Abstract
The purpose of this study was to evaluate the viral frequency, seasonality, and clinical and demographic features of patients hospitalized with acute bronchiolitis. A cross-sectional, descriptive study was performed in 316 infants younger than 2 years of age who were hospitalized for acute viral bronchiolitis. Respiratory tract infection agents were investigated with polymerase chain reaction (PCR). A total of 316 infants were included in this study. Of the 316 infants, at least one respiratory tract pathogen was detected in 75% (237/316). Respiratory syncytial virus (RSV) was the most common virus identified in 127 infants (40.1%) followed by rhinovirus (n = 78, 24.6%). In this study, where viral agents were determined via PCR in patients who were followed-up due to the diagnosis of acute bronchiolitis, RSV was detected as the most common agent, as in other studies. In almost half of the RSV-positive patients, RSV was accompanied by a second or third agent.
Keywords: acute bronchiolitis, infant, respiratory viruses, etiology, seasonality, clinical features
Introduction
Acute bronchiolitis (AB), which is the most common acute lower respiratory system disease in infants, is often caused by a viral infection. It is especially the leading cause of hospitalization in infants under 6 months of age.1,2 Epidemic peaks of AB are frequently seen during the winter season. Respiratory syncytial virus (RSV) is usually the cause of 50% to 80% of the cases, but other viruses including adenovirus, influenza virus, and parainfluenza virus have also been reported to cause AB as the sole pathogen or as coinfection with or without RSV.3,4 With various polymerase chain reaction (PCR) techniques, possible new agents like rhinovirus, human metapnomovirus, human bocavirus, Bordatella pertussis, and atypical pathogens were also described as the leading causes of AB.5-7 Having a cardiovascular disease, chronic pulmonary disease, immunodeficiency, and premature birth increase the risk of AB-associated respiratory failure, or even death.8 The World Health Organization has reported that RSV is the causative pathogen for over 30 million new acute lower respiratory infection episodes in children under 5 years of age and it gives rise to more than 3.4 million hospital admissions and 160 000 deaths every year.9,10
The diagnosis of AB is made based on typical history with wheezing and characteristic clinical features such as tachypnea, nasal flaring, chest retractions, and wheezing and/or rales followed by a viral upper respiratory infection in infants. The American Academy of Pediatrics (AAP) 2006 Clinical Practice Guidelines for the Diagnosis and Management of Bronchiolitis described AB as the first episode of wheezing in children under 24 months of age who have respiratory findings during the viral infection episode.11 Chest radiographs and laboratory studies may be thought of on clinical suspicion after evaluating the differential diagnosis for secondary or comorbid bacterial infection, complications, or other conditions. Viral diagnosis methods including antigen detection or immunofluorescence of nasal secretion wash or nasal aspiration, rapid antigen tests, and PCR are only suggested for identifying specific viral agents in children with bronchiolitis if the results will determine discontinuation of palivizumab prophylaxis, initiation or continuation/discontinuation of antibiotic therapy.12-15
Majority of studies have recently researched the burden of respiratory viral tract infection agents in AB with larger groups. In these studies, epidemiological, clinical, and risk factors of AB have also been defined. So, it can be said that AB is frequent in infancy and that there is an increase in the number of admissions to hospitals and bronchiolitis-related morbidity.
The purpose of this study was to evaluate the frequency of pathogens and to determine the differences in clinical and microbiological features among patients under 24 months of age, who were hospitalized with AB in Ege University Children’s Hospital.
Materials and Methods
Patients and Sample Collection
Ege University Children’s Hospital, a tertiary care pediatric teaching hospital, is a 200-bed hospital located in heavily populated area of Izmir. It serves nearly 10 000 inpatients per year that are mostly from middle to low socioeconomic status. This study was approved by the institutional review board of Ege University Children’s Hospital and was conducted during a 3-year period. A prospective cross-sectional study of children younger than 24 months who were hospitalized with AB was conducted between October 2013 and October 2016 at the General Pediatrics ward of Children’s Hospital. The ethics committee of Ege University approved the study.
Infants who were hospitalized with AB were recruited with informed, written, parental consent. According to the recommendations of AAP guidelines, the diagnosis of AB was based on at least 2 of the following signs: chest retractions, tachypnea, and wheezing or rales on auscultation following viral upper respiratory tract infection in children less than 24 months of age for the first time.11 Excluded were infants who hospitalized within 2 weeks before the current admission, those who developed nosocomial AB, or those who had a known history of bronchopulmonary dysplasia or chronic lung disease or congenital heart disease or any immunodeficiency. Infants who were born prematurely were also excluded from the study. Detailed demographic data including age, gender, smoke exposure, and epidemiological data were collected with a structured questionnaire and from medical files of all patients on admission.
On hospital admission, clinical parameters and severity of the disease including wheezing, retraction, respiratory rate, and general situation were evaluated and assessed with Wang respiratory score.16 Each parameter was given points from 0 to 3 and then the child’s points were added up to describe the severity of AB; points between 0 and 3 showed mild bronchiolitis, between 4 and 8 moderate, and points between 9 and 12 indicated severe bronchiolitis. Complete blood count (white blood cell count and percentage of lymphocytes) and C-reactive protein were measured at admission.
Respiratory Organisms Detection Assay
A nasal smear was obtained from each infant and tested for the presence of RSV, influenza virus types A and B, adenovirus, parainfluenza viruses, human rhinovirus, human coronavirus, human metapneumovirus, and human bocavirus with multiplex reverse-transcription PCR methods (RealAccurate, Respiratory RT PCR, PathoFinder, Netherlands, and Seeplex RV15 ACE Detection, Seegene, South Korea). Nasal samples were obtained more commonly by a nurse or sometimes a research assistant on all subjects within 48 hours of admission using a standardized protocol.17 Samples were frozen at −20°C and transported on ice to the Department of Clinical Microbiology and Virology Laboratory of our university for viral nucleic acid amplification.
Statistical Analyses
Data analyses were performed using SPSS version 21.0. The quantitative data including percentage, mean, and standard deviations were calculated by simple descriptive analyses. Means ± SD were used to summarize the demographic data and the baseline characteristics of the cases. All analyses were conducted using SPSS (SPSS Inc, Chicago, IL).
Results
Demographic and Clinical Data of the Study Population With Acute Bronchiolitis
Between April 2013 and April 2016, a total of 316 infants hospitalized with AB were included in this prospective study. The mean age was 7 ± 6.5 months; 201 (63.6%) were male (Table 1). The most frequent age range of infants with AB was 1 to 6 months, accounting for 63.6% of all included cases. Most of the patients had cough and runny nose on admission to hospital. Nearly half of the infants diagnosed with AB were exposed to passive smoking. There was a history of atopy in 12% of all the infants.
Table 1.
Demographic and Clinical Data of Infants Hospitalized With Acute Bronchiolitis.
| Age, mean ± SD, months | 7 ± 6.5 |
| Age distribution, n (%) | |
| 1-6 months | 201 (63.6) |
| 7-15 months | 69 (21.8) |
| 16-24 months | 46 (14.6) |
| Gender | |
| Male, n (%) | 201 (63.6) |
| Female, n (%) | 115 (36.4) |
| Personal history of atopy, n (%) | 40 (12.7) |
| Passive smoking exposure, n (%) | 144 (45.6) |
Abbreviation: SD, standard deviation.
Table 2 shows the clinical data and laboratory findings, disease severity scores, hospitalization rates, and duration of hospitalizations of the infants. The most common complaint of the patients was cough (100%), and fever was present only in 88 (27.8%) of all the cases on admission to hospital. On examination, the percentage of infants with wheezing was 58. Ninety-six (30.4%) infants needed oxygen supplementation, and the mean length of hospitalization duration was 7.5 days. More than half of the infants had moderate bronchiolitis and the mean respiratory score was 5.3.
Table 2.
Clinical Features and Laboratory Measurements of Infants Diagnosed With Acute Bronchiolitis.
| Clinical Characteristics | |
|---|---|
| Fever, n (%)a | 88 (27.8) |
| Wheezing, n (%) | 183 (58) |
| Respiratory score, mean ± SD | 5.3 ± 2.2 |
| Respiratory score, n (%) | |
| Mild | 73 (23.1) |
| Moderate | 209 (66.1) |
| Severe | 34 (10.8) |
| Supplemental oxygen therapy, n (%) | 96 (30.4) |
| Hospitalization duration, mean ± SD, days | 7.5 ± 5.1 |
| Laboratory Findings | |
| White blood cell/mm3, mean ± SD | 11 370 ± 4900 |
| Lymphocyte count (%), mean ± SD | 44 ± 18 |
| C-reactive protein, mg/dL, mean ± SD | 1.9 ± 1.1 |
Abbreviation: SD, standard deviation.
The percentages were calculated by dividing the value over the total number of patients tested (N = 316).
Prevalence of Respiratory Agents and Seasonal Patterns in Infants With Acute Bronchiolitis
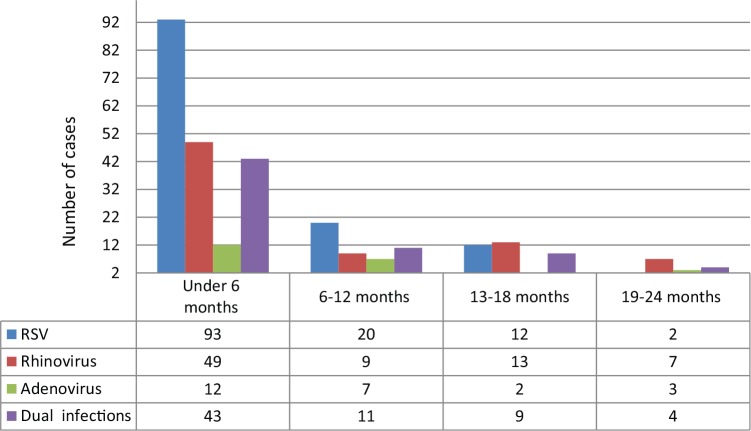
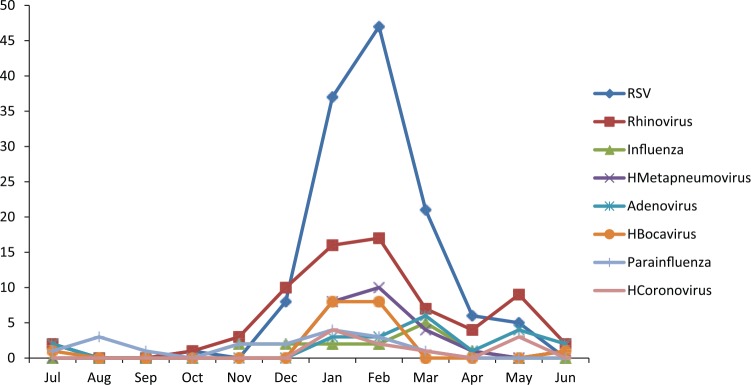
Acute bronchiolitis was diagnosed in 316 infants younger than 24 months of age. Overall, at least one respiratory virus was detected in 75% (237/316) of the cases at Ege University Children’s Hospital. The results of the incidence of respiratory viruses in infants hospitalized with AB during the study period are shown in Table 3. RSV was identified in 127 (40.1%) of the 316 infants hospitalized for AB, and RSV was the most common agent. Of the 127 RSV-positive infants, 76 (59.8%) were sole pathogen for RSV and 51 infants had RSV-coinfection, which was responsible for respiratory tract infection. The second prevalent viral agent was rhinovirus, identified in 78 (24.6%) of the subjects, and as a single pathogen in 35 (44.8%). Furthermore, in this study, influenza virus types A and B (n = 28/316, 8.8%), human metapneumovirus (n = 27/316, 8.5%), adenovirus (n = 23/316, 7.2%), human bocavirus (n = 20/316, 6.3%), parainfluenza virus (n = 18/316, 5.6%), and human coronavirus (n = 10/316, 3.1%) were determined (Table 3). A single virus in 156/316 (49.3%), dual infections in 67/316 (21.2%), triple infections in 13/316 (4.1%), and quadruple infections in 1/316 (0.3%) were detected. Of the 81 infants with multiple agents, RSV (51 cases) was the most common virus followed by rhinovirus (n = 43, 53%), influenza virus (n = 18, 22.2%), human metapneumovirus (n = 16, 19.7%), PIV (n = 10, 12.3%), adenovirus (n = 16, 19.7%), human bocavirus (n = 13, 16%), parainfluenza virus (n = 10, 12.3%), and human coronavirus (n = 8, 9.8%). Among dual co-infections, the combination of RSV and rhinovirus had the the highest number (n = 15, 22.3%). The age distribution of infants is shown in Figure 1 for RSV, rhinovirus, adenovirus, and dual infections. In particular, most viral agents were identified in children of all months, whereas RSV was detected in infants aged under 6 months (n = 93/127, 73.2%) and in 15.7% (n = 20/127) of the 127 RSV-positive infants aged under 1 year of age, which was the case in most of our patients. The monthly incidence of bronchiolitis in infants due to common respiratory pathogens is shown in Figure 2. The majority of the cases were admitted to hospital in the winter time with a peak occurring in January, February, and March; detection rates were also high during these months (26.2%, 25.3%, and 17%, respectively). Other respiratory virus epidemics were seen also during the winter season (Figure 3). Fifteen cases infected with parainfluenza virus, adenovirus, and human bocavirus were seen in the summer time.
Table 3.
Respiratory Pathogens Identified in Infants With Acute Bronchiolitis.
| Pathogen | n (%) | Sole Pathogen, n (%) |
|---|---|---|
| Respiratory syncytial virus | 127 (40.1) | 76 (59.8) |
| Rhinovirus | 78 (24.6) | 35 (44.8). |
| Influenza virus | 28 (8.8) | 10 (35.7) |
| Human metapneumovirus | 27 (8.5) | 11 (40.7) |
| Adenovirus | 23 (7.2) | 7 (30.4) |
| Human bocavirus | 20 (6.3) | 7 (35) |
| Parainfluenza virus | 18 (5.6) | 8 (44.4) |
| Human coronavirus | 10 (3.1) | 2 (20) |
Figure 1.
The age distribution of respiratory syncytial virus, rhinovirus, adenovirus, and dual infections.
Figure 2.
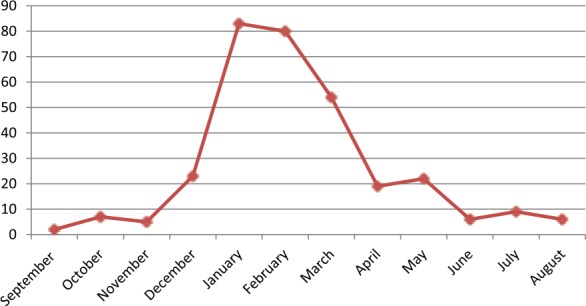
Monthly distribution of infants with acute bronchiolitis.
Figure 3.
Monthly distribution of respiratory viruses.
Discussion
The current study of the infants hospitalized with bronchiolitis has proved once again that AB is an important cause of hospitalization in infants, mostly younger than 6 months of age. Using multiplex real-time PCR technique we demonstrated that at least one respiratory viral pathogen was responsible for bronchiolitis in 75% of our cases.
Various studies have shown that RSV is the most common etiologic pathogen with a rate of 50% to 80%.18 Almost 30% to 40% of cases are related to other viruses including adenovirus, coronavirus, parainfluenza, influenza, and rhinovirus. In recent years, new human respiratory viruses like human metapneumovirus, human bocavirus, and new human coronaviruses have also been reported as possible pathogens causing AB.19 Today, various viral diagnostic tests allow us to examine the epidemiological differences/clinical characteristics of respiratory viruses and to provide information on their presence. Multiplex PCR has been the most commonly used method due to some significant advantages like permitting simultaneous amplification of several viruses in a single reaction and facilitating cost-effective diagnosis.20 In this study, we evaluated via multiplex PCR technique the variety and burden of viral agents causing AB in infants under 24 months of age.
The findings are in agreement with the results of several studies previously reported, in which RSV was diagnosed in 50% to 80% of infants admitted to hospital with acute bronchiolitis.21-26 RSV was the most common virus identified in 127 infants (40.1%) in our study. Stempel et al26 showed that RSV incidence was 77%, and Antunes et al27 found this as 58.1% in the RSV epidemic season. In our study, RSV was the most frequently identified respiratory virus, accounting for 40.1% of all respiratory viruses. In addition, 24.6% of the infants in our study were positive for rhinovirus, followed by influenza virus (8.8%), human metapneumovirus (8.5%), adenovirus (7.2%), human bocavirus (6.3%), parainfluenza virus (5.6%), and human coronavirus (3.1%). Human rhinoviruses are the most common human respiratory pathogens and are responsible for most upper respiratory infections (eg, common cold). They may also cause severe lower respiratory tract infections, including pneumonia and bronchiolitis.28 Additionally, human rhinoviruses are known as a major pathogen for asthma exacerbations in children and adults. Recently, some studies have shown rhinoviruses to be the second most commonly seen pathogen for AB. A study conducted in the United States found a prevalence of 25.6% for rhinovirus,25 similar to our study. However, other studies reported a lower incidence rate of human rhinoviruses in acute bronchiolitis.24,29,30
Our study showed that the respiratory viral agents exhibited seasonal patterns with the number of RSV and rhinovirus cases peaking in the winter season similar to that already published in previous studies.31 It has been reported that the seasonal distribution of respiratory agents may occur due to meteorological conditions, spreading of infectious pathogens, and pathogen transmission by the host behavior due to different meteorological conditions.32,33 Another important theory on pathogen activity is total sunshine exposure. It is hypothesized that ultraviolet light radiation could affect the spread of RSV by inactivating it.33,34 The city of Izmir in Turkey, located in the west, has Mediterranean climate in which summers are hot and dry and winters are warm and rainy. This climatic feature could explain the seasonal pattern of respiratory viruses in our study.
In keeping with previous studies, we found that the respiratory viruses could occur as co-infection with other respiratory viruses, within a range of 19% to 35% dual, triple, or more, dual being the most frequent.26,35 Ong et al36 showed in their study that RSV bronchiolitis associated with other pathogens was present in 10% of the infants. In a multicenter study, it was reported that the co-infection rate was 9%.25 A study by Papadopoulos et al37 stated that 19.5% of 119 infants were dual-infected, 69% of whom had RSV together with rhinovirus. In this study, we identified co-infection in 81 (25.6%) infants with bronchiolitis, and more than half of 25.6% showed the same dual combination.
In the 1960s, it was reported that the mortality in infants less than 1 year of age diagnosed with bronchiolitis was 4% to 7% for those who were born prematurely and who had an underlying cardiopulmonary disease or immunodeficiency.11 Today, the mortality rate of AB is low due to the fact that the infants are monitored by some preventive measures such as palivizumab and improved health services. However, there is an increase in the number of infants applying to hospital and hospitalization. Hall et al38 reported that the yearly incidence of hospitalization due to RSV bronchiolitis for infants aged under 6 months was 17, emergency department visits were 55, and office visits were 132 per 1000 children. Our study has a number of limitations. First, we only investigated the hospitalized patients with acute bronchiolitis, and not the infants who applied to the emergency services or outpatients policlinics, so our study group was small in number. Second, we did not identify other possible agents such as atypical or bacterial pathogens that could cause acute bronchiolitis. Third, a 3-year study period was not adequate compared to other long-term studies.
Conclusions
Our study showed that the causative agents of acute bronchiolitis are quite varied in inpatient infants in Ege University Children’s Hospital during a period of 3 years. Molecular diagnostic techniques uncovered a high frequency of viruses and viral combination infections among patients hospitalized with AB. RSV remains the most frequent causative pathogen, followed by rhinovirus. Even if the results of diagnostic methods for RSV show that the most common pathogen is single, multiple viral infections should also be considered for AB. The present study also emphasized the potential significance of other viruses such as influenza virus, human metapneumovirus, adenovirus, human bocavirus, parainfluenza virus, and human coronavirus in infants hospitalized with acute bronchiolitis, as sole or co-pathogen.
Author Contributions
ŞG: Contributed to conception and design; contributed to analysis; drafted the manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
ZK: Drafted the manuscript.
GK: Contributed to conception and design.
CÇ: Contributed to acquisition, analysis, or interpretation.
AA: Contributed to conception or design.
Acknowledgments
The authors would like to thank the study staff at the Microbiology Laboratory for conducting the study and the infants and their families for participating in the study. In addition, we wish to formally acknowledge Dr Aslı Süner who helped the statistical analyses.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. Bronchiolitis-associated hospitalizations among US children, 1980-1996. JAMA. 1999;282:1440-1446. [DOI] [PubMed] [Google Scholar]
- 2. Boyce TG, Mellen BG, Mitchel EF, Jr, Wright PF, Griffin MR. Rates of hospitalization for respiratory syncytial virus infection among children in Medicaid. J Pediatr. 2000;137:865-870. [DOI] [PubMed] [Google Scholar]
- 3. Center for Disease Prevention and Control. Acute bronchiolitis-associated outpatient visits and hospitalizations among American Indian and Alaska native children—United States, 1990-2000. MMWR Morb Mortal Wkly Rep. 2003;52:707-710. [PubMed] [Google Scholar]
- 4. Miller EK, Gebretsadik T, Carroll KN, et al. Viral etiologies of infant bronchiolitis,croup and upper respiratory illness during 4 consecutive years. Pediatr Infect Dis J. 2013;32:950-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pientong C, Ekalaksananan T, Teeratakulpisarn J, Ruangsiripiyakul H, Uppala R. Atypical bacterial pathogen infection in children with acute bronchiolitis in northeast Thailand. J Microbiol Immunol Infect. 2011;44:95-100. [DOI] [PubMed] [Google Scholar]
- 6. Korppi M, Leinonen M, Mäkelä PH, Launiala K. Mixed infection is common in children with respiratory adenovirus infection. Acta Paediatr Scand. 1991;80:413-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jartti T, Jartti L, Ruuskanen O, Söderlund-Venermo M. New respiratory viral infections. Curr Opin Pulm Med. 2012;18:271-278. [DOI] [PubMed] [Google Scholar]
- 8. Wainwright C. Acute viral bronchiolitis in children—a very common condition with few therapeutic options. Paediatr Respir Rev. 2010;11:39-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. World Health Organization. WHO Consultation on respiratory syncytial virus (RSV) vaccine development. http://www.who.int/immunization/research/meetings_workshops/rsv_vaccine_development/en/. Accessed June 5, 2017.
- 10. World Health Organization. Research and Development. http://www.who.int/immunization/research/meetings_workshops/rsv_vaccine_development/en/. Accessed June 5, 2017.
- 11. American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis. Diagnosis and management of bronchiolitis. Pediatrics. 2006;118:1774-1793. [DOI] [PubMed] [Google Scholar]
- 12. Ahluwalia G, Embree J, McNicol P, Law B, Hammond GW. Comparison of nasopharyngeal aspirate and nasopharyngeal swab specimens for respiratory syncytial virus diagnosis by cell culture, indirect immunofluorescence assay, and enzyme-linked immunosorbent assay. J Clin Microbiol. 1987;25:763-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Macfarlane P, Denham J, Assous J, Hughes C. RSV testing in bronchiolitis: which nasal sampling method is best? Arch Dis Child. 2005;90:634-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bordley WC, Viswanathan M, King VJ, et al. Diagnosis and testing in bronchiolitis: a systematic review. Arch Pediatr Adolesc Med. 2004;158:119-126. [DOI] [PubMed] [Google Scholar]
- 15. Harris JA, Huskins WC, Langley JM, Siegel JD; Pediatric Special Interest Group of the Society for Healthcare Epidemiology of America. Health care epidemiology perspective on the October 2006 recommendations of the Subcommittee on Diagnosis and Management of Bronchiolitis. Pediatrics. 2007;120:890-892. [DOI] [PubMed] [Google Scholar]
- 16. Wang EE, Milner RA, Navas L, Maj H. Observer agreement for respiratory signs and oximetry in infants hospitalized with lower respiratory infections. Am Rev Resp Dis. 1992;145:106-115. [DOI] [PubMed] [Google Scholar]
- 17. Bezerra PG, Britto MC, Correia JB, et al. Viral and atypical bacterial detection in acute respiratory infection in children under five years. PLoS One. 2011;6:18928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Black-Payne C. Bronchiolitis. In: Hilman BC, ed. Pediatric Respiratory Disease: Diagnosis and Treatment. Philadelphia, PA: WB Saunders; 1993:205-218. [Google Scholar]
- 19. Berry M, Gamieldien J, Fielding BC. Identification of new respiratory viruses in the millennium. Viruses. 2015;7:996-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liolios L, Jenney A, Spelman D, Kotsimbos T, Catton M, Wesselingh S. Comparison of a multiplex reverse transcription-PCR-enzyme hybridization assay with conventional viral culture and immunofluorescence techniques for the detection of seven viral respiratory pathogens. J Clin Microbiol. 2001;39:2779-2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. García CG, Bhore R, Soriano-Fallas A, et al. Risk factors in children hospitalized with RSV bronchiolitis versus non-RSV bronchiolitis. Pediatrics. 2010;126:1453-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Purcell K, Fergie J. Lack of usefulness of an abnormal white blood cell count for predicting a concurrent serious bacterial infection in infants and young children hospitalized with respiratory syncytial virus lower respiratory tract infection. Pediatr Infect Dis J. 2007;26:311-315. [DOI] [PubMed] [Google Scholar]
- 23. Calvo C, Pozo F, García-García ML, Lopez-Valero M, Pérez-Breña P, Casas I. Detection of new respiratory viruses in hospitalized infants with bronchiolitis: a three-year prospective study. Acta Paediatr. 2010;99:883-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Midulla F, Scagnolari C, Bonci E, et al. Respiratory syncytial virus, human bocavirus and rhinovirus bronchiolitis in infants. Arch Dis Child. 2010;95:35-41. [DOI] [PubMed] [Google Scholar]
- 25. Mansbach JM, Piedra PA, Teach SJ, et al. Prospective, multicenter study of viral etiology and hospital length-of-stay in children with severe bronchiolitis Arch Pediatr Adolesc Med. 2012;166:700-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stempel HE, Martin ET, Kuypers J, Englund JA, Zerr DM. Multiple viral respiratory pathogens in children with bronchiolitis. Acta Paediatr. 2009;98:123-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Antunes H, Rodrigues H, Silva N, et al. Etiology of bronchiolitis in a hospitalized pediatric population: prospective multicenter study. J Clin Virol. 2010;48:134-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ruuskanen O, Waris M, Ramilo O. New aspects on human rhinovirus infections. Pediatr Infect Dis J. 2013;32:553-555. [DOI] [PubMed] [Google Scholar]
- 29. Huguenin A, Moutte L, Renois F, et al. Broad respiratory virus detection in infants hospitalized for bronchiolitis by use of a multiplex RT-PCR DNA microarray system. J Med Virol. 2012;84:979-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Teeratakulpisarn J, Pientong C, Ekalaksananan T, Ruangsiripiyakul H, Uppala R. Rhinovirus infection in children hospitalized with acute bronchiolitis and its impact on subsequent wheezing or asthma: a comparison of etiologies. Asian Pac J Allergy Immunol. 2014;32:226-234. [DOI] [PubMed] [Google Scholar]
- 31. Manoha C, Espinosa S, Aho SL, Huet F, Pothier P. Epidemiological and clinical features of hMPV, RSV and RVs infections in young children. J Clin Virol. 2007;38:221-226. [DOI] [PubMed] [Google Scholar]
- 32. du Prel JB, Puppe W, Gröndahl B, et al. Are meteorological parameters associated with acute respiratory tract infections? Clin Infect Dis. 2009;49:861-868. [DOI] [PubMed] [Google Scholar]
- 33. Chen ZR, Ji W, Wang YQ, et al. Etiology of acute bronchiolitis and the relationship with meteorological conditions in hospitalized infants in China. J Formos Med Assoc. 2014;113:463-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yusuf S, Piedimonte G, Auais A, et al. The relationship of meteorological conditions to the epidemic activity of respiratory syncytial virus. Epidemiol Infect. 2007;135:1077-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ray CG, Minnich LL, Holberg CJ, et al. Respiratory syncytial virus-associated lower respiratory illnesses: possible influence of other agents. The Group Health Medical Associates. Pediatr Infect Dis J. 1993;12:15-24. [PubMed] [Google Scholar]
- 36. Ong GM, Wyatt DE, O’Neill HJ, McCaughey C, Coyle PV. A comparison of nested polymerase chain reaction and immunofluorescence for the diagnosis of respiratory infections in children with bronchiolitis, and the implications for a cohorting strategy. J Hosp Infect. 2001;49:122-128. [DOI] [PubMed] [Google Scholar]
- 37. Papadopoulos NG, Moustaki M, Tsolia M, et al. Association of rhinovirus infection with increased disease severity in acute bronchiolitis. Am J Respir Crit Care Med. 2002;165:1285-1289. [DOI] [PubMed] [Google Scholar]
- 38. Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360:588-598. [DOI] [PMC free article] [PubMed] [Google Scholar]