Abstract
Microinjection of morphine into the periaqueductal gray (PAG) produces antinociception. In vitro slice recordings indicate that all PAG neurons are sensitive to morphine either by direct inhibition or indirect disinhibition. We tested the hypothesis that all PAG neurons respond to opioids in vivo by examining the extracellular activity of PAG neurons recorded in lightly anesthetized and awake rats. Spontaneous activity was less than 1 Hz in most neurons. Noxious stimuli (heat, pinch) caused an increase in activity in 57% and 75% of the neurons recorded in anesthetized and awake rats, respectively. The same noxious stimuli caused a decrease in activity in only 17% and 6% of neurons recorded in anesthetized and awake rats. Systemic administration of morphine caused approximately equal numbers of neurons to increase, decrease, or show no change in activity in lightly anesthetized rats. In contrast, administration of morphine caused an increase in the activity of 22 of the 27 neurons recorded in awake rats. No change in activity was evident in the remaining five neurons. Changes in activity caused by morphine were surprisingly modest (a median increase from 0.7 to 1.3 Hz). The small inconsistent effects of morphine are in stark contrast to the large changes produced by morphine on the activity of rostral ventromedial medulla (RVM) neurons or the widespread inhibition and excitation of PAG neurons treated with opioids in in vitro slice experiments. The relatively modest effects of morphine in the present study suggest that morphine produces antinociception by causing small changes in the activity of many PAG neurons.
Keywords: opioid, antinociception, pain modulation, electrophysiology
INTRODUCTION
The periaqueductal gray (PAG) is a complex midbrain structure involved in a wide range of behaviors (Depaulis and Bandler, 1991). Opioids are known to contribute to both the antinociceptive and locomotor effects mediated by the PAG (Morgan et al., 1998). Microinjection of morphine into any region of the PAG produces antinociception (Yaksh et al., 1976; Jensen and Yaksh, 1986), suggesting that a large population of PAG neurons is sensitive to opioids. In vitro slice recordings support this view by showing that all PAG neurons appear to respond to opioids (Chieng and Christie, 1994a,b; Vaughan and Christie, 1997). Opioids directly inhibit GABAergic neurons by binding to mu-opioid receptors and disinhibit PAG neurons receiving input from these GABAergic neurons (Vaughan et al., 1997). Despite the view that every PAG neuron is either directly inhibited or indirectly excited by opioids, there are no published reports examining the effects of opioids on PAG neurons in an intact rat.
There are a number of studies examining the activity of PAG neurons in response to noxious stimuli (Eickhoff et al., 1978; Sanders et al., 1980; Nakahama et al., 1981; Handwerker and Sack, 1982; Heinricher et al., 1987). These extracellular recordings reveal distinct subsets of PAG neurons that are excited and inhibited by noxious stimuli, although the number of responsive neurons is surprisingly low (<20%) in some of these studies (Sanders et al., 1980; Heinricher et al., 1987).
There are also a number of studies examining the effect of opioids on neurons in the rostral ventromedial medulla (RVM), a major output target for PAG-mediated antinociception (Zambotti et al., 1982; Morgan et al., 1992, 2008; Heinricher and Tortorici, 1994). Three classes of RVM neuron have been identified based on changes in activity associated with nociceptive reflexes (On-, Off-, and Neutral cells) (Fields et al., 1983). Administration of opioids at doses that produce antinociception inhibits the activity of On-cells, enhances the activity of Off-cells, and has no effect on Neutral cells (Barbaro et al., 1986). The present study tested the hypothesis that morphine will inhibit or enhance the activity of every PAG neuron recorded. Surprisingly, administration of morphine produced relatively small changes in the activity of a subset of PAG neurons, suggesting that antinociception is caused by small changes in the activity of many PAG neurons.
EXPERIMENTAL PROCEDURES
Experiment 1: anesthetized rats
Recordings of PAG neurons were conducted in 22 male Sprague–Dawley rats. All rats were anesthetized with methohexital throughout the experiment and euthanized immediately afterward. This and the subsequent experiment were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. This experiment was approved by the Institutional Animal Care and Use Committee at Washington State University.
Each rat was initially anesthetized with pentobarbital (55 mg/kg, i.p.). A cannula was implanted into the right jugular vein in order to maintain anesthesia with continuous infusion of methohexital. The rat was placed in a stereotaxic frame with the head held in a horizontal position. A section of the skull over the right caudal PAG was removed. The dura mater was retracted and a stainless steel recording electrode (Frederick Haer Inc.) was lowered into the right PAG. A metal needle in the trapezius muscle was used to ground the circuit. The rat’s tail or hindpaw was pinched periodically to identify the presence of nociceptive reflexes as the pentobarbital wore off. The search for PAG neurons began 30 min after the return of nociceptive reflexes. Once a reflex was detected, continuous infusion of methohexital (15–30 mg/kg/h) was initiated to maintain the rat at a constant level of anesthesia that allowed for nociceptive reflexes, but no spontaneous movements.
The electrode was advanced in 3-μm steps until the activity of a single neuron could be distinguished from background activity. The waveform of the action potential was digitized and analyzed online during the experiment and subsequently offline to insure isolation (DataWave Technologies, Loveland, Colorado). Occasionally, the action potentials of two distinct neurons were recorded with the same electrode. One to five neurons were recorded in each rat in response to the tail withdrawal reflex. Morphine was applied during the last of these cell recordings. The tail withdrawal test consisted of placing the tail in 52 °C water and measuring the latency for a withdraw reflex. Antinociception was defined a priori as a latency exceeding 13 s because this value was sure to exceed any baseline tail withdrawal latency while also limiting exposure of the tail to prolonged heat. The tail was removed from the water by the experimenter if no withdrawal occurred within 13 s. A mechanical transducer was attached to the tail to synchronize the tail withdrawal reflex to neural activity. At least two tail withdrawal tests, separated by 3 min, were conducted for each neuron.
Morphine (5 mg/kg, s.c.) was administered following at least two baseline tail withdrawal trials. A second morphine injection was administered if the tail withdrawal reflex was not inhibited by the third tail withdrawal test (approximately 8 min after the first morphine injection). Antinociception was defined as inhibition of the tail withdrawal reflex on two consecutive tests. The opioid receptor antagonist naloxone (1 mg/kg, s.c.) was injected following the induction of antinociception, and two additional tail withdrawal tests were conducted to assess reinstatement of nociception.
Spontaneous firing rate was determined during the 30 s prior to placing the tail in hot water. To determine whether noxious heat altered cell activity, firing rate during the 2 s immediately prior to the tail withdrawal reflex (i.e., stimulus-evoked activity) was compared to the spontaneous firing rate. An increase or decrease in stimulus-evoked activity was defined as at least a 20% change on two consecutive tail withdrawal trials. Mean neural activity on the two morphine-induced antinociception trials were divided by mean neural activity during the last two baseline trials to determine the percent change in activity. A change in activity following morphine administration was defined as a 20% increase or decrease from baseline activity. Given that neuronal firing rates do not conform to a normal distribution, data are presented using medians, not means.
Following testing, an electrolytic lesion was made to mark the recording site. A lethal dose of methohexital was administered through the jugular cannula. The brain was removed and placed in formalin for at least 2 days. Coronal sections (100 μm) through the PAG were cut with a vibratome and placed on a slide. The location of the PAG lesion was visualized at 10× and plotted on coronal sections from the atlas of Paxinos and Watson (2005). The other recording sites along the electrode tract were reconstructed based on the distance between recording sites.
Experiment 2: awake rats
The activity of PAG neurons was recorded in six male Long-Evans rats implanted with 8–12 tetrodes per rat. Recording tetrodes, constructed from four twisted 20-μm lacquer-coated tungsten wires (California Fine Wire), were aligned in two rows of four or six with approximately 0.5 mm between tetrodes, and mounted on an array of four independently adjustable microdrives (2–3 tetrodes/microdrive; custom made). Tetrode tips were gold-plated to reduce impedance to 0.2–0.4 MΩ (tested at 1 kHz). The number of rats used was kept to a minimum by simultaneously recording from multiple neurons in each rat. This experiment was approved by the Institutional Animal Care and Use Committee at the University of Washington.
Each rat was placed in an induction chamber and deeply anesthetized under isoflurane (4% mix with oxygen at a flow rate of 1 L/min). The rat was placed in a stereotaxic instrument (David Kopf Instruments) with the skull in a horizontal position, and anesthesia was maintained throughout surgery by isoflurane (1–2.5%) delivered via a nosecone. A hole was drilled through the right side of the skull and the dura mater retracted. The tetrode array was unilaterally implanted targeting the right PAG (7.0–7.5 mm posterior from Bregma, 1.5 mm lateral to the midsagittal suture, and 5.5 mm ventral from the dural surface). Tetrodes were implanted at a 10-degree angle from the sagittal plane in order to avoid the mid-sagittal sinus. A ground wire was inserted through a small hole in the left frontal bone of the skull. Dental cement was used to secure the drive assembly to anchoring screws in the skull. The analgesic meloxicam (Metacam,1 mg/kg) and antibiotic enrofloxacin (Baytril; 5 mg/kg) were administered subcutaneously immediately following surgery. The rat was maintained on a heating pad throughout surgery and placed under a heat lamp following surgery. Rats were allowed to recover for at least one week before testing. Food and water were available ad libitum throughout the experiment except during test sessions. Rats were weighed and health status evaluated daily.
Rats were transported to a room adjacent to the recording room at least 30 min before testing. The electronic interface board of the microdrive was connected to a preamplifier, and the outputs were connected to a Cheetah digital data acquisition system (Neuralynx). Once connected, the rat was placed in a large plastic container (35 × 50 cm) for electrophysiological recordings. Single-unit signals were filtered (0.6–6.0 kHz) then digitized at 16 kHz. Neuronal spikes were recorded for 2 ms after the voltage deflection exceeded a predetermined threshold at 500–7000× amplification. Input to the computer from each electrode was limited to large amplitude action potentials by setting the threshold to ignore background activity. If no clearly defined neurons could be distinguished, the headstage was unplugged and the tetrodes advanced. Testing occurred at least one day after a pair of tetrodes was moved to allow for stable recordings.
Following identification and isolation of single-unit activity, an experimenter entered the room to assess evoked activity by gently rubbing the rat’s back and pinching the tail. Application of these stimuli and injections were kept to a minimum to limit the effects of stress on cell activity. Electrophysiological recording was then halted, and the rat was picked up and injected with morphine (5 mg/kg, i.p.) or saline (1 ml/kg, i.p.). The rat was returned to the plastic container for 30 min to assess changes in neural activity. Each rat was tested with saline in the morning and morphine in the afternoon. This order was necessary to prevent morphine-induced confounds on subsequent recordings. At least 2 h separated the two test sessions. Each rat was injected with morphine a single time.
Following testing, rats were placed in a chamber with 4% isoflurane until deeply anesthetized. The final position of each tetrode was marked by passing a 15-μA current through the tetrode tips for 15 s. The rat was then injected with a lethal dose of sodium pentobarbital (100 mg/kg, i.p.) and transcardially perfused with 0.9% saline and 10% formalin. Brains were stored in a 10% formalin–30% sucrose solution at 4 °C for 72 h. The brains were frozen, and then cut in coronal sections (40 μm) on a freezing microtome. The sections were then mounted on gelatin-coated slides, stained with Cresyl violet, and examined under light microscopy. Only cells corresponding to lesion within the PAG were included in data analysis.
Data were sorted offline (Plexon) using standard action potential dimensions to distinguish the activity of individual neurons (Jo et al., 2013; Jo and Mizumori, 2015). Further custom analysis of the sorted units was performed with Matlab software (Mathworks). The firing rates in the 3 s before and during the back rub and tail pinch were compared to determine whether a 20% increase or decrease occurred. Changes in spontaneous activity caused by administration of morphine or saline were determined by comparing mean firing rate over three 10-min increments following morphine administration. A 20% change in activity was used to define an increase or decrease in cell activity. This liberal definition was used because in vitro recordings indicate that opioids produce relatively modest changes in currents (Vaughan, 1998). Given that the firing rates across neurons did not follow a normal distribution (a few cells had high firing rates), data were analyzed using nonparametric statistics. Parametric statistics were used following conversion of data to percent change in activity.
RESULTS
Experiment 1: anesthetized rats
The activity of 42 neurons located either in the PAG (N = 39) or adjacent dorsal raphe nucleus (N = 3) was recorded from 22 rats. Spontaneous activity tended to be quite low (Median = 1.2 Hz). Only two of the 42 neurons had spontaneous firing rates greater than 11 Hz. Placing the tail in 52 °C water evoked a withdrawal reflex in every rat. The mean baseline latency was 7.5 ± 0.3 s. Firing rate increased in the 2 s immediately preceding the tail withdrawal reflex in 24 (57%) of the 42 neurons (Table 1). A decrease in activity occurred in seven of the neurons, and no change was evident in 11 neurons. Even though these groups were defined by whether an increase or decrease occurred, the changes in tail withdrawal-related firing rate were surprisingly modest (Fig. 1).
Table 1.
Percent of neurons showing noxious stimulus-induced changes in activity in anesthetized (Experiment 1) and awake (Experiment 2) rats
| Experiment | Neurons | Increase (%) |
Decrease (%) |
No change (%) |
|---|---|---|---|---|
| Anesthetized | 42 | 57 | 17 | 26 |
| Awake | 16 | 75 | 6 | 19 |
Fig. 1.
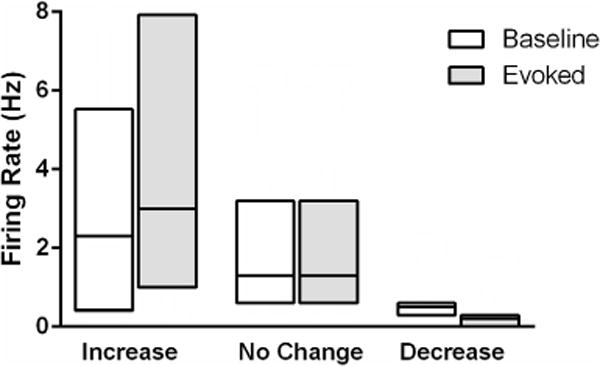
Median change in heat-evoked activity from PAG neurons in anesthetized rats. Neurons were categorized based on whether they showed at least a 20% increase (N = 24), 20% decrease (N = 7), or no change (N = 11) in activity in the 2 s preceding tail withdrawal. The median change in activity was modest despite defining the groups based on whether an increase or decrease was evident. The median is indicated by the line, and the 25th and 75th percentiles by the surrounding box.
The activity of 22 neurons from 19 rats was measured following administration of morphine. Only neurons located within or along the border of the PAG were included in data analysis (Fig. 2A). Systemic administration of morphine inhibited tail withdrawal in all rats, although eight rats required a second injection of morphine because antinociception was not evident within three tail flick tests. An increase in spontaneous activity occurred with the induction of antinociception in eight (36%) of the 22 neurons (Table 2). Administration of naloxone reversed the antinociception, but caused neural activity to return to baseline levels in only one of these eight neurons. Morphine antinociception was associated with at least a 20% decrease in activity in seven neurons (32%). Administration of naloxone reversed morphine inhibition in four of these seven neurons. Administration of morphine had no effect on the activity of another seven neurons (32%). Even with the bias of selecting the groups based on activity, the median change in ongoing activity associated with morphine antinociception was small for each group (Fig. 2B). Moreover, the spontaneous activity preceding each tail withdrawal trial did not consistently predict withdrawal latency. There was a positive correlation (r ⩾ 0.4) between cell activity and tail withdrawal latency in eight neurons, no correlation in six (0.4 < r < −0.4), and a negative correlation in another eight neurons (r ≤ −0.4).
Fig. 2.
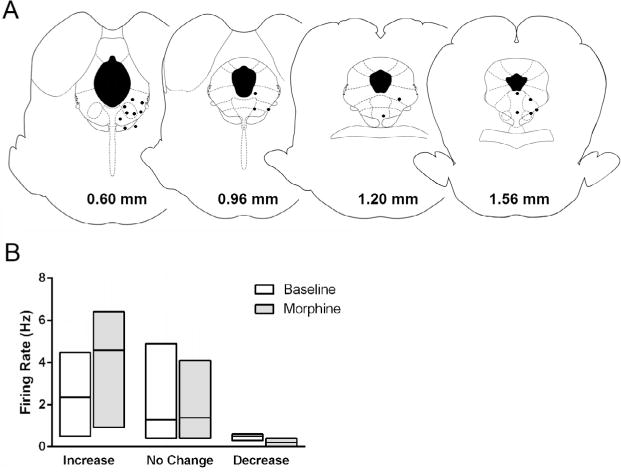
Median change in ongoing activity following morphine antinociception in PAG neurons recorded in anesthetized rats. (A) Location of neurons recorded during administration of morphine to lightly anesthetized rats. Each black dot represents the location of the extracellular recording of the activity of one or two neurons. Coronal images through the PAG are labeled in relation to the interaural line (Paxinos and Watson, 2005). (B) Neurons were categorized based on whether they showed at least a 20% increase (N = 8), 20% decrease (N = 7), or no change (N = 7) in ongoing activity following morphine administration. The bottom and top of each box represents the 25th and 75th percentile surrounding the median represented by the line inside the box.
Table 2.
Percent of neurons showing a change in ongoing activity following morphine or saline administration Initial Median
| Experiment | Neurons | Activity (Hz) | Increase (%) | No change (%) | Decrease (%) |
|---|---|---|---|---|---|
| Anesth.—Morphine | 22 | 0.6 | 36 | 32 | 32 |
| Awake—Morphine | 27 | 0.7 | 81 | 19 | 0 |
| Awake—Saline | 22 | 1.0 | 27 | 41 | 32 |
Morphine antinociception was associated with an increase in heat-evoked activity in 14 of the 22 neurons. Only eight of theses 14 neurons also showed an increase in spontaneous activity following morphine administration, suggesting that the increase in evoked activity was caused by the longer duration the tail was in hot water on trials when morphine inhibited the reflex (7.5 vs. 13.0 s) than a direct effect of morphine. Likewise, administration of naloxone reduced morphine-induced activation in only seven of these 14 neurons. Morphine inhibited heat-evoked activity in four neurons, but administration of naloxone reversed this inhibition in only one case. There was no change in heat-evoked activity in four neurons. The inconsistent effects of morphine when comparing spontaneous and heat-evoked activity, along with the inconsistent reversal of morphine effects by administration of naloxone suggest that morphine has little effect on the heat-evoked activity of PAG neurons.
Experiment 2: awake rats
The activity of 27 PAG neurons was recorded in six rats. Neurons had an anterior to posterior distribution across the PAG (Fig. 3A). Spontaneous activity was very low (Median = 1.3 Hz) during the 10 min used to identify and isolate neurons. Only one of 27 neurons fired at a rate greater than 18 Hz, whereas the firing rate in 12 of the 27 neurons was less than 1 Hz. The effect of gentle rubbing along the back and pinching the tail was assessed in a subset of rats (16 neurons from four rats). Rubbing the back caused an increase in activity in 10 of the 16 neurons with a median increase from a baseline of 1.3 Hz to 3.3 Hz. A decrease in neural activity occurred in three neurons and no change in activity was evident in another three neurons. Tail pinch caused an increase in activity in 12 of the 16 (75%) neurons (Table 1). Median cell activity in these neurons increased from a baseline of 2.3 to 5.1 Hz following pinch. Nine of the twelve were the same neurons activated by rubbing the back (Fig. 3B). Tail pinch caused a decrease in activity in one neuron and had no effect in three other neurons.
Fig. 3.
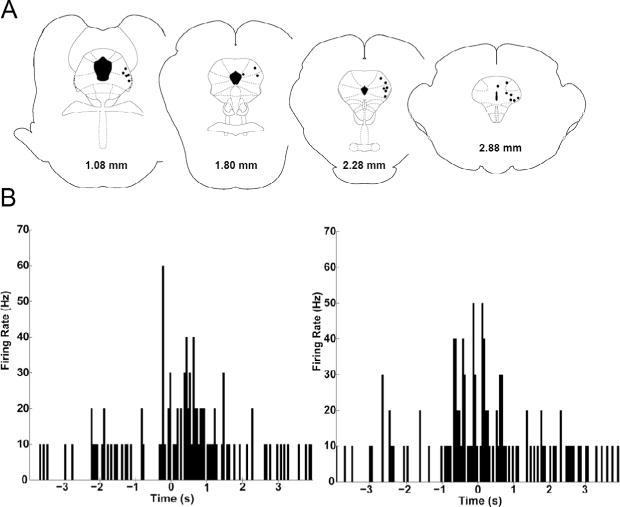
Rub and pinch evoked activation of PAG neurons recorded in awake rats. (A) The location of PAG recording sites in rats tested while awake. Each black dot represents the location where the extracellular activity of one or two neurons was recorded. The location of each coronal section relative to the interaural line is presented at the bottom (Paxinos and Watson, 2005). (B) Representative example of a neuron in which rubbing the rat’s back caused a transient increase in activity (left). A similar increase was evident in 10 of the 16 neurons. Pinching the rat’s tail also caused an increase in neural activity (right). The increase in firing occurred when the tail was touched and increased further at time 0 with the transition to pinch. A similar increase occurred in 12 of the 16 neurons tested. Data are plotted in 100-ms bins for 4 s before and after rub or pinch.
The activity of 22 neurons was recorded following administration of saline. The median spontaneous activity of PAG neurons was low throughout the 30-min saline session. Median firing rate was 1.0 Hz during the first 10 min of the session and 0.9 Hz during the last 10 min. Changes in activity across the 30-min session were distributed in a manner consistent with a normal curve. An increase in activity of at least 20% occurred in six (27%) of the 22 neurons from the first to last 10 min following administration of saline (Table 2). A decrease in activity of at least 20% occurred in seven neurons (32%), and no change in activity occurred (defined as less than a 20% change in activity) in nine neurons following saline administration.
The effect of morphine administration on the spontaneous activity of 27 PAG neurons was assessed. Median activity increased from 0.7 to 1.3 Hz from the first to last 10 min of the morphine session. This shift was driven by an increase in ongoing activity of at least 20% in 22 of 27 (81%) PAG neurons. No change in activity was evident in the five remaining neurons (Table 2). The increase in neural activity caused by morphine compared to saline administration was evident both in terms of the number of neurons showing an increase (Chi Squared = 17.196, p < 0.004) and in the percent change in activity from the first to subsequent 10 min blocks of time (F(1,47) = 13.51, p < 0.001; Fig. 4A). Individual examples showing the gradual increase in firing rate in PAG neurons following morphine administration are displayed in Fig. 4B.
Fig. 4.
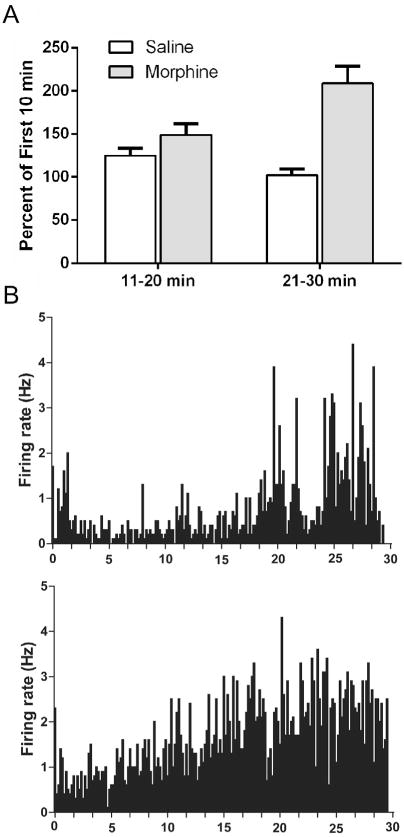
Administration of morphine produced a gradual increase in the spontaneous activity of PAG neurons recorded in awake rats. (A) The mean (±SEM) percent change in firing rate from the first to subsequent 10-min blocks of time following morphine administration (e.g., last 10 min/first 10 min) is shown. Morphine administration caused mean firing rate in the 22 neurons tested to more than double from the first to last 10 min (*p < 0.01), whereas the overall firing rate was relatively consistent across the 30-min session following saline administration. (B) Two representative examples of the increase in neuronal activity caused by morphine administration in awake rats. Neural activity was recorded for 30 min following systemic administration of morphine (5 mg/kg). Spontaneous activity was low in most of the PAG neurons, although handling the rat at the beginning of the trial tended to cause a brief activation. The overall trend was for morphine to produce a gradual increase in activity over the 30-min session. This pattern was evident in 22 of 27 neurons following morphine administration.
DISCUSSION
The present study replicates previous studies examining the effects of noxious stimuli on the activity of PAG neurons, and extends this work in two ways. First, the activity of PAG neurons was recorded in both awake and lightly anesthetized rats; second, this is the first study to examine the effect of morphine on the extracellular responses of intact PAG neurons. Our data show that the activity of PAG neurons in response to noxious stimuli is consistent whether recordings were made while rats were awake or lightly anesthetized with a barbiturate, but administration of morphine was more likely to increase the activity of PAG neurons recorded in awake rats.
The changes in neural activity caused by noxious stimuli is consistent with previous studies—distinct subsets of PAG neurons increase, decrease, or do not change in response to noxious stimuli (Eickhoff et al., 1978; Sanders et al., 1980; Handwerker and Sack, 1982; Heinricher et al., 1987). The change in activity recorded here was associated with withdrawal from the stimulus as described by others (Handwerker and Sack, 1982; Heinricher et al., 1987). Although some neurons showed a decrease in activity following noxious stimulation, activation of PAG neurons was the most common response in our study and those cited above. Although we used different noxious stimuli in rats tested while anesthetized (heat) and awake (pinch), the percentage of neurons showing an increase, decrease, and no response was consistent (see Table 1). Our result showing activation of PAG neurons in response to rubbing the back is also consistent with data derived from the cat showing that innocuous stimuli can activate PAG neurons (Nakahama et al., 1981).
The PAG is well known to contribute to opioid antinociception. Microinjection of morphine into any region of the PAG produces a whole-body antinociception (Jacquet and Lajtha, 1974; Yaksh et al., 1976; Jensen and Yaksh, 1986). In vitro whole-cell patch clamp recordings from the PAG indicate that nearly every PAG neuron responds to opioids. Morphine and other mu-opioid receptor agonists such at Met-enkephalin and DAMGO directly inhibit the activity of some PAG neurons and appear to indirectly excite all other PAG others (Chieng and Christie, 1994a,b; Vaughan and Christie, 1997). These widespread effects of opioids are consistent with the widespread distribution of mu-opioid receptors on PAG neurons (Kalyuzhny et al., 1996; Commons et al., 2000; Loyd et al., 2008; Wilson-Poe et al., 2012).
Despite the overwhelming data indicating that nearly all PAG neurons should be sensitive to morphine, the present data show a surprising lack of response to morphine administration. Single-unit extracellular activity of PAG neurons recorded from lightly anesthetized rats showed that systemic administration of morphine sufficient to inhibit the tail flick reflex produced surprisingly small increases and decreases in the activity of different subsets of neurons, and these changes were not consistently reversed by administration of naloxone. It is possible that barbiturate anesthesia may mask some of the effects of morphine in the PAG. However, direct administration of morphine into the PAG of lightly-anesthetized rats has been shown to produce antinociception (Morgan et al., 1992).
We also recorded the activity of 27 PAG neurons in awake, freely behaving rats. The overwhelming effect of morphine in these rats was to produce a gradual and relatively small increase in neural activity. Although in vitro intracellular recordings indicate that opioids directly inhibit the activity of 29% of PAG neurons (Chieng and Christie, 1994b), our extracellular recordings from PAG neurons in awake rats did not reveal a single neuron that was inhibited by morphine administration. Part of this difference could be caused by the sampling bias that limits extracellular recordings to active neurons. Of course, morphine cannot inhibit the activity of neurons that are already inactive so it is unlikely that this sampling bias had much effect. Although systemic morphine administration has direct effects on PAG neurons, changes in the activity of these neurons could be offset by the actions of morphine on inputs to the PAG coming from sources such as the spinal cord.
An increase in neural activity is consistent with in vitro recordings showing that opioid inhibition of GABAergic neurons in the PAG excites other PAG neurons (Chieng and Christie, 1994b; Vaughan et al., 1997). Presumably, there are tonically active neurons in the PAG that are directly inhibited by opioids, although it is possible that opioids preferentially act on the terminals of these neurons, and therefore inhibit transmitter release without inhibiting neural activity. Opioids may also increase neural activity by inhibiting GABA release from the terminals of neurons projecting into the PAG from distant sites. These neurons would not be present in PAG slice recordings.
Despite the differences in methodology in the experiments using lightly-anesthetized and awake rats, the data are mostly consistent. Spontaneous activity was very low except for a few active neurons in each experiment, and the effects of morphine on neuronal activity were small in both experiments. The primary difference in the results of the two experiments was that morphine reduced the activity of 32% of the neurons recorded in lightly-anesthetized rats, whereas none of the neurons recorded in awake rats showed a decrease. This difference could be caused by sampling error, recording bias (e.g., awake rats could have stress-induced suppression of activity in the subset of neurons that would be inhibited by morphine), or differences in the location of the recording sites. Neurons recorded in anesthetized rats were located primarily in the ventrolateral PAG, whereas the neurons recorded in awake rats were located primarily in more lateral and dorsal regions of the PAG. Although all PAG neurons appear to respond to opioids, a greater percentage of output neurons located in the lateral PAG are inhibited by opioids compared to output neurons in the ventrolateral PAG (Osborne et al., 1996). The fact that none of our neurons were inhibited by opioids suggests that we did not record from opioid-sensitive output neurons. Despite this discrepancy in opioid responsiveness in in vitro and in vivo recordings, there are many other anatomical and functional differences between lateral and ventrolateral regions of the PAG that could contribute to differences in neuronal responses to opioids (Carrive and Morgan, 2012).
The surprisingly small changes in the activity of PAG neurons to morphine are especially striking when compared to the response of neurons in the RVM. RVM neurons receive a direct input from the PAG and project to the dorsal horn of the spinal cord (Morgan et al., 2008). Like the PAG (Heinricher et al., 1987), RVM neurons are classified as On-, Off-, and Neutral cells based on changes in activity that precede nociceptive reflexes (Fields et al., 1983). Systemic administration of morphine consistently inhibits the activity of RVM On-cells, prevents the reflex-related pause in activity in Off-cells, and has no effect on Neutral cells (Barbaro et al., 1986). Microinjection of morphine into the PAG causes identical changes in the activity of RVM neurons (Morgan et al., 1992), demonstrating that PAG morphine administration in lightly anesthetized rats produces neural changes sufficient to produce antinociception. Although numerous studies have examined the effects of opioids on RVM neurons, the present study is the first to record the responses of PAG neurons to morphine in intact or lightly anesthetized rats.
CONCLUSIONS
Microinjection of morphine into the PAG produces a potent antinociception in awake or anesthetized rats (Yaksh et al., 1976; Morgan et al., 1998), and in vitro studies indicate that every PAG neuron is either excited or inhibited by opioids (Chieng and Christie, 1994a,b). Thus, the lack of responsiveness of PAG neurons to morphine is difficult to explain. It should be noted, however, that the currents produced by opioids in in vitro experiments are small (Vaughan, 1998), and the antinociceptive effects of opioids in the PAG are agonist specific (Morgan et al., 2014). The lack of a strong neural response to morphine reported both here and in in vitro studies (Vaughan, 1998) indicate that opioids modulate PAG output in small ways, but could have a large effect by altering the overall pattern of activity. That is, morphine seems to produce antinociception by reorganizing the computational output of the PAG by making many small changes in neural activity.
Acknowledgments
Technical assistance from Shauna School is appreciated. V.L.T. contributed by designing the awake recording experiment, collecting and analyzing data, making figures, and editing the manuscript. S.J.Y.M. contributed by designing the awake recording experiment and editing the manuscript. M.M. M. contributed by designing the experiments, collecting and analyzing data, and writing the manuscript.
Funding: Supported in part by funds provided by the State of Washington Initiative Measure No. 171 to M.M.M., the University of Washington Royalty Research Fund to S.J.Y.M., and the National Institutes of Health to M.M.M. [grant number DA015498], S.J.Y.M. (NIMH grant 58755), and V.L.T. (NIA grant 5T32AG000057-39).
Abbreviations
- PAG
periaqueductal gray
- RVM
rostral ventromedial medulla
References
- Barbaro NM, Heinricher MM, Fields HL. Putative pain modulating neurons in the rostral ventral medulla: reflex-related activity predicts effects of morphine. Brain Res. 1986;366:203–210. doi: 10.1016/0006-8993(86)91296-5. [DOI] [PubMed] [Google Scholar]
- Carrive P, Morgan MM. Periaqueductal gray. In: Paxinos G, Mai JM, editors. The human nervous system. 3rd. Boston: Academic Press; 2012. pp. 368–401. [Google Scholar]
- Chieng B, Christie MJ. Hyperpolarization by opioids acting on mu-receptors of a sub-population of rat periaqueductal gray neurones in vitro. Brit J Pharmacol. 1994a;113:121–128. doi: 10.1111/j.1476-5381.1994.tb16183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chieng B, Christie MJ. Inhibition by opioids acting on mu-receptors of GABAergic and glutamatergic postsynaptic potentials in single rat periaqueductal gray neurones in vitro. Brit J Pharmacol. 1994b;113:303–309. doi: 10.1111/j.1476-5381.1994.tb16209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commons KG, Aicher SA, Kow LM, Pfaff DW. Presynaptic and postsynaptic relations of mu-opioid receptors to gamma-aminobutyric acid-immunoreactive and medullary-projecting periaqueductal gray neurons. J Comp Neurol. 2000;419:532–542. doi: 10.1002/(sici)1096-9861(20000417)419:4<532::aid-cne8>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Depaulis A, Bandler R. The midbrain periaqueductal gray matter: functional, anatomical, and neurochemical organization. New York: Plenum Press; 1991. [Google Scholar]
- Eickhoff R, Handwerker HO, McQueen DS, Schick E. Noxious and tactile input to medial structures of midbrain and pons in the rat. Pain. 1978;5:99–113. doi: 10.1016/0304-3959(78)90032-5. [DOI] [PubMed] [Google Scholar]
- Fields HL, Bry J, Hentall I, Zorman G. The activity of neurons in the rostral medulla of the rat during withdrawal from noxious heat. J Neurosci. 1983;3:2545–2552. doi: 10.1523/JNEUROSCI.03-12-02545.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handwerker HO, Sack R. Single unit activity in the rat’s midbrain during nocifensive tail flick reaction. Neurosci Lett. 1982;30:79–84. doi: 10.1016/0304-3940(82)90016-7. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Tortorici V. Interference with GABA transmission in the rostral ventromedial medulla: disinhibition of off-cells as a central mechanism of nociceptive modulation. Neuroscience. 1994;63:533–546. doi: 10.1016/0306-4522(94)90548-7. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Cheng ZF, Fields HL. Evidence for two classes of nociceptive modulating neurons in the periaqueductal gray. J Neurosci. 1987;7:271–278. doi: 10.1523/JNEUROSCI.07-01-00271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquet YF, Lajtha A. Paradoxical effects after microinjection of morphine in the periaqueductal gray matter in the rat. Science. 1974;185:1055–1057. doi: 10.1126/science.185.4156.1055. [DOI] [PubMed] [Google Scholar]
- Jensen TS, Yaksh TL. Comparison of antinociceptive action of morphine in the periaqueductal gray, medial and paramedial medulla in rat. Brain Res. 1986;363:99–113. doi: 10.1016/0006-8993(86)90662-1. [DOI] [PubMed] [Google Scholar]
- Jo YS, Mizumori SJ. Prefrontal regulation of neuronal activity in the ventral tegmental area. Cereb Cortex. 2015 doi: 10.1093/cercor/bhv215. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo YS, Lee J, Mizumori SJ. Effects of prefrontal cortical inactivation on neural activity in the ventral tegmental area. J Neurosci. 2013;33:8159–8171. doi: 10.1523/JNEUROSCI.0118-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyuzhny AE, Arvidsson U, Wu W, Wessendorf MW. Mu-Opioid and delta-opioid receptors are expressed in brainstem antinociceptive circuits: studies using immunocytochemistry and retrograde tract-tracing. J Neurosci. 1996;16:6490–6503. doi: 10.1523/JNEUROSCI.16-20-06490.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyd DR, Wang X, Murphy AZ. Sex differences in mu-opioid receptor expression in the rat midbrain periaqueductal gray are essential for eliciting sex differences in morphine analgesia. J Neurosci. 2008;28:14007–14017. doi: 10.1523/JNEUROSCI.4123-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MM, Heinricher MM, Fields HL. Circuitry linking opioid-sensitive nociceptive modulatory systems in periaqueductal gray and spinal cord with rostral ventromedial medulla. Neuroscience. 1992;47:863–871. doi: 10.1016/0306-4522(92)90036-2. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Whitney PK, Gold MS. Immobility and flight associated with antinociception produced by activation of the ventral and lateral/dorsal regions of the rat periaqueductal gray. Brain Res. 1998;804:159–166. doi: 10.1016/s0006-8993(98)00669-6. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Whittier KL, Hegarty DM, Aicher SA. Periaqueductal gray neurons project to spinally projecting GABAergic neurons in the rostral ventromedial medulla. Pain. 2008;140:376–386. doi: 10.1016/j.pain.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MM, Reid RA, Stormann TM, Lautermilch NJ. Opioid selective antinociception following microinjection into the periaqueductal gray of the rat. Pain. 2014;15:1102–1109. doi: 10.1016/j.jpain.2014.07.008. [DOI] [PubMed] [Google Scholar]
- Nakahama H, Shima K, Aya K, Fujii H. Peripheral somatic activation and spontaneous firing patterns of neurons in the periaqueductal gray of the cat. Neurosci Lett. 1981;25:43–46. doi: 10.1016/0304-3940(81)90098-7. [DOI] [PubMed] [Google Scholar]
- Osborne PB, Vaughan CW, Wilson HI, Christie MJ. Opioid inhibition of rat periaqueductal grey neurones with identified projections to rostral ventromedial medulla in vitro. J Physiol. 1996;490(Pt 2):383–389. doi: 10.1113/jphysiol.1996.sp021152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson SJ. stereotaxic coordinates. Sydney: Academic Press; 2005. The rat brain. [Google Scholar]
- Sanders KH, Klein CE, Mayor TE, Heym C, Handwerker HO. Differential effects of noxious and non-noxious input on neurones according to location in ventral periaqueductal grey or dorsal raphe nucleus. Brain Res. 1980;186:83–97. doi: 10.1016/0006-8993(80)90257-7. [DOI] [PubMed] [Google Scholar]
- Vaughan CW. Enhancement of opioid inhibition of GABAergic synaptic transmission by cyclo-oxygenase inhibitors in rat periaqueductal grey neurones. Brit J Pharmacol. 1998;123:1479–1481. doi: 10.1038/sj.bjp.0701818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan CW, Christie MJ. Presynaptic inhibitory action of opioids on synaptic transmission in the rat periaqueductal grey in vitro. J Physiol. 1997;498(Pt 2):463–472. doi: 10.1113/jphysiol.1997.sp021872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan CW, Ingram SL, Connor MA, Christie MJ. How opioids inhibit GABA-mediated neurotransmission. Nature. 1997;390:611–614. doi: 10.1038/37610. [DOI] [PubMed] [Google Scholar]
- Wilson-Poe AR, Morgan MM, Aicher SA, Hegarty DM. Distribution of CB1 cannabinoid receptors and their relationship with mu-opioid receptors in the rat periaqueductal gray. Neuroscience. 2012;213:191–200. doi: 10.1016/j.neuroscience.2012.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksh TL, Yeung JC, Rudy TA. Systematic examination in the rat of brain sites sensitive to the direct application of morphine: observation of differential effects within the periaqueductal gray. Brain Res. 1976;114:83–103. doi: 10.1016/0006-8993(76)91009-x. [DOI] [PubMed] [Google Scholar]
- Zambotti F, Zonta N, Parenti M, Tommasi R, Vicentini L, Conci F, Mantegazza P. Periaqueductal gray matter involvement in the muscimol-induced decrease of morphine antinociception. N-S Arch Pharmacol. 1982;318:368–369. doi: 10.1007/BF00501180. [DOI] [PubMed] [Google Scholar]


