SUMMARY
Thiazolidinediones (TZDs) are PPARγ activators that exhibit vasculoprotective properties. To determine the vascular function of PPARγ, we analyzed Tie2Cre/flox and SM22Cre/flox mice. Unexpectedly, both knockout strains exhibited a significant reduction of circadian variations in blood pressure and heart rate in parallel with diminished variations in urinary norepinephrine/epinephrine excretion and impaired rhythmicity of the canonical clock genes including Bmal1. PPARγ expression in the aorta exhibited a robust rhythmicity with a more than 20-fold change during the light/dark cycle. Rosiglitazone treatment induced aortic expression of Bmal1 mRNA, and ChIP and promoter assays revealed that Bmal1 is a direct PPARγ target gene. These studies have uncovered a role for vascular PPARγ as a peripheral factor participating in regulation of cardiovascular rhythms.
Keywords: PPARγ, roziglitazone, Cre-loxP, endothelial cells, smooth muscle cells, blood pressure
INTRODUCTION
The internal molecular clock drives circadian rhythms of physiology and behavior in most organisms in order to adapt to a changing environment. Over the past decade, much progress has been made in understanding the molecular basis of circadian rhythms. The master regulator of circadian rhythms is thought to reside in the suprachiasmatic nucleus (SCN), a group of neurons expressing an autoregulatory transcription–translation-based feedback loop. In the simplest form, the heterodimers of the basic helix-loop-helix Per Arnt Sim transcription factors, CLOCK and Bmal1, drive the rhythmic expression of three period genes (mPer1-3) and two Cryptochrome genes (mCry1 and mCry2) through E-box enhancer elements (Reppert and Weaver, 2001; Reppert and Weaver, 2002; Young and Kay, 2001). As the mPer and mCry proteins are translated, they form multimeric complexes that are translocated to the nucleus. In the nucleus, mCry proteins directly interact with CLOCK and/or Bmal1 to inhibit transcription, resulting in the formation of a negative feedback loop (Kume et al., 1999). Emerging evidence suggests that molecular “clocks” also exist in peripheral tissues, and that they operate in the same way as in SCN neurons (Balsalobre et al., 1998; McNamara et al., 2001; Oishi et al., 1998a; Oishi et al., 1998b). Various components of the clock system have been identified in liver, kidney, heart, and blood vessels (Zylka et al., 1998) and even in immortalized rat fibroblast cells that have been kept in culture for more than 25 years (Balsalobre et al., 1998). Approximately 8–10% of the total number of genes expressed in mouse heart and liver exhibit a circadian expression pattern (Storch et al., 2002). However, the majority of these studies have been descriptive in nature. To address the circadian function in individual tissues, it is essential to use a tissue-specific approach such as the Cre-loxP recombination system. This conclusion is reflected in the recent generation of CLOCK floxed mice which offer a unique tool to study molecular clock functions in a tissue-specific manner (Debruyne et al., 2006). The mice were initially used to produce whole-body knockouts of CLOCK which unexpectedly exhibited normal circadian phenotypes (Debruyne et al., 2006) challenging the central role of CLOCK:Bmal1 heterodimers in clock function.
Circadian variations in blood pressure (BP) and heart rate (HR) are among the best recognized circadian rhythms of physiology. In humans, there is a sharp rise in BP before awakening with highest values around mid morning. Many cardiovascular events such as sudden cardiac death, myocardial infarction and stroke display diurnal variations with an increased incidence in the early morning hours which may correlate with the morning surge in BP (Muller, 1999a; Muller, 1999b). On the other hand, the reduction of the decline in nocturnal BP has been suggested to be a strong predictor of cardiovascular events and end-organ damage in both hypertensive patients and in the normotensive population (Ohkubo et al., 2002). As in many other peripheral tissues, the circadian expression of clock genes was well demonstrated in the cardiovascular system (Durgan et al., 2005; Maemura et al., 2000; Nonaka et al., 2001; Portman, 2001; Rudic et al., 2005; Takeda et al., 2007; Young and Kay, 2001), but the biological significance of this phenomenon remains unclear.
Peroxisome proliferator-activated receptor-γ (PPARγ) is a member of the superfamily of nuclear receptor ligand-activated transcription factors and is best known for serving as a molecular target for TZDs including rosiglitazone (RGZ) and pioglitazone that are widely prescribed and highly effective for treatment of type 2 diabetes. Compelling evidence from both pharmacological and genetic studies has established a pivotal role of this nuclear receptor in the control of glucose and lipid metabolism (Berger et al., 2005; Evans et al., 2004). Apart from their metabolic activity, TZDs exert vasculoprotective effects through poorly characterized mechanisms. The present study describes the use of conditional knockout mice to determine the circadian function of vascular PPARγ.
RESULTS
Validation of conditional deletion of PPARγ
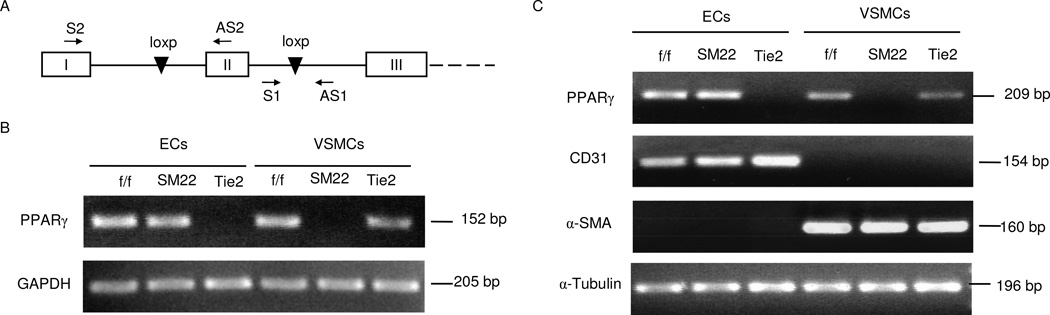
Both Tie2Cre/flox and SM22Cre/flox mice were born at the expected Mendelian ratio and neither mutant had gross morphological abnormalities in adults (Supplemental Table 1). Of note, sporadic alopecia was found in young but not adult Tie2Cre/flox mice. DNA recombination and mRNA expression analyses of the PPARγ gene were evaluated in freshly isolated ECs and vascular smooth muscle cells (VSMCs) from both KO strains together with their floxed controls. Primers S1 and AS1 flank the loxP site and amplify a product of 152 bp from the floxed allele but not the recombined allele (Fig. 1A). Primers S2 and AS2 are located in separate exons and approximately 10 kb apart and the resulting product of 209 bp is derived from PPARγ mRNA but not genomic DNA. Isolation of ECs and VSMCs was based on controlled digestion with collagenase. The SMC marker α-SMA was detected in VSMCs but not ECs. Conversely, the EC marker CD31 was observed in ECs but not VSMCs (Fig. 1B and C), thus confirming the purity of our preparations. Using PCR we verified that the PPARγ floxed allele was deleted in ECs but not VSMCs of Tie2Cre/flox mice and in VSMCs but not ECs of SM22Cre/flox mice (Fig. 1B). RT-PCR analyses established that a similar pattern of PPARγ mRNA expression existed in the two KO strains as that described earlier for DNA recombination (Fig. 1C). Real time-PCR indicated that the aorta from SM22Cre/flox mice and the vena cava from Tie2Cre/flox mice exhibited the greatest reduction of the floxed allele (Fig. S1). These findings likely are due to the large smooth muscle and endothelial compartments in arteries and veins, respectively. Echocardiographic (Supplemental Table 2) and hematoxylin and eosin (HE) analyses (data not shown) indicate the SM22 promoter did not precipitate any cardiac abnormalities in SM22Cre/flox mice. Lastly, because plasma glucose, cholesterol, and triglycerides (Supplemental Table 1), and glucose tolerance (Fig. S2) were similar among Tie2Cre/flox and SM22Cre/flox mice and their respective controls, we believe PPARγ signaling was intact in metabolic tissues such as liver, fat, and skeletal muscles.
Fig. 1.
Validation of PPARγ deletion in the vascular cells. (A) Schematic illustration of primer design for detection of the PPARγ foxed allele (S1 and AS1) and PPARγ mRNA expression (S2 and AS2). (B) PCR analysis of the PPARγ floxed allele in ECs and VSMCs freshly isolated from PPARγf/f (f/f), SM22Cre/flox (SM22), Tie2Cre/flox (Tie2) mice using primers S1 and AS1. Amplification of GAPDH gene served as a loading control. C, RT-PCR analysis of PPARγ mRNA in ECs and VSMCs from the three strains of mice. CD31 and α-SMA are markers of EC and SMC, respectively. α-Tubulin was used as a loading control. Shown are representatives of 2~3 experiments.
Characterization of the circadian phenotype
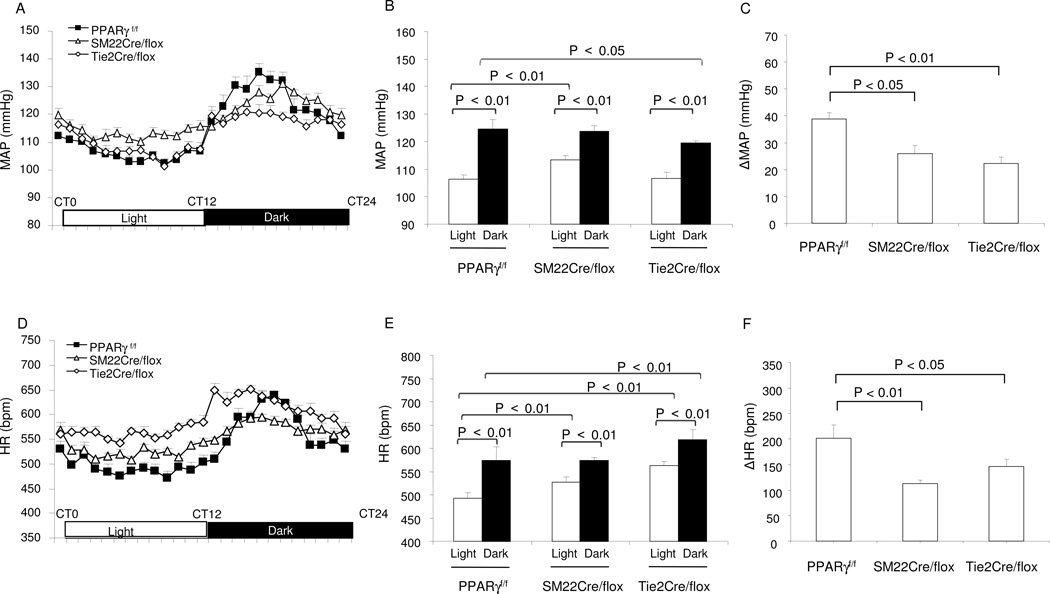
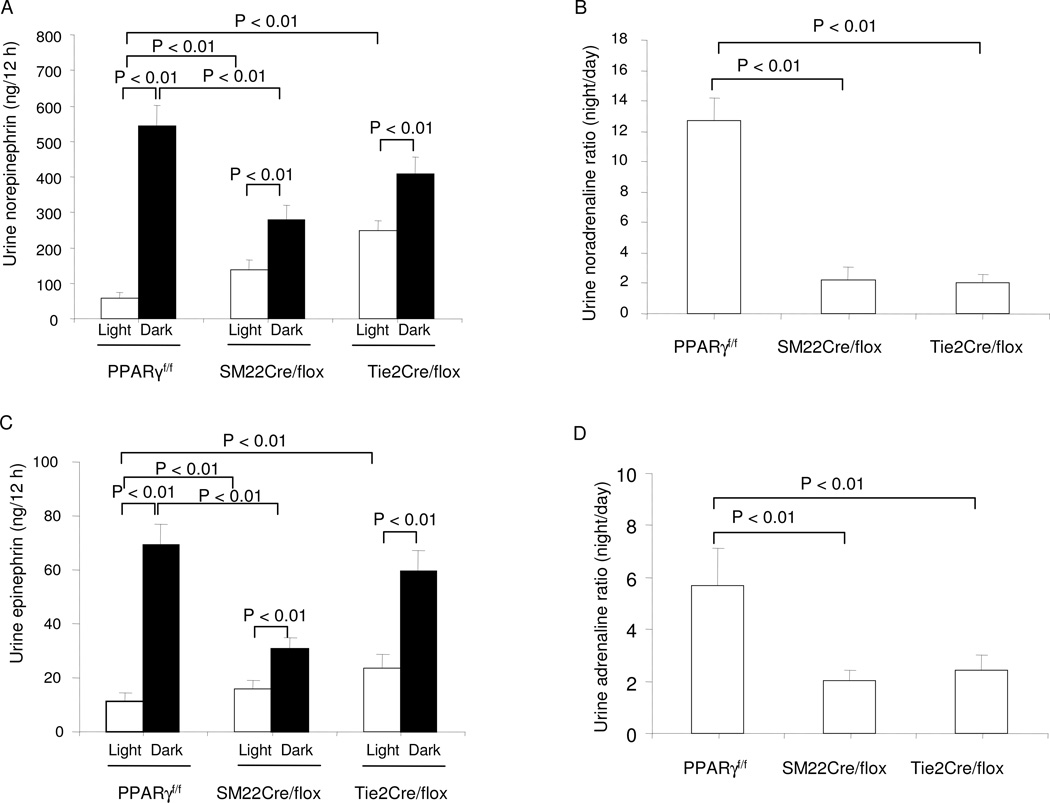
Telemetry was performed to analyze circadian variations in MAP and HR in Tie2Cre/flox and SM22Cre/flox mice. As expected, telemetry data from the floxed mice showed nocturnally activated circadian rhythms of MAP and HR that were significantly higher during the dark vs. light phase (Fig. 2A–B and D–E). When compared to floxed mice, SM22Cre/flox animals exhibited higher MAP and HR during the light cycle but not during the dark cycle. A different pattern was observed in Tie2Cre/flox mice. The changes in MAP and HR in Tie2Cre/flox mice appeared to be dissociated with a reduction of MAP during the dark phase but an increase in HR during both phases relative to floxed mice. Taken together, the diurnal variations of MAP and HR were blunted in the two mutant strains vs. floxed controls (Fig. 2C and F). Given the central role of sympathetic nerve activity in the generation of cardiovascular frequency, we determined urinary norepinephrine (NE) and (epinephrine) Epi during the light and dark phase. The diurnal variations of urine output in either mutant mouse line were not different from their floxed controls (PPARγf/f: day 0.56 ± 0.12 vs. night 1.7 ± 0.32 ml; SM22Cre/flox: day 0.57 ± 0.11 vs. night 1.58 ± 0.26 ml; Tie2Cre/flox: day 0.77 ± 0.15 vs. night 1.45 ml ± 0.2 ml, N=6–7 in each group). In the floxed control mice, urinary NE and Epi excretion exhibited a robust variation (>10 fold) during the light/dark cycle, but this variation was diminished markedly in SM22Cre/flox and Tie2Cre/flox animals (Fig. 3A–D).
Fig. 2.
Altered diurnal rhythms in MAP and HR in SM22Cre/flox and Tie2Cre/flox mice. (A) Hourly recordings of MAP over 24 h. CT0, the beginning of a subjective circadian period (6:00am). (B) The average MAP during light and dark phases. (C) The maximal variations of MAP over 24 h. (D) Hourly recordings of HR over 24 h. (E) The average HR during light and dark phases. (C) The maximal variations of HR over 24 h. N=5–7 in each group. Data are mean ± SE.
Fig. 3.
Altered diurnal rhythms in sympathetic activity in SM22Cre/flox and Tie2Cre/flox mice. (A) 12-h urine output of NE during light and dark phases. (B) Night-to-day ratios of urinary NE. (C) 12-h urine output of Epi. (D) Night-to-day ratios of urinary Epi. N=6–7 in each group. Data are mean ± SE.
Interaction of PPARγ with Bmal1 in the blood vessels
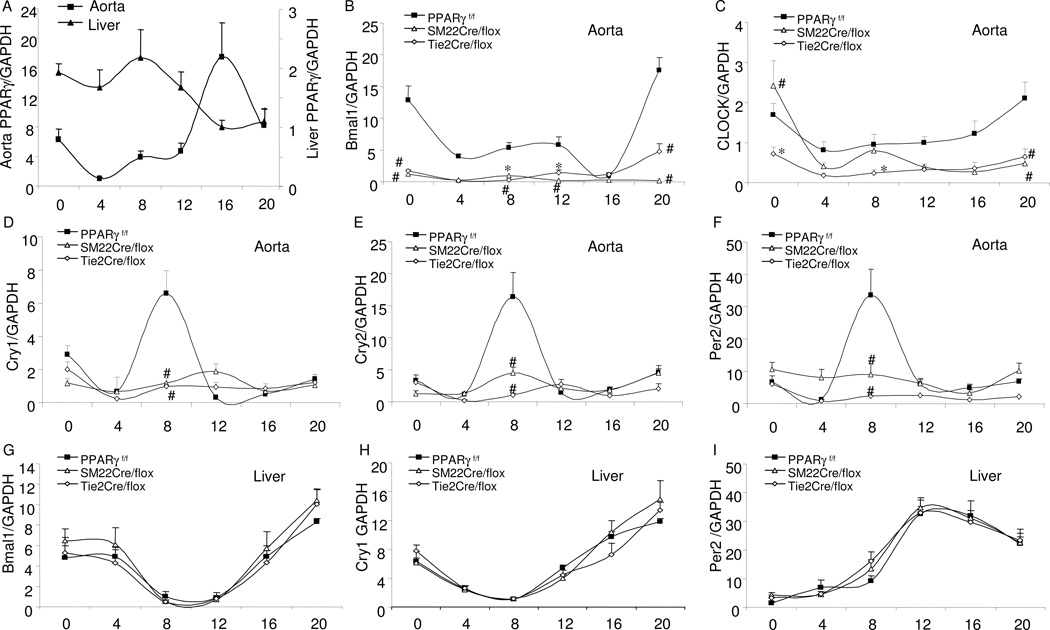
In support of the circadian function of vascular PPARγ, real time RT-PCR analyses of PPARγ expression in the aortae of PPARγf/f mice exhibited a more than 20-fold change during the light/dark cycle with a peak level at CT16 preceding the induction of Bmal1 while hepatic PPARγ exhibited a distinct pattern of diurnal variation with a peak level at CT8 (Fig. 4A). In the floxed mice, aortic expression of canonical clock genes exhibited typical circadian variations with the transcription factors Bmal1 and CLOCK peaking at CT20 and other clock genes Cry1, Cry2, and Per2 at CT8 (Fig. 4B–F). The amplitude of Bmal1 expression was significantly reduced in both SM22Cre/flox and Tie2Cre/flox mice compared to controls, in parallel with the suppressed amplitude of Cry1, Cry2, and Per2 in the mutant strains. Despite the reduced amplitude of the circadian rhythm, significant circadian variations were detected for Bmal1 in SM22Cre/flox mice and for all circadian genes in Tie2Cre/flox mice. A different pattern was observed for CLOCK. The circadian variation of CLOCK was significantly suppressed in Tie2Cre/flox mice but appeared to be well preserved in SM22Cre/flox mice. Since NPAS2 (MOP4) was reported to be able to functionally substitute for CLOCK in the master brain clock in mice to regulate circadian rhythmicity (DeBruyne et al., 2007), we determined NPAS2 expression in the aortae of the SM22Cre/flox and Tie2Cre/flox mice. In the floxed controls, the aortic expression of NPAS2 exhibited robust variations that were significantly blunted in both mutant mice (Fig. S3A). Additionally, in the floxed control mice, the aortic expression of the vasoconstrictor α1d-adrenergic receptor (α1dAR) and vasodilator β1AR exhibited opposite circadian regulation patterns: α1dAR peaked at CT0, decreased at CT8, and remained at low levels at CT 20, whereas β1AR was detected at the lowest levels at CT0 that gradually increased. As compared with the floxed controls, the circadian rhythms of the adrenoceptor subtypes were altered in both mutant mice. In particular, the circadian variations in α1dAR in the aortae were remarkably suppressed in SM22Cre/flox mice.
Fig. 4.
Real time RT-PCR of analysis of gene expressions in the aortae and livers of PPARγf/f, SM22Cre/flox and Tie2Cre/flox mice at 4-h intervals. X-axis represents circadian time (CT0, the beginning of subjective light cycle). (A) PPARγ expression in PPARγf/f mice; (B–I) Expressions of canonical clock genes in aortae and livers in the three mouse strains. N=5 in each time point. Shown are mean ± SE. *, P<0.05 and #, P<0.01 vs. PPARγf/f.
In contrast to the overall blockade of the rhythms of the clock genes in the vasculature of the PPARγ mutant mice, the rhythms in the liver were well preserved (Fig. 4G–I). Indeed, none of the diurnal rhythms of locomotor activity or metabolism were affected in either one of the PPARγ mutant strains (Fig. S4).
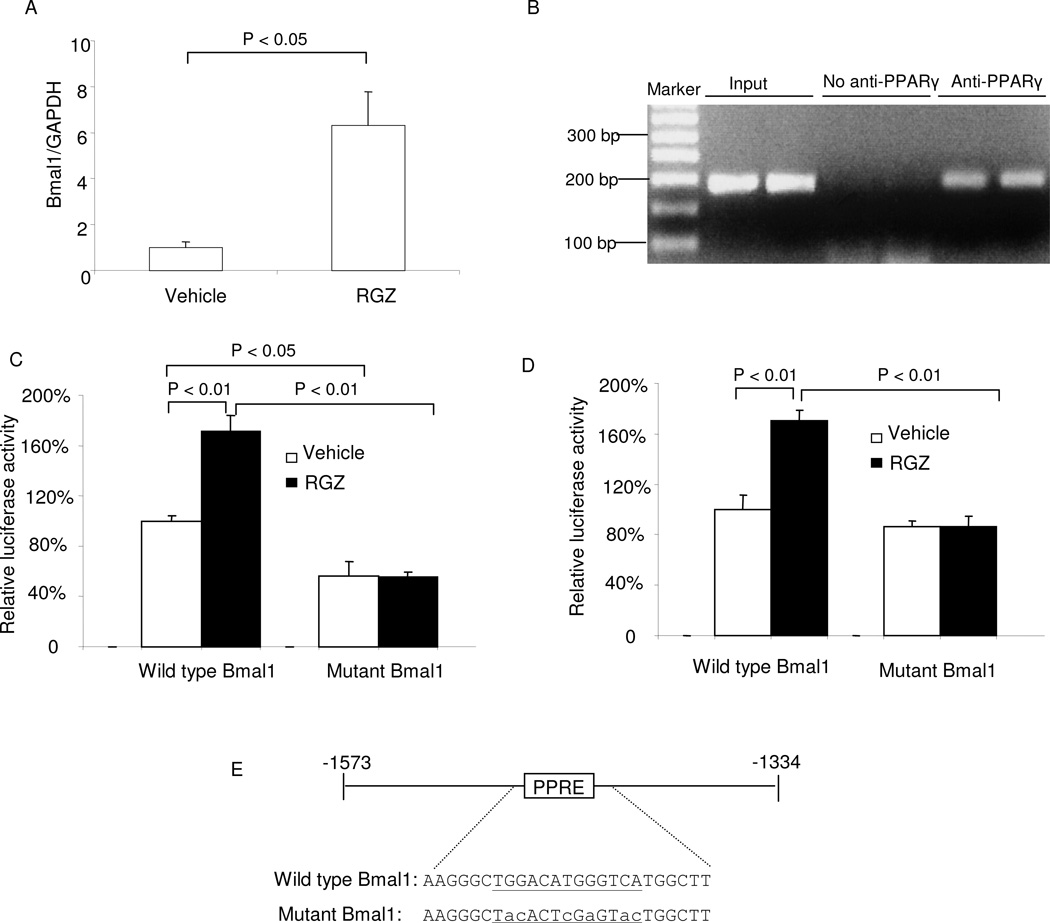
Subsequently, we performed a series of experiments to test whether PPARγ functioned as a direct regulator of Bmal1 in the vasculature. Following treatment with RGZ, the aortic expression of Bmal1 was significantly elevated (Fig. 5A). ChIP assay performed on the aortae of RGZ treated mice revealed a direct physical interaction between PPARγ and the Bmal1 promoter that contains a PPRE site (Fig. 5B). When transfected in SVEC4-10 cells (a mouse endothelial cell line), a 239 bp fragment of mouse Bmal-1 promoter containing the PPRE exhibited a significant increase in promoter activity following exposure to 2 µM RGZ (Fig. 5C). This increase was completely abolished by mutagenesis of the PPRE site (Fig. 5C). Similar results were obtained with M1 cells (a mouse collecting duct cell line) (Fig. 5D). An exception is that mutagenesis of the PPRE site led to reduced baseline luciferase activities in SVEC4-10 but not M1 cells.
Fig. 5.
PPARγ regulates Bmal1 gene transcription. PPARγf/f mice were treated with rosiglitazone (RGZ) at 320 mg/kg diet for 2 days. The thoracic aortae were harvested and subjected to real time RT-PCR (A) and ChIP assay (B). For luciferase assay, mouse SVEC4-10 cells (C) and mouse M1 cells (D) transiently transfected with constructs containing wild-type Bmal1 promoter or Bmal1 promoter with mutated PPRE, and cotransfected with expression vectors for PPARγ, RXRα, and β-galactosidase. In (E), the PPRE site is shown in green and mutated sequences are indicated by lower-case letters. Confluent cells were exposed for 24 h with vehicle or 2 µM RGZ. Luciferase activity was normalized by β-galactosidase activity. In (A), (C), and (D), N = 4–5 per group and data are mean ± SE. In (B), the two lanes in each group represent two separate animals.
Light- and food-sensitivity of the rhythmicity of vascular PPARγ
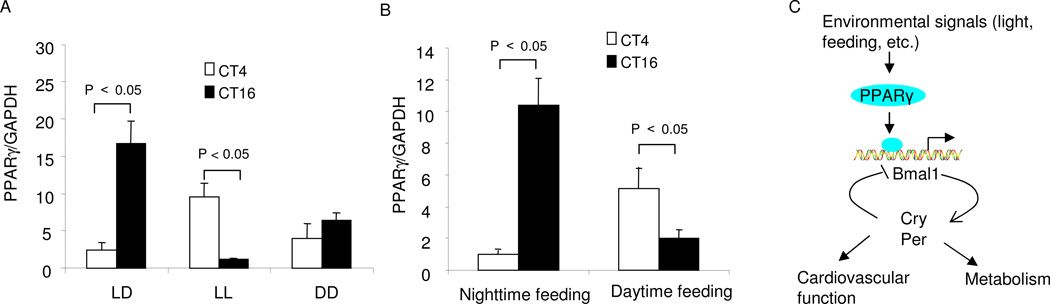
It is thought that the SCN may entrain the rhythmic expression of peripheral clock genes via neural or hormonal pathways (Buijs and Kalsbeek, 2001). To test whether the oscillation of vascular PPARγ is under the influence of the SCN, we compared the diurnal pattern of vascular PPARγ under changing light conditions. Under the regular light/dark cycle, aortic PPARγ exhibited a nocturnally activated expression pattern. In contrast, this rhythm was reversed under constant lightness and diminished under constant darkness (Fig. 6A). Feeding is another important factor that exerts an influence on circadian oscillators in peripheral cells (Damiola et al., 2000). Therefore, we determined the effect of restricted feeding on the rhythmicity of vascular PPARγ under regular light-dark conditions. The pattern of diurnal variations with nighttime feeding (Fig. 6B) was not different from that obtained with unrestricted feeding. As nocturnal animals, mice consume most of their food during the night. In contrast, the diurnal pattern of vascular PPARγ was reversed by the daytime feeding (Fig. 6B).
Fig. 6.
Effects of lightness/darkness and restricted feeding on the cyclic expression of vascular PPARγ. (A) PPARγ mRNA expression in the aortae of PPARγf/f mice kept under regular 12:12 h light/dark cycle (LD), constant lightness (LL), or constant darkness (DD) for 3 days. (B) PPARγ mRNA expression in the aortae of PPARγf/f mice kept under the regular LD cycle and fed only during the light or dark phase. All animals were killed at CT4 and CT16, and aortic PPARγ expression was determined by real time RT-PCR. N=5 in each group. Shown are mean ± SEM. (C) Proposed role of PPARγ in integrating the environmental signals, the clock, the cardiovascular function, and metabolism.
DISCUSSION
Although the SCN is classically viewed as the master regulator of molecular clocks, it has become apparent that a similar system exists in peripheral tissues (e.g., blood vessels). To obtain molecular insights into circadian functions existing in blood vessels, we performed a parallel analysis of hemodynamic parameters in two strains of mice with vascular PPARγ deletions. Our most important finding is that diurnal variations in hemodynamic variables are blunted in both mutants relative to floxed controls. Specifically, radiotelemetry of untethered animals showed that circadian fluctuations in HR and BP were significantly reduced in both SM22Cre/flox and Tie2Cre/flox mice vs. the appropriate controls. These data are the first to provide in vivo evidence that the circadian rhythms of BP and HR are, at least in part, regulated by a PPARγ-dependent, peripheral mechanism intrinsic to the blood vessels. Systemic gene knockout studies have established an essential role for Bmal1, and Cry1 and Cry2 in control of the cardiovascular rhythms but the data obtained are unable to assign this role to a specific tissue type. In agreement with the circadian function of vascular PPARγ, pioglitazone shifts the circadian rhythm of BP from non-dipper to dipper type in patients with type 2 diabetes (Anan et al., 2007). Moreover, upregulation of PPARγ as well as PPARα is associated with restoration of this rhythm in obese insulin resistant mice following a 24-h fast (Verreth et al., 2004).
Sympathetic nerve activity is known to be an important regulator of circadian variations in BP and HR. In particular, mice lacking dopamine β hydroxylase (Dbh) are hypertensive and lose circadian variability in BP (Swoap et al., 2004). In conjunction with the impairment of cardiovascular rhythmicity, the diurnal variations in urinary excretion of NE and Epi taken as estimates of overall sympathetic tone were similarly suppressed in both PPARγ mutants. These findings are similar to those reported to occur in Bmal1−/− (Curtis et al., 2007), and Cry1−/−Cry2−/− (Masuki et al., 2005) mice. Moreover, we observed distinct patterns of circadian regulation of adrenoceptor subtypes in the floxed aortae: α1dAR peaked at CT0 and gradually decreased, whereas β1AR was detected at the lowest levels at CT0 and gradually increased. This coordinated regulation of the adrenoceptor subtypes may be an important determinant of the circadian variation of vascular tone although recent studies have shown maintained cardiovascular rhythmicity in β1/β2AR deficient mice (Kim et al., 2008). As compared with the floxed controls, the circadian rhythms of the adrenoceptor subtypes were altered in both mutant mice. In particular, the circadian variations in α1dAR in the aortae were remarkably suppressed in SM22Cre/flox mice. These findings suggest that dysregulation of adrenoceptor subtypes in the mutant mice may underlie the diminished circadian variations in BP and HR. Future studies are needed to determine whether PPARγ directly regulates expression of vascular adrenoceptors or indirectly affects the expression via Bmal1.
We present compelling evidence supporting PPARγ as a direct regulator of Bmal1 in the blood vessels. First, vascular PPARγ exhibits a robust oscillation that precedes Bmal1. Second, PPARγ deficiency in the blood vessels leads to effective blockade of the rhythmicity as well as the baseline expression of Bmal1 in the aorta. Third, the PPARγ activator RGZ induces aortic expression of Bmal1. Fourth, a ChIP assay reveals a direct physical interaction between PPARγ and Bmal1 promoter. Lastly, the Bmal1 promoter in both vascular and non-vascular cells was responsive to RGZ and this response was abolished by mutagenesis of the PPRE site. It is interesting to note that PPARγ deficiency in either ECs or SMCs produces a similar, nearly complete blockade of rhythmicity of the canonical clock genes. This may suggest a possible interplay between the two types of vascular cells in accomplishing diurnal variations in BP and HR. We suggest that PPARγ in the two types of vascular cells may interact with each other to form a functional unit of the peripheral clock.
The SCN perceives light and entrains the rhythmic expression of the clock genes in peripheral tissues via neural or hormonal pathways (Buijs and Kalsbeek, 2001). The reversal of the diurnal rhythm by changing the light/dark cycle indicates that the rhythmic expression of vascular PPARγ is light-sensitive. These findings represent indirect evidence that the circadian function of vascular PPARγ may be under the influence of the SCN. To further address this possibility, future studies are needed to evaluate expression of vascular PPARγ in SCN-lesioned animals. It was reported that changes in metabolism, such as restricted feeding, can lead to an uncoupling of peripheral oscillators from the central pacemaker (Damiola et al., 2000). In agreement of this notion, changes in feeding time, similar to changes in the photoperiod, reset the phase of rhythmic expression of vascular PPARγ. Overall, these data support a role for vascular PPARγ as a component of the peripheral clock.
Emerging evidence suggests a link between the circadian rhythm and metabolism. A large number of human and animals studies demonstrate that the circadian rhythms have a direct impact on energy metabolism. Perturbations in circadian rhythms in humans are associated with increased risk of obesity and hyperlipidemia (Karlsson et al., 2001). Reduced sleep duration in children is associated with increased risk of being overweight (Lumeng et al., 2007). Remarkably, the disruption of the core molecular clock machinery including Bmal1 and CLOCK leads to hyperphagia and obesity, and metabolic syndrome characterized by hyperleptinemia, hyperlipidemia, hepatic steatosis, and hyperglycemia (Rudic et al., 2004; Turek et al., 2005). PPARγ is a key regulator of glucose and lipid metabolism (Berger et al., 2005; Evans et al., 2004). The identification of the circadian function of PPARγ by the present study has placed this nuclear receptor as a promising mediator between the circadian rhythm and metabolism as illustrated in Fig. 6C. Along this line, a closely related member of the PPAR family, PPARα, which is primarily involved in hepatic lipid metabolism, was reported to regulate the peripheral oscillator of the liver by directly interacting with Bmal1 (Canaple et al., 2006). Interestingly, the same PPRE site at −1519 bp in the Bmal1 promoter confers the response to both PPARα and PPARγ activators. Together, these data suggest that different PPAR subtypes may coordinate their roles in integrating circadian rhythms and various aspects of metabolism by targeting a common component of the peripheral clock, e.g. Bmal1. The complexity of the system comes from the report of Yang et al. who found that among 45 nuclear receptors that are expressed in white and brown adipose tissue, liver, and skeletal muscle, more than half exhibit rhythmicity (Yang et al., 2006), thus suggesting involvement of the nuclear receptor network in this phenomenon.
The present study is limited in that SM22 and Tie2 promoter activities are not exclusively restricted to the vascular cells. For example, SM22 promoter activity has been reported in the developing heart (Miano et al., 2004). Accordingly, a thorough analysis of cardiac morphology and function in SM22Cre/flox mice was performed, but revealed no signs of cardiac hypertrophy or other types of cardiac dysfunction, a phenotype clearly different from mice with cardiomyocyte-specific deletion of PPARγ (Duan et al., 2005). The DNA recombination in the gastrointestinal tract and bladder which contain a rich smooth muscle compartment is expected, but these tissues would appear less relevant to the cardiovascular phenotype. Similarly, although the Tie2 promoter is quite extensively used for targeting ECs (Isermann et al., 2001; Sabrane et al., 2005), Tie2 expression is found in hematopoietic cells (Takakura et al., 1998). The Tie2-Cre model has been used to inactivate PPARγ in hematopoietic and endothelial cells, leading to alopecia and growth retardation, and osteoperosis (Wan et al., 2007a; Wan et al., 2007b). Although obvious gross morphological abnormalities were not noticed in our model, it is difficult to rule out the possibility that some of these confounding factors may affect vascular function.
In summary, mice with conditional deletion of PPARγ using Tie2-Cre and SM22-Cre developed abnormalities in circadian variations in BP and HR, in parallel with a reduction of diurnal variations in the sympathetic nerve activity, and impaired rhythmicity of Bmal1 in the blood vessels. Furthermore, vascular PPARγ exhibits a robust cyclic expression and PPARγ activation stimulates Bmal1 expression. Together, our studies have defined PPARγ as a key component of the vascular clock.
EXPERIMENTAL PROCEDURES
Transgenic mouse lines
PPARγf/f mice contain two loxP sites inserted into introns 1 and 2 of the PPARγ gene flanking the critical exon 2 by homologous recombination in ES cells. The floxed mice were crossed with Tie2-Cre (Kisanuki et al., 2001) and SM22-Cre mice (Holtwick et al., 2002), respectively, to yield mice homologous for the floxed allele and heterozygous for the Cre transgene (termed Tie2Cre/flox and SM22Cre/flox). Each Cre strain has been used to target respective vascular cells (Boucher et al., 2003; Frutkin et al., 2006; Hernando et al., 2007; Miano et al., 2004; Xin et al., 2007). Genotypes were confirmed by PCR analysis as described previously (Nicol et al., 2005; Zhang et al., 2005). All male mice at 3–4 mo of age were maintained under 12:12 h light/dark (LD) cycle (lights on at 6:00am and lights off at 6:00pm) for at least 2 wks before the day of experiment. Mice fed during the day received food from 6:00am to 6:00pm, whereas mice fed during the night received food from 6:00pm to 6:00am. All procedures were in accordance with the guidelines approved by the University of Utah Institutional Animal Care and Use Committee.
Evaluation of DNA recombination of PPARγ
ECs and SMCs from KO mice and floxed controls were freshly isolated as previously described with modifications (Sutliff et al., 1999). Briefly, the thoracic and abdominal aortae were dissected free of adherent tissue, removed from the animal, and opened longitudinally. The layer of endothelium and the tunica media was denuded with forceps, cut into 1-mm pieces, and incubated in a physiological solution containing 0.8% collagenase A at 37 °C. The loosely adhered cells were dislodged by repeated mixing with a pipette. Following a 20-min incubation, these cells were determined to be ECs. After an additional 20-min incubation, the tunica media were collected and determined to be enriched in VSMCs. The purity of these cells was evaluated using the SMC marker α-SMA and the EC marker CD31. Isolated ECs and VSMCs were subjected to a PCR-based analysis of DNA recombination and mRNA expression of PPARγ.
Telemetry recordings
The radiotelemetric device was implanted into male 3–4-month-old PPARγf/f, SM22Cre/flox, and Tie2Cre/flox mice through catheterization of the carotid artery (Model#: TA11PA-C20, DSI, MN) as previously described (Jia et al., 2006). After allowing 1 week for recovery, BP, HR, and activity counts were recorded for at least 48 h to study the circadian variation of these variables.
Analysis of circadian gene expression
PPARγf/f, SM22Cre/flox, and Tie2Cre/flox mice kept under the LD cycle were killed at 4-h intervals over 24 h and thoracic and abdominal aortae, and livers were harvested for real time RT-PCR analysis of PPARγ and canonical clock genes including Bmal1, CLOCK, Cry1-2, and Per2. The primer sequences are listed in Supplemental Table 3. Real time PCR amplification was performed using the SYBR Green Master Mix (Applied Biosystems) and the Prism 7500 Real time PCR Detection System (Applied Biosystems). Cycling conditions were 95°C for 10 min followed by 40 repeats of 95°C for 15 s and 60°C for 1 min. For testing the effect of PPARγ activation on vascular Bmal1 expression, PPARγf/f mice were treated with RGZ at 320 mg/kg diet for 5 days and the aortae were harvested for analysis of Bmal1 expression. The diet was prepared as previously described (Zhang et al., 2005). For testing light-sensitivity of vascular PPARγ expression, PPARγf/f mice were kept under regular LD cycle, constant lightness (LL), or constant darkness (DD) for 3 days, as previously described (Kolker et al., 2003). The animals were killed at CT4 and CT16, and aortae harvested for real time RT-PCR analysis of PPARγ expression. Maximal variations of aortic PPARγ were found between these two circadian time points.
ChIP (chromatin immunoprecipitation) assay
Mouse thoracic and abdominal aortae were harvested, cut into 1-mm pieces, and cross-linked with 1% formaldehyde for 15 min. The tissues were lysed in a SDS lysis buffer containing a protease inhibitor cocktail (Roche) and chromatin was fragmented by sonication. The chromatin fraction was pre-cleared with salmon sperm DNA/protein A agarose and immunoprecipitated with PPARγ antibody (sc-7273X, Santa Cruz Biotechnology Inc.). The immunoprecipitated mouse Bmal1 DNA is amplified by PCR with Bmal1 sense primer 5’-ACTCAGGGGAAGTGGGAAGT-3’ and antisense primer 5’-TGGACCAGCTCGTGTGACTA-3’. The resulting PCR products of 187 bp were separated on a 2% agarose gel and stained with ethidium bromide.
Construction of plasmids
A 239 bp MluI/BglII fragment of mouse Bmal-1 promoter containing a PPRE was amplified by PCR with sense primer, 5’-TGGACGCGTACTCAGGGGAAGTGGGAAGT-3’ and antisense primer, 5’-TGGAGATCTGGCTGGATCCATGAACAAAT-3’. The PCR fragment was constructed into the MluI and BglII site of pGL3-basic vector (Promega). The PPRE mutant plasmid was generated by the overlapping PCR technique and then ligated into the same site of pGL3 plasmid. All constructs were purified using the Hispeed plasmid Maxi kit (Qiagen) and verified by DNA sequencing.
Transfection and luciferase reporter gene assay
M1 cells (a mouse cortical collecting duct cell line) and SVEC4-10 cells (a mouse vascular endothelial cell line) were obtained from ATCC. At 70% confluence, the cells, grown in a 12-well plate, were transfected with the wild-type or mutated Bmal1 promoter/luciferase constructs (400 ng each), and co-transfected with expression plasmids for PPARγ, RXRα, and γ-galactosidase (200 ng each) using Fugene HD (Roche) according to the manufacturer’s protocol. 24 h after transfection, cells were treated for 24 h with 2 µM RGZ or DMSO (vehicle). Luciferase activities were measured with luciferase assay reagent (Promega) using a Lumat LB9501 luminometer (Berthold System, Inc., Pittsburgh) and were normalized by β-galactosidase activity.
Catecholamine assay
Urine was collected in a vial containing 10–15 µl of 6 M HCl during the light phase (CT0-12) and dark phase (CT12-24) and was stored at −20°C before the assay. Urinary Epi and NE were measured by using a commercially available enzyme immunoassay kit (Bi-CAT EIA, ALPCO Diagnostics, Salem, NH) according to the manufacturers’ instructions.
Statistical Analysis
All values are presented as mean ± SE. 2-way ANOVA and Bonferroni post-tests were used for comparisons among multiple groups and the unpaired Student’s t test for comparisons between two groups. Differences were considered to be significant when the P value was less than 0.05.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by Merit Review from the Department of Veterans Affairs as well as RO-1 HL079453 from National Institute of Health (to T. Yang).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Anan F, Masaki T, Fukunaga N, Teshima Y, Iwao T, Kaneda K, Umeno Y, Okada K, Wakasugi K, Yonemochi H, et al. Pioglitazone shift circadian rhythm of blood pressure from non-dipper to dipper type in type 2 diabetes mellitus. Eur J Clin Invest. 2007;37:709–714. doi: 10.1111/j.1365-2362.2007.01854.x. [DOI] [PubMed] [Google Scholar]
- Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. doi: 10.1016/s0092-8674(00)81199-x. [DOI] [PubMed] [Google Scholar]
- Berger JP, Akiyama TE, Meinke PT. PPARs: therapeutic targets for metabolic disease. Trends Pharmacol Sci. 2005;26:244–251. doi: 10.1016/j.tips.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Boucher P, Gotthardt M, Li WP, Anderson RG, Herz J. LRP: role in vascular wall integrity and protection from atherosclerosis. Science. 2003;300:329–332. doi: 10.1126/science.1082095. [DOI] [PubMed] [Google Scholar]
- Buijs RM, Kalsbeek A. Hypothalamic integration of central and peripheral clocks. Nat Rev Neurosci. 2001;2:521–526. doi: 10.1038/35081582. [DOI] [PubMed] [Google Scholar]
- Canaple L, Rambaud J, Dkhissi-Benyahya O, Rayet B, Tan NS, Michalik L, Delaunay F, Wahli W, Laudet V. Reciprocal regulation of brain and muscle Arnt-like protein 1 and peroxisome proliferator-activated receptor alpha defines a novel positive feedback loop in the rodent liver circadian clock. Mol Endocrinol. 2006;20:1715–1727. doi: 10.1210/me.2006-0052. [DOI] [PubMed] [Google Scholar]
- Curtis AM, Cheng Y, Kapoor S, Reilly D, Price TS, Fitzgerald GA. Circadian variation of blood pressure and the vascular response to asynchronous stress. Proc Natl Acad Sci U S A. 2007;104:3450–3455. doi: 10.1073/pnas.0611680104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debruyne JP, Noton E, Lambert CM, Maywood ES, Weaver DR, Reppert SM. A clock shock: mouse CLOCK is not required for circadian oscillator function. Neuron. 2006;50:465–477. doi: 10.1016/j.neuron.2006.03.041. [DOI] [PubMed] [Google Scholar]
- DeBruyne JP, Weaver DR, Reppert SM. CLOCK and NPAS2 have overlapping roles in the suprachiasmatic circadian clock. Nat Neurosci. 2007;10:543–545. doi: 10.1038/nn1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan SZ, Ivashchenko CY, Russell MW, Milstone DS, Mortensen RM. Cardiomyocyte-specific knockout and agonist of peroxisome proliferator-activated receptor-gamma both induce cardiac hypertrophy in mice. Circ Res. 2005;97:372–379. doi: 10.1161/01.RES.0000179226.34112.6d. [DOI] [PubMed] [Google Scholar]
- Durgan DJ, Hotze MA, Tomlin TM, Egbejimi O, Graveleau C, Abel ED, Shaw CA, Bray MS, Hardin PE, Young ME. The intrinsic circadian clock within the cardiomyocyte. Am J Physiol Heart Circ Physiol. 2005;289:H1530–H1541. doi: 10.1152/ajpheart.00406.2005. [DOI] [PubMed] [Google Scholar]
- Evans RM, Barish GD, Wang YX. PPARs and the complex journey to obesity. Nat Med. 2004;10:355–361. doi: 10.1038/nm1025. [DOI] [PubMed] [Google Scholar]
- Frutkin AD, Shi H, Otsuka G, Leveen P, Karlsson S, Dichek DA. A critical developmental role for tgfbr2 in myogenic cell lineages is revealed in mice expressing SM22-Cre, not SMMHC-Cre. J Mol Cell Cardiol. 2006;41:724–731. doi: 10.1016/j.yjmcc.2006.06.067. [DOI] [PubMed] [Google Scholar]
- Hernando E, Charytonowicz E, Dudas ME, Menendez S, Matushansky I, Mills J, Socci ND, Behrendt N, Ma L, Maki RG, et al. The AKT-mTOR pathway plays a critical role in the development of leiomyosarcomas. Nat Med. 2007;13:748–753. doi: 10.1038/nm1560. [DOI] [PubMed] [Google Scholar]
- Holtwick R, Gotthardt M, Skryabin B, Steinmetz M, Potthast R, Zetsche B, Hammer RE, Herz J, Kuhn M. Smooth muscle-selective deletion of guanylyl cyclase-A prevents the acute but not chronic effects of ANP on blood pressure. Proc Natl Acad Sci U S A. 2002;99:7142–7147. doi: 10.1073/pnas.102650499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isermann B, Hendrickson SB, Zogg M, Wing M, Cummiskey M, Kisanuki YY, Yanagisawa M, Weiler H. Endothelium-specific loss of murine thrombomodulin disrupts the protein C anticoagulant pathway and causes juvenile-onset thrombosis. J Clin Invest. 2001;108:537–546. doi: 10.1172/JCI13077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Z, Zhang A, Zhang H, Dong Z, Yang T. Deletion of microsomal prostaglandin E synthase-1 increases sensitivity to salt loading and angiotensin II infusion. Circ Res. 2006;99:1243–1251. doi: 10.1161/01.RES.0000251306.40546.08. [DOI] [PubMed] [Google Scholar]
- Karlsson B, Knutsson A, Lindahl B. Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people. Occup Environ Med. 2001;58:747–752. doi: 10.1136/oem.58.11.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SM, Huang Y, Qin Y, Mizel D, Schnermann J, Briggs JP. Persistence of circadian variation in arterial blood pressure in beta1/beta2-adrenergic receptor-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1427–R1434. doi: 10.1152/ajpregu.00074.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev Biol. 2001;230:230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- Kolker DE, Fukuyama H, Huang DS, Takahashi JS, Horton TH, Turek FW. Aging alters circadian and light-induced expression of clock genes in golden hamsters. J Biol Rhythms. 2003;18:159–169. doi: 10.1177/0748730403251802. [DOI] [PubMed] [Google Scholar]
- Kume K, Zylka MJ, Sriram S, Shearman LP, Weaver DR, Jin X, Maywood ES, Hastings MH, Reppert SM. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell. 1999;98:193–205. doi: 10.1016/s0092-8674(00)81014-4. [DOI] [PubMed] [Google Scholar]
- Lumeng JC, Somashekar D, Appugliese D, Kaciroti N, Corwyn RF, Bradley RH. Shorter sleep duration is associated with increased risk for being overweight at ages 9 to 12 years. Pediatrics. 2007;120:1020–1029. doi: 10.1542/peds.2006-3295. [DOI] [PubMed] [Google Scholar]
- Maemura K, de la Monte SM, Chin MT, Layne MD, Hsieh CM, Yet SF, Perrella MA, Lee ME. CLIF, a novel cycle-like factor, regulates the circadian oscillation of plasminogen activator inhibitor-1 gene expression. J Biol Chem. 2000;275:36847–36851. doi: 10.1074/jbc.C000629200. [DOI] [PubMed] [Google Scholar]
- Masuki S, Todo T, Nakano Y, Okamura H, Nose H. Reduced alpha-adrenoceptor responsiveness and enhanced baroreflex sensitivity in Cry-deficient mice lacking a biological clock. J Physiol. 2005;566:213–224. doi: 10.1113/jphysiol.2005.086728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara P, Seo SP, Rudic RD, Sehgal A, Chakravarti D, FitzGerald GA. Regulation of CLOCK and MOP4 by nuclear hormone receptors in the vasculature: a humoral mechanism to reset a peripheral clock. Cell. 2001;105:877–889. doi: 10.1016/s0092-8674(01)00401-9. [DOI] [PubMed] [Google Scholar]
- Miano JM, Ramanan N, Georger MA, de Mesy Bentley KL, Emerson RL, Balza RO, Jr, Xiao Q, Weiler H, Ginty DD, Misra RP. Restricted inactivation of serum response factor to the cardiovascular system. Proc Natl Acad Sci U S A. 2004;101:17132–17137. doi: 10.1073/pnas.0406041101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JE. Circadian variation and triggering of acute coronary events. Am Heart J. 1999a;137:S1–S8. doi: 10.1016/s0002-8703(99)70390-x. [DOI] [PubMed] [Google Scholar]
- Muller JE. Circadian variation in cardiovascular events. Am J Hypertens. 1999b;12:35S–42S. doi: 10.1016/s0895-7061(98)00278-7. [DOI] [PubMed] [Google Scholar]
- Nicol CJ, Adachi M, Akiyama TE, Gonzalez FJ. PPARgamma in endothelial cells influences high fat diet-induced hypertension. Am J Hypertens. 2005;18:549–556. doi: 10.1016/j.amjhyper.2004.10.032. [DOI] [PubMed] [Google Scholar]
- Nonaka H, Emoto N, Ikeda K, Fukuya H, Rohman MS, Raharjo SB, Yagita K, Okamura H, Yokoyama M. Angiotensin II induces circadian gene expression of clock genes in cultured vascular smooth muscle cells. Circulation. 2001;104:1746–1748. doi: 10.1161/hc4001.098048. [DOI] [PubMed] [Google Scholar]
- Ohkubo T, Hozawa A, Yamaguchi J, Kikuya M, Ohmori K, Michimata M, Matsubara M, Hashimoto J, Hoshi H, Araki T, et al. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama study. J Hypertens. 2002;20:2183–2189. doi: 10.1097/00004872-200211000-00017. [DOI] [PubMed] [Google Scholar]
- Oishi K, Sakamoto K, Okada T, Nagase T, Ishida N. Antiphase circadian expression between BMAL1 and period homologue mRNA in the suprachiasmatic nucleus and peripheral tissues of rats. Biochem Biophys Res Commun. 1998a;253:199–203. doi: 10.1006/bbrc.1998.9779. [DOI] [PubMed] [Google Scholar]
- Oishi K, Sakamoto K, Okada T, Nagase T, Ishida N. Humoral signals mediate the circadian expression of rat period homologue (rPer2) mRNA in peripheral tissues. Neurosci Lett. 1998b;256:117–119. doi: 10.1016/s0304-3940(98)00765-4. [DOI] [PubMed] [Google Scholar]
- Portman MA. Molecular clock mechanisms and circadian rhythms intrinsic to the heart. Circ Res. 2001;89:1084–1086. [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Molecular analysis of mammalian circadian rhythms. Annu Rev Physiol. 2001;63:647–676. doi: 10.1146/annurev.physiol.63.1.647. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- Rudic RD, McNamara P, Curtis AM, Boston RC, Panda S, Hogenesch JB, Fitzgerald GA. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2004;2:e377. doi: 10.1371/journal.pbio.0020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudic RD, McNamara P, Reilly D, Grosser T, Curtis AM, Price TS, Panda S, Hogenesch JB, FitzGerald GA. Bioinformatic analysis of circadian gene oscillation in mouse aorta. Circulation. 2005;112:2716–2724. doi: 10.1161/CIRCULATIONAHA.105.568626. [DOI] [PubMed] [Google Scholar]
- Sabrane K, Kruse MN, Fabritz L, Zetsche B, Mitko D, Skryabin BV, Zwiener M, Baba HA, Yanagisawa M, Kuhn M. Vascular endothelium is critically involved in the hypotensive and hypovolemic actions of atrial natriuretic peptide. J Clin Invest. 2005;115:1666–1674. doi: 10.1172/JCI23360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, Weitz CJ. Extensive and divergent circadian gene expression in liver and heart. Nature. 2002;417:78–83. doi: 10.1038/nature744. [DOI] [PubMed] [Google Scholar]
- Sutliff RL, Hoying JB, Kadambi VJ, Kranias EG, Paul RJ. Phospholamban is present in endothelial cells and modulates endothelium-dependent relaxation. Evidence from phospholamban gene-ablated mice. Circ Res. 1999;84:360–364. doi: 10.1161/01.res.84.3.360. [DOI] [PubMed] [Google Scholar]
- Swoap SJ, Weinshenker D, Palmiter RD, Garber G. Dbh(−/−) mice are hypotensive, have altered circadian rhythms, and have abnormal responses to dieting and stress. Am J Physiol Regul Integr Comp Physiol. 2004;286:R108–R113. doi: 10.1152/ajpregu.00405.2003. [DOI] [PubMed] [Google Scholar]
- Takakura N, Huang XL, Naruse T, Hamaguchi I, Dumont DJ, Yancopoulos GD, Suda T. Critical role of the TIE2 endothelial cell receptor in the development of definitive hematopoiesis. Immunity. 1998;9:677–686. doi: 10.1016/s1074-7613(00)80665-2. [DOI] [PubMed] [Google Scholar]
- Takeda N, Maemura K, Horie S, Oishi K, Imai Y, Harada T, Saito T, Shiga T, Amiya E, Manabe I, et al. Thrombomodulin is a clock-controlled gene in vascular endothelial cells. J Biol Chem. 2007;282:32561–32567. doi: 10.1074/jbc.M705692200. [DOI] [PubMed] [Google Scholar]
- Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verreth W, De Keyzer D, Pelat M, Verhamme P, Ganame J, Bielicki JK, Mertens A, Quarck R, Benhabiles N, Marguerie G, et al. Weight-loss-associated induction of peroxisome proliferator-activated receptor-alpha and peroxisome proliferator-activated receptor-gamma correlate with reduced atherosclerosis and improved cardiovascular function in obese insulin-resistant mice. Circulation. 2004;110:3259–3269. doi: 10.1161/01.CIR.0000147614.85888.7A. [DOI] [PubMed] [Google Scholar]
- Wan Y, Chong LW, Evans RM. PPAR-gamma regulates osteoclastogenesis in mice. Nat Med. 2007a;13:1496–1503. doi: 10.1038/nm1672. [DOI] [PubMed] [Google Scholar]
- Wan Y, Saghatelian A, Chong LW, Zhang CL, Cravatt BF, Evans RM. Maternal PPAR gamma protects nursing neonates by suppressing the production of inflammatory milk. Genes Dev. 2007b;21:1895–1908. doi: 10.1101/gad.1567207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin M, Small EM, van Rooij E, Qi X, Richardson JA, Srivastava D, Nakagawa O, Olson EN. Essential roles of the bHLH transcription factor Hrt2 in repression of atrial gene expression and maintenance of postnatal cardiac function. Proc Natl Acad Sci U S A. 2007;104:7975–7980. doi: 10.1073/pnas.0702447104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Downes M, Yu RT, Bookout AL, He W, Straume M, Mangelsdorf DJ, Evans RM. Nuclear receptor expression links the circadian clock to metabolism. Cell. 2006;126:801–810. doi: 10.1016/j.cell.2006.06.050. [DOI] [PubMed] [Google Scholar]
- Young MW, Kay SA. Time zones: a comparative genetics of circadian clocks. Nat Rev Genet. 2001;2:702–715. doi: 10.1038/35088576. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhang A, Kohan DE, Nelson RD, Gonzalez FJ, Yang T. Collecting duct-specific deletion of peroxisome proliferator-activated receptor gamma blocks thiazolidinedione-induced fluid retention. Proc Natl Acad Sci U S A. 2005;102:9406–9411. doi: 10.1073/pnas.0501744102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylka MJ, Shearman LP, Weaver DR, Reppert SM. Three period homologs in mammals: differential light responses in the suprachiasmatic circadian clock and oscillating transcripts outside of brain. Neuron. 1998;20:1103–1110. doi: 10.1016/s0896-6273(00)80492-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.