Abstract
The mucosal adjuvant effect of synthetic double-stranded RNA polyriboinosinic polyribocytidylic acid [poly(I:C)] against influenza virus was examined under intranasal coadministration with inactivated hemagglutinin (HA) vaccine in BALB/c mice and was shown to have a protective effect against both nasal-restricted infection and lethal lung infection. Intranasal administration of vaccine from PR8 (H1N1) with poly(I:C) induced a high anti-HA immunoglobulin A (IgA) response in the nasal wash and IgG antibody response in the serum, while vaccination without poly(I:C) induced little response. Intracerebral injection confirmed the safety of poly(I:C). In addition, we demonstrated that administration of poly(I:C) with either A/Beijing (H1N1) or A/Yamagata (H1N1) vaccine conferred complete protection against PR8 challenge in this mouse nasal infection model, suggesting that poly(I:C) possessed cross-protection ability against variant viruses. To investigate the mechanism of the protective effect of poly(I:C), mRNA levels of Toll-like receptors and cytokines were examined in the nasal-associated lymphoid tissue after vaccination or virus challenge. Intranasal administration of HA vaccine with poly(I:C) up-regulated expression of Toll-like receptor 3 and alpha/beta interferons as well as Th1- and Th2-related cytokines. We propose that poly(I:C) is a new effective intranasal adjuvant for influenza virus vaccine.
When developing a vaccine, both prophylactic effectiveness and safety should be considered. It has been reported that the respiratory tract (RT) mucosal immune system is usually the first immunological barrier against influenza virus infection (16, 17, 36) and a primary site of influenza virus infection. The influenza virus causes annual epidemics of influenza by altering the antigenic properties of its surface hemagglutinin (HA), which is involved in binding of sialic acids to the surface of susceptible cells (23). Inactivated vaccines against the influenza virus have been administered parenterally to induce serum anti-HA immunoglobulin G (IgG) antibodies (Abs) that are highly protective against homologous virus infection but are less effective against heterologous virus infection (19, 23). In contrast, a number of studies have shown that the mucosal immunity acquired by natural infection, which is mainly due to the secreted form of IgA (s-IgA) in the RT, is more effective and cross-protective against virus infections than systemic immunity induced by parenteral vaccines in humans (4, 5, 11, 18, 23) and mice (15, 36). In this regard, induction of s-IgA at the RT has a great advantage in protecting against unpredictable epidemics of influenza.
We have demonstrated that intranasal immunization with an inactivated vaccine together with cholera toxin B subunits (CTB) containing a trace amount of holotoxin (CTB*) induces not only s-IgA with strong cross-protection against infection by variant viruses belonging to the same subtype in the upper RT but also serum IgG with weak cross-protection against variant virus infection in the lower RT in mice (26, 30-32). These findings were consistent with those of previous reports (13, 20, 22). Although CTB* is an effective adjuvant to produce s-IgA, it has some side effects, such as nasal discharge in humans. Several attempts to reduce the side effects have been carried out by introduction of mutations in CTB (8) or using physiological adjuvants, such as complement component C3d (37). Therefore, there is a need for an adjuvant that is both as effective as CTB* and safe for human use for the clinical application of intranasal influenza virus vaccine.
Double-stranded RNA (dsRNA) acts as a molecular mimic associated with viral infection, because most viruses produce dsRNA during their replication (10). It has also been shown that mammalian Toll-like receptor 3 (TLR3) recognizes dsRNA and activates the NF-κB (1) pathway, resulting in activation of alpha/beta interferon (IFN-α/β), which enhances the primary antibody response against subcutaneous immunization of soluble materials (14). The adjuvant activity of IFN-α/β seems to play an important role in bridging the gap between innate and adaptive immunity (14).
In the present study, we demonstrated that the mucosal adjuvant activity of intranasal administration of synthetic dsRNA polyriboinosinic polyribocytidylic acid [poly(I:C)] with inactivated influenza virus HA vaccine induced cross-protective immune responses against homologous and heterologous variant influenza virus infection.
MATERIALS AND METHODS
HA vaccines and influenza viruses.
HA vaccines (split-product virus vaccines) were prepared from influenza viruses, including A/Puerto Rico/3/334 (A/PR8; H1N1), A/Yamagata/120/86 (A/Yamagata; H1N1), A/Beijing/262/95 (A/Beijing; H1N1), A/Guizhou/54/89 (A/Guizhou; H3N2), B/Ibaraki/2/85 (B/Ibaraki), B/Aichi/5/88 (B/Aichi), and B/Yamagata/16/88 (B/Yamagata) strains according to the method of Davenport et al. (6) at the Kitasato Institute (Saitama, Japan). These viruses were grown in allantoic cavities from 10- to 11-day-old fertile chicken eggs, purified, and disintegrated with ethyl ether. The vaccine contained all proteins from virus particles. However, the major component of the vaccine was HA molecules (about 30% of total protein). The PR/8 virus used for the challenge experiments was adapted to mice by subculturing 148 times in ferrets, 596 times in mice, and 73 times in 10-day-old fertile chicken eggs.
Preparation of adjuvants.
Cholera toxin B subunits containing a trace amount of holotoxin were prepared by adding 0.1% CT (holotoxin) to CTB obtained from Sigma (St. Louis, Mo.). Synthetic double-stranded RNA poly(I:C) was kindly provided by Toray Industries, Inc. (Kamakura, Kanagawa, Japan). Heat-denatured double-stranded RNA poly(I:C), which was boiled at 95°C for 5 min and cooled immediately on ice, was used as a negative control.
Immunization with vaccine and virus challenge.
Female BALB/c mice (Japan SLC Inc., Hamamatsu, Japan), age 6 to 8 weeks at the time of immunization, were used in all experiments. All animal experiments were carried out in accordance with the Guides for Animal Experiments Performed at NIID and approved by the Animal Care and Use Committee of the National Institute of Infectious Diseases.
Five mice for each experimental group were anesthetized with diethyl ether and immunized primarily by dropping 5 μl of phosphate-buffered saline (PBS) containing 1 μg of HA vaccine with 0.1 to 10 μg of poly(I:C) into each nostril. Four weeks later, they were reimmunized in the same manner with or without the same adjuvant.
According to a modification of the procedure of Yetter and coworkers (27, 29, 38), each mouse was anesthetized and infected by intranasal administration of 1 μl of PBS containing virus suspension with 1,000 PFU of mouse-adapted PR8 virus into each nostril. As 1 μl of the virus suspension remained in the local nasal area and could not enter the lung tissue, the initial viral infection was limited to the nasal area. To examine cross-reactivity of poly(I:C) treatment against variant influenza virus subtypes, the mice were immunized intranasally with various vaccines (1 μg) together with poly(I:C) (3 μg) and boosted with each vaccine alone 4 weeks later. Two weeks after the second immunization, the immunized mice were challenged by upper RT infection with the A/PR8 virus, and 3 days later nasal wash and serum specimens were collected for virus and Ab titration.
Measurement of the virus titer and anti-PR8 HA antibodies.
Serum, nasal wash fluid, and bronchoalveolar wash fluid were collected for measurement of virus titer and antibodies against PR8 HA from mice that were sacrificed under anesthesia with chloroform. The levels of IgA and IgG Abs against HA molecules purified from the A/PR8 viruses were determined by enzyme-linked immunosorbent assay (ELISA) as described previously (29). Briefly, ELISA was conducted sequentially from the solid phase (EIA plate; Costar, Cambridge, Mass.) with a ladder of reagents consisting of the following: first, HA molecules purified from the A/PR8 virus according to the procedure of Phelan et al. (21); second, nasal wash fluid, bronchoalveolar wash fluid, or serum; third, either goat anti-mouse IgA Ab (α-chain specific; Amersham) or goat anti-mouse IgG antibody (γ-chain-specific anti-IgG antibody; Amersham), or anti-mouse IgG1 and IgG2a (BD Pharmingen, San Diego, Calif.) conjugated with biotin; fourth, streptavidin conjugated with alkaline phosphatase (Life Technologies, Rockville, Md.); and fifth, p-nitrophenylphosphate. The chromogen produced was measured by determining the absorbance at 405 nm with an ELISA reader. A twofold serial dilution of either purified HA-specific IgA (320 ng/ml) or HA-specific monoclonal IgG (160 ng/ml) was used as a standard as described previously (2). The binding kinetics of the standard HA-specific monoclonal IgG were comparable to purified HA-specific IgG from immunized mice. In the IgG subtype assay, HA-specific monoclonal IgG1 and normal mouse serum were used as controls. The HA-specific monoclonal antibody was recognized exclusively by anti-mouse IgG1 antibody but not by anti-mouse IgG2a antibody. No HA-specific IgG1 or IgG2a was detected in normal mouse serum. To examine whether poly(I:C) is effective for protection against influenza virus-induced lethal pneumonia, mice were challenged with a lethal dose (1,000 PFU in 20 μl) of PR8 virus at 2 weeks after the second immunization. The survival rate of the mice was estimated at 2 weeks after the viral challenge. The viral titer of the lung wash fluid was examined 3 days after the challenge (see Fig. 2). The antibody concentrations of unknown specimens were determined from the standard regression curve constructed for each assay with a programmed SJeia Autoreader (model er-8000; Sanko Jun-yaku, Tokyo, Japan).
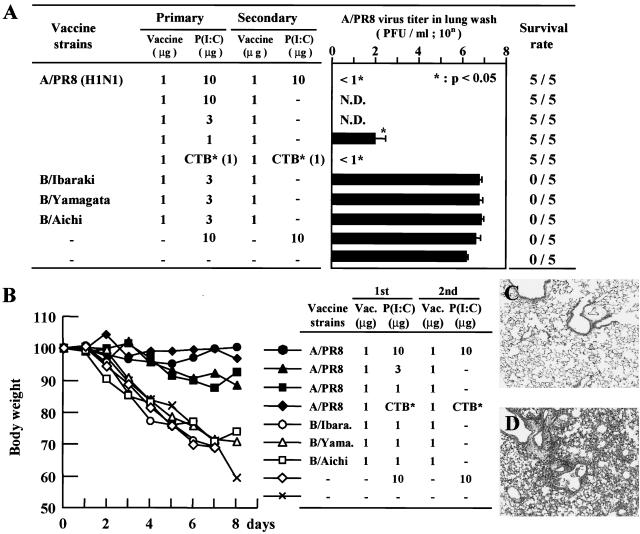
FIG. 2.
(A) Virus titers in the lung wash fluids from the mice that received primary intranasal immunization with 1 to 10 μg of poly(I:C) as an adjuvant. Secondary immunization was performed 4 weeks after primary immunization with or without the adjuvant. The mice were infected intranasally with 1,000 PFU (40 LD50) of PR8 influenza virus 2 weeks after the second immunization. The survival rate of each experimental group is shown on the right side of the bar graph. Each column represents the mean ± SD. N.D., not determined. (B) Body weight change after virus challenge. Each point represents the relative ratio for initial body weight (mean) of 5 mice for each day after the challenge. (C) Histopathological finding of a lung immunized intranasally with A/PR8 vaccine with poly(I:C) followed by 1,000 PFU (40 LD50) of A/PR8 virus at 8 days after the challenge (×40; H&E). (D) Histopathological finding of a lung immunized intranasally with B/Aichi vaccine with poly(I:C) followed by 1,000 PFU (40 LD50) of A/PR8 virus at 8 days after the challenge (×40; H&E).
Virus neutralization by antisera was determined as previously described (3). Briefly, virus was mixed with inactivated antisera from naive or vaccinated mice at 37°C for 1 h, and the mixture was added to Madin-Darby canine kidney (MDCK) cells in a total volume of 200 μl. The neutralizing capacity of antisera was measured by comparing the reduction in the number of infected cells per sample to sera from age-matched naive mice. Inhibition of virus was assessed by the additional reduction in infectivity beyond the background of naive-mouse antisera. Inhibition was measured by 50% inhibition of virus infection (beyond nonspecific inhibition). Samples were run in duplicate assays on the same day and averaged, and data are presented as the average per group.
The virus titer was measured as follows: aliquots of 200 μl of serial 10-fold dilutions of the nasal wash fluid were inoculated into MDCK cells in six-well plates. After a 1-h incubation, each well was overlaid with 2 ml of agar medium according to the method of Tobita and coworkers (34, 35). The number of plaques in each well was counted at 2 days after inoculation. All of the experiments were repeated independently at least three times. The data are presented as means ± standard deviations (SD).
RNA isolation, cDNA synthesis, and real-time PCR.
The expression of TLR3 and TLR4 in nasal-associated lymphoid tissues (NALTs) in vaccinated or influenza virus-infected mice was examined. Mice were inoculated with influenza virus or intranasally administered influenza virus HA vaccine with or without poly(I:C). The NALTs were collected sequentially up to 72 h after administration. The mRNA levels of TLR3 and TLR4 in the NALTs were measured by real-time quantitative PCR after reverse transcription. Total RNA was extracted from the NALTs in mice by using an SV-Total RNA isolation kit (Promega, Madison, Wis.) and cDNA was synthesized using Omniscript reverse transcriptase (QIAGEN, Valencia, Calif.) according to the manufacturer's instructions.
Real-time quantitative PCR was performed using the ABI PRISM 7900 sequence detection system (Applied Biosystems, Foster City, Calif.) with a QuantiTect Probe PCR kit (QIAGEN), TaqMan probes (Applied Biosystems), and primers (Sigma Genosys, Ishikari, Japan) listed in Table 1 designed with Primer Express (Applied Biosystems). The system uses two dye layers to detect the presence of target and control sequences. The FAM (6-carboxyfluorescein) dye layer yields results for quantification of the cytokine target mRNA. The cytokine and TLR targets examined were IFN-α, IFN-β, IFN-γ, interleukin-4 (IL-4), IL-6, IL-12 p40, TLR-3, and TLR-4. PCR was carried out in a volume of 20 μl; initial denaturation at 50°C for 2 min and 95°C for 15 min was followed by 45 cycles of 94°C for 15 s and 60°C for 1 min. For each sample, PCR was performed in duplicate. cDNA levels were determined using the standard curve of cycle thresholds. All data obtained were normalized to the β-actin cDNA level.
TABLE 1.
Primers for quantitative PCRa
| Target | Sequences |
|---|---|
| TLR3 | |
| Forward | CCC AGC TCG ATG TTT CCT ACA |
| Reverse | CAG GCT TGG GAG ATA GGA GAA G |
| Probe | CAA CCT CCA TGA TGT CGG CAA CGG |
| TLR4 | |
| Forward | CAT GGA ACA CAT GGC TGC TAA |
| Reverse | GTA ATT CAT ACC CCT GGA AAG GAA |
| Probe | CTA TAG CAT GGA CCT TAC CGG GCA GAA GG |
| IFN-α | |
| Forward | CTG CTA GTG ATG AGC TAC TGG TCA A |
| Reverse | GGG TCA AGG CTC TCT TGT TCC T |
| Probe | CTG CTC CCT AGG ATG TGA CCT GCC TCA |
| IFN-β | |
| Forward | GCT CCT GGA GCA GCT GAA TG |
| Reverse | TCC GTC ATC TCC ATA GGG ATC T |
| Probe | TCA ACC TCA CCT ACA GGG CGG ACT TC |
| IFN-γ | |
| Forward | AGC CAG ATT ATC TCT TTC TAC CTC AGA |
| Reverse | GCA ATA CTC ATG AAT GCA TCC TTT |
| Probe | CAG GCC ATC AGC AAC AAC ATA AGG GTC |
| IL-4 | |
| Forward | CGC CAT GCA CGG AGA TG |
| Reverse | CGA GCT CAC TCT CTG TGG TGT T |
| Probe | TGC CAA ACG TCC TCA CAG CAA CGA |
| IL-6 | |
| Forward | CCA GAA ACC GCT ATG AAG TTC CT |
| Reverse | CAC CAG CAT CAG TCC CAA GA |
| Probe | TCT GCA AGA GAC TTC CAT CCA GTT GCC |
| IL-12p40 | |
| Forward | AGC TCG CAG CAA AGC AAG AT |
| Reverse | TGG AGA CAC CAG CAA AAC GA |
| Probe | TGT CCT CAG AAG CTA ACC ATC TCC TG |
| β-Actin | |
| Forward | CAC CGA TCC ACA CAG AGT ACT TG |
| Reverse | CAG TGC TGT CTG GTG GTA CCA |
| Probe | CAG TAA TCT CCT TCT GCA TCC TGT CAG CAA |
Probes labeled with FAM (5′) and TAMRA (6-carboxytetramethylrhodamine) (3′).
Effects of intracerebral injection of poly(I:C).
Seven-week-old BALB/c female mice were injected intracerebrally with poly(I:C) or CTB* at various doses [0.25, 2.5, or 25 μg for poly(I:C) and 2.5, 10, or 25 μg for CTB*] in 25 μl of PBS, and their mortality and body weights were monitored for 7 days. PBS was used as a negative control.
Antigen-specific T-cell response.
Antigen-specific T-cell responses were measured as previously described (24). Spleens were harvested from mice 1 week after the boost vaccination. After the preparation of a single cell suspension, T cells were purified by depletion of CD11b+ (Mac-1), CD45R+ (B220), DX5+, and Ter-119+ cells by using a magnetic cell sorting system (MACS; Miltenyi Biotec, Bergisch, Germany). To prepare antigen-presenting cells, normal BALB/c mouse splenocytes were depleted of CD90 (Thy1.2)+ cells by MACS and irradiated at 2,000 cGy.
Purified T cells taken from the spleen (105 cells/well) were cultured with irradiated antigen-presenting cells (5 × 105 cells/well) in the presence or absence of PR8 vaccine (0.1, 1, or 10 μg/ml). Four days after the cultivation, the level of cytokine concentration in the culture supernatant was measured by ELISA using a mouse IFN-γ immunoassay kit (Biosource International, Camarillo, Calif.) according to the manufacturer's instruction. T-cell proliferation was monitored by the incorporation of [3H]thymidine (18.5 kBq/well; ICN Biomedicals, Costa Mesa, Calif.) added 8 h prior to cell harvest. The cells were harvested on a 96-well microplate bonded with a GF/B filter (Packard Instruments, Meriden, Conn.). Incorporated radioactivity was counted by a microplate scintillation counter (Packard Instruments).
Histopathological examination.
Excised lung, brain, and nasal tissues were fixed with 10% neutral-buffered formalin, and nasal tissue was decalcified in the EDTA solution. After fixation, tissues were embedded in paraffin by conventional methods and stained with hematoxylin and eosin (H&E).
Statistics.
Comparisons between the groups were made with the t test for paired observations, and a P of <0.05 was considered significant.
RESULTS
Intranasal immunization of HA vaccine with poly(I:C) protects against influenza virus infection.
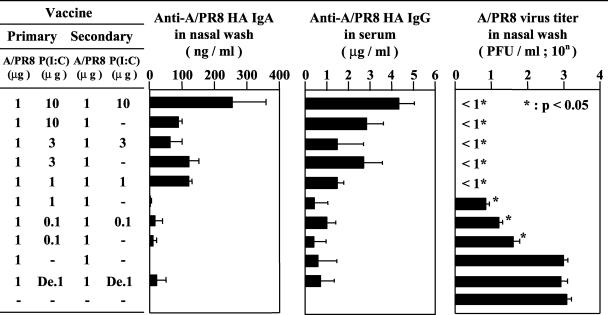
To estimate the mucosal adjuvant efficacy of poly(I:C) for influenza virus HA vaccine, the antibody response against PR8 HA molecules was examined in mice immunized intranasally with 1 μg of PR8 vaccine with different amounts (0 to 10 μg) of poly(I:C). The mice received the primary immunization with vaccine and poly(I:C) at 6 weeks before infection, and secondary immunization was performed with either vaccine with poly(I:C) or vaccine alone at 2 weeks prior to infection (Fig. 1). The highest concentration of anti-HA IgA Ab in the nasal wash fluid was observed in animals given repeated immunization with 1 μg of vaccine and 10 μg of poly(I:C). The concentration of anti-HA IgA Ab of mice immunized with vaccine and 1 μg of poly(I:C) twice was similar to that of mice that received primary immunization with vaccine and 3 μg of poly(I:C) followed by secondary immunization with vaccine alone.
FIG. 1.
Anti-PR8 HA-specific antibody titer and PR8 virus titer. Anti-PR8 HA-specific IgA and IgG responses in BALB/c mice that received primary intranasal immunization with 0.1 to 10 μg of poly(I:C) as an adjuvant. Secondary immunization was performed 4 weeks after primary immunization with or without the adjuvant. The nasal wash and serum samples were collected 2 weeks after the second immunization. The antibody titers of five mice from each group were measured by ELISA. The same groups of mice were infected intranasally with 1,000 PFU of PR8 influenza virus at 2 weeks after the second immunization. Nasal wash fluids were collected 3 days after virus challenge. The virus titers were measured by plaque assay. De, heat-denatured poly(I:C). Each column represents the mean ± SD. The virus titers were statistically compared to those of nonimmunized mice.
The concentration of anti-HA IgG in the serum was also measured in the same experimental groups. The highest level of anti-HA IgG was also observed in mice that were inoculated twice with vaccine and 10 μg of poly(I:C). The serum IgG responses seemed to parallel the IgA response in the nasal wash. Nasal IgA and serum IgG levels were markedly low in mice immunized with 1 μg of denatured poly(I:C) (Fig. 1). The antibody titers of IgG1 and IgG2a in mice immunized with HA vaccine and poly(I:C) were comparable to those in mice inoculated with CTB* combined with HA vaccine (data not shown).
Next, we examined the protective effect of intranasal administration of HA vaccine with poly(I:C) against influenza virus infection. In control mice, virus titers were 103.1 PFU/ml in the nasal wash fluid at 3 days after infection with 1,000 PFU of influenza virus (Fig. 1). Mice immunized with HA vaccine alone showed no protective effect in the nasal wash compared with controls (Fig. 1). On the other hand, mice immunized with vaccine with either 1 μg of poly(I:C) twice or 3 μg of poly(I:C) once at the first immunization showed complete protection against viral infection (Fig. 1). In mice immunized with vaccine with either 0.1 or 1 μg of poly(I:C), significant reductions in virus titers in the nasal wash fluid were observed compared with mice immunized with vaccine alone. Thus, intranasal administration of HA vaccine with poly(I:C) adjuvant protected mice against influenza virus infection, and the protective effect of poly(I:C) was not observed after denaturation. The results were consistent with production of IgA and IgG antibodies.
We next investigated whether poly(I:C) is effective for protection against influenza virus-induced lethal pneumonia in mice. At 2 weeks after lethal virus challenge (40 50% lethal doses [LD50] of the A/PR8 virus), no mice survived in the nonimmunized group or the groups immunized by various influenza B virus with poly(I:C), which showed marked body weight reduction (Fig. 2A and B). In contrast, no death occurred in the group of mice immunized with various amounts of poly(I:C) without body weight loss (Fig. 2A and B). The viral titer of the lung wash fluid of the nonimmunized group and the groups immunized by various influenza B virus with poly(I:C) was around 106.2 PFU/ml, while those of the mice vaccinated with either poly(I:C) or CTB* were below the level of detection (Fig. 2A). We next examined the pathological findings of the lungs in the group immunized with A/PR8 (Fig. 2C) and B/Aichi (Fig. 2D). The lung specimens of mice immunized with A/PR8 vaccine followed by 40 LD50 A/PR8 virus infection demonstrated bronchi and alveoli without any inflammatory change at 8 days after the virus challenge. In the mice immunized with B/Aichi vaccine followed by the same amount of A/PR8 virus, marked pneumonia with destruction of bronchial mucosa and interstitial infiltration of inflammatory cells was recognized in the lung specimen at 8 days after the challenge. A similar pathological change was observed in the group immunized by various influenza B virus with poly(I:C) (data not shown). Thus, it seems that the lung virus titer and the pathological change were well correlated, suggesting that the complete inhibition of lung virus titer by intranasal vaccination with poly(I:C) reflects complete protection against lethal influenza virus pneumonia.
Cross-protective effect of influenza virus HA vaccine with poly(I:C).
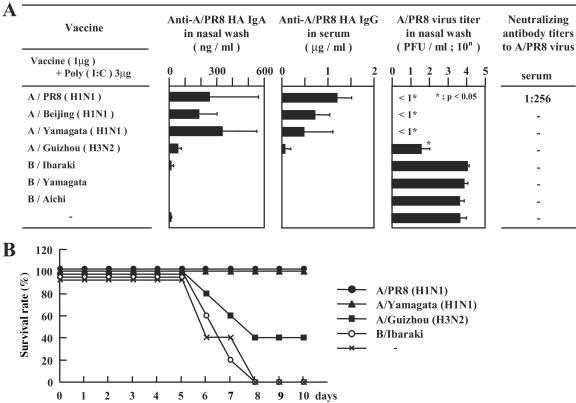
We next characterized the cross-protective effect of intranasal vaccination with poly(I:C) against various influenza virus subtypes. Mice received primary immunization with 1 μg of various vaccines with 3 μg of poly(I:C) and secondary immunization with vaccine alone. Both the IgA antibody titer (>200 ng/ml) in the nasal wash fluid and IgG antibody (>1 μg/ml) in the serum were markedly high in mice immunized with A/PR8 virus vaccine, resulting in the disappearance of PR8 virus in the nasal wash fluid (Fig. 3A). Immunization with A/Beijing (H1N1) and A/Yamagata (H1N1) vaccines induced relatively high levels of nasal IgA and serum IgG against A/PR8 HA and also conferred complete protection against A/PR8 virus (Fig. 3A). Mice immunized with A/Guizhou (H3N2) virus vaccine showed low responses of A/PR8 HA-reactive IgA in the nasal wash fluid and IgG in the serum (Fig. 3A), resulting in low protective efficiency against A/PR8 virus challenge (Fig. 3A). Next, the neutralization activity to A/PR8 virus was examined in vitro by using the sera from the same group of the mice. The mice immunized by A/PR8 vaccine had neutralized antibody against A/PR8 (1:256); however, no neutralizing activity was recognized in the sera from the mice vaccinated by A/Beijing (H1N1), A/Yamagata (H1N1), A/Guizhou (H3N2), and B influenza viruses (Fig. 3A). To examine the cross-protective effects of poly(I:C) combined influenza virus vaccine to lethal influenza virus challenge, the mice immunized with vaccine from various strains including A/PR8 (H1N1), A/Yamagata (H1N1), A/Guizhou (H3N2), and B/Ibaraki were challenged by a lethal dose (40 LD50) of A/PR8 (H1N1) virus. All the mice immunized with poly(I:C) combined with A/PR8 (H1N1) and A/Yamagata (H1N1) vaccine survived, while the survival rate of mice immunized with poly(I:C) combined with A/Guizhou (H3N2) was 40% at 10 days after the challenge. No mouse survived in the groups of unimmunized or B/Ibaraki vaccine-immunized mice at 8 days after the challenge (Fig. 3B). Taken together, these observations indicate that intranasal vaccine with poly(I:C) results in the cross-protective immune responses against homologous or heterologous viruses infection in the upper RT and against lethal influenza virus challenge.
FIG. 3.
(A) Cross-protective antibody responses against PR8 HA in the mice immunized intranasally with A/PR8 (H1N1), A/Beijing (H1N1), A/Yamagata (H1N1), A/Guizhou (H3N2), B/Ibaraki, B/Yamagata, and B/Aichi vaccine with poly(I:C) as an adjuvant. Secondary immunization was performed 4 weeks after primary immunization without the adjuvant. The same groups of mice were infected with 1,000 PFU in 2 μl of PR8 influenza virus 2 weeks after the second immunization. The nasal wash fluid was collected 3 days after virus challenge. The virus titer was measured by plaque assay. Each column represents the mean ± SD. The serum collected at 2 weeks after the booster was analyzed for the presence of neutralizing antibodies against homologous or heterologous influenza virus. Inhibition of the virus was assessed by the additional reduction in infectivity beyond the background of naive mice. Samples were run in duplicate, and data are presented per group, where the ability to inhibit 50% of infection at the indicated dilution is shown. The dash indicates lack of reduction of infectivity. (B) The survival curve of the mice immunized with poly(I:C) and the various vaccines after the lethal A/PR8 (H1N1) challenge. Mice were immunized with 3 μg of A/PR8 (H1N1), A/Yamagata (H1N1), A/Guizhou (H3N2), and B/Ibaraki vaccine with 10 μg of poly(I:C) as an adjuvant. Secondary immunization was performed 4 weeks after primary immunization with the same amount of vaccine with the adjuvant. The survival rates of the mice until 10 days after the virus challenge are presented in a line graph.
Induction of antigen-specific T-cell response by intranasal administration of vaccine with poly(I:C).
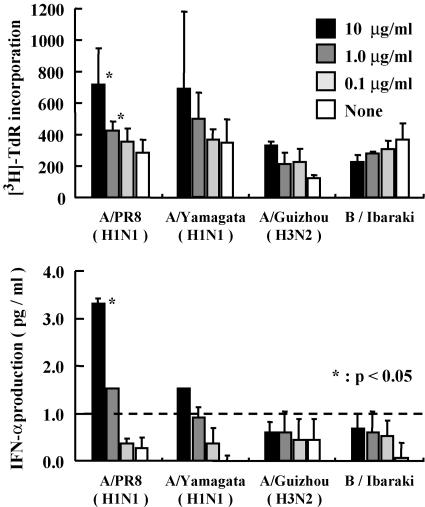
To examine whether intranasally administered influenza virus vaccine induces T-cell response against homologous or heterologous influenza viruses, mice were immunized by A/PR8 (H1N1), A/Yamagata (H1N1), A/Guizhou (H3N2), or B/Ibaraki vaccine with poly(I:C). T cells collected from the spleens of these mice 1 week after the booster immunization were enriched and cultured with irradiated antigen-presenting cells in the presence or absence of A/PR8 vaccine at 0.1, 1, or 10 μg/ml. The T cells from the mice immunized with A/PR8 and A/Yamagata viruses proliferated in an antigen dose-dependent manner; however, no proliferative effect was recognized in the mice vaccinated with A/Guizhou and B/Ibaraki virus (Fig. 4A). These results suggest that the intranasally administered influenza virus vaccine with poly(I:C) does induce T-cell activation with homologous antigen.
FIG. 4.
In vitro responses of A/PR8 (H1N1) influenza virus-specific T cells derived from mice vaccinated with A/PR8 (H1N1), A/Yamagata (H1N1), A/Guizhou (H3N2), and B/Ibaraki viruses. The mice were intranasally administered 1 μg of each vaccine with 10 μg of poly(I:C) and then boosted with the same dose of the reagents at 3 weeks after priming. Spleens were isolated at 1 week after the boost and stimulated with T-cell-depleted splenocytes that had been pulsed with the indicated concentration of A/PR8 vaccine. These cells were cultured for 4 days and [3H]thymidine was added 8 h prior to the harvest. (B) Production of IFN-γ in the culture supernatant of the cells prepared in the same manner as the cells shown in Fig. 4A. The results are represented as a means of two independent experiments.
We also examined the IFN-γ production in the supernatant of T cells derived from the spleens of the mice immunized with A/PR8 vaccine in vitro (Fig. 4B). We found that the A/PR8 (H1N1) vaccine induced IFN-γ production from the T cells derived from the mice immunized with A/PR8 (H1N1) and A/Yamagata (H1N1) virus in an antigen dose-dependent manner (Fig. 4B); however, the T cells from the mice immunized with A/Guizhou (H3N2) and B/Ibaraki viruses did not produce a significant amount of IFN-γ in response to A/PR8 (H1N1). These results suggest that T-cell responses against heterologous influenza viruses were weak and intranasal administration of influenza virus vaccine with poly(I:C) induces systemic antigen-specific T-cell responses.
Induction of TLR3 expression by intranasal administration of vaccine with poly(I:C).
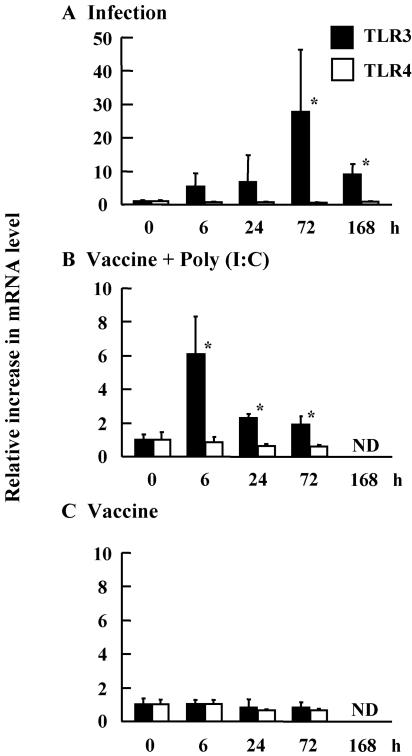
To define the mechanism by which intranasal administration of poly(I:C) with influenza virus HA vaccine functions as a mucosal adjuvant, we examined mRNA expression levels of Toll-like receptors 3 and 4, receptors for double-stranded RNA, and lipopolysaccharide, respectively. The expression of TLR3 in the NALT was up-regulated 30-fold in influenza virus-infected mice (Fig. 5A) and sixfold in mice vaccinated with poly(I:C) (Fig. 5B). The up-regulation of TLR3 in the vaccinated mice persisted for at least 72 h (Fig. 5B); meanwhile, TLR4 was not changed in these animals. The TLR responses were not detected in the mice treated with vaccine alone (Fig. 5C). These results suggested that up-regulation of TLR3 in the NALTs could enhance the adjuvant effect of poly(I:C).
FIG. 5.
Expression of TLR3 and TLR4 mRNAs in the NALTs. Total RNAs were extracted from the NALTs of mice infected with 1,000 PFU of A/PR8 (A) and intranasally immunized with the HA vaccine with poly(I:C) (B) and the HA vaccine alone (C). To determine the mRNA expression levels of TLR3 or TLR4 in the NALTs, real-time quantitative RT-PCR was performed (n = 3). *, P < 0.05 versus the pretreated group (0 h). ND, not determined.
Induction of IFN and Th1- and Th2-related cytokines by intranasal administration of vaccine with poly(I:C).
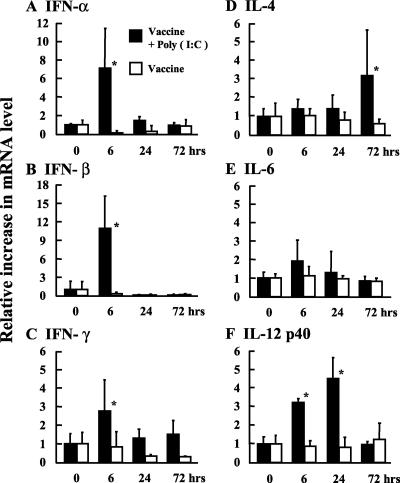
We next investigated the expression of interferons and cytokines in the NALTs after administration of influenza virus vaccine with poly(I:C). Rapid induction of IFN-α, IFN-β, and IFN-γ (at 6 h after vaccination) and return to the basal levels at 24 h were observed. However, vaccine without poly(I:C) did not induce expression of these interferons (Fig. 6A to C). The mRNA expression levels of cytokines in the NALTs were also examined. IL-4 mRNA expression was increased at 72 h after vaccination with poly(I:C). In addition, IL-12 p40 was up-regulated from 6 to 24 h following vaccination with poly(I:C) (Fig. 6D to F). These observations suggested that up-regulation of IL-4 and IL-12 p40 results in production of anti-HA-specific immunoglobulins.
FIG. 6.
Expression of cytokine mRNAs in the NALTs. Total RNA was extracted from the NALTs of mice intranasally treated with 1 μg of PR8 vaccine with or without poly(I:C). The mRNA levels of IFN-α (A), IFN-β (B), IFN-γ (C), IL-4 (D), IL-6 (E), and IL-12 p40 (F) in the NALTs were determined with real time RT-PCR (n = 3). *, P < 0.05 versus the pretreated group (0 h).
Safety of intranasal and intracerebral injection of poly(I:C).
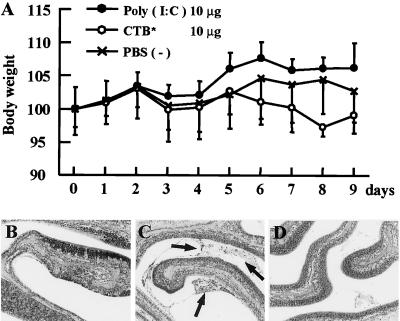
To examine the safety of poly(I:C) in intranasal administration, an excess amount of poly(I:C) and bacteria-derived adjuvant CTB* were intranasally given to mice daily for 9 days. The body weight of the mice administered 10 μg of poly(I:C) was not significantly changed, while that of the mice administered 10 μg of CTB* was relatively decreased (Fig. 7A). Histopathological examination revealed that the nasal areas of the mice administered poly(I:C) had no pathological changes, as well as those of the PBS-treated mice (Fig. 7B and D). Meanwhile, mucus exudation with inflammatory cells was recognized in the nasal areas of the mice treated with CTB* (Fig. 7C).
FIG. 7.
(A) Body weight of mice with intranasal administration of 10 μg of poly(I:C) or 10 μg of CTB* daily for 9 days. Each point represents the mean relative ratio to initial body weight (mean ± SD [%]) of 5 mice in each day. (B to D) Histopathological findings of the nasal cavities of the mice intranasally administered 10 μg of poly(I:C) (B), 10 μg of CTB* (C), and PBS (D) daily for 9 days (×100; H&E). Black arrows indicate the mucus exudation with inflammatory cells.
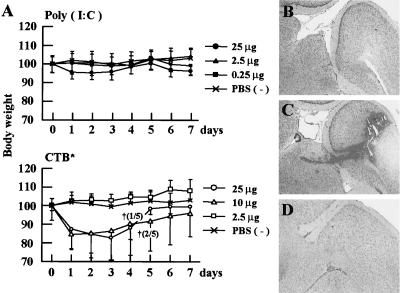
As the nasal cavity is anatomically connected to the brain via the olfactory nerves, we also examined the effects of intracerebral administration of poly(I:C) and CTB*. One mouse administered 10 μg of CTB* and 2 mice administered 25 μg of CTB* died with marked reduction of body weight (<15%) on day 4 after intracerebral injection. On the other hand, all the mice intracerebrally injected various doses (0.25 to 25 μg) of poly(I:C) survived for 7 days without body weight change (Fig. 8A). Histological examination of the brains demonstrated that cerebral hemorrhage was detected in the mice injected with 10 μg of CTB* (Fig. 8C); however, no significant change was recognized in the poly(I:C)-treated group (Fig. 8B) and PBS-treated group (Fig. 8D). This suggests that intranasal and intracerebral administrations of poly(I:C) are harmless to mice.
FIG. 8.
(A) Body weight of mice after intracerebral injection of various doses [poly(I:C), 0.25, 2.5, and 25 μg; CTB*, 2.5, 10, and 25 μg] of poly(I:C) and CTB*. Each point represents the relative ratio to initial body weight (mean ± SD [%]) of 5 mice in each day. Histopathological findings of the brains injected with 10 μg of poly(I:C) (B), 10 μg of CTB* (C), and PBS (D) at day 4 after injection (×100; H&E).
DISCUSSION
In the present study, we clearly demonstrated that poly(I:C) is an effective mucosal adjuvant when administered intranasally with influenza virus vaccine. It has been reported that effective immunization strategies to protect against influenza virus infection involve induction of mucosal immune responses at the nasal mucosal epithelium, which is the initial target of virus infection (9, 28). For effective protection against influenza virus infection at the mucosa, bacterial toxin-derived adjuvants, such as CTB or Escherichia coli heat-labile enterotoxin (LT), have been administered in conjunction with immunization (28, 30, 33). To reduce the toxicity of bacterial toxin-derived adjuvants, mutant toxins (7, 8) or low doses of CTB with trace amounts (0.1%) of holotoxin (25) were applied, and the bacterial toxins became harmless for experimental animal models. However, there are still problems associated with application of bacterial toxin-derived adjuvants to human vaccines. An effective prophylactic method against influenza virus infection in humans must be both safe and effective. We have demonstrated that a synthetic dsRNA adjuvant could induce high titers of anti-HA antibodies comparable to those seen after inoculation of CTB*, when administered intranasally once with the vaccine at the first immunization followed by a booster inoculation of vaccine alone. The immune response was detected in both nasal wash fluid and serum, which resulted in complete protection against influenza viral challenge in both the upper RT-restricted influenza model and the lung infection pneumonia model. In the two-dose immunization regimen, PR8 HA vaccine combined with 1 μg of poly(I:C) at the first immunization conferred complete protection against lethal lung infection (40 LD50) of PR8 influenza virus.
The advantage of nasal vaccination against influenza virus infection is the induction of secretory IgA at the mucosal epithelium, resulting in efficient production of cross-protective immunity compared with serum IgG (26). In fact, we observed the cross-protective effect of nasal vaccination of poly(I:C)-combined vaccination. In the present study, we used HA vaccines from various strains of influenza virus, including A/PR8 (H1N1), A/Beijing (H1N1), A/Yamagata (H1N1), and A/Guizhou (H3N2), with 3 μg of poly(I:C) at the first immunization in the two-dose regimen. Among these vaccines, anti-PR8 HA secretory IgA in the nasal wash fluid and anti-PR8 HA IgG in the serum were detected in the mice immunized with the same H1N1 virus strains. However, the neutralizing activity of the serum against A/PR8 virus was exclusively recognized in the mice immunized with homologous virus (Fig. 3). Although we have examined the neutralizing activity in the nasal wash fluid, no neutralizing activity in the nasal wash fluid was detected in any group. The concentration of anti-A/PR8 HA IgA in the nasal wash fluid is much lower than that of anti-A/PR8 HA IgG in the serum, because the nasal wash fluid was diluted by PBS for collection from the nasal mucosa. It seems that the concentration of anti-A/PR8 HA IgA is much lower than the physiological concentration in the nasal mucosa; therefore, the neutralizing activity in the nasal wash fluid is not detected. However, mice that have the cross-reactive IgA to A/PR8 virus in the nasal wash fluid were completely protected against viral challenge from not only homologous but also heterologous viruses. Even mice immunized with influenza virus vaccine against the H3N2 strain produced low doses of secretory IgA and serum IgG, which represented a 102-fold reduction of viral titer after A/PR8 influenza virus challenge. We observed the antigen-specific T-cell responses and their weak cross-reactivity in the mice treated with heterologous viruses (Fig. 4). Thus, both homologous and heterologous protection against A/PR8 influenza virus challenge may be achieved by production of cross-reactive IgA.
The mechanism of the adjuvant effect of dsRNA is still unclear. As denatured poly(I:C) did not cause any adjuvant effect, the double-stranded structure of poly(I:C) seemed to play a pivotal role in production of IgA and IgG following intranasal immunization with influenza virus HA vaccine. It is known that dsRNA is recognized by TLR3 and RNA helicase, retinoic acid-inducible gene I, and induces activation of NF-κB and production of IFN-α/β (1, 39). Early administration of IFN-α/β during an immune response markedly increases primary antibody response against soluble antigens (14). We demonstrated up-regulation of TLR3 expression but not of TLR4 in the NALTs following influenza virus infection or intranasal administration of vaccine with poly(I:C) (Fig. 5). The peak of up-regulation of TLR3 at 72 h after infection corresponded to that of influenza virus replication in the nasal area at 72 h postinfection. Because the up-regulated TLR3 is the receptor for the poly(I:C) adjuvant, we presume that the up-regulation of TLR3 enhances the signals transduced from poly(I:C). As the influenza virus itself up-regulates the expression of TLR3, we expect the vaccine with poly(I:C) mimics the state of viral infection so that the protective immune response can be elicited. Moreover, administration of poly(I:C) induced expression of IFN-α/β and Th1- and Th2-related cytokines, such as IFN-γ, IL-12 p40, and IL-4. It has recently been reported that the exposure of naive B cells to the cytokine IL-4 and/or antigen leads to a state of “priming,” in which subsequent aggregation of major histocompatibility complex class II molecules induces the mobilization of calcium ions and cell proliferation (12). A significant production of IL-4 in the NALTs immunized intranasally with vaccine with poly(I:C) may contribute to the priming of naive B cells together with the antigen. Thus, poly(I:C) might act to bridge the gap between innate and adaptive immunity.
Prophylactic agents, including vaccines, should be sufficiently safe. As the nasal cavity and the forebrain have direct communication via the olfactory nerve, the safety of nasal administration of the vaccine with adjuvant for the central nervous system should be confirmed. We demonstrated the safety of poly(I:C) to the central nervous system by direct intracerebral injection. Here, we propose that intranasal administration of influenza virus HA vaccine with poly(I:C) by the two-dose regimen is an effective and safe vaccination method.
Acknowledgments
We are grateful to U. Suzuki (Kitasato Institute) and T. Tanaka (Toray Industries, Inc.) for providing us the materials. We are grateful to A. Harashima for technical assistance.
REFERENCES
- 1.Alexopoulou, L., A. C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413:732-738. [DOI] [PubMed] [Google Scholar]
- 2.Asahi, Y., T. Yoshikawa, I. Watanabe, T. Iwasaki, H. Hasegawa, Y. Sato, S. Shimada, M. Nanno, Y. Matsuoka, M. Ohwaki, Y. Iwakura, Y. Suzuki, C. Aizawa, T. Sata, T. Kurata, and S. Tamura. 2002. Protection against influenza virus infection in polymeric Ig receptor knockout mice immunized intranasally with adjuvant-combined vaccines. J. Immunol. 168:2930-2938. [DOI] [PubMed] [Google Scholar]
- 3.Bower, J. F., X. Yang, J. Sodroski, and T. M. Ross. 2004. Elicitation of neutralizing antibodies with DNA vaccines expressing soluble stabilized human immunodeficiency virus type 1 envelope glycoprotein trimers conjugated to C3d. J. Virol. 78:4710-4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clements, M. L., S. O'Donnell, M. M. Levine, R. M. Chanock, and B. R. Murphy. 1983. Dose response of A/Alaska/6/77 (H3N2) cold-adapted reassortant vaccine virus in adult volunteers: role of local antibody in resistance to infection with vaccine virus. Infect. Immun. 40:1044-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Couch, R. B., and J. A. Kasel. 1983. Immunity to influenza in man. Annu. Rev. Microbiol. 37:529-549. [DOI] [PubMed] [Google Scholar]
- 6.Davenport, F. M., A. V. Hennessy, F. M. Brandon, R. G. Webster, C. D. Barrett, Jr., and G. O. Lease. 1964. Comparisons of serologic and febrile responses in humans to vaccination with influenza A viruses or their hemagglutinins. J. Lab. Clin. Med. 63:5-13. [PubMed] [Google Scholar]
- 7.Hagiwara, Y., T. Iwasaki, H. Asanuma, Y. Sato, T. Sata, C. Aizawa, T. Kurata, and S. Tamura. 2001. Effects of intranasal administration of cholera toxin (or Escherichia coli heat-labile enterotoxin) B subunits supplemented with a trace amount of the holotoxin on the brain. Vaccine 19:1652-1660. [DOI] [PubMed] [Google Scholar]
- 8.Hagiwara, Y., K. Komase, Z. Chen, K. Matsuo, Y. Suzuki, C. Aizawa, T. Kurata, and S. Tamura. 1999. Mutants of cholera toxin as an effective and safe adjuvant for nasal influenza vaccine. Vaccine 17:2918-2926. [DOI] [PubMed] [Google Scholar]
- 9.Hasegawa, H., S. Kadowaki, H. Takahashi, T. Iwasaki, S. Tamura, and T. Kurata. 2000. Protection against influenza virus infection by nasal vaccination in advance of sublethal irradiation. Vaccine 18:2560-2565. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs, B. L., and J. O. Langland. 1996. When two strands are better than one: the mediators and modulators of the cellular responses to double-stranded RNA. Virology 219:339-349. [DOI] [PubMed] [Google Scholar]
- 11.Johnson, P. R., S. Feldman, J. M. Thompson, J. D. Mahoney, and P. F. Wright. 1986. Immunity to influenza A virus infection in young children: a comparison of natural infection, live cold-adapted vaccine, and inactivated vaccine. J. Infect. Dis. 154:121-127. [DOI] [PubMed] [Google Scholar]
- 12.Jordan, M. B., D. M. Mills, J. Kappler, P. Marrack, and J. C. Cambier. 2004. Promotion of B cell immune responses via an alum-induced myeloid cell population. Science 304:1808-1810. [DOI] [PubMed] [Google Scholar]
- 13.Kris, R. M., R. Asofsky, C. B. Evans, and P. A. Small, Jr. 1985. Protection and recovery in influenza virus-infected mice immunosuppressed with anti-IgM. J. Immunol. 134:1230-1235. [PubMed] [Google Scholar]
- 14.Le Bon, A., G. Schiavoni, G. D'Agostino, I. Gresser, F. Belardelli, and D. F. Tough. 2001. Type i interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity 14:461-470. [DOI] [PubMed] [Google Scholar]
- 15.Liew, F. Y., S. M. Russell, G. Appleyard, C. M. Brand, and J. Beale. 1984. Cross-protection in mice infected with influenza A virus by the respiratory route is correlated with local IgA antibody rather than serum antibody or cytotoxic T cell reactivity. Eur. J. Immunol. 14:350-356. [DOI] [PubMed] [Google Scholar]
- 16.Mestecky, J., and J. R. McGhee. 1987. Immunoglobulin A (IgA): molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv. Immunol. 40:153-245. [DOI] [PubMed] [Google Scholar]
- 17.Murphy, B. R. 1994. Mucosal immunity to viruses., p. 333. In P. L. Ogra, M. E. Lamm, J. R. McGhee, J. Mestecky, W. Strober, and J. Biendnstock (ed.), Handbook of mucosal immunology. Academic Press, Inc., New York, N.Y.
- 18.Murphy, B. R., and M. L. Clements. 1989. The systemic and mucosal immune response of humans to influenza A virus. Curr. Top. Microbiol. Immunol. 146:107-116. [DOI] [PubMed] [Google Scholar]
- 19.Murphy, B. R., and R. G. Webster. 1996. Orthomyxoviruses, p. 1397-1445. In B. N. Fields, D. M. Knipe, P. M. Howley, R. M. Chanock, M. S. Hirsch, J. L. Melnick, T. P. Monath, B. Roizman, and S. E. Straus (ed.), Fields virology. Lippincott-Raven, Philadelphia, Pa.
- 20.Nedrud, J. G., X. P. Liang, N. Hague, and M. E. Lamm. 1987. Combined oral/nasal immunization protects mice from Sendai virus infection. J. Immunol. 139:3484-3492. [PubMed] [Google Scholar]
- 21.Phelan, M. A., R. E. Mayner, D. J. Bucher, and F. A. Ennis. 1980. Purification of influenza virus glycoproteins for the preparation and standardization of immunological potency testing reagents. J. Biol. Stand. 8:233-242. [DOI] [PubMed] [Google Scholar]
- 22.Ramphal, R., R. C. Cogliano, J. W. Shands, Jr., and P. A. Small, Jr. 1979. Serum antibody prevents lethal murine influenza pneumonitis but not tracheitis. Infect. Immun. 25:992-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Renegar, K. B., and P. A. Small, Jr. 1994. Passive immunization: systemic and mucosal, p. 347. In P. L. Ogra, M. E. Lamm, J. R. McGhee, J. Mestecky, W. Strober, and J. Biendnstock (ed.), Handbook of mucosal immunology. Academic Press, Inc., New York, N.Y.
- 24.Takasuka, N., H. Fujii, Y. Takahashi, M. Kasai, S. Morikawa, S. Itamura, K. Ishii, M. Sakaguchi, K. Ohnishi, M. Ohshima, S. Hashimoto, T. Odagiri, M. Tashiro, H. Yoshikura, T. Takemori, and Y. Tsunetsugu-Yokota. 2004. A subcutaneously injected UV-inactivated SARS coronavirus vaccine elicits systemic humoral immunity in mice. Int. Immunol. 16:1423-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tamura, S., K. Ishihara, K. Miyata, C. Aizawa, and T. Kurata. 1995. Mechanism of enhancement of the immune responses to influenza vaccine with cholera toxin B subunit and a trace amount of holotoxin. Vaccine 13:339-341. [DOI] [PubMed] [Google Scholar]
- 26.Tamura, S., Y. Ito, H. Asanuma, Y. Hirabayashi, Y. Suzuki, T. Nagamine, C. Aizawa, and T. Kurata. 1992. Cross-protection against influenza virus infection afforded by trivalent inactivated vaccines inoculated intranasally with cholera toxin B subunit. J. Immunol. 149:981-988. [PubMed] [Google Scholar]
- 27.Tamura, S., T. Iwasaki, A. H. Thompson, H. Asanuma, Z. Chen, Y. Suzuki, C. Aizawa, and T. Kurata. 1998. Antibody-forming cells in the nasal-associated lymphoid tissue during primary influenza virus infection. J. Gen. Virol. 79(Pt. 2):291-299. [DOI] [PubMed] [Google Scholar]
- 28.Tamura, S., H. Kurata, H. Funato, T. Nagamine, C. Aizawa, and T. Kurata. 1989. Protection against influenza virus infection by a two-dose regimen of nasal vaccination using vaccines combined with cholera toxin B subunit. Vaccine 7:314-320. [DOI] [PubMed] [Google Scholar]
- 29.Tamura, S., K. Miyata, K. Matsuo, H. Asanuma, H. Takahashi, K. Nakajima, Y. Suzuki, C. Aizawa, and T. Kurata. 1996. Acceleration of influenza virus clearance by Th1 cells in the nasal site of mice immunized intranasally with adjuvant-combined recombinant nucleoprotein. J. Immunol. 156:3892-3900. [PubMed] [Google Scholar]
- 30.Tamura, S., Y. Samegai, H. Kurata, T. Nagamine, C. Aizawa, and T. Kurata. 1988. Protection against influenza virus infection by vaccine inoculated intranasally with cholera toxin B subunit. Vaccine 6:409-413. [DOI] [PubMed] [Google Scholar]
- 31.Tamura, S., A. Yamanaka, M. Shimohara, T. Tomita, K. Komase, Y. Tsuda, Y. Suzuki, T. Nagamine, K. Kawahara, H. Danbara, et al. 1994. Synergistic action of cholera toxin B subunit (and Escherichia coli heat-labile toxin B subunit) and a trace amount of cholera whole toxin as an adjuvant for nasal influenza vaccine. Vaccine 12:419-426. [DOI] [PubMed] [Google Scholar]
- 32.Tamura, S. I., H. Asanuma, Y. Ito, Y. Hirabayashi, Y. Suzuki, T. Nagamine, C. Aizawa, T. Kurata, and A. Oya. 1992. Superior cross-protective effect of nasal vaccination to subcutaneous inoculation with influenza hemagglutinin vaccine. Eur. J. Immunol. 22:477-481. [DOI] [PubMed] [Google Scholar]
- 33.Tamura, S. I., Y. Samegai, H. Kurata, K. Kikuta, T. Nagamine, C. Aizawa, and T. Kurata. 1989. Enhancement of protective antibody responses by cholera toxin B subunit inoculated intranasally with influenza vaccine. Vaccine 7:257-262. [DOI] [PubMed] [Google Scholar]
- 34.Tobita, K. 1975. Permanent canine kidney (MDCK) cells for isolation and plaque assay of influenza B viruses. Med Microbiol. Immunol. (Berlin) 162:23-27. [DOI] [PubMed] [Google Scholar]
- 35.Tobita, K., A. Sugiura, C. Enomote, and M. Furuyama. 1975. Plaque assay and primary isolation of influenza A viruses in an established line of canine kidney cells (MDCK) in the presence of trypsin. Med. Microbiol. Immunol. (Berlin) 162:9-14. [DOI] [PubMed] [Google Scholar]
- 36.Underdown, B. J., and J. M. Schiff. 1986. Immunoglobulin A: strategic defense initiative at the mucosal surface. Annu. Rev. Immunol. 4:389-417. [DOI] [PubMed] [Google Scholar]
- 37.Watanabe, I., T. M. Ross, S. Tamura, T. Ichinohe, S. Ito, H. Takahashi, H. Sawa, J. Chiba, T. Kurata, T. Sata, and H. Hasegawa. 2003. Protection against influenza virus infection by intranasal administration of C3d-fused hemagglutinin. Vaccine 21:4532-4538. [DOI] [PubMed] [Google Scholar]
- 38.Yetter, R. A., S. Lehrer, R. Ramphal, and P. A. Small, Jr. 1980. Outcome of influenza infection: effect of site of initial infection and heterotypic immunity. Infect. Immun. 29:654-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoneyama, M., M. Kikuchi, T. Natsukawa, N. Shinobu, T. Imaizumi, M. Miyagishi, K. Taira, S. Akira, and T. Fujita. 2004. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5:730-737. [DOI] [PubMed] [Google Scholar]