Abstract
The Multimatrix® (MMX®) preparation MMX® is a recently obtained drug formulation developed to facilitate release of high concentrations of active drugs into the colon, with a homogeneous distribution along all colonic segments, particularly the most distal ones; the distal colonic tracts, indeed, are the most difficult to reach in significant amounts when a drug is given orally. The MMX® formulation is characterized by a lipophilic matrix dispersed in a hydrophilic structure. Indeed, in the last few years, MMX® technology has been widely used in the development of various drugs for the treatment of inflammatory and infectious gastrointestinal diseases localized in the colon. In particular, MMX® mesalamine, budesonide and parnaparin formulations have been investigated in patients with ulcerative colitis, and the first two have reached worldwide registration for the treatment of this disease. Moreover, MMX®-rifamycin is being positively tested in the treatment of colonic bacterial infections, including traveler’s diarrhea. MMX® technology is, thus, proving to be a very effective formulation for the treatment of various colonic diseases.
This effectiveness has been related not only to specific colonic delivery, but also to its ability to act in a once-daily dosage, thus favouring patients’ adherence to prescribed schedules of treatment. The effective delivery of the active molecule to the site of need in the colon is also associated with very low systemic absorption and very low rates of adverse events (AEs). In this paper, we have reviewed all clinical trials performed with an MMX®-bound drug and all possible real-life reports, in order to give an overall evaluation of MMX®.
Keywords: budesonide, mesalazine, MMX® technology, ulcerative colitis
Introduction
Significant progress in the technology of colonic drug delivery has been recently obtained by the development of the Multimatrix® (MMX®) approach, which comprises lipophilic and hydrophilic excipients enclosed within a gastro-resistant, pH-dependent coating.1,2 The mechanism of drug release obtained by this pharmaceutical formulation concerns the gastro-resistant coating, which avoids the release of the embedded compound until the tablet is exposed to a pH of seven or higher, which is normally reached in the terminal ileum. After reaching this site, the activity of the tablet core, which consists of hydrophilic excipients (thought to drive the tablet to swell into a viscous gel mass, slowing the release of the drug) and lipophilic excipients (thought to slow the penetration of aqueous fluids into the tablet core), results in a homogenous and prolonged exposure of the whole colonic mucosa to the embedded substance(s). In this way, the MMX® delivery system guarantees that active drugs play their therapeutic role directly on the colonic mucosa and minimizes the systemic absorption of the drug.1
In fact, early pharmacokinetic studies clearly demonstrated the properties of the aforementioned MMX® system: in a first study, 12 healthy male volunteers took a single dose of 1200 mg of mesalamine, enclosed in one MMX®-system tablet, together with a radioactive tracer. Gamma-scintigraphy scans were performed to check tablet dissolution within the gastrointestinal tract; in this study, tablet disintegration started in the ascending or transverse colon, continued throughout the large bowel and the radioactive signal was then detected homogenously in the whole colon, including the most distal part.3 A second study replicated the experimental design and results of the first study, using budesonide 9 mg instead of mesalamine 1200 mg as the delivered compound.4
Since then, this delivery system, conjugated with different therapeutic molecules, has been used to treat conditions in which high colonic drug concentrations are advisable/needed in order to obtain therapeutic success, whereas absorption and systemic delivery of the compound is best avoided.
Ulcerative colitis
Ulcerative colitis (UC) is a chronic, relapsing and remitting inflammatory disorder affecting the colonic mucosa; inflammation typically starts in the rectum and can variably extend proximally to involve portions of, or the entire, large intestine.5 However, in the vast majority of patients, the involvement is confined to the more distal parts of the colon, which account for 60–85% of all UC cases at diagnosis.6 The highest incidence appears to occur during the age range of 20–30 years, although there is some evidence for a second peak in incidence later in life.7 Patients typically experience relapses and remissions throughout the course of their disease; mainly depending on disease extent, UC patients may experience rectal bleeding, diarrhea mostly with bloody stools, urgency, tenesmus and abdominal pain. These symptoms significantly affect the patients’ quality of life and cause loss of work and productivity.8
Endoscopic examination of the colon in patients with UC reveals loss of vascular pattern, erythema, granularity, friability, erosion and ulceration.5,7 The histopathologic features of UC include intense neutrophil and lympho-plasmacellular infiltration of the mucosa, epithelial crypt destruction and distortion, cryptitis and cryptic abscesses, as well as extensive mucosal erosions.
As the etiopathogenesis of UC is still unclear, there is no cure for the condition. Current therapeutic strategies are aimed at inducing and maintaining remission, suppressing inflammation and obtaining mucosal healing, a complete resolution of the visible alterations or lesions, which is associated with reductions in the number of hospitalizations, and the need for colectomy in patients with UC.9,10
A few drugs used in patients with inflammatory bowel disease (IBD), such as mesalamine and low-bioavailability corticosteroids that are administered orally, pass through the stomach and, avoiding absorption as much as possible in the proximal tracts of the bowel, should, indeed, reach the areas of active inflammation. Unfortunately, because of suboptimal delivery systems, they are often significantly absorbed in the small bowel and reach insufficient concentrations at the affected site, thus limiting their efficacy in the distal part of the colon.11 In order to respond to the aforementioned unmet clinical need, the MMX® system has been introduced in IBD therapy and conjugated to different therapeutic tools. In fact, this new delivery technology has been used to modify some commonly used drugs, including mesalamine and budesonide, as well as new potential anti-inflammatory compounds, such as low-molecular-weight heparins (LMWHs). Thus, mesalamine-MMX®, budesonide-MMX® and parnaparin-MMX® were tested for their utility in the management of IBD,1,2 and the first two arrived in clinical practice. The most important clinical implication of MMX® technology in UC patients is represented by an increased adherence to the therapy; the single daily dose is, in fact, much better tolerated compared with multiple doses. This leads to a greater treatment compliance and to a lower risk of complications.
Multimatrix® mesalamine
Current United States12 and European13 guidelines recommend treatment with 5-aminosalicylates (5-ASAs) as first-line therapy for inducing and maintaining remission in patients with mildly to moderately active UC14. Oral mesalamine, taken once daily, is as effective as multiple daily doses both in induction and in remission therapy.15 The 5-ASA compounds are generally well tolerated; headache, nausea, diarrhea and abdominal pain are the most common, still very rare, side effects. Nephrotoxicity is extremely rare in patients on 5-ASA medications, with a mean incidence of 0.3% per person-year. In most cases, renal failure is caused by an acute or chronic interstitial nephritis, which is idiosyncratic and unrelated to 5-ASA formulation and dose.16 Despite its rarity, nephrotoxicity has to be taken into account in all patients treated with mesalamine, and scheduled controls of renal function are suggested in IBD treatment guidelines. MMX® mesalamine uses a high-strength (1.2 g mesalamine) tablet with the patented MMX® system technology (Mesavancol®, Lialda®) to allow high-dose, prolonged release of the drug throughout the colon, and it only requires once-daily dosing.17
The first phase II clinical trial by Prantera et al. was an 8-week, randomized, double-blind, double-dummy, multicenter study evaluating the efficacy of MMX® mesalamine 3.6 g/day (1.2 g given three times daily) compared with a 4 g mesalamine enema/day in 79 patients with mild-to-moderate UC. There was no difference in achieving clinical remission among the two groups of patients (60% for MMX® mesalamine versus 50% for mesalamine enemas) at 8 weeks.1
A second trial compared three different MMX® mesalamine doses (1.2, 2.4 and 4.8 g once daily): at week 8, 31% of patients with 2.4 g daily dosing and 18% with 4.8 g daily dosing were in remission. The 1.2 g dose did not confer any clinical benefit (no patients in remission at 8 weeks).18
The efficacy of MMX® mesalamine in the induction of remission in mild-to-moderate UC was then investigated in two phase III (SPD476-301 and SPD476-302), 8-week, randomized, double-blind, placebo-controlled, multicenter studies.19,20 In the first one, 280 patients were randomized to MMX® mesalamine 2.4 g (1.2 g twice daily), MMX® mesalamine 4.8 g (once daily) or placebo.19 In the second trial, 343 patients were randomized to MMX® mesalamine 2.4 g (once daily), MMX® mesalamine 4.8 g (once daily), placebo or Asacol 2.4 g (0.8 g three times daily).20 In both trials, MMX® mesalamine was superior to placebo in inducing remission in patients with mild-to-moderate UC. There were no differences in inducing remission among the different doses of MMX® mesalamine tested in these trials.
With regard to maintaining remission, MMX® mesalamine was tested in two 12-month randomized, multicenter clinical trials. In the first study, by Kamm et al.,21 459 patients were enrolled and randomized to receive MMX® mesalamine 1.2 g twice daily or 2.4 g once daily for 12 months and there were no differences in maintaining remission across the two groups of patients (remission was maintained in >70% of the patients). In another European study, by Prantera et al., 22 334 patients were randomized to receive MMX® mesalamine 2.4 g once daily or Asacol® 2.4 g/day (1.6 g in the morning and 0.8 g in the evening), and in this study, MMX® mesalamine was as effective as Asacol® in maintaining disease remission in left-sided colitis. In these studies, the compliance was high in patients treated with MMX® mesalamine once-daily dose, while in patients treated with other mesalamine formulations, nonadherence to treatment was associated with a higher rate of disease recurrence. MMX® mesalamine was tolerated as well as placebo or Asacol®, and headache was the most common adverse event (AE) reported. Serious AEs, included aggravated UC, melena, acute pancreatitis and nephrolithiasis, and have an incidence of 1.1–1.7% in patients treated with MMX® mesalamine at different doses. There were no notable differences between treatment groups with regard to the number and types of AEs experienced.21,22
Multimatrix® budesonide
Glucocorticoids have been used in the management of UC for decades.23 Current guidelines recommend corticosteroids when treatment with 5-ASA medications has been unsuccessful; their use is limited to the induction of remission since they have no role in maintenance therapy, due to both inefficacy and AEs.2 Corticosteroids are also available in topical formulations for the induction of remission in proctitis or left-sided mild colitis, as well as in oral compounds for moderate-to-severe colitis.12,13
Despite their efficacy for the treatment of UC, systemic corticosteroids are not recommended as a first-line therapy due to significant toxicity; major AEs derive from the suppression of the hypothalamic–pituitary–adrenal axis and involve metabolism, bone, central nervous system, skin, arterial pressure and the gastrointestinal tract. However, the most serious side effect is the increased risk of infection and, it is worth noting that an increase in mortality has also been reported.24,25 In order to potentially overcome this problem, low bioavailability steroids, such as budesonide and beclomethasone diproprionate, have been introduced in the treatment of IBDs.
Budesonide is a second-generation corticosteroid with a high local anti-inflammatory effect and low systemic bioavailability after oral administration because of extensive (90%) first-pass hepatic metabolism.26 It is a potent glucocorticoid with high affinity for the glucocorticoid receptor, strong anti-inflammatory potency and excellent solubility. Budesonide produces an anti-inflammatory effect through downregulation of cytokines with an important role in the inflammation pathways, including tumor necrosis factor-α (TNF-α), interleukin 1 (IL-1) and 6 (IL-6), as well as the key nuclear factor kappa-B (NF-κB).27,28 In contrast to systemic corticosteroids, budesonide has reduced systemic AEs; the most common side effects associated with budesonide are Cushingoid features or hypokalemia due to effects on the endocrine organs.29 However, the incidence of these systemic side effects is much lower than in patients treated with systemic corticosteroids, due to localized delivery and high first-pass metabolism with limited systemic bioavailability.30
Budesonide is currently available in different oral formulations:31
(1) Controlled ileocolonic-release formulation (CIR), characterized by a pH and time-dependent release: a coated-capsule preparation facilitates delivery of the medication to the terminal ileum and ascending colon. This formulation contains granules that are coated to protect dissolution in gastric juice but that dissolve at a pH ⩾ 5.5 when the granules reach the duodenum.
(2) pH-dependent-release formulation: a pH-dependent-release formulation that contains enteric-coated pellets with a diameter of 1 mm, which are coated with an acrylic polymer resistant to a pH < 6, enabling release in the ileum and ascending colon.
Both controlled-ileal-release and pH-dependent-release formulations may be used for induction of remission of mild-to-moderate Crohn’s disease (CD), involving the ileum or ascending colon.
(3) Multimatrix (MMX®) formulation with particular efficacy in the treatment of patients with mild-to-moderate, left-sided UC.32
Indeed, the MMX® system should be able to maximize budesonide anti-inflammatory action in the whole colon, overcoming the low-bioavailability features of budesonide, itself determined by the hepatic metabolism activity.
As a consequence, the effectiveness of budesonide-MMX® was first investigated in a pilot study on 36 patients with active left-sided colitis. The primary endpoint of the study was remission, with or without Crohn’s disease activity (CAI) reduction by 50% after 4 weeks. A total of 47% of the patients in the budesonide-MMX® 9 mg tablet group achieved the primary endpoint versus 33.3% of patients on placebo, without suppression of adrenocortical functions and without important toxicity. The CAI reduction was significant with budesonide (p < 0.0001) tablets and not with placebo (p = 0.1).33
Later, these preliminary results were confirmed in two phase III, multicenter, randomized, double-blind, double-dummy, placebo-controlled studies.34,35 In the study, CORE I, patients were randomly assigned to groups that were given budesonide-MMX® (9 mg or 6 mg), mesalamine (2.4 g, as reference) or placebo for 8 weeks. The primary endpoint was remission at week 8. Patients treated with budesonide-MMX® at a dose of 9 mg, but not 6 mg, had higher combined clinical and endoscopic remission rates when compared with placebo.34 In the CORE II study, 410 patients were randomly assigned to receive once-daily oral budesonide-MMX® 9 mg, budesonide-MMX® 6 mg, controlled ileal-release budesonide (Entocort® 9 mg) or placebo once daily for 8 weeks. Budesonide-MMX® 9 mg was associated with numerically higher rates of clinical and endoscopic improvement versus placebo. The rate of histological healing and proportion of patients with symptom resolution were significantly higher for budesonide-MMX® 9 mg than placebo.35
The favorable AE profile of budesonide-MMX® in UC patients was demonstrated in the CORE I and II studies. In both studies, the most commonly reported AEs in patients receiving budesonide-MMX® were headache and nausea, and the incidence of serious AEs was low and similar across all treatment groups. The incidence of AEs was similar in the three groups: budesonide-MMX® 9 mg (10.2%), 6 mg (7.5%) and placebo (10.5%) [35]. In a pooled analysis of CORE I and II, the incidence rates of predefined potential glucocorticoid-related adverse effects (acne, fluid retention, flushing, hirsutism, insomnia, mood changes, moon face, sleep changes, striae rubrae) were comparable for budesonide-MMX® 9 mg (10.2%), 6 mg (7.5%) and placebo (10.5%). In the 1-year budesonide-MMX® 6 mg maintenance study, the safety profile of budesonide-MMX® was comparable with that of placebo.
Few studies have evaluated the role of budesonide in the maintenance of remission in patients with UC. The efficacy of budesonide-MMX® in maintaining clinical remission was evaluated in two phase III studies or an open-label study.34,36 Budesonide-MMX® 6 mg was not significantly different from placebo in maintaining clinical remission but the probability of clinical relapse at 12 months was reduced and the median time until clinical relapse was longer in the budesonide-MMX® group when compared to the placebo group. The incidence of AEs was similar between treatment groups (21.0%) and placebo (21.3%). Budesonide’s role as maintenance therapy in UC patients is still very limited and more studies comparing it with mesalamine are required.
In view of these results, in patients with mild-to-moderate UC, budesonide-MMX® can be integrated into treatment algorithms for the induction of remission in patients who are refractory to 5-aminosalicylic acid before initiation of treatment with conventional corticosteroids.24,37
Multimatrix® parnaparin
Unfractioned heparin (UFH) and low-molecular-weight heparins (LMWHs), apart in anticoagulant and antithrombotic activities, are also involved in modulation of cytokine production and of T-lymphocyte cytotoxic activity,38 as well as inhibition of leukocyte adhesion, activation and trafficking.39
Based on these findings, heparin has been proposed for the treatment of IBD. On the basis of previous experimental studies using animal models, the first open-label, noncomparative, dose-titration study was conducted by Pastorelli et al., to assess the safety and gain preliminary data regarding the efficacy of three different oral doses (70, 140 and 210 mg once daily) of parnaparin-MMX® in the treatment of distal mild-to-moderate UC, resistant to mesalamine.40 Although the study population was very small, parnaparin-MMX® induced clinical remission in 7 of the 10 patients enrolled; no serious AEs occurred throughout the study. All patients showed an improvement in quality of life.40
Based on these encouraging preliminary results, a multicenter, double-blind, randomized, placebo-controlled, proof-of-concept trial was conducted to assess the efficacy and tolerability of 8-week daily oral administration of 210 mg of parnaparin sodium compared with placebo in patients with mild-to-moderate UC treated with stable doses of 5-ASA.41 After 8 weeks of treatment, clinical remission was achieved in 86.3% of the parnaparin-MMX® group and in 63.3% of the placebo group (p = 0.01). In conclusion, parnaparin-MMX® seems to be an effective and safe oral adjunctive therapy for patients not adequately controlled by oral aminosalycilates.
Travelers’ diarrhea
One of the potentially largest applications of this technology could be its use with antibiotics. Rifamycin SV-MMX® has been formulated as a tablet for the treatment of colonic bacterial infections, including traveler’s diarrhea (TD), usually contracted from contaminated food and less commonly from water.42 DuPont et al. showed in a randomized, double-blind, phase III study on adult travelers experiencing acute diarrhea that, RIF-MMX® 400 mg twice a day shortened the duration of TD, compared with placebo, and was well tolerated.43 Aside of the brilliant therapeutic results of this approach, the unique pharmacokinetic properties of the drug appear to offer evidence that TD pathogens work at colon level.43
Conclusion
In an era increasingly characterized by the search for an optimal characterization of patients and personalized medicine, the development of drugs with specific deliveries pointing to a given organ is essential. This is particularly true for diseases such as IBD, where patients and conditions may be extremely heterogeneous for onset, features and clinical course, occurrence of intestinal and extraintestinal complications and response to different therapies. To this end, the development of the MMX® technology appears to be an important step forward in the targeting of the colon and in the treatment of many diseases affecting it.
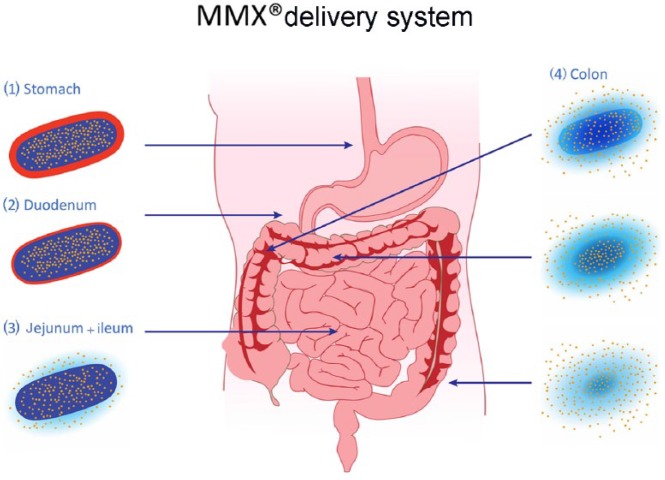
The capacity of the MMX® formulation to effectively carry drugs down through the gastrointestinal tract to the colon has been convincingly shown in pharmacokinetic studies (Figure 1). This type of delivery is characterized not only by the precise targeting of the organ, but also by a very low incidence of AEs due to a very low rate of absorption. Furthermore, the possibility to use once-a-day administration for mesalazine preparations, which was first reported for the mesalazine-MMX® formulation, has been shown to lead to an increased adherence of patients; this is a pivotal characteristic for a drug used in disease conditions in which life-long administration and multiple daily pills are typical features.
Figure 1.
MMX® technology release throughout the gastrointestinal tract. The gastro-resistant coating (1–2) avoids the release of the drug until the tablet is exposed to a pH ⩾ 7, normally reached in the terminal ileum (3). After reaching this site, the activity of the tablet core results in a homogeneous and prolonged exposure of the whole colonic mucosa to the embedded drug (4).
So far, the MMX® preparations have been integrated in the use of common drug for the treatment of UC and this particular formulation improves both compliance and quality of life for patients. Now that this technology has been developed and proved effective, many possible colonic diseases could be targeted, possibly with similar efficacy.
Footnotes
Funding: This work was supported in part by Ferring Spa.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Silvia Nardelli, Department of Gastroenterology, ‘Sapienza’ Università di Roma, Viale dell’Università 37, 00161, Roma, Italy.
Laura Francesca Pisani, Gastroenterology and Gastrointestinal Endoscopy Unit, IRCCS Policlinico San Donato, San Donato Milanese, Italy.
Gian Eugenio Tontini, Gastroenterology and Gastrointestinal Endoscopy Unit, IRCCS Policlinico San Donato, San Donato Milanese, Italy.
Maurizio Vecchi, Gastroenterology and Gastrointestinal Endoscopy Unit, IRCCS Policlinico San Donato, San Donato Milanese, Italy Department of Biomedical Sciences for Health, Università di Milano, Milan, Italy.
Luca Pastorelli, Gastroenterology and Gastrointestinal Endoscopy Unit, IRCCS Policlinico San Donato, San Donato Milanese, Italy Department of Biomedical Sciences for Health, Università di Milano, Milan, Italy.
References
- 1. Prantera C, Viscido A, Biancone L, et al. A new oral delivery system for 5-ASA: preliminary clinical findings for MMX. Inflamm Bowel Dis 2005; 11: 421–427. [DOI] [PubMed] [Google Scholar]
- 2. Fiorino G, Fries W, De La Rue SA, et al. New drug delivery systems in inflammatory bowel disease: MMX and tailored delivery to the gut. Curr Med Chem 2010; 17: 1851–1857. [DOI] [PubMed] [Google Scholar]
- 3. Brunner M, Greinwald R, Kletter K, et al. Gastrointestinal transit and release of 5-aminosalicylic acid from 153Sm-labelled mesalazine pellets vs. tablets in male healthy volunteers. Aliment Pharmacol Ther 2003; 17: 1163–1169. [DOI] [PubMed] [Google Scholar]
- 4. Brunner M, Ziegler S, Di Stefano AF, et al. Gastrointestinal transit, release and plasma pharmacokinetics of a new oral budesonide formulation. Br J Clin Pharmacol 2006; 61: 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ordás I, Eckmann L, Talamini M, et al. Ulcerative colitis. Lancet 2012; 380: 1606–1619. [DOI] [PubMed] [Google Scholar]
- 6. Vecchi M, Saibeni S, Devani M, et al. Review article: diagnosis, monitoring and treatment of distal colitis. Aliment Pharmacol Ther 2003; 17(Suppl. 2): 2–6. [DOI] [PubMed] [Google Scholar]
- 7. Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012; 142: 46–54. e42; quiz e30. [DOI] [PubMed] [Google Scholar]
- 8. De Boer AG, Bennebroek Evertsz’ F, Stokkers PC, et al. Employment status, difficulties at work and quality of life in inflammatory bowel disease patients. Eur J Gastroenterol Hepatol 2016; 28: 1130–1136. [DOI] [PubMed] [Google Scholar]
- 9. Walsh A, Palmer R, Travis S. Mucosal healing as a target of therapy for colonic inflammatory bowel disease and methods to score disease activity. Gastrointest Endosc Clin N Am 2014; 24: 367–378. [DOI] [PubMed] [Google Scholar]
- 10. Pagnini C, Menasci F, Festa S, et al. ‘Mucosal healing’ in ulcerative colitis: between clinical evidence and market suggestion. World J Gastrointest Pathophysiol 2014; 5: 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wilding IR. A scintigraphic study to evaluate what happens to Pentasa® and Asacol® in the human gut. Practical Gastroenterol 1999; 23: 1–8. [Google Scholar]
- 12. Kornbluth A, Sachar DB. and Practice Parameters Committee of the American College of Gastroenterology. Ulcerative colitis practice guidelines in adults: American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol 2010; 105: 501–523; quiz 524. [DOI] [PubMed] [Google Scholar]
- 13. Dignass A, Lindsay JO, Sturm A, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: current management. J Crohns Colitis 2012; 6: 991–1030. [DOI] [PubMed] [Google Scholar]
- 14. Ford AC, Khan KJ, Achkar JP, et al. Efficacy of oral vs. topical, or combined oral and topical 5-aminosalicylates, in ulcerative colitis: systematic review and meta-analysis. Am J Gastroenterol 2012; 107: 167–176; author reply 177. [DOI] [PubMed] [Google Scholar]
- 15. Ford AC, Khan KJ, Sandborn WJ, et al. Once-daily dosing vs. conventional dosing schedule of mesalamine and relapse of quiescent ulcerative colitis: systematic review and meta-analysis. Am J Gastroenterol 2011; 106: 2070–2077; quiz 2078. [DOI] [PubMed] [Google Scholar]
- 16. Gisbert JP, González-Lama Y, Maté J. 5-Aminosalicylates and renal function in inflammatory bowel disease: a systematic review. Inflamm Bowel Dis 2007; 13: 629–638. [DOI] [PubMed] [Google Scholar]
- 17. Baker DE. MMX mesalamine. Rev Gastroenterol Disord 2006; 6: 146–152. [PubMed] [Google Scholar]
- 18. D’Haens G, Hommes D, Engels L, et al. Once-daily MMX mesalazine for the treatment of mild-to-moderate ulcerative colitis: a phase II, dose-ranging study. Aliment Pharmacol Ther 2006; 24: 1087–1097. [DOI] [PubMed] [Google Scholar]
- 19. Lichtenstein GR, Kamm MA, Boddu P, et al. Effect of once- or twice-daily MMX mesalamine (SPD476) for the induction of remission of mild to moderately active ulcerative colitis. Clin Gastroenterol Hepatol 2007; 5: 95–102. [DOI] [PubMed] [Google Scholar]
- 20. Kamm MA, Sandborn WJ, Gassull M, et al. Once-daily, high-concentration MMX mesalamine in active ulcerative colitis. Gastroenterology 2007; 132: 66–75; quiz 432–433. [DOI] [PubMed] [Google Scholar]
- 21. Kamm MA, Lichtenstein GR, Sandborn WJ, et al. Randomised trial of once- or twice-daily MMX mesalazine for maintenance of remission in ulcerative colitis. Gut 2008;57: 893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Prantera C, Kohn A, Campieri M, et al. Clinical trial: ulcerative colitis maintenance treatment with 5-ASA: a 1-year, randomized multicentre study comparing MMX with Asacol. Aliment Pharmacol Ther 2009; 30: 908–918. [DOI] [PubMed] [Google Scholar]
- 23. Truelove SC, Witts LJ. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J 1955; 2: 1041–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Danese S, Siegel CA, Peyrin-Biroulet L. Review article: integrating budesonide-MMX into treatment algorithms for mild-to-moderate ulcerative colitis. Aliment Pharmacol Ther 2014; 39: 1095–1103. [DOI] [PubMed] [Google Scholar]
- 25. Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infections and mortality in association with therapies for Crohn’s disease: TREAT registry. Clin Gastroenterol Hepatol 2006; 4: 621–630. [DOI] [PubMed] [Google Scholar]
- 26. Ryrfeldt A, Andersson P, Edsbäcker S, et al. Pharmacokinetics and metabolism of budesonide, a selective glucocorticoid. Eur J Respir Dis Suppl 1982; 122: 86–95. [PubMed] [Google Scholar]
- 27. Sherlock ME, Seow CH, Steinhart AH, et al. Oral budesonide for induction of remission in ulcerative colitis. Cochrane Database Syst Rev 2010; 10: CD007698. [DOI] [PubMed] [Google Scholar]
- 28. Silverman J, Otley A. Budesonide in the treatment of inflammatory bowel disease. Expert Rev Clin Immunol 2011; 7: 419–428. [DOI] [PubMed] [Google Scholar]
- 29. O’Donnell S, O’Morain CA. Therapeutic benefits of budesonide in gastroenterology. Ther Adv Chronic Dis 2010; 1: 177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schoon EJ, Bollani S, Mills PR, et al. Bone mineral density in relation to efficacy and side effects of budesonide and prednisolone in Crohn’s disease. Clin Gastroenterol Hepatol 2005; 3: 113–121. [DOI] [PubMed] [Google Scholar]
- 31. Lichtenstein GR. Budesonide multi-matrix for the treatment of patients with ulcerative colitis. Dig Dis Sci 2016; 61: 358–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sherlock ME, MacDonald JK, Griffiths AM, et al. Oral budesonide for induction of remission in ulcerative colitis. Cochrane Database Syst Rev 2015; 10: CD007698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. D’Haens GR, Kovács A, Vergauwe P, et al. Clinical trial: preliminary efficacy and safety study of a new Budesonide-MMX® 9 mg extended-release tablets in patients with active left-sided ulcerative colitis. J Crohns Colitis 2010; 4: 153–160. [DOI] [PubMed] [Google Scholar]
- 34. Sandborn WJ, Travis S, Moro L, et al. Once-daily budesonide-MMX® extended-release tablets induce remission in patients with mild-to-moderate ulcerative colitis: results from the CORE I study. Gastroenterology 2012; 143: 1218–1226. e1–e2. [DOI] [PubMed] [Google Scholar]
- 35. Travis SP, Danese S, Kupcinskas L, et al. Once-daily budesonide-MMX in active, mild-to-moderate ulcerative colitis: results from the randomised CORE II study. Gut 2014; 63: 433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sandborn WJ, Danese S, D’Haens G, et al. Induction of clinical and colonoscopic remission of mild-to-moderate ulcerative colitis with budesonide MMX 9 mg: pooled analysis of two phase III studies. Aliment Pharmacol Ther 2015; 41: 409–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Biancone L, Annese V, Ardizzone S, et al. Safety of treatments for inflammatory bowel disease: clinical practice guidelines of the Italian Group for the Study of Inflammatory Bowel Disease (IG-IBD). Dig Liver Dis. Epub ahead of print 17 January 2017. DOI: 10.1016/j.dld.2017.01.141. [DOI] [PubMed] [Google Scholar]
- 38. Elsayed E, Becker RC. The impact of heparin compounds on cellular inflammatory responses: a construct for future investigation and pharmaceutical development. J Thromb Thrombolysis 2003; 15: 11–18. [DOI] [PubMed] [Google Scholar]
- 39. Papa A, Danese S, Gasbarrini A, et al. Review article: potential therapeutic applications and mechanisms of action of heparin in inflammatory bowel disease. Aliment Pharmacol Ther 2000; 14: 1403–1409. [DOI] [PubMed] [Google Scholar]
- 40. Pastorelli L, Saibeni S, Spina L, et al. Oral, colonic-release low-molecular-weight heparin: an initial open study of parnaparin-MMX for the treatment of mild-to-moderate left-sided ulcerative colitis. Aliment Pharmacol Ther 2008; 28: 581–588. [DOI] [PubMed] [Google Scholar]
- 41. Celasco G, Papa A, Jones R, et al. Clinical trial: oral colon-release parnaparin sodium tablets (CB-01-05 MMX) for active left-sided ulcerative colitis. Aliment Pharmacol Ther 2010; 31: 375–386. [DOI] [PubMed] [Google Scholar]
- 42. Farrell DJ, Putnam SD, Biedenbach DJ, et al. In vitro activity and single-step mutational analysis of rifamycin SV tested against enteropathogens associated with traveler’s diarrhea and Clostridium difficile. Antimicrob Agents Chemother 2011; 55: 992–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. DuPont HL, Petersen A, Zhao J, et al. Targeting of rifamycin SV to the colon for treatment of travelers’ diarrhea: a randomized, double-blind, placebo-controlled phase III study. J Travel Med 2014; 21: 369–376. [DOI] [PubMed] [Google Scholar]