Abstract
Background. Although Burkitt lymphoma (BL) is the most common childhood lymphoma in sub-Saharan Africa, Hodgkin lymphoma (HL) and other non-Hodgkin lymphomas occur. Diagnosing non-jaw mass presentations is challenging with limited pathology resources. Procedure. We retrospectively analyzed 114 pediatric lymphomas in Lilongwe, Malawi, from December 2011 to June 2013 and compared clinical versus pathology-based diagnoses over two time periods. Access to pathology resources became more consistent in 2013 compared with 2011-2012; pathology interpretations were based on morphology only. Results. Median age was 8.4 years (2.1-16.3). The most common anatomical sites of presentation were palpable abdominal mass 51%, peripheral lymphadenopathy 35%, and jaw mass 34%. There were 51% jaw masses among clinical diagnoses versus 11% in the pathology-based group (P < .01), whereas 62% of pathology diagnoses involved peripheral lymphadenopathy versus 16% in the clinical group (P < .01). The breakdown of clinical diagnoses included BL 85%, lymphoblastic lymphoma (LBL) 9%, HL 4%, and diffuse large B-cell lymphoma (DLBCL) 1%, whereas pathology-based diagnoses included HL 38%, BL 36%, LBL 15%, and DLBCL 11% (P < .01). Lymphoma diagnosis was pathology confirmed in 19/66 patients (29%) in 2011-2012 and 28/48 (60%) in 2013 (P < .01). The percentage of non-BL diagnoses was consistent across time periods (35%); however, 14/23 (61%) non-BL diagnoses were pathology confirmed in 2011-2012 versus 16/17 (94%) in 2013. Conclusions. Lymphomas other than Burkitt accounted for 35% of childhood lymphoma diagnoses. Over-reliance on clinical diagnosis for BL was a limitation, but confidence in non-BL diagnoses improved with time as pathology confirmation became standard. Increased awareness of non-BL lymphomas in equatorial Africa is warranted.
Keywords: Burkitt lymphoma, non-Hodgkin lymphoma, Hodgkin lymphoma, Africa, low- and middle-income countries, pathology, pediatric oncology, global health
Introduction
Identified as a lymphoma in Uganda in the 1960s, Burkitt lymphoma (BL) is the most common overall childhood cancer throughout the malaria-endemic regions of Africa.1-3 Whereas the classical presentation with a rapidly progressing jaw mass is clinically distinct, abdominal mass presentations are impossible to definitively diagnose without pathology evaluation. With a paucity of pathology resources throughout sub-Saharan Africa, it is evident that childhood malignancies are often diagnosed on clinical grounds or remain undiagnosed.4,5
Over the past 50 years, the majority of endemic BL research studies have relied on clinical or cytology-based diagnoses from fine needle aspiration (FNA).6-24 Although this is usually sufficient for the classic jaw mass BL presentation, the overlap in similarity of the morphological appearance of BL with the other common non-Hodgkin lymphomas (NHLs) of childhood—lymphoblastic lymphoma (LBL) and diffuse large B-cell lymphoma (DLBCL)—as well as with other small round blue cell solid tumors of childhood, renders this approach fallible.25,26 Although the classic cytological appearance of BL with cytoplasmic vacuoles is characteristic, many cases lack these features. In high-income countries, definitive diagnosis of all lymphomas is established with a complete array of diagnostic tools, including morphology, immunohistochemical stains, flow cytometry, and molecular and cytogenetic studies.27
The spectrum of pediatric lymphomas occurring in regions of Africa with high rates of endemic BL is not well described. Data from a multicenter International Network of Cancer Treatment and Research (INCTR) study across sub-Saharan Africa represent the most comprehensive report. Backed by expert international hematopathology review of 132 lymphoma cases, INCTR data demonstrated 72% BL, 13% Hodgkin lymphoma (HL), 7% DLBCL, and 8% other NHL.28 Mutalima et al29 reported 330 lymphomas from Blantyre, Malawi, demonstrating 86% BL, 11% other NHL, and 3% HL—just >60% were pathology confirmed.29
In the United States and Europe, the epidemiological distribution of pediatric lymphoma is well established. HL is the most common overall.30,31 Among NHLs, BL is most common (~40%), followed by LBL (~25%), DLBCL (~15%-20%), anaplastic large cell lymphoma (~5%-10%), primary mediastinal B-cell lymphoma (~5%),31-34 and other rare NHLs (<5%).35 Because clinical presentations and therapeutic strategies for the various lymphomas are distinct, determining the precise histology is critical.
Although there are a few institutions in sub-Saharan Africa attempting to provide comprehensive pediatric oncology treatment, severe limitations in resources remain a critical problem.5 Lacking capacity to treat all childhood cancers, several hospitals have attempted to provide single-agent cyclophosphamide regimens aimed at treating low-stage BL (ie, disease limited to the jaws).16,17,36-39 This approach was a logical first step toward the provision of care and treatment for children with cancer because of a few factors: (1) the predominance of BL in sub-Saharan Africa, (2) limitations in access to chemotherapy, supportive care, and diagnostic resources, and (3) the established success of curing low-stage BL with cyclophosphamide monotherapy. At Kamuzu Central Hospital (KCH) in Malawi’s capital city Lilongwe, this was the treatment paradigm for 20 years.15
In 2011, the pediatric oncology program at KCH was expanded with the goal of attempting curative treatment of the most common childhood malignancies—lymphomas account for the majority of these curable diseases in Malawi.4,29,40,41 The concurrent development of the KCH Pathology Laboratory gradually improved access to pathology services over the study period and greatly enhanced the pediatric oncology program.42,43 Acknowledging the challenges of confidently establishing non-BL diagnoses amid severe limitations in resources, our goal was to compare the breakdown of BL versus non-BL diagnoses using clinical and pathology-based methods and describe the evolution of our experience with increasing access to timely pathology evaluations.
Methods
Located in Malawi’s central region, KCH is a public tertiary care facility serving a catchment area of approximately 5 to 8 million people. Malawi is endemic for malaria and has an estimated human immunodeficiency virus (HIV) prevalence of 10%.44 This study is a retrospective analysis of medical records of children and adolescents (<18 years) newly diagnosed with lymphoma at KCH between December 2011 and June 2013. Data were extracted from medical records and standardized treatment flow sheets; patient health information was de-identified and protected for confidentiality.
Diagnostic and Staging Workup
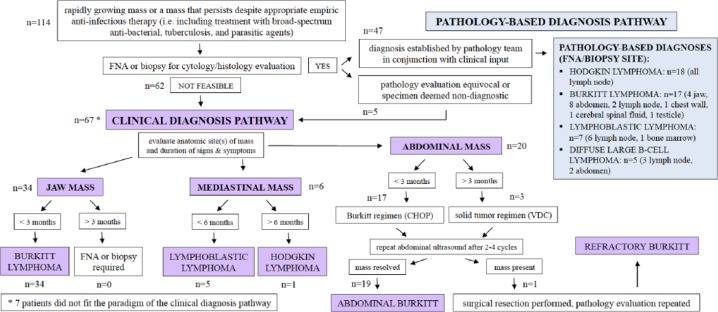
Standard clinical evaluation included physical exam, complete blood count, point-of-care testing for HIV, chest X-ray (CXR), and abdominal ultrasound. Clinical diagnoses were established based on a constellation of clinical factors, including patterns of anatomical site(s) of presentation, duration of symptoms, and evaluation of treatment response (Figure 1). The well-described phenomenon of a rapidly progressing jaw mass established a clinical diagnosis of BL in the absence of pathology confirmation. Otherwise, clinical diagnoses relied on the established clinical patterns associated with the common pediatric lymphoma entities in high-income countries.
Figure 1.
Clinical and pathology-based diagnostic schema.a
Abbreviations: FNA, fine needle aspiration; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone; VDC, vincristine, doxorubicin, cyclophosphamide.
aSeven patients did not fit the diagnosis pathway: 3 with Burkitt lymphoma are described in the text; 1 with diffuse large B-cell lymphoma presented with cervical lymphadenopathy, abdominal mass, massive ascites, and peritoneal fluid cytology evaluation that was suspicious for diffuse large B-cell lymphoma; 2 with Hodgkin lymphoma presented with abdominal masses—one with prolonged duration of massive lymphadenopathy and the other with a chest wall mass—and 1 with lymphoblastic lymphoma presented with bulging peripheral lymphadenopathy and severe pancytopenia, which may have represented a patient with acute lymphoblastic leukemia versus lymphoma.
A diagnostic pathology laboratory was opened at KCH in July 2011, and services were gradually increased in subsequent years.42,43 In 2011-2012, slide review took place remotely by a pathologist located in Blantyre, Malawi, or through an occasional rotation of volunteers from Pathologists Overseas.42 Therefore, although the laboratory was able to process FNA and biopsy specimens, the time required to finalize an interpretation was dependent on pathologist availability and varied from 1 to several weeks.42 Considering this limitation, the clinical utility of delayed pathology evaluation for patients with the aggressive clinical scenarios characteristic of childhood NHL was limited. Bolstered by the addition of a full-time pathologist in late 2012, routine pathology services became incorporated into the pediatric oncology program in January 2013.42 From that point onward, except for classic cases of BL or Reed-Sternberg cells (HL), the majority of slides were additionally evaluated at weekly telepathology conferences, including pathologists in Malawi and the United States.42 FNA and tissue biopsy specimens were processed and evaluated for morphology only. Sites of FNA/biopsy are delineated for each diagnosis in Figure 1.
A major clinical dilemma surfaced in patients with abdominal masses without other anatomical sites of involvement. In the context of very limited surgical support, intra-abdominal biopsies were not feasible/safe, and those patients who were established as not having solid tumors were clinically diagnosed with BL. Discernment of solid tumor versus abdominal lymphoma was based on (1) pattern of intra-abdominal organ involvement on ultrasound (ie, primary kidney or adrenal masses favored Wilms tumor and neuroblastoma, respectively), (2) reported duration of symptoms (shorter duration of <3 months favored lymphoma), (3) response to initial cycles of chemotherapy (complete resolution of mass favored lymphoma), and (4) morphological appearance of FNA cytology when available. Upfront surgical resection was not feasible because of the limitations in surgical support; therefore, all patients with abdominal mass received upfront chemotherapy. Some patients who were initially diagnosed as having abdominal lymphoma eventually demonstrated persistence of the abdominal mass after 2 to 4 cycles of chemotherapy and were ultimately identified as having either a refractory lymphoma or a solid tumor on eventual surgical resection. Solid tumors were excluded.
Mediastinal masses, although uncommon as the primary site of presentation in pediatric BL/DLBCL,27,45-47 are common in HL, and are the most common anatomical site in LBL.33,47,48 Thus, patients presenting with mediastinal masses lacking pathology confirmation were categorized as either HL or LBL based on duration of symptoms (duration >6 months favored HL).
We utilized the St Jude staging classification for NHL and the Ann Arbor classification for HL. Without access to computed tomography (CT) imaging, staging was based on physical exam and CXR only. Abdominal ultrasonography results were inconsistent and often unreliable when used solely for staging assessment; therefore, the presence of abdominal mass was identified by exam only. Mediastinal masses were identified via CXR. Central nervous system (CNS) involvement was based on exam only (ie, cranial nerve palsy, lower-extremity weakness, or bowel/bladder dysfunction). Starting in 2013, routine cytological evaluation of cerebral spinal fluid from the initial lumbar puncture provided additional staging information. Bone marrow aspirations were not routinely performed; bone marrow involvement was determined based on presence of 2 or more cell lines suppressed on peripheral blood count. Bulky disease in HL was defined as mediastinal mass greater than one-third the thoracic diameter on CXR and/or extra-mediastinal nodal aggregates greater than 6 cm in diameter.
Therapeutic and Supportive Care Strategies
Treatment regimens utilized the consistently available chemotherapeutic agents, which included cyclophosphamide, doxorubicin, vincristine, methotrexate, bleomycin, and oral prednisone. The upfront chemotherapy regimen varied according to diagnosis and risk stratification (Table 1). We used a modified INCTR 03-06 BL protocol for patients with stage I/II BL as well as the initial 10 patients with stage III/IV BL.20 After 9 of 10 patients with stage III/IV BL relapsed with INCTR 03-06, and because of the fact that the chemotherapeutic agents (ifosfamide and etoposide) in the INCTR second-line salvage regimen were unavailable, patients with DLBCL and stage III/IV BL were, thereafter, treated with a modified CHOP regimen (Table 1). We chose not to intensify with high-dose methotrexate because of concerns about its safety in our resource-limited setting.17 Lacking availability of lymphoblastic leukemia–specific agents (ie, mercaptopurine, cytarabine, asparaginase) and with the challenges of delivering prolonged maintenance phase therapy, patients with LBL received a prolonged CHOP regimen. Patients with HL received a modified ABVE-PC(doxorubicin, bleomycin, vincristine, etoposide, prednisone, and cyclophosphamide) protocol without etoposide. Radiation therapy was not available.
Table 1.
Disease-Specific Chemotherapy Regimens for Pediatric Lymphomas at Kamuzu Central Hospital.a
| Lymphoma | Protocol | Number of Cycles | Frequency |
|---|---|---|---|
| Stage I/II | Modified INCTR 03-06 protocol | 6 | 14 Days |
| Burkitt lymphoma | Cyclophosphamide 1200 mg/m2 ×1 | ||
| Vincristine 1.4 mg/m2 ×1 | |||
| Methotrexate 75 mg/m2 ×1 | |||
| Prednisone 1-2 mg/kg/d ×5 | |||
| DLBCL and | Modified CHOP protocolb | 6 | 21 Days |
| Stage III/IV | Cyclophosphamide 800 mg/m2 ×1 | ||
| Burkitt lymphoma | Doxorubicin 40 mg/m2 ×1 | ||
| Vincristine 2 mg/m2 ×1 | |||
| Prednisone 1-2 mg/kg/d ×5 | |||
| Lymphoblastic | Modified CHOP protocolb | 8 | 21 Days |
| Lymphoma | Cyclophosphamide 800 mg/m2 ×1 | ||
| Doxorubicin 40 mg/m2 ×1 | |||
| Vincristine 2 mg/m2 ×1 | |||
| Prednisone 1-2 mg/kg/d ×5 | |||
| Hodgkin | Modified ABVE-PC protocol | 6-8 | 21 Days |
| Lymphoma | Doxorubicin 40 mg/m2 ×1 | ||
| Bleomycin 10 U/m2 ×1 | |||
| Vincristine 2 mg/m2 ×1 | |||
| Etoposide unavailable | |||
| Cyclophosphamide 800 mg/m2 ×1 | |||
| Prednisone 1-2 mg/kg/d ×5 |
Abbreviations: INCTR, International Network for Cancer Treatment and Research; DLBCL, diffuse large B-cell lymphoma; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone.
All patients with non-Hodgkin lymphoma received intrathecal methotrexate plus hydrocortisone—2 doses per cycle for patients with central nervous system involvement and 1 dose per cycle otherwise.
An initial 1-week COP reduction prephase (cyclophosphamide 300-400 mg/m2, vincristine 2 mg/m2, prednisone 1-2 mg/kg for 5 days) was given to mitigate risk for tumor lysis syndrome.
General guidelines for chemotherapy included delaying and/or dose-adjusting chemotherapy for absolute neutrophil count <1.0 × 109/L and/or platelet count <100 × 109/L. Whole blood transfusions were consistently available; however, access to platelet transfusions was rare. Resources for supportive care have been previously described.49 All patients received at least 10 to 14 days of oral allopurinol to treat/prevent tumor lysis syndrome (TLS). Laboratory evaluations of TLS were not available. We generally maintained a low threshold to empirically treat infection/febrile illness with the available antibiotics. Treatment was free of charge, and financial support for transportation was uniformly provided. Definitions of treatment abandonment were according to international guidelines.50,51 Only those patients who clinically demonstrated disease progression and subsequently died were defined as having died from disease. All other deaths, where patients died on therapy, were considered treatment-related deaths.
Statistical Analysis and Ethical Considerations
Demographic, disease, and treatment information were described by standard descriptive statistics. Fisher’s exact test was used for differences in categorical variables, whereas the Kruskal-Wallis test was used for median values. Survival is reported using standard Kaplan-Meier curves and log-rank test evaluated differences in survivorship functions between groups. Patients who abandoned treatment were presumed to have died and included as deaths in measurements of survival. Statistical significance was considered at a 2-sided α-level of .05. All statistical analyses were done using STATA/IC, version 14 (StataCorp LP, College Station, TX). Ethics committee approval was obtained for this retrospective review from both the Malawi National Health Sciences Research Committee and the Baylor College of Medicine Institutional Review Board for Human Subjects Research. Data were anonymized and de-identified prior to analysis to maintain strict protection of confidentiality. Because of the retrospective nature of this study, informed consent was not obtained for the review of medical records.
Results
Overall, there were 114 patients newly diagnosed with lymphoma, accounting for 44% of all childhood malignancies diagnosed at KCH during the evaluation period (overall n = 258). The median age was 8.4 years (range = 2.1-16.3); there were 41 (36%) girls (Table 2). Pathology confirmation was achieved in 47 (41%) patients: 39 via FNA plus 8 (7 HL, 1 BL) from biopsies. HIV testing was performed in all patients; 4 (4%) were HIV infected (3 BL, 1 HL).
Table 2.
Clinical Characteristics and Treatment Outcomes of Children and Adolescents With Lymphoma Categorized by Mode of Diagnosis.
| Overall (n = 114) | Clinical Diagnosis (n = 67) | Pathology Diagnosis (n = 47) | P Value | |
|---|---|---|---|---|
| Median age in years (range) | 8.4 (2.1-16.3) | 7.6 (2.2-16.3) | 9.9 (2.1-15.3) | .01 |
| Female gender, n (%) | 41 (36%) | 26 (39%) | 15 (32%) | .29 |
| Anatomical sites of disease involvement, n (%) | ||||
| Abdominal mass | 58 (51%) | 34 (51%) | 24 (51%) | .56 |
| Peripheral LAD | 40 (35%) | 11 (16%) | 29 (62%) | <.01 |
| Jaw mass | 39 (34%) | 34 (51%) | 5 (11%) | <.01 |
| CNS involvement | 18 (16%) | 12 (18%) | 6 (13%) | .61 |
| Periorbital mass | 15 (13%) | 11 (16%) | 4 (9%) | .27 |
| Mediastinal mass | 13 (11%) | 6 (9%) | 7 (15%) | .38 |
| Cytopenias | 11 (10%) | 4 (6%) | 7 (15%) | .20 |
| Lymphoma diagnosis, n (%) | ||||
| Burkitt lymphoma | 74 (65%) | 57 (85%) | 17 (36%) | <.01 |
| Diffuse large B-cell lymphoma | 6 (5%) | 1 (1%) | 5 (11%) | |
| Lymphoblastic lymphoma | 13 (11%) | 6 (9%) | 7 (15%) | |
| Hodgkin lymphoma | 21 (18%) | 3 (4%) | 18 (38%) | |
| HIV infected, n (%) | 4 (3.5%) | 3 (4%) | 1 (2%) | .45 |
Abbreviation: LAD, lymphadenopathy; CNS, central nervous system; HIV, human immunodeficiency virus.
Overall, the most common clinical presentation was palpable abdominal mass (51%) followed by peripheral lymphadenopathy (35%) and jaw mass (34%). There were 51% jaw masses among clinical diagnoses versus 11% in the pathology-based group (P < .01) and 62% peripheral lymphadenopathy among pathology-based diagnoses versus 16% in the clinical group (P < .01; Table 2). Consequently, the breakdown of diagnoses in the 2 groups differed significantly. Among 67 clinical diagnoses, there were 57 patients with BL (85%), 6 with LBL (9%), 3 with HL (4%), and 1 with DLBCL (1%). In the pathology-based group (n = 47), there were 18 HLs (38%), 17 BLs (36%), 7 LBLs (15%), and 5 DLBCLs (11%); P < .01 (Table 2). Clinical diagnoses of BLs (n = 57) were established by characteristic jaw mass (n = 34) or were diagnoses in patients without jaw mass (20 primary abdominal tumors, 2 periorbital masses, and 1 paraspinal mass with lower-extremity weakness). They all demonstrated rapid improvement with initiation of chemotherapy.
The most common sites of FNA/biopsy were lymph node (n = 29), abdominal mass (n = 11), and jaw mass (n = 4; Figure 1). Five patients had cytology evaluations performed that failed to render a definitive diagnosis, including 1 cervical lymph node FNA, 1 abdominal mass FNA, and 3 evaluations of malignant effusions (2 ascites, 1 pleural).
There were 19 pathology-based diagnoses out of a total of 66 patients (29%) in 2011-2012 compared with 28 pathology-based diagnoses out of 48 patients (60%) in 2013 (P = .003; Table 3). The percentage of non-BL diagnoses (35%) was consistent across the two time periods (Table 3); however, 14/23 (61%) non-BL diagnoses were pathology confirmed in 2011-2012 versus 16/17 (94%) in 2013 (Table 4). Although the majority of patients with BL were diagnosed clinically, the percentage of pathology-confirmed BLs also increased from 12% (5/43) in 2011-2012 to 39% (12/31) in 2013.
Table 3.
Breakdown of Lymphoma Diagnoses Categorized by Time Period.
| Overall (n = 114) | 2011-2012 (n = 66) | 2013 (n = 48) | P Value | |
|---|---|---|---|---|
| Pathology confirmed, n (%) | 47 (41%) | 19 (29%) | 28 (60%) | .003 |
| Lymphoma diagnosis, n (%) | ||||
| Burkitt lymphoma | 74 (65%) | 43 (65%) | 31 (65%) | .21 |
| Diffuse large B-cell lymphoma | 6 (5%) | 1 (2%) | 5 (10%) | |
| Lymphoblastic lymphoma | 13 (11%) | 8 (12%) | 5 (10%) | |
| Hodgkin lymphoma | 21 (18%) | 14 (21%) | 7 (15%) | |
Table 4.
Breakdown of Clinical Versus Pathology-Based Lymphoma Diagnoses Across Time Periods.
| Time Period | Clinical Diagnosis | Pathology Diagnosis | Percentage Pathology Confirmed |
|---|---|---|---|
| 2011-2012 | |||
| BL (n = 43) | 38 | 5 | 12% |
| HL (n = 14) | 3 | 11 | 79% |
| LBL (n = 8) | 6 | 2 | 25% |
| DLBCL (n = 1) | 0 | 1 | 100% |
| 2013 | |||
| BL (n = 31) | 19 | 12 | 39% |
| HL (n = 7) | 0 | 7 | 100% |
| LBL (n = 5) | 0 | 5 | 100% |
| DLBCL (n = 5) | 1 | 4 | 80% |
Abbreviations: BL, Burkitt lymphoma; HL, Hodgkin lymphoma; LBL, lymphoblastic lymphoma; DLBCL, diffuse large B-cell lymphoma.
In all, 20 patients (19 BL, 1 DLBCL) received clinical diagnoses in 2013 for the following reasons: having abdominal masses that were not accessible for FNA (n = 7), clinical discretion to initiate treatment based on classic presentations of rapidly progressive jaw masses representing BL (n = 9), concern over the safety of performing FNA on a periorbital mass (n = 1), and 3 patients with cytology evaluations who failed to render a diagnosis.
Table 5 demonstrates clinical characteristics of patients categorized by lymphoma diagnosis. The abdominal cavity was the predominant anatomical site in patients with BL and DLBCL. Mediastinal mass was most common (77%) in LBL, whereas 95% of patients with HL presented with peripheral lymphadenopathy. Overall, stage I/II BL limited to the jaw/neck represented 22% of all lymphoma diagnoses, and their 18-month overall survival (OS) was 49% (95% CI = 25-68), which trended better than the 18-month OS of 26% (95% CI = 15-40; P = .09) for patients with stage III/IV BL and DLBCL. With a median follow-up of 16 months (range = 3-31), 12- and 18-month OS rates for the entire cohort were 36% (95% CI = 27-46) and 29% (95% CI = 18-41), respectively. It is notable that the 18-month OS for patients with LBL was 0% (Table 5).
Table 5.
Clinical Characteristics of Children and Adolescents With Lymphoma Categorized by Disease.
| Overall (n = 114) | Burkitt (n = 74) | DLBCL (n = 6) | LBL (n = 13) | Hodgkin (n = 21) | P Value | |
|---|---|---|---|---|---|---|
| Median age in years (range) | 8.4 (2.1-16.3) | 7.6 (2.2-16.3) | 11 (2.1-14.1) | 9 (4-12.8) | 11.5 (3.3-15.3) | .03 |
| Female gender, n (%) | 41 (36%) | 27 (36%) | 2 (33%) | 6 (46%) | 6 (29%) | .78 |
| Clinical presentation, n (%) | ||||||
| Abdominal mass | 58 (51%) | 40 (54%) | 4 (67%) | 8 (62%) | 6 (29%) | .13 |
| Peripheral LAD | 40 (35%) | 7 (9%) | 4 (67%) | 9 (69%) | 20 (95%) | <.01 |
| Jaw mass | 39 (34%) | 39 (53%) | 0 | 0 | 0 | <.01 |
| CNS involvement | 18 (16%) | 16 (22%) | 1 (17%) | 1 (8%) | 0 | .06 |
| Periorbital mass | 15 (13%) | 14 (19%) | 1 (17%) | 0 | 0 | .04 |
| Mediastinal mass | 13 (11%) | 0 | 0 | 10 (77%) | 3 (14%) | <.01 |
| Cytopenias | 11 (10%) | 4 (5%) | 1 (17%) | 4 (31%) | 2 (10%) | .02 |
| Pathology confirmation, n (%) | 47 (41%) | 17 (23%) | 5 (83%) | 7 (54%) | 18 (86%) | <.01 |
| HIV infected, n (%) | 4 (4%) | 3 (4%) | 0 | 0 | 1 (5%) | 1.00 |
| Clinical staging, n (%) | —a | |||||
| Stage I/II | 37 (32%) | 25 (34%) | 1 (17%) | 0 | 11 (52%) | |
| Stage III | 46 (40%) | 29 (39%) | 3 (50%) | 8 (62%) | 6 (29%) | .03 |
| Stage IV | 30 (26%) | 20 (27%) | 2 (33%) | 5 (38%) | 3 (14%) | |
| Median symptom duration, months (range) | 2 (0.5-36) | 1.5 (0.5-4) | 2.5 (1.5-3.5) | 2 (1-4) | 9 (3-36) | <.01 |
| Overall survival | ||||||
| 12-Month (95% CI) | 36% (27-46) | 38% (27-49) | 15% (2-39) | 46% (24-65) | .2 | |
| 18-Month (95% CI) | 29% (20-38) | 33% (22-44) | 0 | 38% (17-59) | ||
| Median survivor follow-up, months (range) | 16 (3-31) | 18 (3-31) | N/A | 13 (5-26) | ||
| Treatment abandonment, n (%) | 18 (16%) | 13 (16%) | 1 (8%) | 4 (19%) | .72 | |
| Number of deaths, n (%) | 57 (50%) | 36 (45%) | 12 (92%) | 10 (48%) | .03 | |
| Deaths from disease | 46 (40%) | 28 (35%) | 12 (92%) | 7 (33%) | .01 | |
| Treatment-related deaths | 7 (6%) | 6 (8%) | 0 | 1 (5%) | .91 | |
| Deaths in complete remission | 4 (4%) | 2 (3%) | 0 | 2 (10%) | .22 | |
| Median time to death, months (range) | 7 (0.1-26) | 5.5 (0.1-26) | 9 (1-17) | 9 (0.1-23) | .51 | |
Abbreviations: DLBCL, diffuse large B-cell lymphoma; LBL, lymphoblastic lymphoma; LAD, lymphadenopathy; CNS, central nervous system; HIV, human immunodeficiency virus.
One patient with Hodgkin lymphoma abandoned treatment prior to staging, and 8 of 11 stage I/II patients were stage II with bulky disease. Note that for outcome results, Burkitt and DLBCL patients were analyzed as a combined group of patients with mature B-cell non-Hodgkin lymphoma.
Discussion
This retrospective observational cohort of children and adolescents with lymphoma in Lilongwe, Malawi, demonstrates that approximately one-third of diagnoses are not BL, shifting the lens to focus on increasing the awareness of lymphomas beyond endemic BL. Enhanced by improving pathology services to confirm the non-BL diagnoses over the study period, these data emphasize the importance of not only pathology services, but also collaboration between clinicians and pathologists in this resource-limited setting.
The availability of pathology services evolved over the study period to eventually, in 2013, provide routine diagnostic support for the confirmation of non-BL diagnoses. In comparing the 2 time periods, there were 35% non-BL diagnoses in both 2011-2012 and 2013; the major difference in time periods is that all but one (94%) of the non-BL diagnoses were pathology confirmed in 2013 compared with only 61% in 2011-2012. The 35% non-BL diagnoses in our cohort compares with 28% and 14% non-BL childhood lymphoma diagnoses from data reported by the INCTR and Blantyre, Malawi, respectively.28,29 With those proportions of non-BL childhood lymphoma diagnoses occurring throughout the region, we advocate for increased awareness of HL, LBL, and DLBCL with the hope of improving treatment outcomes for all children with lymphoma in sub-Saharan Africa.
In comparing clinical versus pathology-based diagnoses, the breakdown of lymphoma diagnoses contrasted in accordance with the selection bias of clinical presentations in the two groups. There was a significant predominance of peripheral lymphadenopathy in the pathology-based group, primarily because palpable lymphadenopathy is more amenable to FNA and biopsy. That, coupled with the relatively indolent clinical presentation characteristic of HL, theoretically enabled increased uptake of pathologically confirmed diagnoses of HL. In contrast, the distinct nature of a jaw mass that is rapidly growing in size lends itself to an increased overreliance in the clinical confidence in diagnosing BL and decreased numbers of pathology-based BL diagnoses.
With the gradual increase in capacity of the KCH Pathology Laboratory over the study period duration, the uptake of pathology-based diagnoses increased. Although the pathology laboratory was operational starting in July 2011, delays in diagnostic interpretation because of limited availability of pathologists imposed a major limitation on the clinical utility of FNA/biopsy in patients with the aggressive clinical course characteristic of childhood NHL. For that reason, in 2011-2012, there were few pathology-based diagnoses of NHL, and those obtained were mainly by virtue of coincidentally presenting at a time of pathologist availability.
With significant limitations in surgical support for biopsy, a major clinical dilemma surfaced in patients presenting with primary abdominal tumors. Palpable abdominal mass was the most common anatomical site of presentation in the cohort. Other cohorts have similarly demonstrated high numbers of abdominal BL presentations in Africa, including our more recent prospective cohort of pathologically confirmed BL from KCH.22,52 Increased access to more advanced imaging studies (ie, CT or improved ultrasonography), diagnostic laboratory studies (such as lactate dehydrogenase, uric acid, and Epstein-Barr viral load monitoring), and radiology-guided biopsy procedures may improve uptake of pathologically confirmed diagnoses for abdominal lymphomas.12,53
Only 22% of patients presented with stage I/II BL localized to the jaw/neck —the subgroup of patients who historically achieved encouraging outcomes with cyclophosphamide monotherapy.6,10,15-17,19,21 Reflecting on the experience at KCH from the previous 20 years, where cyclophosphamide was the only chemotherapy agent available, it appears that a small fraction of lymphoma patients would have had a realistic chance to achieve curative outcomes based on this historical paradigm. The remainder of patients had advanced-stage BL, HL, LBL or DLBCL—and although they received intensified treatment regimens, their long-term outcomes were suboptimal, and there were no survivors among children with LBL. These disappointing results highlight the importance of not only establishing accurate diagnoses, but also providing optimized disease-specific, risk-stratified treatment regimens.54,55 It is clear that LBL requires an acute lymphoblastic leukemia-like protocol—a challenge that, to date, has been difficult to surmount in sub-Saharan Africa, however one with successful precedent established in other low-/middle-income countries.56-59
The pattern of pediatric lymphomas in the context of the HIV epidemic in sub-Saharan Africa has not been well described.60 Although NHL was the most common HIV-related malignancy in children in the United States and Europe,61-66 there does not seem to be an acute increase in pediatric lymphoma incidence in the regions of Africa hardest hit by HIV.22,29,67,68 Our data indirectly confirm this observation because HIV-related lymphomas only accounted for 4 cases in the cohort. For contextual comparison, there were 45 children diagnosed with HIV-related Kaposi sarcoma in Lilongwe during the same time period.40
Ultimately, the ability to accurately determine pathological diagnoses of lymphoma in sub-Saharan Africa will provide the best opportunity to select the appropriate disease-specific and risk-stratified treatment regimens. The difficulty in making definitive diagnoses of lymphoma based on cell morphology alone is universally acknowledged; this challenge is more pronounced in sub-Saharan Africa, where the overlap in morphological appearance of BL with LBL and DLBCL can result in diagnostic uncertainty even in centers where cytology is routinely available.25,26 In our experience, collaboration between clinicians and pathologists provided increased confidence in pathology-based diagnoses. Prime examples arise in patients with cytology that is suspicious of both BL and LBL; clinical presentation with mediastinal mass and lymphadenopathy will render a diagnosis of LBL, whereas clinical features of an abdominal mass with or without other sites of involvement will render a diagnosis of BL.
Limitations to the study include its retrospective nature, the modest number of pathology-confirmed diagnoses, lack of uniform long-term follow-up, and the limitations of clinical staging information. Recognizing the hardships that populations living in rural poverty endure, delayed presentation to our tertiary care facility occurs and results in encountering extreme clinical presentations that are all included in this retrospective cohort. This may cause a bias toward worse outcomes in comparison with prospective cohorts where such critically ill patients may not meet eligibility criteria. However, this experience captures the reality and scope of challenges encountered in attempting to accurately diagnose and cure childhood lymphoma in sub-Saharan Africa. We hope that it may serve as a foundation on which more sophisticated prospective trials in pediatric lymphoma in Africa can be built. A prospective characterization of lymphoma at KCH is currently under way to more accurately define the spectrum of disease, with a focus on increased access to immunohistochemical stains.69 A critical pathology-specific challenge to overcome is the inability to perform immunohistochemical stains on cytology samples. Developing strategies to produce cell blocks from FNA specimens or improve capacity to obtain tissue biopsies has become an important focus.
In conclusion, a variety of childhood lymphomas occur in Malawi beyond endemic BL. Pathology services are essential to definitively confirm the true spectrum of childhood lymphomas in the region. Increased awareness of non-BL lymphomas is warranted, and continued improvements in diagnostic, therapeutic, and supportive care resources are needed to bridge the gap in curative outcomes for pediatric lymphoma in Africa compared with the rest of the world.70 With the highest incidence rates of lymphoma worldwide occurring in Africa, we advocate for increased awareness and efforts to implement stronger educational, training, diagnostic, and treatment programs throughout the continent.4,41
Author Contributions
NKE: Contributed to conception and design; contributed to acquisition, analysis, and interpretation; drafted the manuscript; critically revised the manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
MM: Contributed to acquisition; critically revised the manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
IM: Contributed to acquisition; critically revised the manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
SG: Contributed to analysis and interpretation; critically revised the manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
CS: Contributed to analysis; critically revised the manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
PW: Contributed to interpretation; critically revised the manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
MM: Contributed to acquisition; critically revised the manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
MC: Contributed to acquisition; critically revised the manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
WK: Contributed to acquisition; critically revised the manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
JV: Contributed to acquisition; critically revised the manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
YF: Contributed to acquisition and interpretation; critically revised the manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
NM: Contributed to acquisition; critically revised the manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
GL: Contributed to acquisition; critically revised the manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
CK: Contributed to acquisition; Critically revised the manuscript; Gave final approval; Agrees to be accountable for all aspects of work ensuring integrity and accuracy.
RK: Contributed to acquisition; Critically revised the manuscript; Gave final approval; Agrees to be accountable for all aspects of work ensuring integrity and accuracy.
KW: Contributed to interpretation; critically revised the manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
MK: Contributed to interpretation; critically revised the manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
JS: Contributed to interpretation; critically revised the manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
MS: Contributed to interpretation; critically revised the manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
CA: Contributed to interpretation; critically revised the manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
PM: Contributed to conception and design; contributed to interpretation; critically revised the manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
PK: Contributed to conception and design; contributed to interpretation; critically revised the manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
Acknowledgments
We honor the courage and strength of our patients and their families, fighting a brave battle against cancer in the setting of severe poverty. We express sincere gratitude for the many collaborating individuals, institutions, and their leadership that made this work possible—the Baylor College of Medicine Children’s Foundation Malawi inclusive of the Tingathe Outreach Program, the Baylor International Pediatric AIDS Initiative at Texas Children’s Hospital, the Texas Children’s Cancer and Hematology Centers and the Global HOPE Program, Kamuzu Central Hospital, and the University of North Carolina Project–Malawi. The work of the pediatric oncology program at the Baylor College of Medicine Children’s Foundation Malawi and Kamuzu Central Hospital in Lilongwe, Malawi, was supported in part by a grant from the United States Agency for International Development through the Tingathe Program via cooperative agreement number 674-A-00-10-00093-00 and philanthropic contributions to purchase chemotherapy from ConocoPhillips. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Burkitt D, O’Conor GT. Malignant lymphoma in African children: I. A clinical syndrome. Cancer. 1961;14:258-269. [DOI] [PubMed] [Google Scholar]
- 2. van den Bosch CA. Is endemic Burkitt’s lymphoma an alliance between three infections and a tumour promoter? Lancet Oncol. 2004;5:738-746. [DOI] [PubMed] [Google Scholar]
- 3. Molyneux EM, Rochford R, Griffin B, et al. Burkitt’s lymphoma. Lancet. 2012;379:1234-1244. [DOI] [PubMed] [Google Scholar]
- 4. Gopal S, Wood WA, Lee SJ, et al. Meeting the challenge of hematologic malignancies in sub-Saharan Africa. Blood. 2012;119:5078-5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kruger M, Hendricks M, Davidson A, et al. Childhood cancer in Africa. Pediatr Blood Cancer. 2014;61:587-592. [DOI] [PubMed] [Google Scholar]
- 6. Ziegler JL, Morrow RH, Jr, Fass L, Kyalwazi SK, Carbone PP. Treatment of Burkitt’s tumor with cyclophosphamide. Cancer. 1970;26:474-484. [DOI] [PubMed] [Google Scholar]
- 7. Ziegler JL, Bluming AZ, Magrath IT, Carbone PP. Intensive chemotherapy in patients with generalized Burkitt’s lymphoma. Int J Cancer. 1972;10:254-261. [DOI] [PubMed] [Google Scholar]
- 8. Magrath IT, Lwanga S, Carswell W, Harrison N. Surgical reduction of tumour bulk in management of abdominal Burkitt’s lymphoma. Br Med J. 1974;2:308-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Olweny CL, Katongole-Mbidde E, Kaddu-Mukasa A, et al. Treatment of Burkitt’s lymphoma: randomized clinical trial of single-agent versus combination chemotherapy. Int J Cancer. 1976;17:436-440. [DOI] [PubMed] [Google Scholar]
- 10. Nkrumah FK, Perkins IV. Burkitt’s lymphoma: a clinical study of 110 patients. Cancer. 1976;37:671-676. [DOI] [PubMed] [Google Scholar]
- 11. Ziegler JL, Magrath IT, Olweny CL. Cure of Burkitt’s lymphoma: ten-year follow-up of 157 Ugandan patients. Lancet. 1979;2:936-938. [DOI] [PubMed] [Google Scholar]
- 12. Magrath I, Lee YJ, Anderson T, et al. Prognostic factors in Burkitt’s lymphoma: importance of total tumor burden. Cancer. 1980;45:1507-1515. [DOI] [PubMed] [Google Scholar]
- 13. Olweny CL, Katongole-Mbidde E, Otim D, Lwanga SK, Magrath IT, Ziegler JL. Long-term experience with Burkitt’s lymphoma in Uganda. Int J Cancer. 1980;26:261-266. [DOI] [PubMed] [Google Scholar]
- 14. Orem J, Mulumba Y, Algeri S, et al. Clinical characteristics, treatment and outcome of childhood Burkitt’s lymphoma at the Uganda Cancer Institute. Trans R Soc Trop Med Hyg. 2011;105:717-726. [DOI] [PubMed] [Google Scholar]
- 15. Kazembe P, Hesseling PB, Griffin BE, Lampert I, Wessels G. Long term survival of children with Burkitt lymphoma in Malawi after cyclophosphamide monotherapy. Med Pediatr Oncol. 2003;40:23-25. [DOI] [PubMed] [Google Scholar]
- 16. Traore F, Coze C, Atteby JJ, et al. Cyclophosphamide monotherapy in children with Burkitt lymphoma: a study from the French-African Pediatric Oncology Group (GFAOP). Pediatr Blood Cancer. 2011;56:70-76. [DOI] [PubMed] [Google Scholar]
- 17. Hesseling P, Broadhead R, Mansvelt E, et al. The 2000 Burkitt lymphoma trial in Malawi. Pediatr Blood Cancer. 2005;44:245-250. [DOI] [PubMed] [Google Scholar]
- 18. Hesseling PB, Njume E, Kouya F, et al. The Cameroon 2008 Burkitt lymphoma protocol: improved event-free survival with treatment adapted to disease stage and the response to induction therapy. Pediatr Hematol Oncol. 2012;29:119-129. [DOI] [PubMed] [Google Scholar]
- 19. Marjerrison S, Fernandez CV, Price VE, Njume E, Hesseling P. The use of ultrasound in endemic Burkitt lymphoma in Cameroon. Pediatr Blood Cancer. 2012;58(3):352-355. [DOI] [PubMed] [Google Scholar]
- 20. Ngoma T, Adde M, Durosinmi M, et al. Treatment of Burkitt lymphoma in equatorial Africa using a simple three-drug combination followed by a salvage regimen for patients with persistent or recurrent disease. Br J Haematol. 2012;158:749-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Depani S, Banda K, Bailey S, Israels T, Chagaluka G, Molyneux E. Outcome is unchanged by adding vincristine upfront to the Malawi 28-day protocol for endemic Burkitt lymphoma. Pediatr Blood Cancer. 2015;62:1929-1934. [DOI] [PubMed] [Google Scholar]
- 22. Stanley CC, Westmoreland KD, Heimlich BJ, et al. Outcomes for paediatric Burkitt lymphoma treated with anthracycline-based therapy in Malawi. Br J Haematol. 2016;173:705-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Buckle G, Maranda L, Skiles J, et al. Factors influencing survival among Kenyan children diagnosed with endemic Burkitt lymphoma between 2003 and 2011: a historical cohort study. Int J Cancer. 2016;139:1231-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Magrath I. Lessons from clinical trials in African Burkitt lymphoma. Curr Opin Oncol. 2009;21:462-468. [DOI] [PubMed] [Google Scholar]
- 25. Orem J, Sandin S, Weibull CE, et al. Agreement between diagnoses of childhood lymphoma assigned in Uganda and by an international reference laboratory. Clin Epidemiol. 2012;4:339-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ogwang MD, Zhao W, Ayers LW, Mbulaiteye SM. Accuracy of Burkitt lymphoma diagnosis in constrained pathology settings: importance to epidemiology. Arch Pathol Lab Med. 2011;135:445-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Miles RR, Arnold S, Cairo MS. Risk factors and treatment of childhood and adolescent Burkitt lymphoma/leukaemia. Br J Haematol. 2012;156:730-743. [DOI] [PubMed] [Google Scholar]
- 28. Naresh KN, Raphael M, Ayers L, et al. Lymphomas in sub-Saharan Africa: what can we learn and how can we help in improving diagnosis, managing patients and fostering translational research? Br J Haematol. 2011;154:696-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mutalima N, Molyneux EM, Johnston WT, et al. Impact of infection with human immunodeficiency virus-1 (HIV) on the risk of cancer among children in Malawi: preliminary findings. Infect Agent Cancer. 2010;5(5):1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ries LAG, Smith MA, Gurney JG, et al. Cancer Incidence and Survival Among Children and Adolescents: United States SEER Program 1975-1995. Bethesda, MD: National Cancer Institute, SEER Program; 1999. [Google Scholar]
- 31. Allen CE, Kelly KM, Bollard CM. Pediatric lymphomas and histiocytic disorders of childhood. Pediatr Clin North Am. 2015;62:139-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Minard-Colin V, Brugieres L, Reiter A, et al. Non-Hodgkin lymphoma in children and adolescents: progress through effective collaboration, current knowledge, and challenges ahead. J Clin Oncol. 2015;33:2963-2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hochberg J, Waxman IM, Kelly KM, Morris E, Cairo MS. Adolescent non-Hodgkin lymphoma and Hodgkin lymphoma: state of the science. Br J Haematol. 2009;144:24-40. [DOI] [PubMed] [Google Scholar]
- 34. Gross TG, Termuhlen AM. Pediatric non-Hodgkin lymphoma. Curr Hematol Malig Rep. 2008;3(3):167-173. [DOI] [PubMed] [Google Scholar]
- 35. O’Suoji C, Welch JJ, Perkins SL, et al. Rare Pediatric Non-Hodgkin Lymphomas: A Report From Children’s Oncology Group Study ANHL 04B1. Pediatr Blood Cancer. 2016;63:794-800. [DOI] [PubMed] [Google Scholar]
- 36. Hesseling PB, Molyneux E, Tchintseme F, et al. Treating Burkitt’s lymphoma in Malawi, Cameroon, and Ghana. Lancet Oncol. 2008;9:512-513. [DOI] [PubMed] [Google Scholar]
- 37. Hesseling P, Molyneux E, Kamiza S, Israels T, Broadhead R. Endemic Burkitt lymphoma: a 28-day treatment schedule with cyclophosphamide and intrathecal methotrexate. Ann Trop Paediatr. 2009;29:29-34. [DOI] [PubMed] [Google Scholar]
- 38. Hesseling P, Israels T, Harif M, Chantada G, Molyneux E; Pediatric Oncology in Developing Countries. Practical recommendations for the management of children with endemic Burkitt lymphoma (BL) in a resource limited setting. Pediatr Blood Cancer. 2013;60:357-362. [DOI] [PubMed] [Google Scholar]
- 39. Magrath I. Towards curative therapy in Burkitt lymphoma: the role of early African studies in demonstrating the value of combination therapy and CNS prophylaxis. Adv Hematol. 2012;2012:130680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mtete I, Mutai M, Mehta PS, et al. Treating a wide spectrum of childhood malignancies despite limitations in diagnositc and therapeutic resources in Central Malawi [abstract O-220:S201]. Paper presented at: The 47th Congress of the International Society of Paediatric Oncology (SIOP); October 8-11, 2015; Cape Town, South Africa. [Google Scholar]
- 41. Linet MS, Brown LM, Mbulaiteye SM, et al. International long-term trends and recent patterns in the incidence of leukemias and lymphomas among children and adolescents ages 0-19 years. Int J Cancer. 2016;138:1862-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gopal S, Krysiak R, Liomba NG, et al. Early experience after developing a pathology laboratory in Malawi, with emphasis on cancer diagnoses. PLoS One. 2013;8:e70361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gopal S, Krysiak R, Liomba G. Building a pathology laboratory in Malawi. Lancet Oncol. 2013;14:291-292. [DOI] [PubMed] [Google Scholar]
- 44. UNAIDS. Malawi country report 2014. http://www.unaids.org/en/regionscountries/countries/malawi. Accessed June 7, 2017.
- 45. Reiter A, Klapper W. Recent advances in the understanding and management of diffuse large B-cell lymphoma in children. Br J Haematol. 2008;142:329-347. [DOI] [PubMed] [Google Scholar]
- 46. El-Mallawany NK, Cairo MS. Advances in the diagnosis and treatment of childhood and adolescent B-cell non-Hodgkin lymphoma. Clin Adv Hematol Oncol. 2015;13:113-123. [PubMed] [Google Scholar]
- 47. Hochberg J, El-Mallawany NK, Abla O. Adolescent and young adult non-Hodgkin lymphoma. Br J Haematol. 2016;173:637-650. [DOI] [PubMed] [Google Scholar]
- 48. El-Mallawany NK, Frazer JK, Van Vlierberghe P, et al. Pediatric T- and NK-cell lymphomas: new biologic insights and treatment strategies. Blood Cancer J. 2012;2:e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. El-Mallawany NK, Kamiyango W, Slone JS, et al. Clinical factors associated with long-term complete remission versus poor response to chemotherapy in HIV-infected children and adolescents with Kaposi sarcoma receiving bleomycin and vincristine: a retrospective observational study. PLoS One. 2016;11:e0153335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mostert S, Arora RS, Arreola M, et al. Abandonment of treatment for childhood cancer: position statement of a SIOP PODC Working Group. Lancet Oncol. 2011;12:719-720. [DOI] [PubMed] [Google Scholar]
- 51. Weaver MS, Arora RS, Howard SC, et al. A practical approach to reporting treatment abandonment in pediatric chronic conditions. Pediatr Blood Cancer. 2015;62:565-570. [DOI] [PubMed] [Google Scholar]
- 52. Ogwang MD, Bhatia K, Biggar RJ, Mbulaiteye SM. Incidence and geographic distribution of endemic Burkitt lymphoma in northern Uganda revisited. Int J Cancer. 2008;123:2658-2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Westmoreland KD, Montgomery ND, Stanley CC, et al. Plasma Epstein-Barr virus DNA for pediatric Burkitt lymphoma diagnosis, prognosis, and response assessment in Malawi. Int J Cancer. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Harif M, Barsaoui S, Benchekroun S, et al. Treatment of B-cell lymphoma with LMB modified protocols in Africa: report of the French-African Pediatric Oncology Group (GFAOP). Pediatr Blood Cancer. 2008;50:1138-1142. [DOI] [PubMed] [Google Scholar]
- 55. Gross TG, Biondi A. Paediatric non-Hodgkin lymphoma in low and middle income countries. Br J Haematol. 2016;173:651-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chagaluka G, Carey P, Banda K, et al. Treating childhood acute lymphoblastic leukemia in Malawi. Haematologica. 2013;98:e1-e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hunger SP, Sung L, Howard SC. Treatment strategies and regimens of graduated intensity for childhood acute lymphoblastic leukemia in low-income countries: a proposal. Pediatr Blood Cancer. 2009;52:559-565. [DOI] [PubMed] [Google Scholar]
- 58. Anderson JR, Jenkin RD, Wilson JF, et al. Long-term follow-up of patients treated with COMP or LSA2L2 therapy for childhood non-Hodgkin’s lymphoma: a report of CCG-551 from the Childrens Cancer Group. J Clin Oncol. 1993;11:1024-1032. [DOI] [PubMed] [Google Scholar]
- 59. Tubergen DG, Krailo MD, Meadows AT, et al. Comparison of treatment regimens for pediatric lymphoblastic non-Hodgkin’s lymphoma: a Childrens Cancer Group study. J Clin Oncol. 1995;13:1368-1376. [DOI] [PubMed] [Google Scholar]
- 60. Rees CA, Keating EM, Lukolyo H, et al. Mapping the epidemiology of Kaposi sarcoma and non-Hodgkin lymphoma among children in sub-Saharan Africa: a review. Pediatr Blood Cancer. 2016;63:1325-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Biggar RJ, Frisch M, Goedert JJ. Risk of cancer in children with AIDS. AIDS-Cancer Match Registry Study Group. JAMA. 2000;284:205-209. [DOI] [PubMed] [Google Scholar]
- 62. Pollock BH, Jenson HB, Leach CT, et al. Risk factors for pediatric human immunodeficiency virus-related malignancy. JAMA. 2003;289:2393-2399. [DOI] [PubMed] [Google Scholar]
- 63. Kest H, Brogly S, McSherry G, Dashefsky B, Oleske J, Seage GR., III Malignancy in perinatally human immunodeficiency virus-infected children in the United States. Pediatr Infect Dis J. 2005;24:237-242. [DOI] [PubMed] [Google Scholar]
- 64. Granovsky MO, Mueller BU, Nicholson HS, Rosenberg PS, Rabkin CS. Cancer in human immunodeficiency virus-infected children: a case series from the Children’s Cancer Group and the National Cancer Institute. J Clin Oncol. 1998;16:1729-1735. [DOI] [PubMed] [Google Scholar]
- 65. Caselli D, Klersy C, de Martino M, et al. Human immunodeficiency virus-related cancer in children: incidence and treatment outcome: report of the Italian Register. J Clin Oncol. 2000;18:3854-3861. [DOI] [PubMed] [Google Scholar]
- 66. Evans JA, Gibb DM, Holland FJ, Tookey PA, Pritchard J, Ades AE. Malignancies in UK children with HIV infection acquired from mother to child transmission. Arch Dis Child. 1997;76:330-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tukei V, Kekitiinwa A, Beasley R. Prevalence and outcome of HIV-associated malignancis among children. AIDS. 2011;25:1789-1793. [DOI] [PubMed] [Google Scholar]
- 68. Chintu C, Athale UH, Patil PS. Childhood cancers in Zambia before and after the HIV epidemic. Arch Dis Child. 1995;73:100-104; discussion 104-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Montgomery ND, Liomba NG, Kampani C, et al. Accurate real-time diagnosis of lymphoproliferative disorders in Malawi through clinicopathologic teleconferences: a model for pathology services in sub-Saharan Africa. Am J Clin Pathol. 2016;146:423-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bollard CM, Lim MS, Gross TG; COG Non-Hodgkin Lymphoma Committee. Children’s Oncology Group’s 2013 blueprint for research: non-Hodgkin lymphoma. Pediatr Blood Cancer. 2013;60:979-984. [DOI] [PMC free article] [PubMed] [Google Scholar]