informed consent. The trial was registered at www.clinicaltrials.gov as NCT01428258.
Participants consumed their preferred AA-MF resulting in the use of 8 different AA-MF for the urine study and 14 different AA-MF for the plasma study. The GMP-MF were donated by Cambrooke Therapeutics and contained Glytactin™, a proprietary formulation of ~70% GMP (cGMP-20, Arla Foods Ingredients) and ~30% supplemental AAs (Arg, His, Leu, Trp, Tyr). For the urine study, participants recorded all nutritional intake for 48–72h, starting 24–48h prior to and during the final days of each diet treatment. For the plasma study, participants recorded all nutritional intake on a consecutive 3-d food record during the final days of each treatment. Intake of macronutrients and amino acids was estimated from the food records using Food Processor SQL (Version 10.12.0, ESHA) for medical foods and natural foods; natural foods are defined as all food and beverages consumed that are not PKU medical foods.
Metabolomics analysis was performed on fasting plasma samples obtained at the final study visits after each subject consumed a low-Phe diet with AA-MF or GMP-MF for 3-wk each at home. There was a 3-wk washout period between treatments during which subjects consumed their usual AA-MF (6). The 18 plasma samples were chosen to include the 9 subjects who consented to provide 24-hr urine samples for both AA-MF and GMP-MF treatments and to represent the median plasma Phe response from the total sample of 30 subjects (6). Urine samples were collected after subjects followed their low-Phe diet combined with AA-MF and GMP-MF for 3-wk (n=4) or 1-wk (n=5); seven of the nine subjects completed the urine collection with the AA-MF treatment first Plasma and urine samples were processed and stored frozen(−70°C) until analyzed.
2.2. Amino Acids and Neurotransmitter Metabolites in Blood and Urine
The fasting plasma amino acid profiles were determined using a Hitachi L-8900 amino acid analyzer (15). Concentrations of dopamine, norepinephrine and epinephrine were determined in 24-hr urine samples using standardized techniques in a commercial clinical laboratory (LabCorp; Dublin, OH, USA). The concentration of the serotonin metabolite 6-sulfatoxymelatonin, used as a biomarker to reflect serotonin synthesis in the CNS (16), was analyzed in 24-hr urine samples using a commercial ELISA (EK-M6S, Buhlmann Laboratories AG, Switzerland) (17). Concentrations of neurotransmitter metabolites in urine were assessed per mg creatinine and per 24-hr which yielded similar statistical significance.
2.3. Metabolomics
The non-targeted metabolomics analysis on plasma and urine samples from PKU subjects was carried out by Metabolon, Inc. (Durham, NC, USA). Compounds were identified by comparison to Metabolon’s library of authenticated standards or recurrent unknown entities. The data obtained for urine samples were normalized to osmolality before statistical analysis. The analysis identified significantly different levels of 130 known biochemical compounds in plasma (total of 797 compounds identified) and 103 compounds in urine (total of 652 compounds identified) with ingestion of GMP-MF compared with AA-MF. The metabolomics data discussed in this paper are included as Supplementary Table 1 (plasma) and Supplementary Table 2 (urine).
2.4. Statistical Analysis
Statistical analyses were performed using SAS version 9.4 and assumptions of normality and equal variance were tested (SAS Institute Inc., Cary, N.C.). Most analyses for urinary biomarker excretion, nutrient intake and blood Phe concentrations used PROC MIXED. ANOVA was used to test for main effects for treatment (AA-MF or GMP-MF), genotype (classical or variant PKU), sex (male or female) and their interactions. When data were skewed, effects due to treatment or genotype were analyzed separately using the Kruskal-Wallis test. Each biochemical in the Metabolon analyses in the original scale was rescaled to set the median equal to 1 based on all samples; ANOVA was conducted to determine differences in scaled intensity or fold change for each biochemical when comparing treatment, genotype and treatment times genotype interaction. P values < 0.05 are considered significant. Data are presented as means ± SE for concentrations and metabolomics scaled intensity values. Power calculations were not performed since there is no literature in the PKU population on the effect change of the variables we studied; this is a pilot study.
3. Results
3.1. Characteristics of the Participants
The sample size of 18 participants (8 males and 10 females) included 3 minors, ages 15–17 yr and 15 adults, aged 18–49 yr, Table 1. Eleven participants had classical PKU and 7 participants had variant PKU based on a positive response to sapropterin dihydrochloride and/or a comparison of their genotype with available databases http://www.pahdb.mcgill.ca and http://www.biopku.org. Two of the subjects classified with variant PKU elected to take sapropterin dihydrochloride and maintained a stable dose during the study. The sample size of 9 participants who provided 24-hr urine collections (4 males and 5 females) included 3 minors aged 15–17 yr and 6 adults, aged 19–35 yr; four had classical and five had variant PKU. Final plasma Phe concentration was higher with GMP-MF compared with AA-MF (1.17 scaled intensity GMP-MF relative to AA-MF, p= 0.096), but not significantly different (6).
Table 1.
Characteristics of PKU subjects.
| Subject | Age | Mutation | Pheb (μmol/L) | Tyrb (μmol/L) | Trpb (μmol/L) |
|---|---|---|---|---|---|
| Classicala | |||||
| Female | |||||
| 1 | 34 | L242F; R408W | 829–1009 | 23–29 | 15–19 |
| 2 | 28 | F55>Lfs; R408W | 583–1160 | 30–55 | 18–28 |
| 3 | 23 | R408W; IVS12 | 459–649 | 17–40 | 36–45 |
| 4 | 26 | R408W; IVS7+3G>C | 535–624 | 34–42 | 22–29 |
| 5 | 28 | E280K; IVS12+1G>T | 626–852 | 20–39 | 37–39 |
| Male | |||||
| 6 | 32 | IVS1+5G>T; IVS12+1G>A | 418–1086 | 38–42 | 32–41 |
| 7 | 35 | R408W; R261Q | 378–754 | 43–47 | 17–20 |
| 8 | 18 | R408W; Y356X | 417–851 | 38–57 | 5–31 |
| 9 | 29 | F55>Lfs; IVS5+1G>A | 921–1152 | 39–46 | 24–57 |
| 10 | 18 | R408W; IVS12+1G>T | 284–600 | 35–36 | 19–24 |
| 11 | 44 | R261Q; IVS10-11G>A | 1059–1240 | 27–33 | 21–25 |
| Variant | |||||
| Female | |||||
| 12 | 34 | E280K; E390G | 541–583 | 20–28 | 21–25 |
| 13 | 15 | R157N; L348V | 597–652 | 31–38 | 19–25 |
| 14 | 15 | R158Q; R408W | 640–823 | 26–34 | 18–22 |
| 15 | 49 | L48S; 195_K96delinsK | 180–297 | 47–53 | 32–35 |
| 16 | 24 | 165T; IVS10-11G>A | 451–532 | 34–59 | 30–32 |
| Male | |||||
| 17 | 34 | R408W; IVS12+1G>A | 178–821 | 28–37 | 19–26 |
| 18 | 17 | R68S; IVS12+1G>T | 206–263 | 28–39 | 30–39 |
Subjects classified as having a variant form of PKU displayed a phenylalanine hydroxylase genotype and/or response to sapropterin dihydrochloride that was consistent with a milder or variant form of PKU. Mutation names are defined at http://www.pahdb.mcgill.ca and http://www.biopku.org.
The range of fasting plasma concentrations of Phe, Tyr and Trp reflect three determinations over a period of 8 weeks while subjects were following their usual diet with amino acid medical food.
Urine samples were obtained from subjects 1, 2, 6, 8, 12, 13, 14, 17, and 18. Subjects 13 and 14 maintained stable doses of sapropterin dihydrochloride during the study.
Dietary intake of Phe, Tyr and Trp showed differences, although intake of total protein (~80 g/day) and protein equivalents from AA-MF and GMP- MF (~53 g/day) were not significantly different (6). Intake of Phe was significantly higher with GMP-MF compared with AA-MF due to the residual Phe contained in Glytactin, whereas the AA-MF did not contain Phe, Table 2. Dietary intake of LNAA (includes Tyr, Trp, Leu, Ile, Val, His, Met, and Thr) was higher with GMP-MF compared with AA-MF (p=0.126) in conjunction with significantly higher intakes of Thr, that occur at high levels within the GMP peptide, and supplemental Leu. Our research demonstrates that at least 100–150 mg Leu per g protein equivalent in GMP is needed to maintain a normal plasma profile of branched chain amino acids in PKU subjects (7, 18). The complete intake profile of 18 dietary amino acids for subjects consuming AA-MF and GMP-MF for 3-wk each (6) is available in Stroup et al (19).
Table 2.
Intake of amino acids in subjects with PKU consuming a low-Phe diet in combination with AA-MF and GMP-MF.
| Medical Foods (g/day) | Natural Foods (g/day) | Whole Diet (g/day) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| AA-MF | GMP-MF | p | AA-MF | GMP-MF | p | AA-MF | GMP-MF | p | |
|
|
|||||||||
| Phenylalanine | 0.00 ± 0.00 | 0.09 ± 0.01 | <0.001 | 1.06 ± 0.20 | 1.19 ± 0.22 | 0.443 | 1.06 ± 0.20 | 1.29 ± 0.22 | 0.191 |
| Tryptophan | 1.28 ± 0.10 | 0.81 ± 0.04 | <0.001 | 0.39 ± 0.18 | 0.41 ± 0.14 | 0.811 | 1.67 ± 0.18 | 1.22 ± 0.14 | <0.001 |
| Tyrosine | 6.06 ± 0.39 | 4.18 ± 0.36 | <0.001 | 1.14 ± 0.55 | 1.22 ± 0.43 | 0.725 | 7.20 ± 0.55 | 5.39 ± 0.51 | <0.001 |
| LNAA | 28.6 ± 1.84 | 31.1 ± 1.87 | 0.244 | 7.33 ± 2.14 | 8.35 ± 1.90 | 0.445 | 35.9 ± 2.19 | 39.4 ± 2.05 | 0.126 |
| Isoleucine | 3.71 ± 0.26 | 3.89 ± 0.22 | 0.543 | 0.97 ± 0.24 | 1.13 ± 0.23 | 0.366 | 4.68 ± 0.27 | 5.02 ± 0.22 | 0.281 |
| Leucine | 6.60 ± 0.45 | 10.8 ± 0.63 | <0.001 | 1.69 ± 0.37 | 2.04 ± 0.40 | 0.297 | 8.29 ± 0.41 | 12.8 ± 0.57 | <0.001 |
| Threonine | 3.24 ± 0.26 | 5.95 ± 0.36 | <0.001 | 0.86 ± 0.21 | 0.99 ± 0.19 | 0.387 | 4.09 ± 0.29 | 6.95 ± 0.32 | <0.001 |
Values are mean ± SEM based on 3-d food record, n=18. Large neural amino acids (LNAA) include Tyr, Trp, Leu, Ile, Val, His, Met and Thr. AA medical foods (AA-MF), GMP medical foods (GMP-MF). Natural foods are defined as all foods and beverages that are not medical foods.
The higher concentrations of Tyr and Trp in AA-MF resulted in ~50% greater daily intake of Tyr and Trp, Table 2. The frequency of AA-MF intake was significantly lower than GMP-MF (2.8 vs 3.4 servings/day), resulting in significantly larger intake of Tyr and Trp at each meal that included AA-MF compared with GMP-MF, Figure 1. Overall, there was a pattern of consuming larger quantities of Tyr and Trp from AA-MF at fewer meals during the day compared with GMP-MF. Tyr comprises 3–5% and Trp comprises ~2% of average reference proteins (20). Assuming intake of 80 g protein per day for a typical adult, typical Tyr intake would be 2.4–4 g/day, compared with 7.2 g for the AA-MF treatment and 4.2 g for the GMP-MF treatment. Trp intake would be 1.6 g/day, compared with 1.7 g for the AA-MF treatment and 1.2 g for the GMP-MF treatment.
Figure 1.
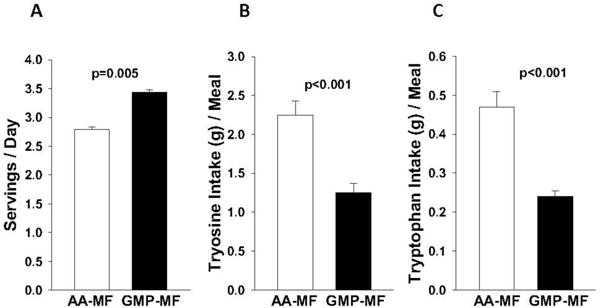
Frequency of dietary intake of amino acid medical foods (AA-MF) and glycomacropeptide medical foods (GMP-MF) (A), Tyr from AA-MF and GMP-MF (B), and Trp from AA-MF and GMP-MF (C). Values are mean ± SE, n=18; determined from food records.
3.2. Metabolomics Evidence of Microbiome-Associated Compounds
The metabolomics analysis identified 40 of 797 known biochemical compounds in plasma as microbiome-associated meaning they are either exclusively synthesized or contributed by intestinal bacteria, as well as by human metabolism. With ingestion of AA-MF compared with GMP-MF, there was evidence of differential plasma levels of 7 of the 40 microbiome-associated biochemical compounds. Significant differences in the plasma profile of secondary bile acids, which are known to be modified by intestinal bacteria, is strong evidence of alterations in the intestinal microbiome with ingestion of GMP-MF and AA-MF in human PKU (21), Supplementary Table 1. Metabolomics analysis identified 45 of 652 known compounds in urine as microbiome-associated and 7 of these compounds showed differential levels with AA-MF compared with GMP-MF.
3.3. Tyrosine and Neurotransmitter Metabolites
To evaluate changes in neurotransmitters derived from Tyr, chemical analysis of 24-hour urine samples were conducted. Daily excretion of the catecholamines, which included dopamine, norepinephrine and epinephrine, was not significantly different due to MF intake or genotype, Figure 2. Male subjects showed a trend for higher urinary excretion of epinephrine compared with female subjects (p=0.10).
Figure 2.
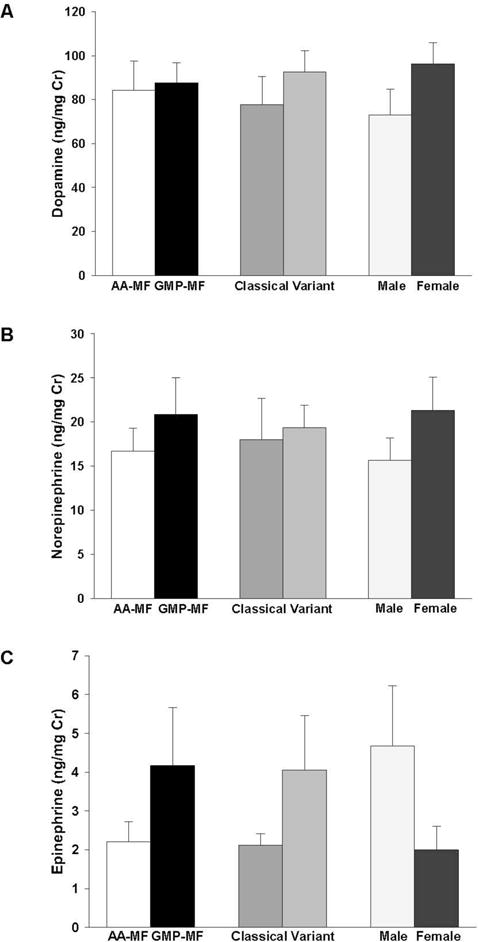
Urinary excretion of catecholamines, dopamine (A), norepinephrine (B), and epinephrine (C). Values are mean ± SE, ng/mg creatinine. Concentrations were measured in 24-hr urine collections from 9 PKU subjects (4 classical and 5 variant) consuming a low-Phe diet in combination with AA-MF or GMP-MF.
Metabolomics analysis indicated no significant differences in relative plasma levels of Tyr and 15 metabolites derived from Tyr including thyroxin and the final degradative products of the dopamine pathway, vanillactate and vanillylmandelate, Supplemental Table 1. A compound formed by methylation of L-DOPA, 3-methoxyltyrosine, was significantly higher with AA-MF compared with GMP-MF. This compound is associated with side effects in patients with Parkinson’s disease treated with chronic L-DOPA therapy (22).
Metabolomics analysis of 24-hr urine samples provided insights regarding the metabolic fate of the higher Tyr intake from AA-MF in conjunction with similar plasma Tyr levels, Figure 3. Urinary excretion of the microbiome-associated compounds tyramine and phenol sulfate (14) were 50–90% higher with ingestion of AA-MF compared with GMP-MF. Consistent with similar concentrations of catecholamines excreted in urine, metabolomics analysis indicated that levels of vanillylmandelate in urine were not significantly different with intake of AA-MF and GMP-MF. Thus, monoamine neurotransmitters derived from Tyr metabolism were not significantly different with AA-MF and GMP-MF, despite 50% higher Tyr intake from AA-MF. Moreover, tyrosine intake showed a positive, significant correlation with fasting plasma tyrosine concentration with ingestion of GMP-MF, but not with ingestion of AA-MF (GMP-MF, r=0.730, P=0.026; AA-MF, r=0.283, P=0.461; n=9).
Figure 3.
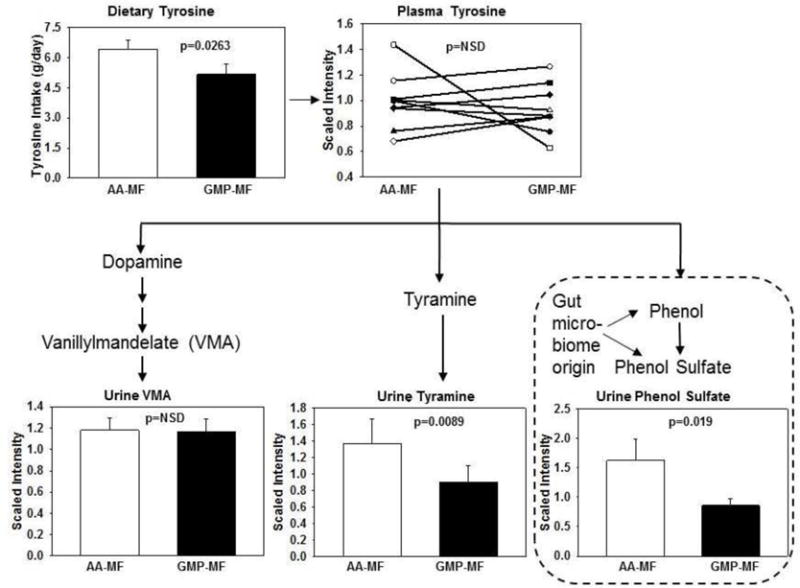
Metabolomics analysis of Tyr metabolism. Values are mean scaled intensity ± SE for metabolites of Tyr in 24-hr urine collections from 9 PKU subjects (4 classical and 5 variant) consuming a low-Phe diet in combination with AA-MF or GMP-MF. Mean scaled plasma Tyr levels and mean ± SE dietary Tyr intake determined from food records are shown, n=9. Plasma Tyr and urine vanillylmandelate are not significantly different, P>0.05. Compounds within dashed lines are synthesized by intestinal microbes.
3.4. Tryptophan Metabolites
Metabolomics analysis showed differences in relative levels of metabolites derived from Trp with ingestion of AA-MF and GMP-MF. Trp is primarily metabolized by the kynurenine pathway or converted to serotonin in the gastrointestinal tract (23), Figure 4. Plasma serotonin levels were ~3-fold higher in PKU subjects with variant compared with classical PKU (P<0.004) consistent with greater synthesis of serotonin in the gastrointestinal tract. Subjects with variant PKU showed significantly higher plasma serotonin levels with ingestion of GMP-MF compared with AA-MF, whereas subjects with classical PKU showed no effect of MF treatment on plasma serotonin.
Figure 4.
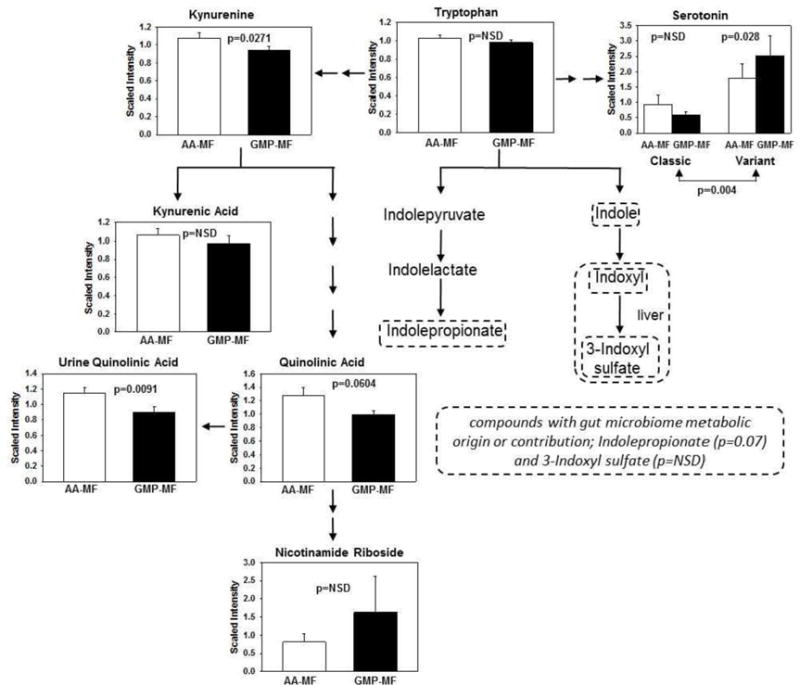
Metabolomics analysis of Trp which is primarily metabolized by the kynurenine pathway or converted to serotonin in the gastrointestinal tract. Values are mean ± SE scaled intensity for metabolites of Trp in plasma from 18 PKU subjects consuming a low-Phe diet in combination with AA-MF or GMP-MF. Levels of quinolinic acid in urine are shown. Compounds within dashed lines are synthesized by intestinal microbes, either exclusively or in combination with human metabolism.
Plasma levels of kynurenine were significantly higher with AA-MF compared with GMP-MF (p=0.027) reflecting diversion of Trp from serotonin synthesis. Downstream metabolites of kynurenine responded differently to MF. Plasma kynurenic acid levels were not different, whereas quinolinic acid a compound with neurotoxic effects (24), showed a trend for higher levels with AA-MF compared with GMP-MF (p=0.0604). Moreover, urinary excretion of quinolinic acid was significantly higher with AA-MF compared with GMP-MF (p=0.01). Quinolinic acid is a precursor to nicotinamide riboside and nicotinomide adenosine dinucleotide. Plasma levels of nicotinamide riboside were not significantly different, although urinary excretion was 2.4-fold higher with ingestion of GMP-MF compared with AA-MF (p=0.01). Dietary intake of niacin was not significantly different with GMP-MF and AA-MF. Levels of indolic compounds synthesized from Trp by intestinal bacteria, including the renal toxins 3-indoxy sulfate (25) and indolepropionate, were not significantly different with ingestion of AA-MF compared with GMP-MF.
In order to isolate the effects of AA-MF and GMP-MF on levels of serotonin made specifically in the CNS instead of the intestine, we measured the concentration of 6-sulfatoxymelatonin in urine, a serotonin metabolite synthesized in the pineal body (26). There was no significant difference in 6-sulfatoxymelatonin excretion with consumption of AA-MF compared with GMP-MF, Figure 5A. There was large variation due to differences in dietary intake of foods contributing serotonin and melatonin, and interaction of genotype and sex, Figure 5B. Females with classical PKU showed higher 6-sulfatoxymelatonin excretion compared with females with variant PKU, whereas males with classical PKU showed lower 6-sulfatoxymelatonin excretion compared with males with variant PKU. Subjects 1 and 2 consumed higher quantities of foods that contribute melatonin and serotonin (27, 28), including avocado, tomato, banana, and small amounts of beer, with the AA-MF treatment compared with the GMP-MF treatment.
Figure 5.
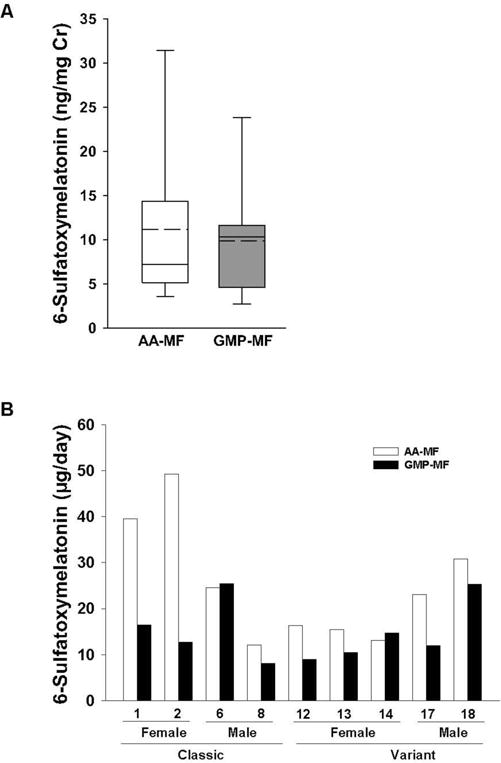
Urinary excretion of 6-sulfatoxymelatonin, a serotonin metabolite synthesized in the pineal body. The box and whisker figure illustrates the variation in 6-sulfatoxymelatonin excretion with ingestion of a low-Phe diet in combination with AA-MF or GMP-MF (A). The box represents the middle 50% of 9 subjects (25th–75th percentile) with a solid line showing the median value and a dashed line showing the mean value. Concentrations of 6-sulfatoxymelatonin for each subject with ingestion of AA-MF or GMP-MF are shown (B). Concentrations were measured in aliquots of 24-hr urine and are expressed as ng/mg creatinine or as ug/day for individual subjects.
4. Discussion
PKU is associated with reduced levels of the monoamine neurotransmitters because high Phe concentrations presumably competitively inhibit transport of the precursor amino acids Tyr and Trp from blood into the brain (9, 12). Emerging evidence suggests that another contributing factor includes changes in the intestinal microbiota with the synthetic PKU diet (4, 13), which may result in degradation of Tyr and Trp, thus reducing their bioavailability for synthesis of neurotransmitters. We demonstrate that larger quantities of Tyr consumed at fewer daily meals with AA-MF compared with GMP-MF, result in greater degradation of Tyr to potentially harmful compounds synthesized by intestinal bacteria. Thus, Tyr from AA-MF has reduced bioavailability compared with Tyr from GMP-MF due, in part, to greater degradation of Tyr by intestinal microbes. We did not observe differential degradation of Trp by intestinal microbes suggesting similar bioavailability of Trp, possibly because intake of Trp from medical foods, unlike Tyr, is not excessive compared to a typical diet.
Dietary intake of Tyr and Trp from AA-MF, although significantly higher compared with GMP-MF, was associated with similar performance on standardized neuropsychological tests emphasizing executive function (Delis-Kaplan Executive Function System and the Cambridge Neuropsychological Test Automated Battery) (19). Similar levels of neurotransmitters may reflect higher intakes of the LNAA, Leu, Thr and Ile, with GMP-MF compared with AA-MF (6, 19). These LNAA may compete with Phe at LAT1 to reduce Phe transport into brain, resulting in greater availability of Tyr and Trp for neurotransmitter synthesis. In support of this, specific supplementation with Leu and Ile has been shown to decrease Phe concentrations in the brain of PKU mice (29), and we have reported a 20% decrease in brain Phe concentrations in Pahenu2 mice fed diets comprised of GMP compared with amino acids (30).
Urinary excretion of 6-sulfatoxymelatonin, a suggested biomarker of serotonin synthesis in the CNS, showed large variation. Higher intake of foods that contribute melatonin and serotonin, such as avocadoes, bananas, tomatoes, beer, and coffee (27, 28), which we did not control for, could account for the high variation in urinary excretion of 6-sulfatoxymelatonin observed in this study and by Yano et al (26).
Serotonin is synthesized primarily (more than 90%) by the gastrointestinal tract and exerts diverse functions including regulation of enteric reflexes, platelet aggregation and immune responses (23). The 3-fold higher plasma serotonin levels in subjects with variant compared with classical PKU in response to GMP-MF, with similar excretion of 6-sulfatoxymelatonin, confirms greater synthesis of serotonin in the gut, not the brain. Intestinal microbes regulate host serotonin synthesis, with increased release of serotonin from intestinal cells in response to microbial production of short chain fatty acids (23). Thus, given evidence that prebiotic GMP increases microbial production of short chain fatty acids (4, 5), higher serotonin levels in subjects with variant PKU consuming GMP-MF suggests that the PKU genotype has differential effects on the intestinal microbiota. This interesting observation warrants further investigation.
Metabolomics analysis with the ability to detect microbiome-associated compounds, suggests that differences in the composition of the intestinal microbiota occur with ingestion of GMP-MF compared with AA-MF. This conclusion is supported by the demonstrated prebiotic properties of GMP (4, 5), differences in plasma serotonin (23), and dramatic changes in the plasma profile of secondary bile acids, but not the primary bile acids, with GMP-MF compared with AA-MF (21). Although many of the compounds synthesized by intestinal microbes confer benefits consistent with symbiosis, such as short chain fatty acids and indolepropionate, other compounds synthesized from Tyr and Trp may have negative effects (31). For example, tyramine formed by decarboxylation of Tyr by both bacteria and human metabolism is associated with headaches (32) and shows cytotoxic effects in human intestinal cells at physiologic concentrations (33). Compounds noted to be harmful to the kidneys and synthesized by intestinal bacteria, referred to as “colon-derived uremic solutes,” include phenolic compounds formed from Tyr, such as phenol sulfate, and indolic compounds formed from Trp, such as 3-indoxyl sulfate (25, 34). Although we observe significantly higher levels of tyramine and phenol sulfate with ingestion of AA-MF compared with GMP-MF, we cannot conclude from the current metabolomics analysis that the levels are high enough to have a negative impact.
Interestingly, a greater proportion of Trp is metabolized via the kynurenine pathway, instead of the serotonin pathway, in PKU subjects consuming AA-MF compared with GMP-MF. The kynurenine pathway is the major route for Trp metabolism in most mammalian tissues and compounds in this pathway are associated with the pathophysiology of inflammation-related neuropsychiatric diseases and the focus of drug development efforts (24). Increased synthesis of kynurenine, which occurred with AA-MF relative to GMP-MF, is associated with an inflammatory response and consistent with our observation of increased inflammatory cytokines with a diet containing AA compared with a GMP-based diet in murine PKU (4). From kynurenine, the pathway branches to form kynurenic acid, a neuroprotective metabolite that shows similar levels with AA-MF and GMP-MF. A second branch in the kynurenine pathway leads to formation of quinolinic acid, a neurotoxic metabolite that shows higher levels with AA-MF compared with GMP-MF in both plasma and urine (24). Unbalanced kynurenine metabolism that results in increased quinolinic acid production may induce oxidative stress, apoptosis and mitochondrial dysfunction, resulting in neuronal loss. Increased quinolinic acid production is implicated in aging and neurodegenerative diseases, such as Al heimer’s disease and Parkinson’s disease (24). Despite lower Trp intake from GMP-MF, nicotinamide metabolites synthesized from Trp that are important in neurological function, were not significantly different with medical food. Further research is needed to understand the metabolism of Trp via the kynurenine pathway in PKU.
Strengths of our study include a controlled crossover design. Moreover, this is the first investigation of the bioavailability of Tyr and Trp from medical foods, and the first use of metabolomics to assess compounds synthesized by intestinal microbes in PKU. Limitations include the small sample size of 9–18 subjects. For the urine study, the treatment order was not randomized and there was variation in the length of dietary treatment before collection of 24-hr urine samples.
5. Conclusion
The data support our hypothesis that degradation of Tyr by intestinal microbes contributes to the apparent decrease in the bioavailability of Tyr from AA-MF compared with GMP-MF. The prebiotic properties of GMP in conjunction with consumption of smaller amounts of Tyr distributed more frequently across the day appear to account for the higher bioavailability of Tyr from GMP-MF. Routine Tyr supplementation in an attempt to increase catecholamine neurotransmitters may have negative effects on the intestinal microbiota and there is no evidence to suggest improved neuropsychological performance in individuals with PKU given Tyr supplementation (35). Further research is need to understand how changes in the intestinal microbiota affect health for individuals with PKU.
Supplementary Material
Highlights.
Ingestion of PKU medical foods comprised primarily of amino acids (AA-MF) compared with glycomacropeptide (GMP-MF) alters compounds synthesized by intestinal microbes in plasma and urine.
The bioavailability of Tyr from prebiotic GMP-MF is greater compared with AA-MF; this reflects less degradation of Tyr by intestinal microbes and consumption of smaller amounts of Tyr distributed more frequently across the day with GMP-MF.
A greater proportion of Trp is metaboli ed via the kynurenine pathway, instead of the serotonin pathway, with consumption of AA-MF compared with GMP-MF.
Routine Tyr supplementation may have negative effects on the intestinal microbiota and is not supported by evidence of improved neuropsychological performance in PKU.
Acknowledgments
Funding: This work was supported by Department of Health and Human Services grants R01 FD003711 from the FDA Office of Orphan Product Development to Ney, P30-HD-03352, T32 DK007665 to Ney, and by the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), grant UL1TR000427. Cambrooke Therapeutics, Inc. donated the GMP medical foods used in this study and contributed funds, along with Arla Foods Ingredients, for the metabolomics analysis. They had no role in the design or conduct of the study or in the collection, analysis or interpretation of the data.
Abbreviations
- AA-MF
amino acid medical foods
- CNS
central nervous system
- GMP
glycomacropeptide
- GMP-MF
glycomacropeptide medical foods
- LNAA
large neutral amino acid
- LAT1
LNAA transporter 1
- PAH
phenylalanine hydroxylase
- PKU
phenylketonuria
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This trial is registered at www.clinicaltrials.gov as NCT01428258.
Conflict of Interest: DMN is a co-inventor on U.S. Patent 8,604,168 B2, “Glycomacropeptide Medical Foods for Nutritional Management of Phenylketonuria and other Metabolic Disorders,” which is held by the Wisconsin Alumni Research Foundation and licensed to Cambrooke Therapeutics, LLC. DMN is a consultant to Arla Foods Ingredients. BMS, MKC, SGM, GMR, FR, and HLL have no conflicts of interest to declare. FR has received consulting fees from Cambrooke Therapeutics. The remaining authors declare no conflict of interest related to this research.
References
- 1.Flydal MI, Martinez A. Phenylalanine hydroxylase: function, structure, and regulation. IUBMB Life. 2013;65(4):341–9. doi: 10.1002/iub.1150. [DOI] [PubMed] [Google Scholar]
- 2.MacLeod EL, Ney DM. Nutritional management of phenylketonuria. Ann Nestle. 2010;68:58–69. doi: 10.1159/000312813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh RH, Rohr F, Frazier D, Cunningham A, Mofidi S, Ogata B, Splett PL, Moseley K, Huntington K, Acosta PB, et al. Recommendations for the nutrition management of phenylalanine hydroxylase deficiency. Genet Med. 2014;16(2):121–31. doi: 10.1038/gim.2013.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sawin EA, De Wolfe TJ, Aktas B, Stroup BM, Murali SG, Steele JL, Ney DM. Glycomacropeptide is a prebiotic that reduces Desulfovibrio bacteria, increases cecal short-chain fatty acids, and is anti-inflammatory in mice. Am J Physiol Gastrointest Liver Physiol. 2015;309(7):G590–601. doi: 10.1152/ajpgi.00211.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ntemiri ACF, O’Callaghan TF, Stanton C, Ross RP, O’Toole PW. Glycomacropeptide sustains microbial diversity and promotes specific taxa in an artificial colon model of elderly gut microbiota. J Agric Food Chem. 2017 doi: 10.1021/acs.jafc6b05434. [DOI] [PubMed] [Google Scholar]
- 6.Ney DM, Stroup BM, Clayton MK, Murali SG, Rice GM, Rohr F, Levy HL. Glycomacropeptide for nutritional management of phenylketonuria: a randomized, controlled, crossover trial. Am J Clin Nutr. 2016;104(2):334–45. doi: 10.3945/ajcn.116.135293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Calcar SC, Macleod EL, Gleason ST, Etzel MR, Clayton MK, Wolff JA, Ney DM. Improved nutritional management of phenylketonuria by using a diet containing glycomacropeptide compared with amino acids. Am J Clin Nutr. 2009;89(4):1068–77. doi: 10.3945/ajcn.2008.27280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Enns GM, Koch R, Brumm V, Blakely E, Suter R, Jurecki E. Suboptimal outcomes in patients with PKU treated early with diet alone: revisiting the evidence. Mol Genet Metab. 2010;101(2–3):99–109. doi: 10.1016/j.ymgme.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 9.de Groot MJ, Hoeksma M, Blau N, Reijngoud DJ, van Spronsen FJ. Pathogenesis of cognitive dysfunction in phenylketonuria: review of hypotheses. Mol Genet Metab. 2010;99(Suppl 1):S86–9. doi: 10.1016/j.ymgme.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 10.Burlina AB, Bonafe L, Ferrari V, Suppiej A, Zacchello F, Burlina AP. Measurement of neurotransmitter metabolites in the cerebrospinal fluid of phenylketonuric patients under dietary treatment. J Inherit Metab Dis. 2000;23(4):313–6. doi: 10.1023/a:1005694122277. [DOI] [PubMed] [Google Scholar]
- 11.van Spronsen FJ, de Groot MJ, Hoeksma M, Reijngoud DJ, van Rijn M. Large neutral amino acids in the treatment of PKU: from theory to practice. J Inherit Metab Dis. 2010;33(6):671–6. doi: 10.1007/s10545-010-9216-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lykkelund C, Nielsen JB, Lou HC, Rasmussen V, Gerdes AM, Christensen E, Guttler F. Increased neurotransmitter biosynthesis in phenylketonuria induced by phenylalanine restriction or by supplementation of unrestricted diet with large amounts of tyrosine. Eur J Pediatr. 1988;148(3):238–45. doi: 10.1007/BF00441411. [DOI] [PubMed] [Google Scholar]
- 13.de Oliveira FPMR, Dobbler PT, Mai V, Pylro VS, Waugh SG, Vairo F, Refosco LF, Roesch LFW, Schwartz IVD. Phenylketonuria and gut microbiota: a controlled study based on next generation sequencing. PloS ONE. 2016;11(6) doi: 10.1371/journal.pone.0157513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, Siuzdak G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci U S A. 2009;106(10):3698–703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stroup BM, Held PK, Williams P, Clayton MK, Murali SG, Rice GM, Ney DM. Clinical relevance of the discrepancy in phenylalanine concentrations analyzed using tandem mass spectrometry compared with ion-exchange chromatography in phenylketonuria. Molecular Genetica and Metabolism Reports. 2016;6:21–6. doi: 10.1016/j.ymgmr.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yano S, Moseley K, Azen C. Large neutral amino acid supplementation increases melatonin synthesis in phenylketonuria: a new biomarker. J Pediatr. 2012;162(5):999–1003. doi: 10.1016/j.jpeds.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsing AW, Meyer TE, Niwa S, Quraishi SM, Chu LW. Measuring serum melatonin in epidemiologic studies. Cancer Epidemiol Biomarkers Prev. 2010;19(4):932–7. doi: 10.1158/1055-9965.EPI-10-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ney DM. Balancing branched chain amino acids in medical foods for inherited disorders of amino acid metabolism. EC Nutrition. 2016;6(2):69–71. [Google Scholar]
- 19.Stroup BM, Murali SG, Nair N, Sawin EA, Rohr F, Levy HL, Ney DM. Effects of a low-phenylalanine diet combined with amino acid medical foods or glycomacropeptide medical foods on dietary intake of amino acids and neuropsychological function in subjects with phenylketonuria. Data in Brief. 2017 doi: 10.1016/j.dib.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Etzel MR. Manufacture and use of dairy protein fractions. J Nutr. 2004;134(4):996S–1002S. doi: 10.1093/jn/134.4.996S. [DOI] [PubMed] [Google Scholar]
- 21.Staley C, Weingarden AR, Khoruts A, Sadowsky MJ. Interaction of gut microbiota with bile acid metabolism and its influence on disease states. Appl Microbiol Biotechnol. 2017;101(1):47–64. doi: 10.1007/s00253-016-8006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee ES, Chen H, King J, Charlton C. The role of 3-O-methyldopa in the side effects of L-dopa. Neurochem Res. 2008;33(3):401–11. doi: 10.1007/s11064-007-9442-6. [DOI] [PubMed] [Google Scholar]
- 23.Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Nagler CR, Ismagilov RF, Mazmanian SK, Hsiao EY. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161(2):264–76. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujigaki H, Yamamoto Y, Saito K. L-Tryptophan-kynurenine pathway enzymes are therapeutic target for neuropsychiatric diseases: Focus on cell type differences. Neuropharmacology. 2017;112(Pt B):264–74. doi: 10.1016/j.neuropharm.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Meyer TW, Hostetter TH. Uremic solutes from colon microbes. Kidney Int. 2012;81(10):949–54. doi: 10.1038/ki.2011.504. [DOI] [PubMed] [Google Scholar]
- 26.Yano S, Moseley K, Fu X, Azen C. Evaluation of Tetrahydrobiopterin Therapy with Large Neutral Amino Acid Supplementation in Phenylketonuria: Effects on Potential Peripheral Biomarkers, Melatonin and Dopamine, for Brain Monoamine Neurotransmitters. PLoS One. 2016;11(8):e0160892. doi: 10.1371/journal.pone.0160892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Islam J, Shirakawa H, Nguyen TK, Aso H, Komai M. Simultaneous analysis of serotonin, tryptophan and tryptamine levels in common fresh fruits and vegetables in Japan using fluorescence HPLC. Food Biosci. 2016;13:56–9. doi: 10.1016/j.fbio.2015.12.006. [DOI] [Google Scholar]
- 28.Peuhkuri K, Sihvola N, Korpela R. Dietary factors and fluctuating levels of melatonin. Food Nutr Res. 2012;56 doi: 10.3402/fnr.v56i0.17252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Vliet D, Bruinenberg VM, Mazzola PN, van Faassen MH, de Blaauw P, Pascucci T, Puglisi-Allegra S, Kema IP, Heiner-Fokkema MR, van der Zee EA, et al. Therapeutic brain modulation with targeted large neutral amino acid supplements in the Pah-enu2 phenylketonuria mouse model. Am J Clin Nutr. 2016;104(5):1292–300. doi: 10.3945/ajcn.116.135996. [DOI] [PubMed] [Google Scholar]
- 30.Ney DM, Hull AK, van Calcar SC, Liu X, Etzel MR. Dietary glycomacropeptide supports growth and reduces the concentrations of phenylalanine in plasma and brain in a murine model of phenylketonuria. J Nutr. 2008;138(2):316–22. doi: 10.1093/jn/138.2.316. [DOI] [PubMed] [Google Scholar]
- 31.Chilloux J, Neves AL, Boulange CL, Dumas ME. The microbial-mammalian metabolic axis: a critical symbiotic relationship. Curr Opin Clin Nutr Metab Care. 2016;19(4):250–6. doi: 10.1097/MCO.0000000000000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khan MZ, Nawaz W. The emerging roles of human trace amines and human trace amine-associated receptors (hTAARs) in central nervous system. Biomed Pharmacother. 2016;83:439–49. doi: 10.1016/j.biopha.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 33.Linares DM, del Rio B, Redruello B, Ladero V, Martin MC, Fernandez M, Ruas-Madiedo P, Alvarez MA. Comparative analysis of the in vitro cytotoxicity of the dietary biogenic amines tyramine and histamine. Food Chem. 2016;197(Pt A):658–63. doi: 10.1016/j.foodchem.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 34.Smith EA, Macfarlane GT. Formation of Phenolic and Indolic Compounds by Anaerobic Bacteria in the Human Large Intestine. Microb Ecol. 1997;33(3):180–8. doi: 10.1007/s002489900020. [DOI] [PubMed] [Google Scholar]
- 35.Webster D, Wildgoose J. Tyrosine supplementation for phenylketonuria. Cochrane Database Syst Rev. 2013;(6):CD001507. doi: 10.1002/14651858.CD001507.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


