Abstract
Issues:
Polyphenolic compounds, especially flavonoids, are known as the most common chemical class of phytochemicals, which possess a multiple range of health-promoting effects. Flavonoids are ubiquitous in nature. They are also present in food, providing an essential link between diet and prevention of several diseases.
Approach:
Chrysin (CH), a natural flavonoid, was commonly found in propolis and honey and traditionally used in herbal medicine. A growing body of scientific evidence has shown that CH possesses protective effects against toxic agents in various animal tissues, including brain, heart, liver, kidney, and lung.
Key Findings:
This study found that CH may be effective in disease management induced by toxic agents. However, due to the lack of information on human, further studies are needed to determine the efficacy of CH as an antidote agent in human.
Conclusion:
The present article aimed to critically review the available literature data regarding the protective effects of CH against toxic agent–induced toxicities as well as its possible mechanisms.
Keywords: chrysin, toxic agents, antioxidant
Introduction
General
Flavonoids are the main group of plant secondary metabolites with extensive vital activities desired for human health. The investigation on flavonoids has been going on with developing interest as they act through physiological mechanisms and a lot of signaling pathways involved in many medical disorders. Flavonoids are also the main polyphenolic ingredients that express a wide range of biological activities, such as anti-inflammatory, antithrombotic, antioxidant, antiallergic, antibacterial, analgesic, and vasodilatory effects.1-9
Chrysin (CH) belongs to the flavonoids and has been used as traditional medicine from ancient.10,11 Chrysin has been shown to be the main ingredient of some medicinal plants, such as Radix scutellariae,12 Lactarius deliciosus (L. ex Fr.) S.F. Gray., Passiflora incarnate L., S. ramosissima M. Pop., Cytisus multiflorus (L’Her. Ex Aiton) Sweet, Scutellaria immaculate Nevski ex Juz., Suillus bellinii (Inzenga) Watling., Centaurea omphalotricha (Batt.) Willk., Passiflora coerulea L. Desmos cochinchinensis Lour., Pelargonium crispum (P.J.Bergius) L Her, Oroxylum indicum (L.) Vent., or propolis, honey,13 many fruits,14,15 passion flowers such as P. caerulea L16 and even mushrooms.17
The beneficial effect of CH is connected to its bioavailability and solubility. Chrysin has poor intestinal absorption, and its maximum concentration in serum is 12 to 64 nM.18 Chrysin has been demonstrated to be a very potent flavonoid acting a huge number of pharmacological activities, such as antiasthmatic activity through the suppression of inducible nitric oxide synthase (iNOS) and nuclear factor-κB (NF-κB),19 inhibition of histone deacetylase20 and DNA topoisomerases,21 cardioprotective activity via improving post-ischemic functional recovery,22 anti-inflammatory activity via blocking histamine release and pro-inflammatory cytokine expression,10,23 prevention of osteoporosis by activation of estrogen receptor /mitogen-activated protein kinase,24 anticancer activity by endorsing the cell death induced by tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) and increasing TRAIL-induced degradation of caspases-3 and caspases-8,10,25 inhibition of TNF-α and interleukin (IL)-1β,26 renoprotective activity by glucose-induced renal tubular cell migration with diminishing matrix metalloproteinase-2 activity,27 suppressive effect on vascular endothelial growth factor-induced angiogenesis,28 antihypercholesterolemic activity,29 antidiabetogenic, antihypertensive,5,13 and preventing metastatic progression in breast cancer cells.30 In addition to all these pharmacological activities of CH, it has also been shown to possess protective effects and diseases managements against toxic agent–induced toxicities through various mechanisms. The protective effects of CH in different tissues, including brain, heart, liver, kidney, and lung have been reported against some toxic materials.29-34 However, unlike other flavonoids, the therapeutic benefits of CH in the act of toxin materials remain nascent in current literature.
Therefore, this review aimed to provide an updated overview of studies on the protective effect of CH against injuries induced by natural and chemical agents in various tissues.
Chemistry and structural activity of CH (5,7-dihydroxy-2-phenyl-4H-chromen-4-one; 5,7-dihydroxyflavone), a type of naturally occurring flavonoid, belongs to a class of chemicals called flavonoids that have 15-carbon skeleton natural polyphenolic compounds. Chrysin possesses 2 benzene rings (A, B) and a third, oxygen-containing (C) ring. Chrysin possesses a 2 to 3 carbon double bond, a carbonyl group at carbon 4, and lack a 3 carbon hydroxyl group. Based on these structures, CH is categorized as flavones. Chrysin also possesses hydroxyl groups at carbons 5 and 7 (Figure 1). Unlike many flavonoids that possess either 2 hydroxy (C3′, C4′-diortho hydroxyl) or 1 (most commonly at C-4′) functional group in ring-B, CH does not have any oxygenation in this ring. Other natural derivatives of CH arise due to diversity in ring-A oxygenation as exemplified by the natural common, biologically important flavonoids, such as wogonin, oroxylin A, and baicalein.35 The chemical activity of CH, due to B and C-ring absence of oxygenation, is connected with a lot of biological abilities that range from antitoxic to anti-inflammatory effects. However, differences in the chemical structure of flavones have been shown to influence the antioxidant activity. Existence of 3′, 4′ hydroxylation, a double bond between carbons 2 and 3, and the existence of a carbonyl group on carbon 4 have been reported to be essential to make antioxidant activity in CH.35
Figure 1.
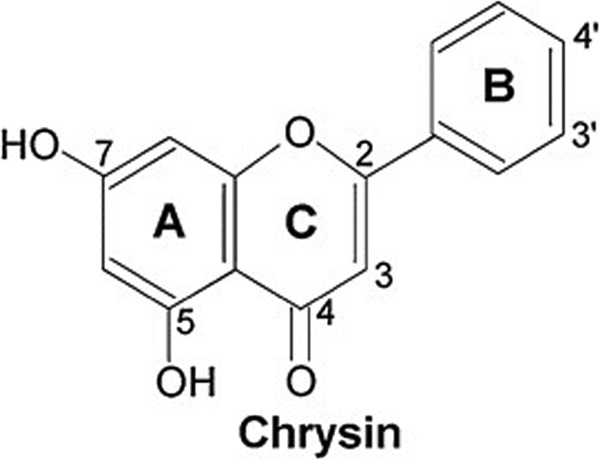
Chemical structures of chrysin.
Safety Study of CH
It was shown that low concentrations of the nutrition flavonoids may be safe for human consumption; however, the higher concentrations have toxic effects on the human body. The recommended daily concentrations of CH are 0.5 to 3 g. However, CH-induced toxicity in trout liver cells36 even in the daily concentrations. It was observed that CH administration disturbed de novo DNA synthesis and decreased cell numbers. The cytotoxicity of CH may be related to the presence of peroxidase-like activity in hepatocytes that caused toxic products of CH.36 Myeloperoxidase (MPO) in neutrophils may be responsible for CH-induced toxicities.37-39
Methods
Online literature search was done using different search engines such as MEDLINE, PubMed, Iran MEDEX, Scopus, and Google Scholar as far back as 1990 to identify articles, editorials, and reviews about antidotal effects of CH against toxic agents.
Protective Effects of CH Against Agent-Induced Hepatotoxicity
Methotrexate
Methotrexate (MTX) is used as an effective chemotherapeutic drug; however, its toxic effects on the liver restrict its clinical use.40 According to the present documents, oxidative stress is a major mechanism involved in the pathogenesis of MTX-induced tissue damage, particularly in the liver.41 Chrysin was found to possess protective effect against MTX hepatotoxicity via the reduction in hepatic injury markers (aspartate aminotransferase [AST], alanine transaminase [ALT], lactate dehydrogenase [LDH]) and elevations in hepatic glutathione (GSH), glutathione peroxidase (GPx), glutathione reductase (GR), superoxide dismutase (SOD), and catalase (CAT) in rats. In addition, CH ameliorated histopathological alterations and apoptosis induced by MTX.41
Tert-butyl hydroperoxide
Tert-butyl hydroperoxide (tBHP), an exogenous inducer of oxidative stress, causes liver toxicity increasing the production of cellular reactive oxygen species (ROS).42 The documents showed CH improved the toxicity of tBHP on liver. Chrypsin increased the protein expression of heme oxygenase 1 (HO-1), glutamate cysteine ligase (GCL), glutamate cysteine ligase catalytic (GCLC), and modifier subunit (GCLM) and increased the intracellular GSH content and the ratio of GSH to oxidized GSH by upregulating HO-1, GCLC, and GCLM gene transcription via the extracellular signal-regulated kinase (ERK2)/Nrf2/ARE signaling pathways in rat primary hepatocytes.43
Cisplatin
Cisplatin (Cis) is an antitumor drug that causes hepatotoxicity via oxidative stress and inflammation.44 It was reported that supplementation with CH can influence on Cis-caused hepatotoxicity. Chrysin improved Cis-induced lipid peroxidation, xanthine oxidase (XO) activity, GSH depletion, decrease in antioxidant (CAT, GR, superoxide dismutas [SOD], GPx, and glucose-6 phosphate dehydrogenase [G6PDD]) and phase II detoxifying (glutathione-S-transferase [GST] and quinone reductase [QR]) enzyme activities. Chrysin also ameliorated the expression of cyclooxygenase-2 (COX-2), iNOS and levels of NF-κB, and TNF-α, and therefore, CH reduces the hepatic tissue damage that is induced by Cis. Chrysin attenuated Cis-induced hepatotoxicity by preventing the oxidative stress and inflammatory response.45
2-Amino-1-methyl-6-phenylimidazo [4, 5-b] pyridine
Numerous studies have been shown that 2-amino-1-methyl-6-phenylimidazo [4, 5-b] pyridine (PhIP), the most abundant heterocyclic amines,46 is a potent mutagen and tumourigenic in animal models. Chrysin supplementation protected the liver against PhIP-induced mutagenic effects by inducing UDP-glucuronosyltransferase and/or inhibiting sulfotransferase in HepG2 cells. The observed effects suggested a feedback mechanism on the antioxidant enzymes triggered by CH.47
Ethanol
Hepatoprotective effects of CH against ethanol on the alteration of alcohol-metabolizing enzymes—alcohol dehydrogenase (ADH), cytochrome P450 2E1 (CYP 2E1), XO—and oxidant/antioxidant status were observed in rats. Chrysin administration prevented liver damages during chronic ethanol consumption by modulating the activities of ADH, CYP 2E1, XO, and CAT enzymes in rats.48 Sathiavelu et al, also suggested that protective effects of CH against free radical–mediated oxidative stress induced liver injury after ethanol consumption in rats. They showed that CH administration to rats with ethanol-induced liver injury significantly decreased the levels of thiobarbituric acid reactive substances (TBARS), lipid hydroperoxides, and conjugated dienes and significantly elevated the activity of SOD, CAT, GPx, GR, GST, and the levels of GSH, vitamin C, and vitamin E in the liver.49
D-galactosamine
D-galactosamine (GalN) is known as a hepatotoxic chemical agent.50 D-galactosamine increased serum hepatic marker enzyme activities (AST, ALT, alkaline phosphatase, and γ-glutamyl transpeptidase) and the lipid peroxidation and decreased the antioxidant capacity of the plasma and liver. It was observed that CH decreased hepatic enzyme activities and lipid peroxidation products, such as TBARS, lipid hydroperoxides, and conjugated dienes, and increased the activities of free radical–scavenging enzymes, such as SOD, CAT, GPx, GR, GST, and the levels of GSH, vitamin C and vitamin E in the liver.51
Streptozotocin
Streptozotocin (STZ, 2-deoxy-2-(3-(methyl-3-nitrosoureido)-D-glucopyranose) is synthesized by Streptomycetes achromogenes and is able to cause insulin-dependent and noninsulin-dependent diabetes mellitus. The protective effects of CH against oxidative damage in the liver of STZ-diabetic rats were assessed. Chrysin ameliorated an elevation of malondialdehyde (MDA) with a reduction in total protein, SOD, catalase (CAT), and GST in the liver of rat that received STZ. This study indicated that CH may be used for recovery from hepatotoxicity induced by STZ via modulating oxidative stress.5 Table 1 shows antidotal effects of CH against hepatotoxic agents.
Table 1.
Antidotal Effects of Chrysin Against Gastrointestinal Toxicity.
| Antidote | Toxin | Experimental Study | Mechanism | References |
|---|---|---|---|---|
| STZ | Rat | Prevention of hepatotoxicity via increasing antioxidant content and decreasing lipid peroxidation | 5 | |
| tBHP | Rat primary hepatocyte | Prevention of oxidative stress-induced hepatotoxicity via upregulating HO-1, GCLC, and GCLM gene transcription via the ERK2/Nrf2/ARE signaling pathways | 43 | |
| Cis | Rat | Prevention of oxidative stress and inflammation-induced hepatotoxicity via increasing antioxidant content and decreasing the expression of COX-2, iNOS and NFκB, and TNF-α | 45 | |
| CH | PhIP | HepG2 cell | Prevention of mutagenic effects via inducing UDGPT and/or inhibition of SULT | 47 |
| Ethanol | Rat | Prevention of hepatotoxicity via modulating the activities of ADH, CYP 2E1, XO, and CAT enzymes | 48 | |
| Ethanol | Rat | Prevention of hepatotoxicity via increasing antioxidant content and decreasing lipid peroxidation | 49 | |
| GalN | Rat | Prevention of hepatotoxicity via increasing antioxidant content and decreasing lipid peroxidation | 51 | |
| DSS | Mice | Prevention of colitis via inhibiting phosphorylation/degradation of IkBa, which correlated with the decrease in the levels of MPO, TNF-α, and IL-6 in the colon and activation of PXR | 52,53 | |
| Cis | Rat | Prevention of jejunal toxicity via attenuating the oxidative stress and apoptotic | 54 | |
| DMH | Rat | Prevention of preneoplastic colorectal lesions via increase in antioxidant content, reduction in nitrosative stress, and inhibition of the cellular proliferation | 55 | |
| MTX | Rat | Prevention of hepatotoxicity via increasing antioxidant content and decreasing lipid peroxidation | 56 | |
| DSS | IEC-6 cell | Prevention of colitis via inhibiting NF-jB activation | 56 |
Abbreviations: ADH, alcohol dehydrogenase; CAT, catalase; COX-2, cyclooxygenase-2; Cis, cisplatin; CH, chrysin; CYP 2E1, cytochrome P450 2E1; DMH, 1,2-dimethylhydrazine; DSS, dextran sodium sulfate; HO-1, heme oxygenase 1; GalN, D-galactosamine; GCLM, glutamate cysteine ligase modifier; GCLC, glutamate cysteine ligase catalytic; iNOS, inducible nitric oxide synthase; IkBa, inhibitor kBa; MTX, methotrexate; NF-κB, nuclear factor-κB; NF-jB, nuclear factor (NF)-jB; PhIP, 2-Amino-1-methyl-6-phenylimidazo [4, 5-b] pyridine; PXR, pregnane X receptor; STZ, streptozotocin; SULT, sulfotransferase; tBHP, tert-butyl hydroperoxide; TNF-α, tumor necrosis factor α; UDGPT, UDP-glucuronosyl transferase; XO, xanthine oxidase.
Protective Effects of CH Against Agent-Induced Colitis
Dextran sodium sulfate
Chrysin could improve colitis induced by dextran sodium sulfate (DSS) and 2, 4, 6 trinitrobenzene sulfonic acid in mice. Chrysin decreased the gene expression of NF-κB, iNOS, intercellular adhesion molecule 1, monocyte chemotactic protein 1, COX-2, TNF-α, and interleukin 6 (IL-6) in the colon mucosa.
Chrysin inhibited the phosphorylation/degradation of inhibitor kBa, which correlated with the decrease in the activity of MPO and the levels of TNF-α and IL-6 in the colon. Chrysin improved colitis with anti-inflammation activity mediated by the suppression of NF-κB activation. Chrysin may also act as a putatively targeted activation of pregnane X receptor, an agonist to prevent experimental colitis in mice.52,53 Chrysin could also alleviate colitis induced by DSS by inhibiting NF-jB activation in vitro. Chrysin inhibited TNFα-induced activation of NF-jB in IEC-6 cells. These findings proposed that CH exerts useful anti-inflammatory effects via the suppression of NF-jB activation.56
Cisplatin
The protective effect of CH against Cis-induced jejunal toxicity was indicated. The mechanism of Cis-induced jejunal toxicity includes oxidative stress and apoptosis via upregulating the expression of caspase-6 and caspase-3. Chrysin improved lipid peroxidation, increase in XO activity, decrease in the levels of GSH, antioxidant enzymes (CAT, GR, GPx, and G6PDD), and phase II detoxifying enzymes (GST and QR) induced by Cis. Chrysin attenuated goblet cell disintegration and enhanced the expression of p53 and apoptotic tissue damage induced by Cis. Histological findings also confirmed the protective effects of CH against Cis-induced damage in the jejunum. The results of the present study demonstrated that oxidative stress and apoptosis are closely associated with Cis-induced toxicity, and CH has shown protective effects against Cis-induced jejunum toxicity possibly via attenuating the oxidative stress and apoptotic tissue damage.54
Dimethylhydrazine
The protective effect of CH on preneoplastic colorectal lesions (aberrant crypt foci) in a rat model of chemical carcinogenesis induced by 1,2-dimethyl hydrazine (DMH) was indicated. Chrysin was effective in attenuating pathological colorectal remodeling and reducing the number of preneoplastic lesions in rats exposed to DMH. Some of these effects might be attributed to the increase in antioxidant content, reduction in nitrosative stress, and inhibition of the cellular proliferation.55 Table 1 shows antidotal effects of CH against agent-induced colitis.
Protective Effects of CH Against Agent-Induced Cardiovascular Toxicity
Triton WR-1339
Triton WR-1339, an inhibitor of lipoprotein lipase, induced hypercholesterolemia in rats. Hypercholesterolemia and oxidative stress accelerated coronary artery disease and progression of atherosclerosis. It was reported that oral administration of CH to hypercholesterolemic rats decreased the serum levels of glucose, lipid profile parameters, and hepatic enzymes and increased enzymatic and nonenzymatic antioxidant parameters.17
Doxorubicin
Doxorubicin (DOX) is one of the most effective chemotherapeutic agents; however, its cardiotoxicity effects limit therapeutic use.57 Chrysin could protect against DOX-induced acute cardiotoxicity via modulating oxidative stress and inflammatory responses as well as inhibiting apoptosis. Mantawy et al indicated that CH ameliorated serum creatine kinase isoenzyme-MB (CK-MB), LDH and myofibrillar disarrangement, GSH, lipid peroxidation, and the activities of antioxidant enzymes (CAT and SOD). Chrysin decreased the expressions of NF-κB, iNOS, and COX-2 and the levels of TNF-α and NO. In addition, CH decreased the expressions of Bax and cytochrome c and caspase-3 activity while increased the expression of Bcl-2.58
Streptozotocin
It was indicated that CH attenuated diabetes-induced impairment in endothelial-dependent relaxation possibly via ameliorating detrimental changes in lipid profile, advanced glycation end products, and NO generation.59 The protective effects of CH against oxidative damage in the serum of STZ diabetic rats were assessed. Chrysin ameliorated an elevation in glucose, MDA, triglyceride (TG), total cholesterol (TC), and low-density lipoprotein cholesterol (LDL-C), and a reduction in high-density lipoprotein cholesterol (HDL-C), total protein, SOD, CAT, and GST in the serum of rat received STZ. This study offered that CH may recover diabetes and its complications by modification of oxidative stress.5
Mitoxantrone
Cardiotoxicity is a major concern of mitoxantrone (MTX) treatment. It has been shown that MTX treatment can result in cardiomyopathy leading to reduced left ventricular ejection fraction and irreversible congestive heart failure.56 Risk increases with cumulative dose.60,61 The MTX treatment in mouse significantly increased the serum levels of CK-MB, indicator of cardiac injury, and withdrawn under CH protection.62 Expression levels of Bcl-2 decreased, while those of Bax and caspase-3 increased following MTX treatment,63 while CH treatment daily reduced Bax and caspase-3 immunopositivity and restored Bcl-2 levels to a value comparable to the control.62 TUNEL (+) cardiomyocyte nuclei of MTX group showed typical signs of apoptosis, which almost completely disappeared in response to CH treatment. In parallel, an irregular distribution and a weak expression of desmin are associated with MTX-induced cardiotoxic effects, which were also restored by CH treatment.62 Therefore, CH inhibits MTX-triggered cardiomyocyte apoptosis via multiple pathways, including decrease in the Bax/Bcl-2 ratio and caspase-3 expression along with the preservation of the desmin disarray. Table 2 shows antidotal effects of CH against agent-induced cardiovascular toxicity.
Table 2.
Antidotal Effects of Chrysin Against Cardiovascular and Renal Toxicity.
| Antidote | Toxin | Experimental Study | Mechanism | References |
|---|---|---|---|---|
| STZ | Rat | Prevention of hyperglycemia and hyperlipidemia via modulating oxidative stress | 5 | |
| CH | Triton WR-1339 | Rat | Prevention of hypercholesterolemia via increasing antioxidant content | 17 |
| Ethanol | Rat | Prevention of renal toxicity via modulating the activities of ADH, CYP 2E1, XO, and antioxidant enzymes | 48 | |
| DOX | Rat | Prevention of cardiotoxicity via modulating oxidative stress and inflammatory responses as well as inhibiting apoptosis | 58 | |
| STZ | Rat | Prevention of diabetes-induced impairment in endothelial-dependent relaxation possibly via ameliorating detrimental changes in lipid profile, AGEs, and NO generation | 59 | |
| Mitoxantrone | Mouse | CH treatment daily reduced Bax and caspase-3 immunopositivity and restored Bcl-2 levels to a value comparable to the control | 56 | |
| Adenine | Rat | Prevention of renal failure via modulating the PPARγ and NF-κB signaling pathways | 60 | |
| TCDD | Rat | Prevention of renal toxicity via increasing the levels of GSH, CAT, GPx, and SOD and decreasing lipid peroxidation | 62 | |
| DOX | Rat | Prevention of renal toxicity via increasing the antioxidant content | 63 | |
| 5-FU | Rat | Prevention of renal toxicity via modulating oxidative stress and apoptotic damage in kidney | 64 | |
| Cis | Rat | Prevention of renal toxicity via increasing the antioxidant content, diminishing the DNA damage | 65 | |
| CCl4 | Rat | Prevention of renal toxicity via increasing the levels of GSH, CAT, GPx, and SOD and decreasing lipid peroxidation and expression of the iNOS gene | 66 |
Abbreviations: AGEs, advanced glycation end products; ADH, alcohol dehydrogenase; CYP 2E1, cytochrome P450 2E1; CCl4, carbon tetrachloride; Cis, cisplatin; CAT, catalase; DOX, doxorubicin; 5-FU, 5-fluorouracil; GSH, glutathione; GPx, glutathione peroxidase; iNOS, inducible nitric oxide synthase; NO, nitric oxide; NF-κB, nuclear factor-κB; PPARγ, proliferator–activated receptor gamma; SOD, superoxide dismutase; STZ, streptozotocin; TCDD, tetrachlorodibenzo-p-dioxin; XO, xanthine oxidase.
Protective Effects of CH Against Chemical-Induced Renal Toxicity
Adenine
Adenine, an organic compound belonging to the purine family, induced renal failure in animal models.67 It was observed that adenine increased plasma levels of creatinine (Cr), urea, neutrophil gelatinase–associated lipocalin and N-Acetyl-β-d glucosaminidase activity, uremic toxin 3-indoxyl sulfate, some inflammatory cytokines, and urinary albumin concentration. In addition, fibrosis and inflammation were observed in the rat kidney. Antioxidant indices such as SOD and CAT, total antioxidant status, and GSH were also decreased after adenine administration. Chrysin was effective against adenine-induced renal failure in rats via modulating the peroxisome proliferator–activated receptor γ and decreasing the expressions of the pro-inflammatory NF-κB signaling pathway.64
Tetrachlorodibenzo-p-dioxin
2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), polychlorinated dibenzo-p-dioxin and the persistent environmental contaminant, was known as nephrotoxic agent.65 Ciftci et al indicated that CH treatment prevented renal toxic effects of TCDD via increasing the levels of GSH, CAT, GPx, and SOD and decreasing lipid peroxidation. It was also determined that exposure to TCDD caused histological damage in kidney tissue, and these effects can be improved with CH treatment.66
Doxorubicin
Doxorubicin is an anticancer agent used in the treatment of many human malignancies. However, its clinical use is limited because of several side effects like nephrotoxicity. Doxorubicin induced nephrotoxicity via decreasing antioxidant content in rats. Chrysin treatment significantly reversed histopathological damages and the levels of serum Cr, blood urea nitrogen (BUN), AST, ALT, and LDH via increasing antioxidant content in kidney tissue.68
5-Fluorouracil
5-Fluorouracil (5-FU) is a potent antineoplastic agent commonly used for the treatment of various malignancies. It has adverse effects such as nephrotoxicity that restrict its wide and extensive clinical usage.69 The protective effect of CH against 5-FU-induced renal toxicity has been indicated. 5-Fluorouracil induced renal toxicity by inducing oxidative stress, activating an apoptotic pathway by upregulation of p53, Bax, and caspase-3 and downregulation of Bcl-2. Chrysin treatment (50 and 100 mg/kg body weight) prevented renal toxicity induced by 5-FU via ameliorating oxidative stress and apoptotic damage in the rat kidney.70
Cisplatin
Cisplatin-induced nephrotoxicity is the main cause for its dose-limited use in the treatment of various cancers and results in acute renal cell injury through the generation of ROS. Sultana et al showed that CH attenuated Cis-induced renal oxidative damage by diminishing the DNA damage and toxicity markers, such as serotonin and BUN, lipid peroxidation, and XO activity, accompanied by an increase in the levels of antioxidant enzyme (CAT, GPx, GR, and GST) and GSH. The data of the present study suggested that CH effectively suppressed Cis-induced renal injury by ameliorating oxidative stress.71
Ethanol
Nephroprotective effects of CH have been indicated against ethanol intake in animal models. Ethanol administration significantly induced CYP 2E1, ADH, and XO in the kidneys, parallel to the increase in levels of MDA, BUN, Cr, and LDH, versus control rats. In addition, ethanol decreased GSH, Gpx, CAT, and GR in the kidney of ethanol-treated rats versus control rats. Chrysin reduced lipid peroxidation in kidney tissue and also decreased BUN, Cr, and LDH. In addition, CH also restored antioxidant enzymes in the kidney of the ethanol-treated rats.48
Carbon tetrachloride
The effect of CH against carbon tetrachloride (CCl4)-induced toxicity in male rats
The administration of CCl4 (2 mL/kg) to rats for 4 days increased significantly the serum levels of ALP and LDH, versus normal rats. In addition, the significant increase in MDA level and the decrease in GSH level of the kidney of the CCl4-administered rats were observed. The activities of CAT, SOD, and Gpx enzymes decreased in the kidney of CCl4-administered rats. Chrysin restored ALP and LDH to levels in CCl4-treated rats near normal. In addition, CH increased the levels of GSH and CAT, SOD, and Gpx and decreased MDA level in the kidney of CCl4-administered rats. The upregulated expression of the iNOS in the kidney of CCl4-exposed untreated rats ameliorated in the CCl4-exposed CH-treated rats. This study indicated that CH prevented the oxidative damage induced by CCl4 in the kidney of rats.72 Table 2 shows antidotal effects of CH against agent-induced renal toxicity.
Protective Effects of CH Against Agent-Induced Respiratory Toxicity
Ovalbumin
Du et al reported that CH inhibited the total inflammatory cell and eosinophil counts in bronchoalveolar lavage fluid and total immunoglobulin E levels in serum of mice exposed to ovalbumin (OVA). Histological examination of lung tissue demonstrated that CH significantly ameliorated OVA-induced lung eosinophilic inflammation and mucus-producing goblet cells in the airway. In addition, CH shifted the immune response toward a T-helper type 1 (Th1) profile via modulating the transcription factors T-bet and GATA-3 in OVA-exposed mice. These data indicated that CH had protective effects in a murine model of asthma due to its anti-inflammatory and immunoregulatory effects.73 Wadibhasme et al also indicated the antiasthmatic effect of CH in an animal model exposed to OVA. It was observed that CH decreased total lung injury score (infiltration of leucocytes, type of inflammatory exudates, the status of bronchi, the perivascular status of lung blood vessels, the integrity of alveoli, and the activation of alveolar macrophages). They suggested that antiasthmatic effects of CH may be related to the modulation of Th1/Th2 via suppressing iNOS and NF-κB.19
Bleomycin
The pulmonary toxicity of bleomycin (BLC), a medication for cancer, has been found.74 Chrysin supplementation restored histological changes such as alveolar congestion, increased connective tissue, infiltration, and the thickness of alveolar wall caused by BLC via the modulating antioxidant system. Increased level of MDA was significantly reversed by CH administration. The reduction in activities of CAT, SOD, and level of GSH was ameliorated by CH supplementation. Furthermore, this study showed that CH significantly prevents BLC-induced lung inflammation and fibrosis in rats.75
Cigarette smoke
The effect of CH on cigarette smoke-induced airway inflammation in mice was indicated. Chrysin pretreatment prevented cigarette smoke–induced airway inflammation, inflammatory cytokine (TNF-α, IL-1β, and IL-8) release, and MPO expression. Chrysin intervention inhibited the expression of phosphorylation ERK and p38 induced by cigarette smoke.34 Table 3 shows antidotal effects of CH against agent-induced respiratory toxicity.
Table 3.
Antidotal Effects of Chrysin Against Respiratory and Neurotoxicity.
| Antidote | Toxin | Experimental Study | Mechanism | References |
|---|---|---|---|---|
| STZ | Rat | Prevention of neurotoxicity via increasing antioxidant content and decreasing lipid peroxidation | 5 | |
| Formalin | Rat | Prevention of pain via decreasing CORT and NA | 13 | |
| CH | OVA | Mice | Protective effects in a murine asthmatic model via inhibiting the total inflammatory cell and eosinophil counts in BALF and IgE levels in serum of sensitized mice, shift the immune response toward a Th1 profile via modulating the transcription factors T-bet and GATA-3 | 68 |
| OVA | Rat | Protective effects in a murine asthmatic model via modulation due to suppression effects on iNOS and NF-κB | 69 | |
| BLC | Rat | Prevention of histological changes such as alveolar congestion, increased connective tissue, infiltration, and the thickness of alveolar via modulating antioxidant and inflammatory system | 71 | |
| CS | Mice | Prevention of airway inflammation, inflammatory cytokines (TNF-α, IL-1β, and IL-8) release, and MPO expression via inhibiting the expression of phosphorylation ERK and p38 | 72 | |
| Formalin and carrageenan | Rat | Prevention of pain and edema via decreasing inflammation due to high binding to COX-2 | 73 | |
| LPS | Microglia cells | Prevention of NO, TNF-α, IL-1β and the expressions of iNOS and COX-2 via inhibiting the c-JNK and NF-kB | 19 | |
| STZ | Rat | Prevention of DACD via modulating oxidative stress, inflammation, and apoptotic cascades | 74 |
Abbreviations: BLC, bleomycin; BALF, bronchoalveolar lavage fluid; CORT, corticosterone; c-JNK, c-Jun N-terminal kinase; CS, cigarette smoke; COX-2, cyclooxygenase-2; DACD, diabetes-associated cognitive decline; ERK, extracellular signal-regulated kinase; IL, interleukin; iNOS, inducible nitric oxide synthase; IgE, immunoglobulin E; LPS, lipopolysaccharide; MPO, myeloperoxidase; NA, noradrenalin; NO, nitric oxide; NF-κB, nuclear factor-κB; OVA, ovalbumin; STZ, streptozotocin; Th1, T-helper type 1; TNF-α, tumor necrosis factor α.
Protective Effects of CH Against Agent-Induced Neurotoxicity
Formalin
Chrysin treatment decreased formalin-induced pain in the rat. Chrysin attenuated the content of corticosterone and noradrenaline in the serum of rat received formalin.13 The anti-inflammatory and analgesic effects of CH were also evaluated on writhing and formalin test and also on carrageenan-induced paw edema. On the writhing test, we observed a significant inhibition of writhing after the administration of CH. On formalin test, CH displayed analgesic and anti-inflammatory effect on both early and late phase and similarly displayed at 4 hours a significant anti-inflammatory effect in carrageenan-induced paw edema. Moreover, in silico analysis of receptor-ligand complex indicated that CH interacts weakly with COX-1 binding site but displayed a significant interaction with COX-2. These data indicated that the CH possesses anti-inflammatory and antinociceptive potential via high binding to COX-2.76
Lipopolysaccharide
The protective effect of CH on the production of pro-inflammatory mediators in lipopolysaccharide (LPS)-stimulated microglia cells has been reported. Chrysin prevented the release of NO and pro-inflammatory cytokines including TNF-α and IL-1β and the expressions of iNOS and COX-2 via inhibiting the activations of signaling molecules involved in neuroinflammation (c-Jun N-terminal kinase and NF-κB). This study suggested that CH has a potential therapeutic agent for neuroinflammation diseases.77
Streptozotocin
The neuroprotection of CH on diabetes-associated cognitive decline (DACD) was evaluated in a rat model of diabetes induced by intraperitoneal injection of STZ. The diabetic rats indicated significant decrease in body weight, the percentage of time spent in target quadrant, and the number of times of crossing platform, parallel to the increases in plasma glucose levels, escape latency, mean path length and oxidative stress (elevated MDA level and reduced levels of CAT, SOD, and GSH), NF-κB p65 unit, TNF-α, IL-1β, IL-6, and caspase-3 in the cerebral cortex and hippocampus. Moreover, CH supplement significantly recovered the behavioral, biochemical and molecular alterations in diabetes. Chrysin remarkably alleviated DACD via modulating oxidative stress, inflammation, and apoptotic cascades.78 Samarghandian et al also showed that CH ameliorated an elevation of MDA with a reduction in total protein, SOD, CAT, and GST in the brain of rat received STZ. They proposed that CH has neuroprotective effects induced by STZ via modulating oxidative stress.5 Table 3 showed antidotal effects of CH against agent-induced neurotoxicity.
Discussion and Conclusions
In this study, several animal studies summarized to find the protective effects of CH against toxicities induced by toxic agents in various tissues. According to the results of different studies, CH acts as an antidote in different intoxications induced by toxic agents.
Methotrexate, tBHP, Cis, PhIP, ethanol, GalN, STZ, DSS, DMH, Triton WR-1339, DOX, MTX, adenine, TCDD, DOX, 5-FU, CCl4, OVA, BLC, cigarette smoke, formalin, LPS are some examples of chemical agents which CH could protect various tissues such as the liver, colon, cardiovascular, kidney, lung, and brain against their toxicities. It is also confirmed that CH with excessive therapeutic effects showed protective effects against some chemical drugs such as chemotherapeutic drugs (MTX, DOX, CDDP, BLL and 5-FU) that have organ toxicities, particularly in overdose. Different mechanisms such as antioxidant, the modulation of cardiac, renal, and liver enzymes, improvement in antioxidant defense systems, inhibition of oxidative stress, inflammation, and apoptosis are involved in CH antidotal effects. In this review article, the different in vitro and animal studies are summarized in order to discover the efficacy of CH in protection against toxicities induced by natural or chemical toxins in different tissues. According to the results of several important investigations, CH acts as an antidote in different intoxications induced by natural toxins.
In conclusion, based on the current review, CH has an extensive spectrum of protective properties against toxicities induced by either natural or chemical toxins. As these findings have not yet been verified by clinical trials on humans, to establish the antidotal effects of CH in human intoxications, human trials should be carried out. Therefore, CH has protective effects against toxicities induced by toxic agents. However, more studies are needed to verify the antidotal effects of CH in human intoxications.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Samarghandian S, Afshari JT, Davoodi S. Honey induces apoptosis in renal cell carcinoma. Pharmacogn Mag. 2011;7(25):46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Samarghandian S, Farkhondeh T, Samini F, Borji A. Protective effects of carvacrol against oxidative stress induced by chronic stress in rat’s brain, liver, and kidney. Biochem Res Int. 2016;2016:2645237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Samarghandian S, Asadi-Samani M, Farkhondeh T, Bahmani M. Assessment the effect of saffron ethanolic extract (Crocus sativus L.) on oxidative damages in aged male rat liver. Der Pharmacia Lettre. 2016;8(3):283–290. [Google Scholar]
- 4. Farkhondeh T, Samarghandian S. Honey and health: a review of recent clinical research. Pharmacognosy Res. 2016. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Samarghandian S, Azimin-Nezhad M, Samini F, Farkhondeh T. Chrysin treatment improves diabetes and its complications in streptozotocin-induced diabetic rat. Can J Physiol Pharmacol. 2016;94(4):388–393. [DOI] [PubMed] [Google Scholar]
- 6. Sarlak N, Sadighara P. Nutritional-qualitative factors changes during ripening of canned olives at different temperatures storage. Carpathian J Food Sci Technol. 2015;7(4):19–27. [Google Scholar]
- 7. Samarghandian S, Azimi-Nezhad M, Samini F. Preventive effect of safranal against oxidative damage in aged male rat brain. Exp Anim. 2015;64(1):65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Samarghandian S, Borji A, Delkhosh MB, Samini F. Safranal treatment improves hyperglycemia, hyperlipidemia and oxidative stress in streptozotocin-induced diabetic rats. J Pharm Pharm Sci. 2013;16(2):352–362. [DOI] [PubMed] [Google Scholar]
- 9. Samini F, Samarghandian S, Borji A, Mohammadi G, bakaian M. Curcumin pretreatment attenuates brain lesion size and improves neurological function following traumatic brain injury in the rat. Pharmacol Biochem Behav. 2013;110:238–244. [DOI] [PubMed] [Google Scholar]
- 10. Samarghandian S, Afshari JT, Davoodi S. Chrysin reduces proliferation and induces apoptosis in the human prostate cancer cell line pc-3. Clinics (Sao Paulo). 2011;66(6):1073–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Samarghandian S, Nezhad MA, Mohammadi G. Role of caspases, Bax and Bcl-2 in chrysin-induced apoptosis in the A549 human lung adenocarcinoma epithelial cells. Anticancer Agents Med Chem. 2014;14(6):901–909. [DOI] [PubMed] [Google Scholar]
- 12. Tong L, Wanb MX, Zhang LH, Zhu YH, Sun H, Bi KS. Simultaneous determination of baicalin, wogonoside, baicalein, wogonin, oroxylin A and chrysin of Radix scutellariae extract in rat plasma by liquid chromatography tandem mass spectrometry. J Pharm Biomed Anal. 2012;70:6–12. [DOI] [PubMed] [Google Scholar]
- 13. Farkhondeh T, Samarghandia S, Azimin-Nezha M, Samin F. Effect of chrysin on nociception in formalin test and serum levels of noradrenalin and corticosterone in rats. Int J Clin Exp Med. 2015;8(2):2465–2470. [PMC free article] [PubMed] [Google Scholar]
- 14. Song WJ, Li YH, Wang JG, Li ZY, Zhang JQ. Characterization of nucleobases and nucleosides in the fruit of Alpinia oxyphylla collected from different cultivation regions. Drug Test Anal. 2014;6(3):239–245. [DOI] [PubMed] [Google Scholar]
- 15. Qing ZJ, Yong W, Hui LY, et al. Two new natural products from the fruits of Alpinia oxyphylla with inhibitory effects on nitric oxide production in lipopolysaccharide activated RAW264.7 macrophage cells. Arch Pharm Res. 2012;35(12):2143–2146. [DOI] [PubMed] [Google Scholar]
- 16. Medina JH, Paladini AC, Wolfman C, et al. Chrysin (5,7-di-OH-flavone), a naturally-occurring ligand for benzodiazepine receptors, with anticonvulsant properties. Biochem Pharmacol. 1990;40(10):2227–2231. [DOI] [PubMed] [Google Scholar]
- 17. Anandhi R, Annadurai T, Anitha TS, et al. Antihypercholesterolemic and antioxidative effects of an extract of the oyster mushroom, Pleurotus ostreatus, and its major constituent, chrysin, in Triton WR-1339-induced hypercholesterolemic rats. J Physiol Biochem. 2013;69(2):313–323. [DOI] [PubMed] [Google Scholar]
- 18. Kao YC, Zhou C, Sherman M, Laughton CA, Chen S. Molecular basis of the inhibition of human aromatase (estrogen synthetase) by flavone and isoflavone phytoestrogens: a site-directed mutagenesis study. Environ Health Perspect. 1998;106(2):85–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wadibhasme PG, Ghaisas MM, Thakurdesai PA. Anti-asthmatic potential of chrysin on ovalbumin-induced bronchoalveolar hyperresponsiveness in rats. Pharm Biol. 2011;49(5):508–515. [DOI] [PubMed] [Google Scholar]
- 20. Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res. 2001;50(6):537–546. [PubMed] [Google Scholar]
- 21. Russo P, Del Bufalo A, Cesario A. Flavonoids acting on DNA topoisomerases: recent advances and future perspectives in cancer therapy. Curr Med Chem. 2012;19(31):5287–5293. [DOI] [PubMed] [Google Scholar]
- 22. Tian SS, Jiang FS, Zhang K, et al. Cardioprotective the leaves of Carya cathayensis Sarg. inhibit vascular endothelial growth factor-induced angiogenesis. Fitoterapia. 2014;92:34–40. [DOI] [PubMed] [Google Scholar]
- 23. Bae Y, Lee S, Kim SH. Chrysin suppresses mast cell-mediated allergic inflammation: involvement of calcium, caspase-1 and nuclear factor-κB. Toxicol Appl Pharmacol. 2011;254(1):56–64. [DOI] [PubMed] [Google Scholar]
- 24. Zeng W, Yan Y, Zhang F, Zhang C, Liang W. Chrysin promotes osteogenic differentiation via ERK/MAPK activation. Protein Cell. 2013;4(7):539–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li X, Wang JN, Huang JM, et al. Chrysin promotes tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) induced apoptosis in human cancer cell lines. Toxicol In Vitro. 2011;25(3):630–635. [DOI] [PubMed] [Google Scholar]
- 26. Bai J, Luo Y, Song Z, et al. Effects and the mechanisms of chrysin on sepsis-associated acute lung injury of rats chrysin inhibits acute lung injury. Life Sci J. 2013;10(3):1052–1058 [Google Scholar]
- 27. Kang MK, Park SH, Choi YJ, Shin D, Kang YH. Chrysin inhibits diabetic renal tubulointerstitial fibrosis through blocking epithelial to mesenchymal transition. J Mol Med. 2015;93(7):759–772. [DOI] [PubMed] [Google Scholar]
- 28. Tsuji PA, Walle T. Benzo[a]pyrene-induced cytochrome P4501A and DNA binding in cultured trout hepatocytes—inhibition by plant polyphenols. Chem Biol Interact. 2007;169(1):25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Anandhi R, Thomas PA, Geraldine P. Evaluation of the anti-atherogenic potential of chrysin in Wistar rats. Mol Cell Biochem. 2014;385(1-2):103–113. [DOI] [PubMed] [Google Scholar]
- 30. Lirdprapamongkol K, Sakurai H, Abdelhamed S, et al. A flavonoid chrysin suppresses hypoxic survival and metastatic growth of mouse breast cancer cells. Oncol Rep. 2013;30(5):2357–2364. [DOI] [PubMed] [Google Scholar]
- 31. Nabavi SF, Braidy N, Habtemariam S, et al. Neuroprotective effects of chrysin: from chemistry to medicine. Neurochem Int. 2015;90:224–231. [DOI] [PubMed] [Google Scholar]
- 32. Balta C, Herman H, Boldura OM, et al. Chrysin attenuates liver fibrosis and hepatic stellate cell activation through TGF-β/Smad signaling pathway. Chem Biol Interact. 2015;240:94–101. [DOI] [PubMed] [Google Scholar]
- 33. Rehman MU, Tahir M, Khan AQ, et al. Chrysin suppresses renal carcinogenesis via amelioration of hyperproliferation, oxidative stress and inflammation: plausible role of NF-κB. Toxicol Lett. 2013;216(2-3):146–158. [DOI] [PubMed] [Google Scholar]
- 34. Shen Y, Tian P, Li D, et al. Chrysin suppresses cigarette smoke-induced airway inflammation in mice. Int J Clin Exp Med. 2015;8(2):2001–2008. [PMC free article] [PubMed] [Google Scholar]
- 35. Harris GK, Qian Y, Leonard SS, Sbarra DC, Shi X. Luteolin and chrysin differentially inhibit cyclooxygenase-2 expression and scavenge reactive oxygen species but similarly inhibit prostaglandin-E2 formation in RAW 264.7 cells. J Nutr. 2006;136(6):1517–1521. [DOI] [PubMed] [Google Scholar]
- 36. Tsuji PA, Walle T. Cytotoxic effects of the dietary flavones chrysin and apigenin in a normal trout liver cell line. Chem Biol Interact. 2008;171(1):37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gardner I, Leeder JS, Chin T, Zahid N, Uetrecht P. A comparison of the covalent binding of clozapine and olanzapine to human neutrophils in vitro and in vivo. Mol Pharmacol. 1998;53(6):999–1008. [PubMed] [Google Scholar]
- 38. Gardner I, Zahid N, MacCrimmon D, Uetrecht JP. A comparison of the oxidation of clozapine and olanzapine to reactive metabolites and the toxicity of these metabolites to human leukocytes. Mol Pharmacol. 1998. b;53(6):991–998. [PubMed] [Google Scholar]
- 39. Kagan VE, Kuzmenko AI, Tyurina YY, Shvedova AA, Matsura T, Yalowich JC. Pro- oxidant and antioxidant mechanisms of etoposide in HL-60 cells: role of myeloperoxidase. Cancer Res. 2001;61(21):7777–7784. [PubMed] [Google Scholar]
- 40. Hagag AA, AbdElaal AM, Elfaragy MS, Hassan SM, Elzamarany EA. Therapeutic value of black seed oil in methotrexate hepatotoxicity in Egyptian children with acute lymphoblastic leukemia. Infect Disord Drug Targets. 2015;15(1):64–71. [DOI] [PubMed] [Google Scholar]
- 41. Ali N, Rashid S, Nafees S, Hasan SK, Sultana S. Beneficial effects of chrysin against methotrexate-induced hepatotoxicity via attenuation of oxidative stress and apoptosis. Mol Cell Biochem. 2014;385(1-2):215–223. [DOI] [PubMed] [Google Scholar]
- 42. Kim EY, Hong KB, Suh HJ, Choi HS. Protective effects of germinated and fermented soybean extract against tert-butyl hydroperoxide-induced hepatotoxicity in HepG2 cells and in rats. Food Funct. 2015;6(11):3512–3521. [DOI] [PubMed] [Google Scholar]
- 43. Huang CS, Lii CK, Lin AH, et al. Protection by chrysin, apigenin, and luteolin against oxidative stress is mediated by the Nrf2-dependent up-regulation of heme oxygenase 1 and glutamate cysteine ligase in rat primary hepatocytes. Arch Toxicol. 2013;87(1):167–178. [DOI] [PubMed] [Google Scholar]
- 44. Ateyya H, Yosef H, Nader MA. Ameliorative effect of trimetazidine on cisplatin- induced hepatotoxicity in rats [published online August 19, 2015] Can J Physiol Pharmacol. 2015:1–6. [DOI] [PubMed] [Google Scholar]
- 45. Rehman MU, Ali N, Rashid S, et al. Alleviation of hepatic injury by chrysin in cisplatin administered rats: probable role of oxidative and inflammatory markers. Pharmacol Rep. 2014;66(6):1050–1059. [DOI] [PubMed] [Google Scholar]
- 46. Han JF, He XY, Herrington JS, White LA, Zhang JF, Hong JY. Metabolism of 2-amino-1- methyl-6-phenylimidazo [4,5-b] pyridine (PhIP) byhuman CYP1B1 genetic variants. Drug Metab Dispos. 2008;36(4):745–752. [DOI] [PubMed] [Google Scholar]
- 47. Uhl M, Ecker S, Kassie F, et al. Effect of chrysin, a flavonoid compound, on the mutagenic activity of 2-amino-1-methyl-6- phenylimidazo[4,5- b]pyridine (PhIP) and benzo(a)pyrene (B(a)P) in bacterial and human hepatoma (HepG2) cells. Arch Toxicol. 2003;77(8):477–484. [DOI] [PubMed] [Google Scholar]
- 48. Tahir M, Sultana S. Chrysin modulates ethanol metabolism in Wistar rats: a promising role against organ toxicities. Alcohol Alcohol. 2011;46(4):383–392. [DOI] [PubMed] [Google Scholar]
- 49. Sathiavelu J, Senapathy GJ, Devaraj R, Namasivayam N. Hepatoprotective effect of chrysin on prooxidant-antioxidant status during ethanol-inducedtoxicity in female albino rats. J Pharm Pharmacol. 2009;61(6):809–817. [DOI] [PubMed] [Google Scholar]
- 50. Khan MA, Gupta A, Kumar S, Ahmad S, Sastry JL. Hepatoprotective activity of a new polyherbal formulation against paracetamol and D-galactosamine induced hepatic toxicity. J Pharm Bioallied Sci. 2015;7(4):246–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pushpavalli G, Kalaiarasi P, Veeramani C, Pugalendi KV. Effect of chrysin on hepatoprotective and antioxidant status in D-galactosamine-inducedhepatitis in rats. Eur J Pharmacol. 2010;631(1-3):36–41. [DOI] [PubMed] [Google Scholar]
- 52. Dou W, Zhang J, Zhang E, et al. Chrysin ameliorates chemically induced colitis in the mouse through modulation of a PXR/NF- κB signaling pathway. J Pharmacol Exp Ther. 2013;345(3):473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shin EK, Kwon HS, Kim YH, Shin HK, Kim JK. Chrysin, a natural flavone, improves murine inflammatory bowel diseases. Biochem Biophys Res Commun. 2009;381(4):502–507. [DOI] [PubMed] [Google Scholar]
- 54. Khan R, Khan AQ, Qamar W, et al. Chrysin abrogates cisplatin induced oxidative stress, p53 expression, goblet cell disintegration and apoptotic responses in the jejunum of Wistar rats. Br J Nutr. 2012;108(9):1574–1585. [DOI] [PubMed] [Google Scholar]
- 55. Sequetto PL, Oliveira TT, Soares IA, et al. The flavonoid chrysin attenuates colorectal pathological remodeling reducing the number and severity of pre-neoplastic lesions in rats exposed to the carcinogen 1,2-dimethylhydrazine. Cell Tissue Res. 2013;352(2):327–339. [DOI] [PubMed] [Google Scholar]
- 56. Pai VB, Nahata MC. Cardiotoxicity of chemotherapeutic agents: incidence, treatment and prevention. Drug Saf. 2000;22(4):263–302. [DOI] [PubMed] [Google Scholar]
- 57. Pecoraro M, Del Pizzo M, Marzocco S, et al. Inflammatory mediators in a short-time mouse model of doxorubicin- induced cardiotoxicity. Toxicol Appl Pharmacol. 2016;293:44–52. [DOI] [PubMed] [Google Scholar]
- 58. Mantawy EM, El-Bakly WM, Esmat A, Badr AM, El-Demerdash E. Chrysin alleviates acute doxorubicin cardiotoxicity in rats via suppression of oxidative stress, inflammation and apoptosis. Eur J Pharmacol. 2014;728:107–118. [DOI] [PubMed] [Google Scholar]
- 59. El-Bassossy HM, Abo-Warda SM, Fahmy A. Chrysin and luteolin attenuate diabetes-induced impairment in endothelial-dependent relaxation: effect on lipid profile, AGEs and NO generation. Phytother Res. 2013;27(11):1678–1684. [DOI] [PubMed] [Google Scholar]
- 60. Mather FJ, Simon RM, Clark GM, Von Hoff DD. Cardiotoxicity in patients treated with mitoxantrone: Southwest Oncology Group phase II studies. Cancer Treat Rep. 1987;71(6):609–613. [PubMed] [Google Scholar]
- 61. Ghalie RG, Edan G, Laurent M, et al. Cardiac adverse effects associated with mitoxantrone (Novantrone) therapy in patients with MS. Neurology. 2002;59(6):909–913. [DOI] [PubMed] [Google Scholar]
- 62. Anghel N, Cotoraci C, Ivan A, et al. Chrysin attenuates cardiomyocyte apoptosis and loss of intermediate filaments in a mouse model of mitoxantrone cardiotoxicity. Histol Histopathol. 2015;30(12):1465–1475. [DOI] [PubMed] [Google Scholar]
- 63. Angsutararux P, Luanpitpong S, Issaragrisil S. Chemotherapy-induced cardiotoxicity: overview of the roles of oxidative stress. Oxid Med Cell Longev. 2015;2015:795602 doi:10.1155/2015/795602. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ali BH, Adham SA, Al Za’abi M, et al. Ameliorative effect of chrysin on adenine-induced chronic kidney disease in rats. PLoS One. 2015;10(4):e0125285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lu CF, Wang YM, Peng SQ, et al. Combined effects of repeated administration of 2,3,7,8-tetrachlorodibenzo-p-dioxin and polychlorinated biphenyls on kidneys of male rats. Arch Environ Contam Toxicol. 2009;57(4):767–776. [DOI] [PubMed] [Google Scholar]
- 66. Ciftci O, Ozdemir I, Vardi N, Beytur A, Oguz F. Ameliorating effects of quercetin and chrysin on 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced nephrotoxicity in rats. Toxicol Ind Health. 2012;28(10):947–954. [DOI] [PubMed] [Google Scholar]
- 67. Ali BH, Inuwa I, Al Za’abi M, et al. Renal and myocardial histopathology and morphometry in rats with adenine - induced chronic renal failure: influence of gum acacia. Cell Physiol Biochem. 2014;34(3):818–828. [DOI] [PubMed] [Google Scholar]
- 68. Rashid S, Ali N, Nafees S, et al. Alleviation of doxorubicin-induced nephrotoxicity and hepatotoxicity by chrysin in Wistar rats. Toxicol Mech Methods. 2013;23(5):337–345. [DOI] [PubMed] [Google Scholar]
- 69. Sarkar C, Chakroborty D, Dasgupta PS, Basu S. Dopamine is a safe antiangiogenic drug which can also prevent 5-fluorouracil induced neutropenia. Int J Cancer. 2015;137(3):744–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rashid S, Ali N, Nafees S, Hasan SK, Sultana S. Mitigation of 5 fluorouracil induced renal toxicity by chrysin via targeting oxidative stress and apoptosis in Wistar rats. Food Chem Toxicol. 2014;66:185–193. [DOI] [PubMed] [Google Scholar]
- 71. Sultana S, Verma K, Khan R. Nephroprotective efficacy of chrysin against cisplatin- induced toxicity via attenuation of oxidative stress. J Pharm Pharmacol. 2012;64(6):872–881. [DOI] [PubMed] [Google Scholar]
- 72. Anand KV, Anandhi R, Pakkiyaraj M, Geraldine P. Protective effect of chrysin on carbon tetrachloride (CCl4)-induced tissue injury in male Wistar rats. Toxicol Ind Health. 2011;27(10):923–933. [DOI] [PubMed] [Google Scholar]
- 73. Du Q, Gu X, Cai J, Huang M, Su M. Chrysin attenuates allergic airway inflammation by modulating the transcription factors T-bet and GATA-3 in mice. Mol Med Rep. 2012;6(1):100–104. [DOI] [PubMed] [Google Scholar]
- 74. Nikbakht J, Hemmati AA, Arzi A, Mansouri MT, Rezaie A, Ghafourian M. Protective effect of gallic acid against bleomycin-induced pulmonary fibrosis in rats. Pharmacol Rep. 2015;67(6):1061–1067. [DOI] [PubMed] [Google Scholar]
- 75. Kilic T, Ciftci O, Cetin A, Kahraman H. Preventive effect of chrysin on bleomycin-induced lung fibrosis in rats. Inflammation. 2014;37(6):2116–2124. [DOI] [PubMed] [Google Scholar]
- 76. Rauf A, Khan R, Raza M, et al. Suppression of inflammatory response by chrysin, a flavone isolated from Potentilla eves titaTh. Wolf. In silico predictive study on its mechanistic effect. Fitoterapia. 2015;103:129–135. [DOI] [PubMed] [Google Scholar]
- 77. Ha SK, Moon E, Kim SY. Chrysin suppresses LPS stimulated proinflammatory responses by blocking NF-κB and JNK activations in microglia cells. Neurosci Lett. 2010;485(3):143–147. [DOI] [PubMed] [Google Scholar]
- 78. Li R, Zang A, Zhang L, et al. Chrysin ameliorates diabetes-associated cognitive deficits in Wistar rats. Neurol Sci. 2014;35(10):1527–1532. [DOI] [PubMed] [Google Scholar]


