Abstract
Objective
Rational development of technology for rapid control of non-compressible torso hemorrhage (NCTH) requires detailed understanding of what is bleeding. Our objectives were to describe the anatomic location of truncal bleeding in patients presenting with NCTH and compare endovascular (ENDO) versus open (OPEN) management.
Methods
Retrospective study of adult trauma patients with NCTH admitted to 4 urban level 1 trauma centers in the Houston and San Antonio metropolitan areas in 2008–2012. Inclusion criteria: named axial torso vessel disruption, AIS chest or abdomen ≥3 with shock (base excess <−4) or truncal operation in ≤90 minutes, or pelvic fracture with ring disruption. Exclusion criteria: isolated hip fractures, falls from standing, or prehospital CPR. After dichotomizing into OPEN, ENDO, and resuscitative thoracotomy (RT) groups based on the initial approach to control NCTH, a mixed-effects Poisson regression with robust error variance (controlling for age, mechanism, ISS, shock, hypotension, and severe head injury as fixed effects and site as a random effect) was used to test the hypothesis that ENDO was associated with reduced in-hospital mortality in NCTH patients.
Results
543 patients with NCTH underwent ENDO (n=166, 31%), OPEN (n=309, 57%), or RT (n=68, 12%). Anatomic bleeding locations were 25% chest, 41% abdomen, and 31% pelvis. ENDO was used to treat relatively few types of vascular injuries, while OPEN and RT injuries were more diverse. ENDO patients had more blunt trauma (95% vs 34% vs 32%); severe injuries (median ISS 34 vs 27 vs 21), and increased time to intervention (median 298 vs 92 vs 51 min), compared to OPEN and RT. Mortality was 15% vs 20% vs 79%. ENDO was associated with decreased mortality compared to OPEN (RR 0.58, 95% CI 0.46–0.73).
Conclusion
Although ENDO may reduce mortality in NCTH patients, significant group differences limit the generalizability of this finding.
Level of Evidence
Level V (Prognostic and Epidemiologic)
Keywords: non-compressible torso hemorrhage, angioembolization, endovascular hemorrhage control
Introduction
Hemorrhage is the leading cause of potentially preventable civilian1,2 and military3 trauma deaths. In contrast to other causes of trauma death, exsanguination occurs rapidly (median of 2–3 hours after presentation).4–8 Although great progress has been made in the rapid control of compressible hemorrhage, especially the use of tourniquets for extremity hemorrhage,9–11 initial management of non-compressible torso hemorrhage (NCTH) from thoracic, abdominal, and pelvic sources still presents a significant challenge.11,12 A retrospective review of 4,596 combat casualty mortalities from 2001–2011 in Afghanistan and Iraq found that 24% of deaths were potentially preventable and related to delayed or lack of hemorrhage control. Of the potentially preventable deaths, 67% were due to NCTH.11 An epidemiologic study of the National Trauma Data Bank found that patients admitted to civilian trauma centers with NCTH and hypotension had nearly 50% mortality.12
Until recently, expeditious open thoracotomy and/or laparotomy were required for patients in hemorrhagic shock. Endovascular interventions were reserved for patients who were stable enough to undergo diagnostic studies revealing the injured vessel. The advent of hybrid operating rooms where open and endovascular techniques can be utilized as needed has made possible the more frequent use of minimally invasive approaches for definitive control of NCTH in severely injured polytrauma patients.13 However, both precise anatomic descriptions of the source(s) of NCTH and comparisons of endovascular versus open techniques for hemorrhage control are lacking. The objective of this study was two-fold: first, to describe the precise anatomic locations of bleeding in a population of adult trauma patients with NCTH, and second, to test the hypothesis that the endovascular (ENDO) versus open (OPEN) approach was associated with reduced mortality in trauma patients presenting with NCTH.
Methods
Study design
Approval was obtained from the institutional review boards of all four study sites: the University of Texas Health Science Center at Houston (UTHealth), Baylor College of Medicine, the University of Texas Health Science Center at San Antonio, and the San Antonio Military Medical Center as well as the Human Research Protections Office of the US Army Medical Research and Materiel Command. The four study sites represent all of the adult level 1 trauma centers for the Houston and San Antonio metropolitan areas; they collectively serve a population of 5.85 million,14,15 or 2.5% of the US adult population.16
Each institutional trauma registry was queried to identify trauma patients who presented between 2008–2012 with NCTH defined as 1) named axial torso vessel disruption, 2) Abbreviated Injury Scale (AIS) chest or abdomen ≥3 with concomitant shock (base excess <−4) or emergent operation (≤90 minutes after presentation), or 3) pelvic fracture with ring disruption. Patients who had an isolated hip fracture or isolated fall from standing were excluded. Demographic information, admission vital signs and laboratory studies, AIS and Injury Severity Score (ISS), transfusion of blood products, ICU-free days, and mortality were obtained from the institutional trauma registry and/or medical record. Cause of death was obtained from the institutional trauma registry. Radiological and/or operative reports were reviewed to describe as specifically as possible the anatomic location of NCTH or the specific vessel injured. Patients were divided into three groups based on the initial approach to hemorrhage control: OPEN (without resuscitative thoracotomy), ENDO (including REBOA), and resuscitative thoracotomy (RT). RT patients were grouped separately due to the ramifications of this therapy. Patients who did not undergo one of these procedures or who had prehospital cardiopulmonary resuscitation (CPR) were excluded. Patients with documented OPEN and ENDO procedures for initial control of one or more sources of NCTH were categorized as the more invasive modality (OPEN). Study data were collected and managed at UTHealth using Research Electronic Data Capture (REDCap),17 a secure, web-based application designed to support data capture for research studies.
Statistical analysis
Statistical analysis was performed using Stata 14.1 (StataCorp LP, College Station, TX). Data are reported as median values with interquartile range (IQR) or proportions as appropriate. Categorical data were analyzed by the Chi-squared test or Fisher’s exact test for categories with ≤5 patients. Nonparametric comparisons of continuous variables were performed using the Kruskal-Wallis test. If significant, post-hoc pairwise comparisons with Holm–Bonferroni adjustments were performed using the Wilcoxon rank-sum test for continuous data and Chi-squared or Fisher’s exact test for categorical data. To test the hypothesis that ENDO was associated with decreased in-hospital mortality compared to OPEN, we used a mixed-effects Poisson regression with robust error variance, which has been described as a method to estimate relative risks for a binary outcome.18 We controlled for age, ISS, mechanism, severe head injury (AIS head ≥3), shock (base excess <−4 mEq/L), and hypotension (SBP <90 mmHg) as fixed effects and site as a random effect. Subgroup analyses were performed based on the body cavity of hemorrhage (chest, abdomen, or pelvis). Missing base excess and systolic blood pressure (SBP) data were imputed with predictive mean matching (20 imputations) using age, ISS, AIS scores, mechanism, and treatment (ENDO versus OPEN versus RT). An alpha level of 0.05 was used for all statistical tests.
Results
Demographics
During the five-year study period, 678 patients with NCTH were included. A total of 135 patients were excluded (56 with prehospital CPR, 54 who did not undergo a procedure for hemorrhage control, 15 who died before an intervention was performed, and 10 with missing procedure data), leaving 543 patients (80%) who underwent ENDO (n=166, 31%), OPEN (n=309, 57%), or RT (n=68, 12%). The number of included patients per site was 203, 171, 128, and 41 respectively. Use of ENDO significantly increased from 21% in 2008 to 41% in 2012 (p<0.01). Only one patient in the ENDO group underwent REBOA. Nine OPEN patients (3%) also underwent an endovascular procedure for initial control of NCTH.
Demographics and admission parameters are summarized in Table 1. Patients in the ENDO group were older, had higher incidence of blunt mechanism, and had higher Injury Severity Scores (ISS), as well as much longer times to intervention than OPEN or RT patients.
Table 1.
Demographics and admission parameters
| Variable | Missing | ENDO (E) (n=166, 31%) | OPEN (O) (n=309, 57%) | Resuscitative thoracotomy (RT) (n=68, 12%) | p |
|---|---|---|---|---|---|
| Age (years) | 0 (0 %) | 38 (24, 52) | 31 (23,42) | 31 (23, 44) | <0.01 |
| Male | 0 (0 %) | 126 (76%) | 255 (83%) | 56 (82%) | 0.20 |
| Blunt | 0 (0 %) | 157 (95%) | 104 (34%) | 22 (32%) | <0.001 |
| AIS head ≥3 | 0 (0 %) | 52 (31%) | 38 (12%) | 15 (22%) | <0.001 |
| AIS chest ≥3 | 0 (0 %) | 116 (70%) | 142 (46%) | 40 (59%) | <0.001 |
| AIS abdomen ≥3 | 0 (0 %) | 127 (77%) | 249 (81%) | 52 (76%) | 0.51 |
| AIS extremity ≥3 | 0 (0 %) | 95 (57%) | 68 (22%) | 29 (29%) | <0.001 |
| ISS | 0 (0 %) | 34 (25, 41) | 21 (16, 30) | 27 (24, 43) | <0.001 |
| ED SBP (mmHg) | 44 (8%) | 107 (85, 129) | 102 (82, 125) | 91 (74, 127) | 0.23 |
| ED hypotension* | 44 (8%) | 48 (30%) | 90 (32%) | 23 (43%) | 0.20 |
| ED GCS | 19 (3%) | 14 (3, 15) | 15 (8, 15) | 8 (3, 14) | <0.001 |
| ED Base excess (mEq/L) | 28 (5%) | −7 (−11, −4) | −7 (−13, −4) | −16 (−21, −12) | <0.001 |
| ED shock† | 28 (5%) | 116 (73%) | 213 (71%) | 54 (95%) | 0.001 |
| ED Hemoglobin (g/dL) | 27 (5%) | 11.9 (10.5, 13.3) | 12.0 (10.4, 13.3) | 10.9 (9.2, 12.6) | <0.01 |
| ED Platelet count (x10^9/L) | 43 (8%) | 227 (172, 289) | 216 (155, 273) | 199 (132, 264) | 0.06 |
| Time to intervention (min) | 95 (18%) | 298 (200, 683) | 92 (61, 163) | 51 (33, 89) | <0.001 |
AIS, abbreviated injury scale; ISS, injury severity score; ED, emergency department; SBP, systolic blood pressure; GCS, Glasgow coma score.
SBP <90 mmHg
base excess <−4 mEq/L
Blood product transfusions and mortality
Outcomes data are summarized in Table 2. The mortality per site was 29%, 30%, 13%, and 37% respectively (p<0.01). Transfusions were performed according to institutional practice without sharing of protocols. ENDO patients received the fewest 24-hour RBCs, while plasma and platelet transfusions were not different between groups. ENDO patients had the highest 24-hour plasma:RBC ratio. Both median 24-hour plasma:RBC (1.00 vs 0.14 vs 0.70 vs 0.63) and platelet:RBC (0.67 vs 0.00 vs 0.28 vs 0.53) varied significantly between sites (both p<0.05).
Table 2.
Outcomes
| Variable | Missing | ENDO (E) (n=166, 31%) | OPEN (O) (n=309, 57%) | Resuscitative thoracotomy (RT) (n=68, 12%) | p |
|---|---|---|---|---|---|
| 24h RBC (units) | 3 (<1%) | 4 (1, 11) | 8 (3, 17) | 14 (7, 22) | <0.001 |
| 24h Plasma (units) | 3 (<1%) | 3 (0, 10) | 4 (0, 12) | 3 (0, 12) | 0.38 |
| 24h Plt (units) | 3 (<1%) | 0 (0, 12) | 0 (0, 6) | 1 (0, 6) | 0.83 |
| 24h Plasma:RBC ratio | 3 (<1%) | 0.86 (0.25, 1.15) | 0.67 (0.20, 1.00) | 0.29 (0, 0.69) | <0.001 |
| 24h Plt:RBC ratio | 3 (<1%) | 0.50 (0, 1) | 0.14 (0, 0.75) | 0.08 (0, 0.38) | 0.20 |
| Rebleeding | 3 (<1%) | 9 (5%) | 9 (3%) | 4 (6%) | 0.16 |
| ICU-free days | 0 (0%) | 18 (4, 25) | 22 (0, 27) | 0 (0, 0) | <0.001 |
| Death | 0 (0%) | 25 (15%) | 63 (20%) | 54 (79%) | <0.001 |
| Causes of death* | 0 (0%) | ||||
| Exsanguination | 10 (40%) | 42 (67%) | 48 (89%) | <0.001 | |
| TBI | 9 (36%) | 6 (10%) | 1 (2%) | <0.001 | |
| Respiratory | 2 (8%) | 1 (2%) | 2 (4%) | 0.34 | |
| Sepsis/MOF | 8 (32%) | 11 (19%) | 3 (6%) | 0.01 | |
| MI/Stroke | 2 (8%) | 3 (5%) | 1 (2%) | 0.42 | |
| Pulmonary embolism | 1 (3%) | 0 (0%) | 0 (0%) | 0.18 | |
| Time to death (hours) | 0 (0%) | 66 (7, 185) | 4 (2, 25) | 2 (1, 6) | <0.001 |
RBC, red blood cells; Plt, platelets; ICU, intensive care unit; MOF, multiple organ failure.
not mutually exclusive
Exsanguination was significantly less frequent as a cause of death in ENDO patients (40% vs 67% vs 89%), although exsanguination was the most commonly cited cause of death for all groups. TBI and sepsis/multiple organ failure (MOF) were the second and third most common causes of death in the ENDO group and were more common compared to the OPEN and RT groups. Time to death was significantly longer in ENDO patients (median 66 vs 4 vs 2 hours). Incidence of rebleeding requiring unplanned interventions and ICU-free days between ENDO and OPEN groups were similar. The procedure performed to address rebleeding (open versus endovascular) was not available.
Description of vascular injury
Anatomic locations of bleeding are summarized in Table 3. The overall distribution of NCTH was chest 25%, abdomen 41%, pelvis 31%, and unspecified 3%. Injuries in the ENDO group were most commonly in the pelvis while injuries in the OPEN and RT groups were most commonly abdominal. In the ENDO group, two anatomic locations of bleeding – the descending thoracic aorta (n=44, 27%) and the internal iliac arteries (n=51, 31%) – accounted for over half of all ENDO interventions. Only 5 ENDO interventions (3%) were for venous bleeding. In contrast, sources of bleeding in the OPEN and RT groups were much more diverse. Many relatively common bleeding sources in the OPEN and RT groups had no representation in the ENDO group.
Table 3.
Anatomic location of vascular injury
| ENDO (E) (n=166, 31%) | OPEN (O) (n=309, 57%) | Resuscitative thoracotomy (RT) (n=68, 12%) | |
|---|---|---|---|
| CHEST | 50 (30.1%) | 66 (21.4%) | 21 (30.9%) |
| Pulmonary artery | 5 (1.6%) | 1 (1.5%) | |
| Ascending aorta | 1 (0.6%) | 7 (2.3%) | 3 (4.4%) |
| Aortic arch | 1 (0.6%) | 1 (0.3%) | 1 (1.5%) |
| Innominate artery | 2 (0.6%) | ||
| Right subclavian artery | 2 (1.2%) | 7 (2.3%) | 1 (1.5%) |
| Intra-thoracic right common carotid artery | 2 (0.6%) | ||
| Intra-thoracic left common carotid artery | 3 (1%) | ||
| Left subclavian artery | 2 (1.2%) | 4 (1.3%) | 1 (1.5%) |
| Intercostal/Internal thoracic arteries | 17 (5.5%) | ||
| Descending thoracic aorta | 44 (26.5%) | 8 (2.6%) | 10 (14.7%) |
| Pulmonary vein | 2 (0.6%) | 1 (1.5%) | |
| Superior vena cava | 1 (0.3%) | 2 (2.9%) | |
| Innominate vein | 3 (1%) | ||
| Subclavian vein | 4 (1.3%) | 1 (1.5%) | |
| ABDOMEN | 37 (22.3%) | 154 (49.8%) | 34 (50.0%) |
| Abdominal aorta | 6 (1.9%) | 4 (5.9%) | |
| Visceral abdominal aorta | 1 (0.3%) | 1 (1.5%) | |
| Celiac artery | 2 (0.6%) | ||
| Common hepatic artery | 7 (4.2%) | 10 (3.2%) | 1 (1.5%) |
| L hepatic artery | 2 (1.2%) | 1 (0.3%) | |
| R hepatic artery | 4 (2.4%) | 1 (0.3%) | |
| Splenic artery | 6 (3.6%) | 4 (1.3%) | 1 (1.5%) |
| Left gastric artery | 1 (0.6%) | 3 (1%) | |
| Gastroepiploic artery | 3 (1%) | ||
| Superior mesenteric artery | 20 (6.5%) | 1 (1.5%) | |
| Ileocolic artery | 1 (0.3%) | ||
| Inferior mesenteric artery | 2 (0.6%) | 1 (1.5%) | |
| Other abdominal visceral artery | 13 (4.2%) | 1 (1.5%) | |
| Renal artery | 14 (8.4%) | 8 (2.6%) | 2 (2.9%) |
| Infra-renal aorta | 1 (0.6%) | 4 (1.3%) | 1 (1.5%) |
| Suprarenal/Subhepatic inferior vena cava | 16 (5.2%) | 6 (8.8%) | |
| Retro-hepatic inferior vena cava | 8 (2.6%) | 2 (2.9%) | |
| Portal vein | 1 (0.6%) | 5 (1.6%) | 2 (2.9%) |
| Splenic vein | 9 (2.9%) | 1 (1.5%) | |
| Superior mesenteric vein | 11 (3.6%) | 1 (1.5%) | |
| Renal vein | 5 (1.6%) | 1 (1.5%) | |
| Gonadal vein | 1 (0.6%) | 4 (1.3%) | |
| Infra-renal inferior vena cava | 14 (4.5%) | 6 (8.8%) | |
| Inferior vena cava, unspecified | 3 (1%) | 2 (2.9%) | |
| PELVIS | 76 (45.8%) | 81 (26.2%) | 10 (14.7%) |
| Common iliac artery | 1 (0.6%) | 7 (2.3%) | 3 (4.4%) |
| External iliac artery | 4 (2.4%) | 12 (3.9%) | 2 (2.9%) |
| Internal iliac artery | 51 (30.7%) | 17 (5.5%) | 5 (7.4%) |
| Internal iliac artery branches | 17 (10.2%) | 13 (4.2%) | |
| Common iliac vein | 18 (5.8%) | ||
| External iliac vein | 11 (3.6%) | ||
| Internal iliac vein | 3 (1.8%) | 3 (1%) | |
| Unknown | 3 (1.8%) | 8 (2.6%) | 3 (4.4%) |
Multivariable analysis
Predictive mean matching with age, ISS, AIS scores, mechanism, and treatment were used to impute missing SBP and base excess values for 65 patients (12%). After imputation, the distribution of SBP and base excess values were unchanged. We constructed a mixed-effects Poisson regression with robust error variance controlling for age, ISS, mechanism, severe head injury (AIS head ≥3), shock (base excess <−4 mEq/L), and hypotension (SBP <90 mmHg) as fixed effects and site as a random effect and found that ENDO was associated with reduced in-hospital mortality compared to OPEN (relative risk [RR] 0.58, 95% confidence interval [CI] 0.46–0.73), while RT was associated with increased mortality compared to OPEN (RR 3.00, 95% CI 1.84–4.90). We found similar results in a model analyzing only patients with non-missing SBP and base excess data and in a model where OPEN patients who underwent a concurrent ENDO procedure (n=9, 3%) were moved to the ENDO group (Supplement).
Because a large proportion of ENDO interventions were performed after blunt thoracic aortic injury (BTAI), possibly for low-grade injuries, we performed a sensitivity analysis using the above covariates and imputed values after excluding patients with BTAI. In this cohort (n=488) ENDO was associated with reduced mortality compared to OPEN (RR 0.67, 95% CI 0.54–0.83).
ENDO vs OPEN in the chest vs abdomen vs pelvis
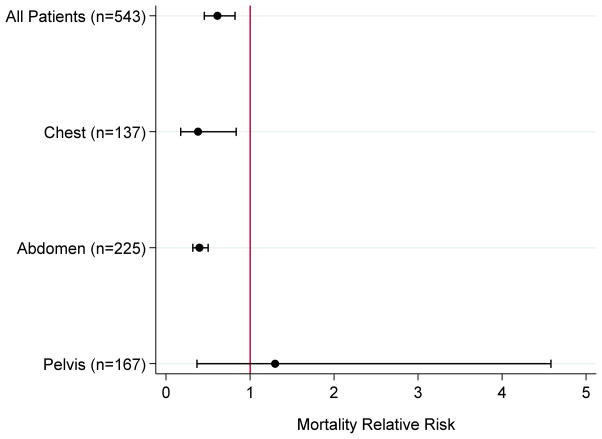
Exploratory analyses of bleeding for each body cavity (chest, abdomen, or pelvis) were performed (Figure 1). Using the same covariates as above, use of ENDO was associated with reduced mortality in the chest (n=137; RR 0.31, 95% CI 0.15–0.64) and abdomen (n=225; RR 0.38, 95% CI 0.34–0.44), but not pelvis (n=167; RR 1.10, 95% CI 0.32–3.79).
Figure 1.
Relative risk of mortality in ENDO versus OPEN groups for all included patients and each anatomic region of bleeding.
Diagnostic studies were performed for all multivariable models (Supplement). In terms of global goodness-of-fit, Chi-squared tests of the deviance statistic were not significant (p>0.05). Visual inspection of deviance residuals versus predicted values revealed no outliers, and all residuals were within two standard deviations of the mean. Due to the dichotomous outcome, the residuals were not expected to be normally distributed. Instead, we used our model to generate simulated data; visual comparison of actual versus simulated residuals did not reveal large deviations.
Discussion
We performed a retrospective study of adult patients with NCTH presenting to four level 1 trauma centers from 2008 to 2012, which collectively represent all of the adult level 1 centers in the Houston and San Antonio metropolitan areas. The most common cause of death of all patients in this study was exsanguination (70%): median time to exsanguination was 2 hours (IQR 1 to 5 hours), consistent with other studies of hemorrhaging trauma patients,4–8 and a testament to the time criticality of hemorrhage control. Using mixed-effects P, we found that ENDO was significantly associated with decreased mortality compared to OPEN in all patients. However, there were significant pre-intervention disparities between ENDO and OPEN patients. Although ENDO patients had higher ISS, OPEN patients had significantly decreased time to intervention, increased 24-hour RBC transfusions despite shorter time to death, and increased incidence of exsanguination, all consistent with the notion that OPEN patients had substantial on-going hemorrhage at admission, while ENDO patients – although in shock – were stable enough to undergo an endovascular procedure. There were no differences in 24-hour plasma or platelet transfusions, but this may have been due to survivor bias.
Given the ubiquity of endovascular therapy in the elective treatment of vascular disease and its successful use in vascular emergencies such as ruptured abdominal aortic aneurysm,19 definitive endovascular treatment of traumatic hemorrhage is becoming increasingly viable.20 One of the first areas where endovascular treatment was identified as potentially advantageous was bleeding from pelvic fractures.21,22 Guidelines from the Eastern Association for the Surgery of Trauma (EAST), published in 2011, recommend pursuing endovascular management for any hemodynamically unstable patient with hemorrhage related to pelvic fractures without significant bleeding from another source (Level I recommendation), while pre-peritoneal packing is recommended as salvage therapy after failure of endovascular therapy (Level III recommendation).23 On the other hand, a more recent guideline from the Western Trauma Association (WTA), published in 2016, describes several “complimentary, and not mutually exclusive, options” including pelvic stabilization, pre-peritoneal packing, REBOA, and endovascular therapy,24 without clear superiority of any one strategy. A recent multicenter prospective observational study of 1,339 patients with pelvic fracture found that angioembolization and external pelvic fixation were the most common hemorrhage control techniques for arterial and venous bleeding respectively.25 Whereas the subgroup with hemorrhagic shock had 32% mortality, a recent single-institution observational study by Burlew et al found that pre-peritoneal packing as first-line therapy for 128 patients with hemorrhagic shock from unstable pelvic fractures was associated with mortality of only 21%,26 challenging the notion that pre-peritoneal packing should be relegated to salvage therapy. In the present study, mortality in patients with hemorrhagic shock from pelvic hemorrhage was 27%. We found that use of ENDO for pelvic hemorrhage was not associated with improved odds of survival on within-group multiple logistic regression. This was likely because ENDO patients with the most significant hemorrhage were bleeding from the pelvis, consistent with EAST guidelines. Among all 166 ENDO patients, 7 patients (4%) exsanguinated within 6 hours of admission, and 6 of these had bleeding from the pelvis.
In the chest and abdomen, current guidelines recommend that endovascular therapy should be reserved for hemodynamically stable patients, while unstable patients should undergo open hemorrhage control.27–32 Specifically, current data suggest that delayed repair of low-grade (1 or 2) blunt thoracic aortic injuries (BTAI) is safe, and endovascular therapy is recommended as first-line treatment by 2015 EAST guidelines.27 Delayed repair of BTAI is justified in the polytrauma patient where more immediate life-saving interventions (e.g. emergent laparotomy or craniotomy) are prioritized, and the aortic injury is temporized by medical blood pressure control prior to definitive repair.27 However, patients with high-grade (3 or 4) blunt aortic injuries should not be considered for delayed repair.28 Current EAST and WTA guidelines recommend endovascular intervention for blunt hepatic29,30 and splenic31,32 injury only in hemodynamically stable patients. In the present study, ENDO was associated with reduced mortality in the chest and abdomen.
We found that <1% of venous injuries were treated by ENDO technique. Disruption of larger and more centrally located veins is required for significant venous hemorrhage due to the lower pressure in the venous system. Patients with injury of large veins often present in profound hemorrhagic shock and are not stable enough to undergo endovascular intervention. At the same time, the endovascular treatment of major venous disruption such as caval33 or portal venous injury34 is not well described.
One impetus for this study is to generate data to inform the rational development of prehospital NCTH control devices or the prehospital implementation of existing devices (e.g. REBOA). Such devices could save many lives in both the civilian and military sectors in much the same way as the extremity tourniquet,35 especially if applied early36 and before the onset of shock.9–11 However, the danger is that any device which occludes or tamponades blood flow may exacerbate hemorrhage proximal to the occlusion. Therefore, it is essential to establish the anatomic source of NCTH (or at least rule out proximal hemorrhage) if such a device is to be used safely. For example, Hurley et al performed a retrospective review of 402 patients who underwent emergent laparotomy at a single level 1 trauma center, and found that 9% of patients would have benefited from prehospital application of the abdominal aortic junctional tourniquet (AAJT), as the bleeding source was distal to the aortic bifurcation.37
The primary limitation of this study was confounding by indication bias. ENDO interventions were performed almost exclusively for arterial bleeding and for predominantly few anatomic locations. As a whole, ENDO patients had significantly longer times to intervention, longer times to death, and decreased incidence of exsanguination – all suggesting that ENDO patients were generally hemodynamically stable enough for an endovascular procedure while OPEN patients more often had substantial ongoing hemorrhage at time of admission. Exceptions included patients with substantial pelvic hemorrhage who underwent ENDO. Indicators of response to initial resuscitation – such as number of blood products transfused within the first few hours after admission, presence of computed tomography (CT) scan prior to definitive hemorrhage control, and perioperative vital signs or laboratory data – could have been used to stratify patients by degree of ongoing hemorrhage, but were unfortunately not available for this retrospective study. The ENDO group likely included patients with low-grade injury who were at low risk for hemorrhage, but we lacked data regarding the grade of blunt arterial injury to exclude these patients. Another limitation is that only 1 patient underwent REBOA during this time period. The use of REBOA is increasing, is safe in high volume-centers,38 and is at least as effective as resuscitative thoracotomy in achieving aortic occlusion without the morbidity of a highly invasive procedure.39 It is unclear what impact REBOA has in the decision-making of endovascular versus open control of NCTH, and this should be investigated in future studies. It is also unknown how many OPEN patients underwent exploration primarily for identification and repair of non-vascular injuries (e.g. bowel injuries) with incidental intraoperative control of hemorrhage. However, there were likely few such patients, given that OPEN patients had evidence of increased on-going hemorrhage as described above. Additionally, we observed a strong association between ENDO and decreased mortality, whereas a significant number of OPEN patients who were at low risk for exsanguination would have diluted this association. Although we sought to identify all adult patients with significant NCTH who survived to hospital presentation, this study does not include those successfully managed at a non-level 1 facility, or those who presented to a non-level 1 facility and died prior to transfer. Comprehensive autopsy studies will be required to definitively address the magnitude of this problem.
In conclusion, use of endovascular techniques for non-elective indications is increasing. In this study of patients with NCTH, endovascular treatment was used predominantly after blunt mechanism, in a much more delayed fashion, and for a narrower range of anatomic injuries compared to open hemorrhage control. Its use seems consistent with current guidelines. Although endovascular treatment in this study was significantly associated with decreased mortality in all patients, significant between-group differences limit the generalizability of this finding.
Supplementary Material
Acknowledgments
Funding: The Non-Compressible Torso Hemorrhage (NCTH) study was sponsored by the U.S. Department of Defense (W81XWH-14-1-0112). REDCap is supported by the National Center for Advancing Translational Sciences (grant number UL1TR000445). RC is supported by a T32 fellowship from National Institute of General Medical Sciences (grant number 5T32GM008792).
Non-Compressible Torso Hemorrhage (NCTH) Study Group:
Clinical Coordinating Center: John B. Holcomb, MD; Charles E. Wade, PhD; Erin E. Fox, PhD; Jeanette M. Podbielski, RN; Jeffrey S. Tomasek, MD; Ronald Chang, MD.
Data Coordinating Center: Stacia M. DeSantis, PhD; Thomas J. Greene, MPH.
Delphi Meeting Attendees: Charlton-Ouw, MD; O. Clark West, MD ; Martin A. Schreiber, MD; Lena Napolitano, MD; Frederic Pieracci, MD; Charles Fox, MD; Steven Shackelford, MD; Matthew Martin, MD; Joseph J. DuBose, MD; Todd Rasmussen, MD; Megan Brenner, MD; Laura L. Moore, MD; C Rodriquez, MD.
NCTH Clinical Sites (listed in order of number of patients enrolled):
McGovern Medical School (a part of the University of Texas Health Science Center at Houston): Garrett Jost, Gerald R. Fortuna, Jr., MD.
University of Texas Health Science Center at San Antonio: Brian J. Eastridge, MD; Rachelle Babbitt-Jonas; Jessica Raley.
Baylor College of Medicine: Ramyar Gilani, MD; Samuel R. Todd, MD; Reginya Knight.
San Antonio Military Medical Center and US Army Institute of Surgical Research: Valerie G. Sams, MD; Kevin K. Chung, MD; Sonya Charo-Griego, RN.
Footnotes
Conflict of Interest: J.B.H is the co-inventor of the Junctional Emergency Treatment Tool (JETT). All other authors report no conflicts of interest.
Disclaimer: The opinions or conclusions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of any sponsor.
This study was presented at the 30th EAST Annual Assembly on 1/13/17.
Author Contributions:
Study inception and design by E.E.F., B.J.E., R.G., K.K.C., J.M.P., C.E.W., and J.B.H. Data acquisition by E.E.F., B.J.E., R.G., K.K.C., S.M.D., J.J.D., J.S.T., G.R.F., V.G.S., S.R.T., and J.M.P. Data analysis by R.C., E.E.F., T.J.G., and S.M.D. All authors contributed to manuscript preparation.
References
- 1.Sauaia A, Moore FA, Moore EE, Moser KS, Brennan R, Read RA, Pons PT. Epidemiology of trauma deaths: a reassessment. J Trauma. 1995;38(2):185–93. doi: 10.1097/00005373-199502000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Tien HC, Spencer F, Tremblay LN, Rizoli SB, Brenneman FD. Preventable deaths from hemorrhage at a level I Canadian trauma center. J Trauma. 2007;62(1):142–6. doi: 10.1097/01.ta.0000251558.38388.47. [DOI] [PubMed] [Google Scholar]
- 3.Kotwal RS, Montgomery HR, Kotwal BM, Champion HR, Butler FK, Jr, Mabry RL, Cain JS, Blackbourne LH, Mechler KK, Holcomb JB. Eliminating Preventable Death on the Battlefield. Arch Surg. 2011;146(12):1350–8. doi: 10.1001/archsurg.2011.213. [DOI] [PubMed] [Google Scholar]
- 4.Holcomb JB, del Junco DJ, Fox EE, Wade CE, Cohen MJ, Schreiber MA, Alarcon LH, Bai Y, Brasel KJ, Bulger EM, et al. PROMMTT Study Group. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: comparative effectiveness of a time-varying treatment with competing risks. JAMA Surg. 2013;148:127–36. doi: 10.1001/2013.jamasurg.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, del Junco DJ, Brasel KJ, Bulger EM, Callcut RA, et al. PROPPR study group. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313:471–82. doi: 10.1001/jama.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oyeniyi BT, Fox EE, Scerbo M, Tomasek JS, Wade CE, Holcomb JB. Trends in 1029 trauma deaths at a level 1 trauma center: Impact of a bleeding control bundle of care. Injury. 2017;48(1):5–12. doi: 10.1016/j.injury.2016.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fox EE, Holcomb JB, Wade CE, Bulger EM, Tilley BC PROPPR Study Group. Earlier Endpoints Are Required for Hemorrhagic Shock Trials among Severely Injured Patients. Shock. doi: 10.1097/SHK.0000000000000788. Epub 2017 Feb 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tisherman SA, Schmicker RH, Brasel KJ, Bulger EM, Kerby JD, Minei JP, Powell JL, Reiff DA, Rizoli SB, Schreiber MA. Detailed description of all deaths in both the shock and traumatic brain injury hypertonic saline trials of the Resuscitation Outcomes Consortium. Ann Surg. 2015;261(3):586–90. doi: 10.1097/SLA.0000000000000837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kragh JF, Jr, Littrel ML, Jones JA, Walters TJ, Baer DG, Wade CE, Holcomb JB. Battle casualty survival with emergency tourniquet use to stop limb bleeding. J Emerg Med. 2011;41(6):590–7. doi: 10.1016/j.jemermed.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 10.Kragh JF, Walters TJ, Baer DG, Fox CJ, Wade CE, Salinas J, Holcomb JB. Survival with emergency tourniquet use to stop bleeding in major limb trauma. Ann Surg. 2009;249(1):1–7. doi: 10.1097/SLA.0b013e31818842ba. [DOI] [PubMed] [Google Scholar]
- 11.Eastridge BJ, Mabry RL, Seguin P, Cantrell J, Tops T, Uribe P, Mallett O, Zubko T, Oetjen-Gerdes L, Rasmussen TE, et al. Death on the battlefield (2001–2011): implications for the future of combat casualty care. J Trauma Acute Care Surg. 2012;73(6 Suppl 5):S431–7. doi: 10.1097/TA.0b013e3182755dcc. [DOI] [PubMed] [Google Scholar]
- 12.Kisat M, Morrison JJ, Hashmi ZG, Efron DT, Rasmussen TE, Haider AH. Epidemiology and outcomes of non-compressible torso hemorrhage. J Surg Res. 2013;184(1):414–21. doi: 10.1016/j.jss.2013.05.099. [DOI] [PubMed] [Google Scholar]
- 13.Holcomb JB, Fox EE, Scalea TM, Napolitano LM, Albarado R, Gill B, Dunkin BJ, Kirkpatrick AW, Cotton BA, Inaba K, et al. Catheter-Based Hemorrhage Control Study Group. Current opinion on catheter-based hemorrhage control in trauma patients. J Trauma Acute Care Surg. 2014;76(3):888–93. doi: 10.1097/TA.0000000000000133. [DOI] [PubMed] [Google Scholar]
- 14.United States Census Bureau. Census 2010 P.L. 94-171 Profile -- Houston-Sugar Land-Baytown, TX Metro Area (US0001977) Washington DC: ProximityOne; 2011. [Accessed 8 November 2016]. http://proximityone.com/cen2010/pl/plUS0001977.htm. [Google Scholar]
- 15.United States Census Bureau. Census 2010 P.L. 94-171 Profile -- San Antonio-New Braunfels, TX Metro Area (US0002360) Washington DC: ProximityOne; 2011. [Accessed 8 November 2016]. http://proximityone.com/cen2010/pl/plUS0002360.htm. [Google Scholar]
- 16.United States Census Bureau. Age and Sex Composition: 2010. Washington DC: Department of Commerce; 2011. [Accessed 8 November 2016]. http://www.census.gov/prod/cen2010/briefs/c2010br-03.pdf. [Google Scholar]
- 17.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yelland LN, Salter AB, Ryan P. Performance of the modified Poisson regression approach for estimating relative risks from clustered prospective data. Am J Epidemiol. 2011;174(8):984–92. doi: 10.1093/aje/kwr183. [DOI] [PubMed] [Google Scholar]
- 19.Ullery BW, Tran K, Chandra V, Mell MW, Harris EJ, Dalman RL, Lee JT. Association of an Endovascular-First Protocol for Ruptured Abdominal Aortic Aneurysms With Survival and Discharge Disposition. JAMA Surg. 2015;150(11):1058–65. doi: 10.1001/jamasurg.2015.1861. [DOI] [PubMed] [Google Scholar]
- 20.Reuben BC, Whitten MG, Sarfati M, Kraiss LW. Increasing use of endovascular therapy in acute arterial injuries: Analysis of the National Trauma Data Bank. J Vasc Surg. 2007;46(6):1222–6. doi: 10.1016/j.jvs.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 21.Agolini SF, Shah K, Jaffe J, Newcomb J, Rhodes M, Reed JF., 3rd Arterial embolization is a rapid and effective technique for controlling pelvic fracture hemorrhage. J Trauma. 1997 Sep;43(3):395–9. doi: 10.1097/00005373-199709000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Panetta T, Sclafani SJ, Goldstein AS, Phillips TF, Shaftan GW. Percutaneous transcatheter embolization for massive bleeding from pelvic fractures. J Trauma. 198;25(11):1021–9. [PubMed] [Google Scholar]
- 23.Cullinane DC, Schiller HJ, Zielinski MD, Bilaniuk JW, Collier BR, Como J, Holevar M, Sabater EA, Sems SA, Vassy WM, Wynne JL. Eastern Association for the Surgery of Trauma practice management guidelines for hemorrhage in pelvic fracture--update and systematic review. J Trauma. 2011;71(6):1850–68. doi: 10.1097/TA.0b013e31823dca9a. [DOI] [PubMed] [Google Scholar]
- 24.Tran TL, Brasel KJ, Karmy-Jones R, Rowell S, Schreiber MA, Shatz DV, Albrecht RM, Cohen MJ, DeMoya MA, Biffl WL, et al. Western Trauma Association Critical Decisions in Trauma: Management of pelvic fracture with hemodynamic instability-2016 updates. J Trauma Acute Care Surg. 2016;81(6):1171–4. doi: 10.1097/TA.0000000000001230. [DOI] [PubMed] [Google Scholar]
- 25.Costantini TW, Coimbra R, Holcomb JB, Podbielski JM, Catalano R, Blackburn A, Scalea TM, Stein DM, Williams L, Conflitti J, et al. Current management of hemorrhage from severe pelvic fractures: Results of an American Association for the Surgery of Trauma multi-institutional trial. J Trauma Acute Care Surg. 2016;80(5):717–23. doi: 10.1097/TA.0000000000001034. [DOI] [PubMed] [Google Scholar]
- 26.Burlew CC, Moore EE, Stahel PF, Geddes AE, Wagenaar AE, Pieracci FM, Fox CJ, Campion EM, Johnson JL, Mauffrey C. Preperitoneal pelvic packing reduces mortality in patients with life-threatening hemorrhage due to unstable pelvic fractures. J Trauma Acute Care Surg. 2017;82(2):233–242. doi: 10.1097/TA.0000000000001324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fox N, Schwartz D, Salazar JH, Haut ER, Dahm P, Black JH, Brakenridge SC, Como JJ, Hendershot K, King DR, et al. Evaluation and management of blunt traumatic aortic injury: A practice management guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg. 2015;78(1):136–46. doi: 10.1097/TA.0000000000000470. [DOI] [PubMed] [Google Scholar]
- 28.Dubose JJ, Azizzadeh A, Estrera AL, Safi HJ. Contemporary management of blunt aortic trauma. J Cardiovasc Surg. 2015;56(5):751–62. [PubMed] [Google Scholar]
- 29.Stassen NA, Bhullar I, Cheng JD, Crandall M, Friese R, Guillamondegui O, Jawa R, Maung A, Rohs TJ, Jr, Sangosanya A, et al. Nonoperative management of blunt hepatic injury: An Eastern Association for the Surgery of Trauma practice management guideline. J Trauma Acute Care Surg. 2012;73(5):S288–93. doi: 10.1097/TA.0b013e318270160d. [DOI] [PubMed] [Google Scholar]
- 30.Kozar RA, Feliciano DV, Moore EE, Moore FA, Cocanour CS, West MA, Davis JW, McIntyre RC., Jr Western Trauma Association critical decisions in trauma: nonoperative management of adult blunt hepatic trauma. J Trauma. 2009;67(6):1144–8. doi: 10.1097/TA.0b013e3181ba361f. [DOI] [PubMed] [Google Scholar]
- 31.Stassen NA, Bhullar I, Cheng JD, Crandall M, Friese R, Guillamondegui O, Jawa R, Maung A, Rohs TJ, Jr, Sangosanya A, et al. Selective nonoperative management of blunt splenic injury: An Eastern Association for the Surgery of Trauma practice management guideline. J Trauma Acute Care Surg. 2012;73(5):S294–S300. doi: 10.1097/TA.0b013e3182702afc. [DOI] [PubMed] [Google Scholar]
- 32.Moore FA, Davis JW, Moore EE, Jr, Cocanour CS, West MA, McIntyre RC., Jr Western Trauma Association (WTA) critical decisions in trauma: management of adult blunt splenic trauma. J Trauma. 2008;65(5):1007–11. doi: 10.1097/TA.0b013e31818a93bf. [DOI] [PubMed] [Google Scholar]
- 33.Castelli P, Caronno R, Piffaretti G, Tozzi M. Emergency endovascular repair for traumatic injury of the inferior vena cava. Eur J Cardiothorac Surg. 2005;28(6):906–8. doi: 10.1016/j.ejcts.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 34.Sundarakumar DK, Smith CM, Lopera JE, Kogut M, Suri R. Endovascular interventions for traumatic portal venous hemorrhage complicated by portal hypertension. World J Radiol. 2013;5(10):381–5. doi: 10.4329/wjr.v5.i10.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ode G, Studnek J, Seymour R, Bosse MJ, Hsu JR. Emergency tourniquets for civilians: Can military lessons in extremity hemorrhage be translated? J Trauma Acute Care Surg. 2015;79(4):586–591. doi: 10.1097/TA.0000000000000815. [DOI] [PubMed] [Google Scholar]
- 36.Scerbo MH, Holcomb JB, Gates K, Mumm J, Wade CE, Love JD, Cotton BA. The trauma center is too late: Severe extremity injuries without a pre-hospital tourniquet have increased death from hemorrhage [abstract]. Proceedings of the 75th Annual Meeting of the American Association for the Surgery of Trauma; 2016 September 14–17; Waikoloa Village, HI. Chicago: AAST; 2016. Poster nr 49. [Google Scholar]
- 37.Hurley MJ, Holcomb JB. Three potential methods for prehospital treatment of abdominal hemorrhage [abstract]. Proceedings of the 11th Annual Meeting of the Academic Surgical Congress; 2016 February 2–4; Las Vegas, NV. Chicago: Association for Academic Surgery; 2016. Quickshot nr 76.01. [Google Scholar]
- 38.Moore LJ, Brenner M, Kozar RA, Pasley J, Wade CE, Baraniuk MS, Scalea T, Holcomb JB. Implementation of resuscitative endovascular balloon occlusion of the aorta as an alternative to resuscitative thoracotomy for noncompressible truncal hemorrhage. J Trauma Acute Care Surg. 2015;79(4):523–30. doi: 10.1097/TA.0000000000000809. [DOI] [PubMed] [Google Scholar]
- 39.DuBose JJ, Scalea TM, Brenner M, Skiada D, Inaba K, Cannon J, Moore L, Holcomb J, Turay D, Arbabi CN, et al. the AAST AORTA Study Group. The AAST prospective Aortic Occlusion for Resuscitation in Trauma and Acute Care Surgery (AORTA) registry: Data on contemporary utilization and outcomes of aortic occlusion and resuscitative balloon occlusion of the aorta (REBOA) J Trauma Acute Care Surg. 2016;81(3):409–19. doi: 10.1097/TA.0000000000001079. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.