Abstract
The urokinase plasminogen activator receptor (uPAR) has been proposed as a potential prognostic factor for various malignancies. The aim of this study is to assess the prognostic value of uPAR expression in neoplastic and stromal cells of patients with pancreatic adenocarcinoma. Urokinase plasminogen activator receptor expression was determined by immunohistochemistry in 122 pancreatic ductal adenocarcinomas. Kaplan-Meier and Cox regression analyses were used to determine the association with survival. Respectively 66%, 82% and 62% of patients with pancreatic cancer expressed uPAR in neoplastic cells, stromal, and in both combined. Multivariate analysis showed a significant inverse association between uPAR expression in both neoplastic and stromal cells and overall survival. The prognostic impact of uPAR in stromal cells is substantial, but not as pronounced as that of uPAR expression in neoplastic cells. This study suggests a role for uPAR as a biomarker to single out higher risk subgroups of patients with pancreatic cancer.
Keywords: Pancreatic adenocarcinoma, immunohistochemistry, survival, uPAR, stroma
Introduction
Pancreatic cancer ranks the fourth leading cause of cancer-related death and is estimated to be the second leading cause of cancer death by 2020.1,2 Complete surgical resection offers the only hope for cure; however, even after successful tumour removal, recurrence rates range from 46% to 89%.3–8 Currently, anatomic resectability and carbohydrate antigen 19-9 (CA 19-9) serum levels are the most commonly used prognostic factors to select optimal treatment strategies for non-metastatic patients with pancreatic cancer, but unfortunately with only modest impact.9,10 Consequently, there is a necessity for novel molecular markers that are able to predict biological behaviour to identify patients requiring more aggressive systemic and/or surgical treatment.
Proteolysis via the plasminogen activation cascade is a crucial biological process involved in cancer cell invasion and metastasis. The urokinase plasminogen activator receptor (uPAR), a glycosyl-phosphatidylinositol–anchored membrane protein, plays a dominant role in this cascade by localizing the urokinase plasminogen activator (uPA) to the cell membrane.11 After binding to uPAR, uPA converts the inactive zymogen plasminogen into plasmin. This active serine protease subsequently activates other proteinases, resulting in the proteolysis of basement membrane proteins and extracellular matrix.12 Considerable evidence indicates that uPAR expression in neoplastic cells, as well as stromal cells, is correlated with shortened survival in various malignancies, including colorectal, breast, and renal carcinoma.13–21
In pancreatic cancer, uPAR expression has been observed in both tumour and surrounding stromal cells. However, it remains unclear which cellular uPAR localization is more immediately involved with tumour behaviour and therefore associated with patient prognosis.15,22 In the present immunohistochemistry study, performed in a large cohort of patients with pancreatic adenocarcinoma, the expression pattern of uPAR in both tumour and stromal cells and its clinical implications were evaluated.
Methods
Patient selection
Retrospectively collected, formalin-fixed and paraffin-embedded tissue blocks were obtained from the archives of the Pathology Department for 137 patients with pancreatic adenocarcinoma, who underwent resection with curative intent during the period from 2001 to 2012 at the Leiden University Medical Centre, Leiden, The Netherlands. Only patients with pancreatic adenocarcinoma were included in this study. None of the patients in this study received chemotherapy and/or radiation prior to surgery. Clinicopathological data were collected from electronic hospital records. Differentiation grade was determined according to the guideline of the World Health Organization, and the TNM stage was defined according to the American Joint Commission on Cancer criteria.23 All samples were non-identifiable and used in accordance with the code for proper secondary use of human tissue as prescribed by the Dutch Federation of Medical Scientific Societies. The use of archived human tissue conformed to an informed protocol that had been reviewed and approved by the institutional review board of the Leiden University Medical Centre, Leiden, The Netherlands.
Immunohistochemistry
Tissue microarrays (TMAs) of pancreatic adenocarcinoma were constructed to perform uniform and simultaneous immunohistochemical staining patterns to limit intra-assay variation. A single representative block was selected for each patient based on haematoxylin-eosin–stained sections. From each donor block, triplicate 2.0-mm cores were punched from areas with clear histopathologic tumour representation and transferred to a recipient TMA block using the TMA Master (3DHISTECH, Budapest, Hungary). From each completed TMA block, 5-µm sections were sliced. The sections were deparaffinized in xylene and rehydrated in serially diluted alcohol solutions, followed by demineralized water according to standard protocols. Endogenous peroxidase was blocked by incubation in 0.3% hydrogen peroxide in phosphate-buffered saline (PBS) for 20 minutes. Antigen retrieval was performed by heat induction at 95°C using PT Link (Dako, Glostrup, Denmark) with a low-pH Envision FLEX target retrieval solution (citrate buffer pH 6.0, Dako). Immunohistochemical staining was performed by incubating TMAs overnight with antibodies against uPAR (ATN-615, provided by Professor A.P. Mazar),24 α-smooth muscle actin (α-SMA) for myofibroblasts (PA5-16697; Thermo Fisher Scientific, Waltham, USA), and vimentin for mesenchymal cells (clone V9; Santa Cruz, Dallas, USA), all at room temperature. All antibodies were used at predetermined optimal dilutions using proper positive and negative control tissue: ATN-615 at 1 µg/mL, PA5-16697 at 0.25 µg/mL, and V9 at 2 µg/mL. Control samples were incubated with PBS instead of the primary antibodies. The sections were washed with PBS, followed by incubation with Envision anti-mouse (K4001; Dako) or Envision anti-Rabbit (K4003; Dako), where applicable, for 30 minutes at room temperature. After additional washing, immunohistochemical staining was visualized using 3,3-diaminobenzidine tetrahydrochloride solution (Dako) for 5 to 10 minutes resulting in brown colour and counterstained with haematoxylin, dehydrated, and finally mounted in Pertex.
Immunohistochemistry evaluation
All stained sections were scanned and viewed at ×200 magnification using the Philips Ultra Fast Scanner 1.6 EA (Philips, Eindhoven, The Netherlands). Evaluation of the immunohistochemical staining of all molecular targets was performed blinded and independently by 2 observers (S.W.L.d.G. and H.A.J.M.P.). In cases of discrepancy, the 2 observers resolved the final score in accordance with a pathologist (H.M.). Immunostaining positivity was determined by a combination of staining intensity and percentage of tumour cells stained. Immunostaining intensity was scored as 0 = negative, 1 = weakly positive, 2 = moderately positive, and 3 = strongly positive. However, in this relatively small cohort, the staining intensity did not contribute substantially to the survival analyses. Therefore, in the final analysis, percentages of uPAR staining in neoplastic cells were dichotomized as low (<50% moderate/strong expression) or high (⩾50% moderate/strong expression).21 As described in a previous study, the staining results for α-SMA were scored, according to the extent of stromal positivity, as low/negative (<50% stroma positive) or high (diffuse expression throughout tumour, >50% stroma positive).25
Statistical analysis
All statistical analyses were conducted using SPSS statistical software (version 23.0; IBM SPSS Inc, Chicago, IL, USA). Baseline characteristics were reported as frequencies, and continuous data were presented as median with interquartile range (IQR) unless indicated otherwise. Comparison of the clinical and pathological characteristics of the 2 cohorts was made using the χ2 test. The Fisher exact test was used when one of the groups counted less than 5. Disease-free survival (DFS) was defined as the time from surgery to the first evidence of local or distant recurrence disease, death from any cause, or lost to follow-up, whatever came first. Overall survival (OS) was defined as the time from the date of surgery to the date of death or lost to follow-up. Kaplan-Meier estimates of the survival function, including P values from the log-rank test were used to graphically compare the time-to-event outcomes based on uPAR expression and to estimate median OS and DFS. Furthermore, univariate and multivariate survival analyses were performed using the Cox proportional hazard regression model. Only variables that were significant on univariate analysis were included in multivariate analyses. Separate multivariate models were employed, one including uPAR expression in neoplastic and stromal cells as different covariates, and another incorporating uPAR expression in both neoplastic and stromal cells as one covariate. In case the proportional hazard assumption was violated, the log-rank test was used, and subsequently, these covariates could not be included in the multivariate regression model.26 Statistical significance was set at P < .05.
Results
Patient and tumour characteristics
Microscopic semi-quantification of uPAR expression in neoplastic and stromal cells was successful in 89% (n = 122) of pancreatic adenocarcinoma. Patient and tumour characteristics are listed in Table 1. The median age was 65 (IQR: 60-72) years, 62 (51%) patients were women and 114 (93%) patients were diagnosed with pancreatic adenocarcinoma located in the head of the pancreas. Primary tumour stage was classified as pT1 in 17 (14%) patients, pT2 in 32 (26%), pT3 in 65 (53%), and pT4 in 8 (7%) patients. In addition, most of the patients had positive nodes (n = 93; 76%) and moderately differentiated tumours (n = 41; 45%). Complete surgical resection (R0) was possible in 83 (68%) cases, and 61 (50%) patients underwent adjuvant chemotherapy after surgical resection of the tumour.
Table 1.
Characteristics of pancreatic adenocarcinoma patients subdivided by uPAR expression in neoplastic and/or stromal cells.
| CHARACTERISTICS | uPAR IN NEOPLASTIC CELLS | P VALUE | uPAR IN STROMAL CELLS | P VALUE | uPAR IN NEOPLASTIC AND STROMAL CELLS | P VALUE | |||
|---|---|---|---|---|---|---|---|---|---|
| LOW (N = 41) |
HIGH (N = 81) |
LOW (N = 22) |
HIGH (N = 100) |
LOW (N = 47) |
HIGH (N = 75) |
||||
| Age, n (%) | |||||||||
| <65 y | 17 (42) | 45 (56) | .141 | 7 (32) | 55 (55) | .049 | 19 (40) | 43 (57) | .069 |
| ⩾65 y | 24 (58) | 36 (44) | 15 (68) | 45 (45) | 28 (60) | 43 (43) | |||
| Sex, n (%) | |||||||||
| Male | 20 (49) | 40 (49) | .950 | 11 (50) | 49 (49) | .932 | 21 (45) | 39 (52) | .431 |
| Female | 21 (51) | 41 (51) | 11 (50) | 51 (51) | 26 (55) | 36 (48) | |||
| Tumour location, n (%) | |||||||||
| Head of pancreas | 38 (93) | 76 (94) | .549 | 21 (96) | 93 (93) | .662 | 44 (94) | 70 (93) | .585 |
| Other | 3 (7) | 5 (6) | 1 (4) | 7 (7) | 3 (6) | 5 (7) | |||
| pT-stage, n (%) | |||||||||
| pT1 | 9 (22) | 8 (10) | .074 | 5 (23) | 12 (12) | .163 | 11 (23) | 6 (8) | .015 |
| pT2 | 14 (34) | 18 (22) | 8 (36) | 24 (24) | 15 (32) | 17 (23) | |||
| pT3 | 16 (39) | 49 (61) | 7 (32) | 58 (58) | 17 (36) | 48 (64) | |||
| pT4 | 2 (5) | 6 (7) | 2 (9) | 6 (6) | 4 (9) | 4 (5) | |||
| pN-stage, n (%) | |||||||||
| pN0 | 8 (20) | 21 (26) | .432 | 4 (18) | 25 (25) | .496 | 10 (21) | 19 (25) | .608 |
| pN1 | 33 (80) | 60 (74) | 18 (82) | 75 (75) | 37 (79) | 56 (75) | |||
| Tumour differentiation, n (%)a | |||||||||
| Well differentiated | 7 (22) | 4 (7) | .093 | 3 (18) | 8 (11) | .426 | 7 (20) | 4 (7) | .136 |
| Moderately differentiated | 14 (44) | 27 (46) | 9 (53) | 32 (43) | 17 (47) | 24 (44) | |||
| Poorly differentiated | 11 (34) | 28 (47) | 5 (29) | 34 (46) | 12 (33) | 27 (49) | |||
| Adjuvant therapy, n (%) | |||||||||
| Yes | 19 (46) | 42 (52) | .565 | 11 (50) | 50 (50) | >.999 | 22 (47) | 39 (52) | .577 |
| No | 22 (54) | 39 (48) | 11 (50) | 50 (50) | 25 (53) | 36 (48) | |||
Abbreviation: uPAR, urokinase plasminogen activator receptor.
Tumour differentiation was only available for 75% (n = 91) of the population; significant P values are bold.
uPAR expression
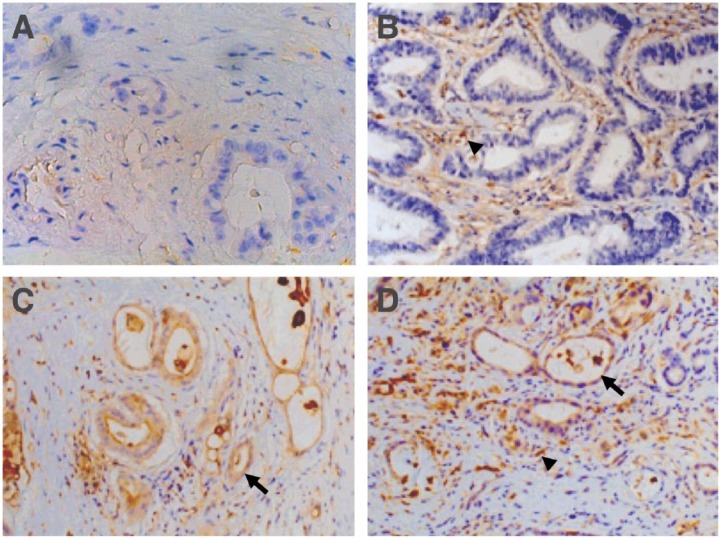
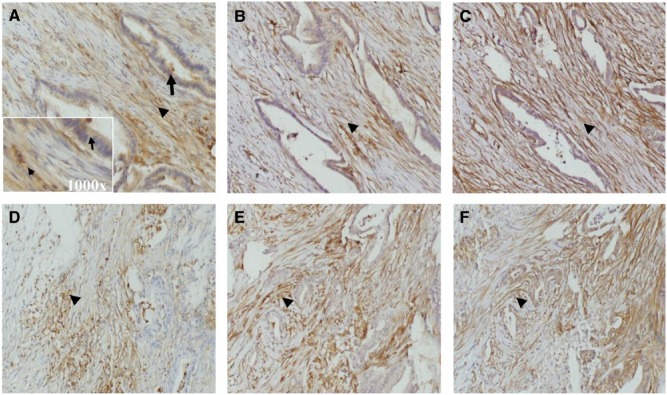
In pancreatic adenocarcinoma, uPAR expression was detected in both neoplastic cells and tumour-associated stroma cells, including myofibroblasts and other mesenchymal cell, as identified by staining for α-SMA and vimentin (Figures 1 and 2). The uPAR expression was elevated in neoplastic cells in 66% of the cases (n = 81) and in tumour-associated cells in 82% (n = 100). A significant correlation (P < .001) was found between uPAR expression in neoplastic and tumour-associated stromal cells. About 62% (n = 75) of the patients with pancreatic adenocarcinoma demonstrated uPAR overexpression in both neoplastic and tumour-associated tumour cells.
Figure 1.
Representative images of pancreatic adenocarcinoma showing urokinase plasminogen receptor (uPAR) expression in neoplastic epithelial (arrow) and stromal cells (arrow head). (A) Low uPAR expression, (B) uPAR expression only in stromal cells, and (C and D) uPAR expression in stromal and neoplastic cells (×200 magnification). uPAR was stained using mouse ATN-15 as primary antibody.
Figure 2.
Representative images of immunohistochemical staining patterns on consecutive tissue sections demonstrating the presence of (A and D) urokinase plasminogen receptor, (B and E) vimentin, and (C and F) α-smooth muscle actin in pancreatic adenocarcinoma (×200 magnification). Arrows and arrow heads indicate, respectively, epithelial and stromal cells. The insert in A represents the ×1000 magnification of the area with arrow and arrow head. uPAR, vimentin were stained with mouse monoclonal antibodies ATN-615 and V9. Smooth muscle actin was detected by rabbit polyclonal antibodies (PA5-16697).
The uPAR expression in stromal cells was significantly associated (P = .049) with age < 65 years, whereas uPAR expression in both neoplastic and stromal cells correlated (P = .015) with more advanced pT-stage. No association was found between baseline clinicopathological characteristics and uPAR expression in either neoplastic or stromal cells (Table 1).
Overall survival
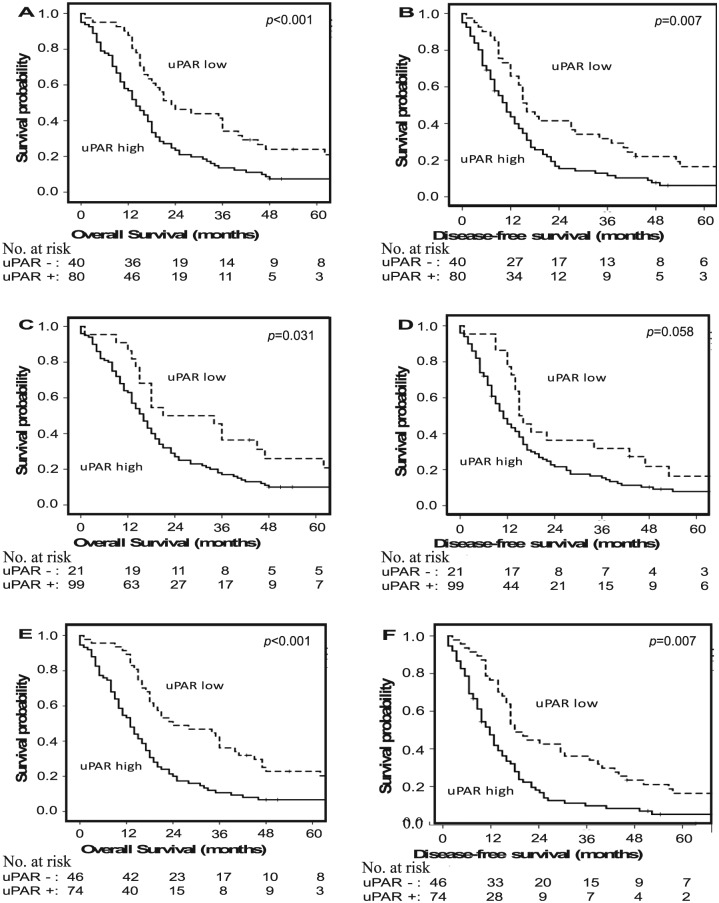
At the time of analysis, 91% (n = 111) of the study population was deceased. The median OS in the overall cohort was 17 (95% confidence interval: 15-19) months. Using univariate analysis, age, sex, tumour location, pT-stage, tumour differentiation, and treatment with adjuvant therapy (log-rank P = .382) were not associated with OS. However, positive lymph nodes, uPAR expression in neoplastic cells (median OS: 14 vs 23 months; Figure 2A), uPAR expression in stromal cells (median OS: 16 vs 21 months; Figure 2C), and uPAR expression in both neoplastic and stromal cells (median OS: 13 vs 24 months; P < .001; Figure 2E) were significantly predictive for OS (Table 2).
Table 2.
Univariate and multivariate Cox proportional hazard regression analyses for the predictive value of uPAR expression on overall survival of patients with pancreatic adenocarcinoma.
| COVARIATES | UNIVARIATE ANALYSIS | MULTIVARIATE ANALYSIS (MODEL 1) | MULTIVARIATE ANALYSIS (MODEL 2) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P VALUE | HR | 95% CI | P VALUE | HR | 95% CI | P VALUE | |
| Age (⩾65 vs <65 y) | 1.11 | 0.76–1.60 | .598 | ||||||
| Sex (male vs female) | 1.38 | 0.95–2.00 | .096 | ||||||
| pT-stage (pT3-4 vs pT1-2) | 1.46 | 0.99–2.17 | .058 | ||||||
| pN-stage (pN1 vs pN0) | 1.86 | 1.16–3.00 | .011 | 1.96 | 1.21–3.15 | .006 | 1.92 | 1.19–3.08 | .007 |
| Tumour differentiationa (well/moderately vs poorly) | 1.24 | 0.80–1.91 | .340 | ||||||
| uPAR in neoplastic cells (high vs low) | 1.93 | 1.28–2.91 | .002 | 1.83 | 1.17–2.85 | .008 | |||
| uPAR in stromal cells (high vs low) | 1.70 | 1.03–2.81 | .036 | 1.31 | 0.76–2.25 | .334 | |||
| uPAR in neoplastic and stromal cells (high vs low) | 2.27 | 1.52–3.40 | <.001 | 2.31 | 1.55–3.45 | <.001 | |||
Abbreviations: CI, confidence interval; HR, hazard ratio; uPAR, urokinase plasminogen activator receptor.
Tumour differentiation was available for 75% (n = 95) of the population; significant P values are bold.
In multivariate analysis, positive lymph nodes and uPAR expression in neoplastic cells were independent prognostic factors for OS, but uPAR expression in stromal cells did not keep its significance. On separate multivariate analysis, positive lymph nodes and uPAR expression in both neoplastic and stromal cells were also significant prognostic factors for OS in pancreatic cancer (Table 2).
Disease-free survival
About 35% (n = 35) of all patients reported local recurrence, 63% (n = 64) distant recurrence, 42% (n = 42) liver metastasis, 22% (n = 22) lung metastasis, and 20% (n = 20) local and distant recurrence. The uPAR expression in stromal cells (P = .018) was associated with the development of liver metastases. No correlations between uPAR expression and specific types of recurrence were found (Table 3).
Table 3.
Recurrence by uPAR expression in patients with pancreatic adenocarcinoma.
| CHARACTERISTICS | uPAR IN NEOPLASTIC CELLS | P VALUE | uPAR IN STROMAL CELLS | P VALUE | uPAR IN NEOPLASTIC AND STROMAL CELLS | P VALUE | |||
|---|---|---|---|---|---|---|---|---|---|
| LOW (N = 34) |
HIGH (N = 67) |
LOW (N = 18) |
HIGH (N = 83) |
LOW (N = 40) |
HIGH (N = 61) |
||||
| Local recurrencea | 13(38%) | 22(33%) | .590 | 7(39%) | 28(34%) | .677 | 17(43%) | 18(30%) | .180 |
| Distant recurrencea | 18 (53%) | 46 (69%) | .121 | 10 (56%) | 54 (65%) | .448 | 21 (53%) | 43 (71%) | .066 |
| Liver metastasisa | 10 (29%) | 32 (48%) | .077 | 3 (17%) | 39 (47%) | .018 | 11 (28%) | 31 (51%) | .020 |
| Lung metastasisa | 7 (21%) | 15 (22%) | .836 | 5 (28%) | 17 (21%) | .497 | 9 (23%) | 13 (21%) | .887 |
| Local and distant recurrencea | 5 (15%) | 15 (22%) | .360 | 4 (22%) | 16 (19%) | .776 | 8 (20%) | 12 (20%) | .968 |
Abbreviation: uPAR, urokinase plasminogen activator receptor.
Recurrence was documented for 83% (n = 101) of the cohort; significant P values are bold.
In univariate analyses, age, sex, tumour location (log-rank P = .727), pT-stage, tumour differentiation, and receipt of adjuvant therapy (log-rank P = .245) did not demonstrate predictive value for DFS. However, positive lymph nodes, uPAR expression in neoplastic cells (median DFS, 11 vs 16 months; Figure 2B), and uPAR expression in both neoplastic and stromal cells (median DFS: 10 vs 16 months; Figure 3F) were significantly associated with poor survival (Table 4). The association of uPAR expression in stromal cells with OS did not reach statistical significance (median DFS: 11 vs 15 months; HR, Figure 2D).
Figure 3.
Kaplan-Meier curves for overall and disease-free survival for patients with pancreatic adenocarcinoma after surgical treatment, stratified by the status of urokinase plasminogen receptor (uPAR) expression in (A and B) neoplastic cells, (C and D) stromal cells, and (E and F) both neoplastic and stromal cells.
Table 4.
Univariate and multivariate Cox proportional hazard regression analyses for the predictive value of uPAR expression on disease-free survival in patients with pancreatic adenocarcinoma.
| COVARIATES | UNIVARIATE ANALYSIS | MULTIVARIATE ANALYSIS (MODEL 1) | MULTIVARIATE ANALYSIS (MODEL 2) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P VALUE | HR | 95% CI | P VALUE | HR | 95% CI | P VALUE | |
| Age (⩾65 vs <65 y) | 1.03 | 0.71–1.49 | .892 | ||||||
| Sex (female vs male) | 1.21 | 0.83–1.76 | .319 | ||||||
| pT-stage (pT3-4 vs pT1-2) | 1.01 | 1.00–1.03 | .153 | ||||||
| pN-stage (pN1 vs pN0) | 1.86 | 1.15–2.99 | .011 | 1.95 | 1.21–3.14 | .006 | 1.91 | 1.19–3.08 | .007 |
| Tumour differentiationa (well/moderately vs poorly) | 1.16 | 0.75–1.80 | .511 | ||||||
| uPAR in neoplastic cells (high vs low) | 1.72 | 1.15–2.58 | .009 | 1.66 | 1.06–2.58 | .025 | |||
| uPAR in stromal cells (high vs low) | 1.58 | 0.97–2.58 | .065 | 1.26 | 0.74–2.15 | .394 | |||
| uPAR in neoplastic and stromal cells (high vs low) | 2.00 | 1.34–2.98 | .001 | 2.05 | 1.37–3.04 | <.001 | |||
Abbreviations: CI, confidence interval; HR. hazard ratio; uPA, urokinase plasminogen activator receptor.
Tumour differentiation was available for 75% (n = 95) of the population; significant P values are bold.
Multivariate analysis showed that positive lymph nodes, uPAR expression in neoplastic cells and uPAR expression in both neoplastic and stromal cells were independently associated with poor DFS (Table 4).
Discussion
Outcomes after resection for pancreatic adenocarcinoma are variable and contingent on both the biology of the disease and the efficacy of the treatment. Determination of proteins or pathways that increase risk of recurrence and impair survival might be helpful to assist with the selection of patients with pancreatic cancer who could benefit from (neo)adjuvant and targeted therapies. The results of this immunohistochemical study reveal a significant inverse correlation between uPAR expression and OS and DFS of patients with pancreatic cancer. The prognostic impact of uPAR in stromal cells is striking, but it is not an independent parameter, such as uPAR expression in neoplastic cells.
Relatively, few studies have analysed tissue expression of uPAR and its association with prognosis in pancreatic cancer.27 In 1997, Cantero et al15 were the first to report worse survival for patients with high uPAR-positive pancreas tumours in a small cohort of 30 patients. Although they noticed uPAR staining in malignant epithelial cells and stroma cells, they did not correlate these separately with survival. More than 10 years later, the level of uPAR messenger RNA (mRNA) was shown not to be correlated with prognosis in a small cohort of 25 patients, whereas in another study with 46 patients, uPAR mRNA appeared to be the strongest biological prognostic marker.28,29 The prognostic association of uPAR with pancreatic cancer was further confirmed by the measurement of high levels of soluble uPAR in urine of these patients.30 Our data, in a relatively large cohort of patients with pancreatic cancer, confirm the association between uPAR and survival. This suggests a role for uPAR as a potential independent indicator for the identification of higher risk patient subgroups, as has been found for other tumour types, including colorectal, breast, and lung cancers.20,31,32
The uPAR enhancement on malignant cells can partly be explained by oncogenic amplification of the PLAUR gene, as has been identified by Ströbel and co-workers in 52% of the cases in a cohort of 50 patients with pancreatic cancer.22 However, uPAR upregulation in neoplastic cells is not dependent solely on gene amplification, as uPAR expression is also upregulated by several oncologic pathways in which transcription factors such as AP1 and PEA3/ETS are involved.11 Furthermore, environmental factors such as tumour necrosis factor-α and interleukins can enhance uPAR expression, which could partly explain the upregulation in tumour stromal cells, such as myofibroblasts, macrophages, and endothelial cells.33 Upregulation of uPAR in these cells has no genetic background and is primarily a response to signals from the cancer cells. The association of uPAR upregulation in stromal cells with survival, as found in this study, turned out not to be independent in multivariate analyses, as has been found in other tumour types.21,34–36 However, in these other tumours, the uPAR-positive stromal cells were often located at the invasive front, which seems not specifically the case for uPAR expressing stromal cells in pancreatic cancer.
Just the presence of uPAR on certain cell types does not contribute to the malignancy of a tumour and could not explain a prognostic relevance. As a receptor, uPAR is strongly dependent on its interaction with other proteins for its functions.11 The most obvious function of uPAR is the stimulation of proteolysis, which does not exist without the presence of plasminogen and uPA and is otherwise tightly regulated by the presence of inhibitors PAI-1 and PAI-2. The chemotactic function of uPAR depends on cleavage by uPA, where again the inhibitors play a regulatory role. Also, uPAR-mediated intracellular signalling relies on the binding of uPA, Vitronectin, and several integrins as ligands. Because uPAR itself does not contain an intracellular domain, these signals are transduced by other, ‘professional’ signalling proteins with transmembrane and intracellular domains such as tyrosine kinase receptors, G protein–coupled receptors, and integrins.37 All these interactions between uPAR and other proteins, plus the shedding of 1 of the 3 domains by uPA influence the 3-dimensional structure of uPAR. Therefore, it is well established that different anti-uPAR antibodies with varying epitope specificity result in different immunohistochemical staining patterns.38 Obviously, part of the discrepancies regarding the prognostic value of uPAR in pancreatic cancer described in the literature may be explained using antibodies targeting different domains within the uPAR protein. In this study, the extensively validated antibody ATN-615 was used, which detects almost all forms of uPAR, probably explaining the abundant presence of uPAR in multiple cell types in comparison with some other studies.24
Another difference with previous studies is the use of a TMA, which might also be the biggest limitation of this study. Although the tumour areas were carefully selected to represent a complete overview of the tumour, the possibility of discrepant patterns of uPAR in comparison with conventional tissue sections is not ruled out. However, previous studies in breast cancer demonstrated that analysis of at least 2 cores on the TMA is comparable with the analysis of whole tissue sections in >95% of cases.39 Another restriction of this study is that patients with metastatic unresectable disease at time of diagnosis could not be included because these patients rarely have adequate tissue for detailed immunohistochemical evaluation. Considering the uPAR distribution in stage and grade, it seems not likely that including these patients with expected bad prognosis would have influenced the analysis dramatically.
Next to a possible application as prognostic marker, uPAR may also hold promise as a selective target for either tumourspecific image-guided surgery or targeted therapy because of its absence in normal pancreatic tissue and chronic pancreatitis.40,41 A preclinical study has indeed demonstrated the ability of uPAR-targeted near-infrared dye–labelled theranostic nanoparticles, to visualize residual disease in pancreatic xenografts.42 Furthermore, uPAR-targeted magnetic iron oxide nanoparticles carrying gemcitabine were able to overcome the tumour stromal barrier and subsequently were able to enhance the efficiency of the drug. This is particularly relevant, as high resistance to therapy is a major challenge in pancreatic cancer care.43,44
In summary, this study demonstrates in a relatively large cohort of patients with pancreatic adenocarcinoma that uPAR expression, in particular determined in stromal cells as well as in cancerous cells, is predictive for unfavourable OS and DFS. Evaluation of uPAR expression, alone or in combination with other predictive factors, may improve the identification of patients who could benefit from more aggressive treatment. Although the combination of uPAR determination in neoplastic cells and stromal cells seemed to have the highest prognostic impact, further studies for better understanding of the mechanisms involved are still necessary.
Acknowledgments
The authors thank R van Vlierberghe, NG Dekker-Ensink, R Keijzer, and CM Jansen for their excellent technical assistance and Dr LJAC Hawinkels for providing antibodies.
Footnotes
PEER REVIEW: Five peer reviewers contributed to the peer review report. Reviewers’ reports totalled 1705 words, excluding any confidential comments to the academic editor.
FUNDING: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was performed within the framework of the Center for Translational Molecular Medicine (CTMM), project MUSIS (grant: 030-202).
DECLARATION OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions
SWLdG, PJKK, HJAMP, and CFMS conceived and designed the experiments. SWLdG and HM analysed the data. SWLdG wrote the first draft of the manuscript. VMB, MCB, PJKK, HJAMP, APM, JM, BAB, and CFMS contributed to the writing of the manuscript. SWLdG, VMB, MCB, APM, PJKK, HJAMP, BAB, HM, CJHvdV, ALV, and CFMS agree with manuscript results and conclusions. All authors reviewed and approved the final manuscript.
Disclosures and Ethics
As a requirement of publication, author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality, and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
REFERENCES
- 1.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 3.Griffin JF, Smalley SR, Jewell W, et al. Patterns of failure after curative resection of pancreatic carcinoma. Cancer. 1990;66:56–61. doi: 10.1002/1097-0142(19900701)66:1<56::aid-cncr2820660112>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 4.Westerdahl J, Andren-Sandberg A, Ihse I. Recurrence of exocrine pancreatic cancer – local or hepatic? Hepatogastroenterology. 1993;40:384–387. [PubMed] [Google Scholar]
- 5.Yeo CJ, Cameron JL, Lillemoe KD, et al. Pancreaticoduodenectomy for cancer of the head of the pancreas. 201 patients. Ann Surg. 1995;221:721–731. doi: 10.1097/00000658-199506000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sperti C, Pasquali C, Piccoli A, Pedrazzoli S. Recurrence after resection for ductal adenocarcinoma of the pancreas. World J Surg. 1997;21:195–200. doi: 10.1007/s002689900215. [DOI] [PubMed] [Google Scholar]
- 7.Fischer R, Breidert M, Keck T, Makowiec F, Lohrmann C, Harder J. Early recurrence of pancreatic cancer after resection and during adjuvant chemotherapy. Saudi J Gastroenterol. 2012;18:118–121. doi: 10.4103/1319-3767.93815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aoyama T, Murakawa M, Katayama Y, et al. Impact of postoperative complications on survival and recurrence in pancreatic cancer. Anticancer Res. 2015;35:2401–2409. [PubMed] [Google Scholar]
- 9.Sho M, Akahori T, Tanaka T, et al. Optimal indication of neoadjuvant chemoradiotherapy for pancreatic cancer. Langenbecks Arch Surg. 2015;400:477–485. doi: 10.1007/s00423-015-1304-0. [DOI] [PubMed] [Google Scholar]
- 10.Bergquist JR, Puig CA, Shubert CR, et al. Carbohydrate antigen 19–9 elevation in anatomically resectable, early stage pancreatic cancer is independently associated with decreased overall survival and an indication for neoadjuvant therapy: a national cancer database study. J Am Coll Surg. 2016;223:52–65. doi: 10.1016/j.jamcollsurg.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Blasi F, Sidenius N. The urokinase receptor: focused cell surface proteolysis, cell adhesion and signaling. FEBS Lett. 2010;584:1923–1930. doi: 10.1016/j.febslet.2009.12.039. [DOI] [PubMed] [Google Scholar]
- 12.Mazar AP. Urokinase plasminogen activator receptor choreographs multiple ligand interactions: implications for tumor progression and therapy. Clin Cancer Res. 2008;14:5649–5655. doi: 10.1158/1078-0432.CCR-07-4863. [DOI] [PubMed] [Google Scholar]
- 13.Grøndahl-Hansen J, Peters HA, van Putten WL, et al. Prognostic significance of the receptor for urokinase plasminogen activator in breast cancer. Clin Cancer Res. 1995;1:1079–1087. [PubMed] [Google Scholar]
- 14.Duggan C, Maguire T, McDermott E, O’Higgins N, Fennelly JJ, Duffy MJ. Urokinase plasminogen activator and urokinase plasminogen activator receptor in breast cancer. Int J Cancer. 1995;61:597–600. doi: 10.1002/ijc.2910610502. [DOI] [PubMed] [Google Scholar]
- 15.Cantero D, Friess H, Deflorin J, et al. Enhanced expression of urokinase plasminogen activator and its receptor in pancreatic carcinoma. Br J Cancer. 1997;75:388–395. doi: 10.1038/bjc.1997.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Bock CE, Wang Y. Clinical significance of urokinase-type plasminogen activator receptor (uPAR) expression in cancer. Med Res Rev. 2004;24:13–39. doi: 10.1002/med.10054. [DOI] [PubMed] [Google Scholar]
- 17.Bhuvarahamurthy V, Schroeder J, Denkert C, et al. In situ gene expression of urokinase-type plasminogen activator and its receptor in transitional cell carcinoma of the human bladder. Oncol Rep. 2004;12:909–913. [PubMed] [Google Scholar]
- 18.Bhuvarahamurthy V, Schroeder J, Kristiansen G, et al. Differential gene expression of urokinase-type plasminogen activator and its receptor in human renal cell carcinoma. Oncol Rep. 2005;14:777–782. [PubMed] [Google Scholar]
- 19.Cozzi PJ, Wang J, Delprado W, et al. Evaluation of urokinase plasminogen activator and its receptor in different grades of human prostate cancer. Hum Pathol. 2006;37:1442–1451. doi: 10.1016/j.humpath.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Hildenbrand R, Schaaf A, Dorn-Beineke A, et al. Tumor stroma is the predominant uPA-, uPAR-, PAI-1-expressing tissue in human breast cancer: prognostic impact. Histol Histopathol. 2009;24:869–877. doi: 10.14670/HH-24.869. [DOI] [PubMed] [Google Scholar]
- 21.Boonstra MC, Verbeek FP, Mazar AP, et al. Expression of uPAR in tumorassociated stromal cells is associated with colorectal cancer patient prognosis: a TMA study. BMC Cancer. 2014;14:269. doi: 10.1186/1471-2407-14-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hildenbrand R, Niedergethmann M, Marx A, et al. Amplification of the urokinase-type plasminogen activator receptor (uPAR) gene in ductal pancreatic carcinomas identifies a clinically high-risk group. Am J Pathol. 2009;174:2246–2253. doi: 10.2353/ajpath.2009.080785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greene FL. The American Joint Committee on Cancer: updating the strategies in cancer staging. Bull Am Coll Surg. 2002;87:13–15. [PubMed] [Google Scholar]
- 24.Li Y, Parry G, Chen L, et al. An anti-urokinase plasminogen activator receptor (uPAR) antibody: crystal structure and binding epitope. J Mol Biol. 2007;365:1117–1129. doi: 10.1016/j.jmb.2006.10.059. [DOI] [PubMed] [Google Scholar]
- 25.Horn LC, Schreiter C, Canzler A, Leonhardt K, Einenkel J, Hentschel B. CD34(low) and SMA(high) represent stromal signature in uterine cervical cancer and are markers for peritumoral stromal remodeling. Ann Diagn Pathol. 2013;17:531–535. doi: 10.1016/j.anndiagpath.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 26.Putter H, Sasako M, Hartgrink HH, van de Velde CJ, van Houwelingen JC. Long-term survival with non-proportional hazards: results from the Dutch Gastric Cancer Trial. Stat Med. 2005;24:2807–2821. doi: 10.1002/sim.2143. [DOI] [PubMed] [Google Scholar]
- 27.Boonstra MC, Verspaget HW, Ganesh S, et al. Clinical applications of the urokinase receptor (uPAR) for cancer patients. Curr Pharm Des. 2011;17:1890–1910. doi: 10.2174/138161211796718233. [DOI] [PubMed] [Google Scholar]
- 28.Warnecke-Eberz U, Prenzel KL, Baldus SE, et al. Significant down-regulation of the plasminogen activator inhibitor 1 mRNA in pancreatic cancer. Pancreas. 2008;36:173–177. doi: 10.1097/MPA.0b013e31815ac538. [DOI] [PubMed] [Google Scholar]
- 29.Xue A, Scarlett CJ, Jackson CJ, Allen BJ, Smith RC. Prognostic significance of growth factors and the urokinase-type plasminogen activator system in pancreatic ductal adenocarcinoma. Pancreas. 2008;36:160–167. doi: 10.1097/MPA.0b013e31815750f0. [DOI] [PubMed] [Google Scholar]
- 30.Sorio C, Mafficini A, Furlan F, et al. Elevated urinary levels of urokinase-type plasminogen activator receptor (uPAR) in pancreatic ductal adenocarcinoma identify a clinically high-risk group. BMC Cancer. 2011;11:448. doi: 10.1186/1471-2407-11-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pedersen H, Brünner N, Francis D, et al. Prognostic impact of urokinase, urokinase receptor, and type 1 plasminogen activator inhibitor in squamous and large cell lung cancer tissue. Cancer Res. 1994;54:4671–4675. [PubMed] [Google Scholar]
- 32.Boonstra MC, Verbeek FP, Mazar AP, et al. Expression of uPAR in tumor-associated stromal cells is associated with colorectal cancer patient prognosis: a TMA study. BMC Cancer. 2014;14:269. doi: 10.1186/1471-2407-14-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tran-Thang C, Kruithof E, Lahm H, Schuster WA, Tada M, Sordat B. Modulation of the plasminogen activation system by inflammatory cytokines in human colon carcinoma cells. Br J Cancer. 1996;74:846–852. doi: 10.1038/bjc.1996.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laerum OD, Ovrebo K, Skarstein A, et al. Prognosis in adenocarcinomas of lower oesophagus, gastro-oesophageal junction and cardia evaluated by uPAR-immunohistochemistry. Int J Cancer. 2012;131:558–596. doi: 10.1002/ijc.26382. [DOI] [PubMed] [Google Scholar]
- 35.Illemann M, Laerum OD, Hasselby JP, et al. Urokinase-type plasminogen activator receptor (uPAR) on tumor-associated macrophages is a marker of poor prognosis in colorectal cancer. Cancer Med. 2014;3:855–864. doi: 10.1002/cam4.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dohn LH, Illemann M, Hoyer-Hansen G, et al. Urokinase-type plasminogen activator receptor (uPAR) expression is associated with T-stage and survival in urothelial carcinoma of the bladder. Urol Oncol. 2015;33:165.e15–165.e24. doi: 10.1016/j.urolonc.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 37.Ferraris GM, Sidenius N. Urokinase plasminogen activator receptor: a functional integrator of extracellular proteolysis, cell adhesion, and signal transduction. Semin Thromb Hemost. 2013;39:347–355. doi: 10.1055/s-0033-1334485. [DOI] [PubMed] [Google Scholar]
- 38.Ahn SB, Chan C, Dent OF, et al. Epithelial and stromal cell urokinase plasminogen activator receptor expression differentially correlates with survival in rectal cancer stages B and C patients. PLoS ONE. 2015;10:e0117786. doi: 10.1371/journal.pone.0117786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kyndi M, Sorensen FB, Knudsen H, et al. Tissue microarrays compared with whole sections and biochemical analyses. A subgroup analysis of DBCG 82 b&c. Acta Oncol. 2008;47:591–599. doi: 10.1080/02841860701851871. [DOI] [PubMed] [Google Scholar]
- 40.Chen Y, Zheng B, Robbins DH, et al. Accurate discrimination of pancreatic ductal adenocarcinoma and chronic pancreatitis using multimarker expression data and samples obtained by minimally invasive fine needle aspiration. Int J Cancer. 2007;120:1511–1517. doi: 10.1002/ijc.22487. [DOI] [PubMed] [Google Scholar]
- 41.de Geus SW, Boogerd LS, Swijnenburg RJ, et al. Selecting tumor-specific molecular targets in pancreatic adenocarcinoma: paving the way for image-guided pancreatic surgery. Mol Imaging Biol. 2016;18:807–819. doi: 10.1007/s11307-016-0959-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang L, Sajja HK, Cao Z, et al. uPAR-targeted optical imaging contrasts as theranostic agents for tumor margin detection. Theranostics. 2013;4:106–118. doi: 10.7150/thno.7409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Warshaw AL, Fernandez-del Castillo C. Pancreatic carcinoma. N Engl J Med. 1992;326:455–465. doi: 10.1056/NEJM199202133260706. [DOI] [PubMed] [Google Scholar]
- 44.Lee GY, Qian WP, Wang L, et al. Theranostic nanoparticles with controlled release of gemcitabine for targeted therapy and MRI of pancreatic cancer. ACS Nano. 2013;7:2078–2089. doi: 10.1021/nn3043463. [DOI] [PMC free article] [PubMed] [Google Scholar]