Abstract
The goal of this work is to understand adsorption-induced deformation of hierarchically structured porous silica exhibiting well-defined cylindrical mesopores. For this purpose, we performed an in situ dilatometry measurement on a calcined and sintered monolithic silica sample during the adsorption of N2 at 77 K. To analyze the experimental data, we extended the adsorption stress model to account for the anisotropy of cylindrical mesopores, i.e., we explicitly derived the adsorption stress tensor components in the axial and radial direction of the pore. For quantitative predictions of stresses and strains, we applied the theoretical framework of Derjaguin, Broekhoff, and de Boer for adsorption in mesopores and two mechanical models of silica rods with axially aligned pore channels: an idealized cylindrical tube model, which can be described analytically, and an ordered hexagonal array of cylindrical mesopores, whose mechanical response to adsorption stress was evaluated by 3D finite element calculations. The adsorption-induced strains predicted by both mechanical models are in good quantitative agreement making the cylindrical tube the preferable model for adsorption-induced strains due to its simple analytical nature. The theoretical results are compared with the in situ dilatometry data on a hierarchically structured silica monolith composed by a network of mesoporous struts of MCM-41 type morphology. Analyzing the experimental adsorption and strain data with the proposed theoretical framework, we find the adsorption-induced deformation of the monolithic sample being reasonably described by a superposition of axial and radial strains calculated on the mesopore level. The structural and mechanical parameters obtained from the model are in good agreement with expectations from independent measurements and literature, respectively.
1. Introduction
All porous materials deform upon adsorption of fluids.1 This phenomenon was already reported nearly a century ago2,3 but found increasing scientific interest in recent years due to the refinements of experimental and computational approaches in this field. For most porous materials adsorption-induced deformation does not exceed the order of per mill in terms of volumetric strain. Nevertheless, similarly to adsorption isotherms, experimentally measured strain isotherms, i.e., the sample strain as a function of the adsorbate gas pressure, contain valuable information about the pore size distribution4,5 and mechanical properties.6−9 Furthermore, there have been first attempts to utilize adsorption-induced deformation for the development of actuators.10,11
Currently, there are two fundamentally different approaches to measure strain isotherms experimentally. The first is the determination of adsorption-induced strain on the macroscopic scale by in situ dilatometry3,6,12−20 or in situ ellipsometry7,21 techniques only applicable to materials available as monoliths of sufficient size or thin transparent films, respectively. The second approach is the investigation of adsorption-induced strains on the microscopic level of the pores or the nonporous backbone by scattering techniques such as small-angle X-ray scattering (SAXS)8,22 or X-ray diffraction (XRD);23 except for large deformations,24 this approach requires materials with ordered porosity or crystalline substructure exhibiting clear scattering peaks, whose shift can be interpreted as strain. Examples where both techniques can be applied to the same material are very rare;25,26 however, the available results clearly indicate that adsorption-induced deformation is not necessarily an isotropic effect but (depending on the sample’s structural and mechanical properties) may be significantly anisotropic. This finding is also supported by a recent dilatometric study on a thin membrane with oriented mesopores.27
With respect to the theoretical understanding of adsorption-induced deformation, a considerable number of studies were performed in recent years, e.g., refs (4, 5, and 28−50). One of the prevailing concepts in these studies is the adsorption stress approach proposed by Ravikovitch and Neimark;29 it was used to elucidate the specifics of adsorption-induced deformation of zeolites,29 carbons,5,34,39 metal–organic frameworks,40 and mesoporous solids.38,43 Although the adsorption stress approach was instrumental for revealing the mechanism of adsorption deformation in pores of different size, it was tacitly assumed that the sample deformation is caused by the stress oriented normal to the pore walls. Contrary, the change of the adsorbent’s surface energy during the adsorption process causes tangential stress in the pore walls; this effect is commonly known as Bangham’s effect or Bangham’s law (see original work51 and additions49,52). With this in mind, the adsorption stress model correctly describes the isotropic pore geometry of a sphere, since there stresses orientated normal and tangential to the pore wall are unequivocally correlated. However, for anisotropic pore geometries such as cylindrical or slit-shaped pores, tangential stresses and normal stresses need to be individually addressed. For the particular case of cylindrical pores, one should therefore expect two different deformation components: one oriented in the radial plane of the pore as predicted by the adsorption stress model,38 and another and likely different one in the axial direction of the pore. Support for the concept of anisotropic stress in nanopores was also given by recent computational studies on the adsorption in micropores.53−57
Given the experimental and theoretical evidence for anisotropic stress in nanopores two questions arise: (i) what kind of adsorption-induced deformation should one expect from an anisotropic pore geometry, if stresses oriented normal and tangential to the pore wall are properly considered? (ii) how should experimental data on adsorption-induced deformation be analyzed, if the sample investigated exhibits anisotropic pore geometry? In this work we aim to answer these questions for the particular case of a hierarchical structured porous silica with well-defined cylindrical mesopores (section 2.1), whose deformation during N2 adsorption at 77 K was measured by in situ dilatometry (section 2.2). The key of our proposed approach is the extension of the adsorption stress model for cylindrical mesopores with calculation of radial and axial stresses within the framework of Derjaguin, Broekhoff, and de Boer theory (section 3.1).58−60 The calculated stresses serve as input quantities for mechanical modeling of mesoporous structures (section 3.2). Here we consider two structural models: (a) simplistic model of an individual cylindrical tube and (b) 3D array of hexagonally ordered cylindrical pores typical for MCM-41,61 SBA-15,62 or hierarchical structured silicas.63 The latter model is investigated by three-dimensional finite element calculations. Finally, the proposed theoretical approach is applied to the experimental data (section 4).
2. Experimental Methods
2.1. Model System—Synthesis and Characterization
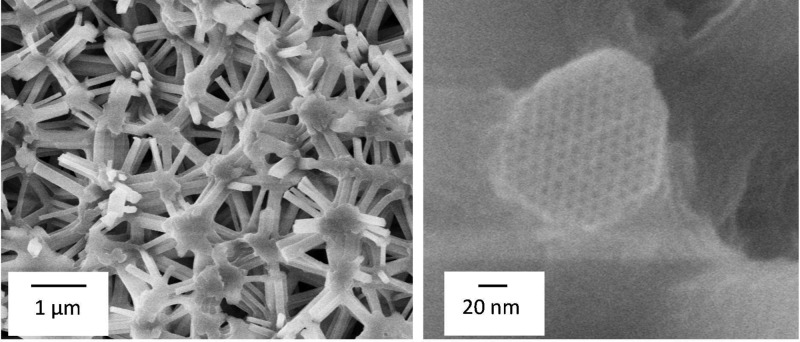
The model system investigated in this work is a hierarchically structured porous silica monolith prepared as a macroscopic cylindrical rod via a sol–gel processing of tetrakis(2-hydroxyethyl)orthosilicate following the protocols given in refs (63) and (64). In the gel state the inner surface of the sample was treated with trimethylchlorosilane (TMCS) and subsequently dried under ambient conditions. Then the sample was calcined at 500 °C for 10 h under ambient conditions to remove all organic components and finally sintered at 950 °C for 15 min at ambient atmosphere to remove microporosity while preserving the mesoporosity. The removal of the microporosity is essential, since microporosity was shown to potentially affect adsorption-induced deformation over the whole relative pressure range even when micropore filling is essentially completed.4 The sample preparation described above is one of very few successful approaches to a material exhibiting well-defined cylindrical mesopores and being available in monolithic form, which is required for in situ dilatometry measurements of adsorption-induced deformation. The sintered sample was investigated by scanning electron microscopy (SEM) (Ultra Plus, Carl-Zeiss NTS) and N2 adsorption analysis at 77 K (ASAP2020, Micromeritics). Furthermore, the macroscopic density of the sample ρ was determined after degassing at 110 °C for 1 d at gas pressures below 10–3 mbar. All characterization results are summarized in Table 1. SEM images of the sample investigated (Figure 1) show a disordered macroporous strut network (Figure 1 left) with the struts containing highly ordered cylindrical mesopores arranged on a hexagonal lattice (Figure 1 right). The N2 adsorption isotherm of the sample was analyzed by the BET theory65 for the specific surface area SBET and by the Gurvich rule66 for the specific mesopore volume VGurvich yielding an average pore diameter d̅meso = 4VGurvich/SBET. Additionally, we calculated the density of the mesoporous struts via ρstrut = 1/(VGurvich + 1/ρsolid), the porosity of the mesoporous struts ϕstrut = VGurvich/(VGurvich + 1/ρsolid), and the porosity of the macroporous network ϕnetwork = 1 – ρ/ρstrut. For the density of the nonporous solid ρsolid we used the value reported in literature for silica sintered at 1000 °C, i.e., (2.1 ± 0.1) g/cm3.67
Table 1. Summary of Structural Parameters of the Model System Investigateda.
| ρ [g/cm3] | SBET [m2/g] | VGurvich [cm3/g] | d̅meso [nm] | ρstrut [g/cm3] | ϕstrut [%] | ϕnetwork [%] |
|---|---|---|---|---|---|---|
| 0.525 ± 0.029 | 203 ± 5 | 0.24 ± 0.01 | 4.7 ± 0.2 | 1.40 ± 0.05 | 34 ± 2 | 62 ± 4 |
That is, the density of the monolithic sample ρ, the specific surface area SBET, the specific pore volume VGurvich, the average mesopore diameter d̅meso, the density of the mesoporous struts ρstrut, the porosity of the mesoporous struts ϕstrut, and the porosity of the macropore network ϕnetwork.
Figure 1.
SEM images of the model system on different length scales.
2.2. In Situ Dilatometry
The in situ dilatometry measurements were performed with a setup consisting of a commercial adsorption instrument (ASAP2020, Micromeritics) and a self-designed sample holder with a built-in dilatometer. Details of the setup are given in refs (5, 20, and 26). It provides the common adsorption isotherm complemented by a strain isotherm εdil(p/p0), i.e., the relative linear length change of the monolithic sample as a function of the relative gas pressure p/p0. The absolute accuracy of the dilatometric setup is about ±0.2 μm corresponding to a strain resolution of ±1.7 × 10–5 for the sample of length L0 = 1.2 cm investigated in this work. Prior to the measurement the sample was degassed at 110 °C for 1 day inside the sample holder to avoid contact of the sample with the ambient atmosphere between degassing and measurement. The analysis gas used was N2 of purity 5.0. During the measurement, the sample holder was placed in a liquid nitrogen bath and the respective saturation pressure of the analysis gas was measured at regular intervals.
3. Theoretical Methods
3.1. Adsorption-Induced Stress
When fluid is adsorbed inside a pore, it exerts pressure on the pore walls and causes deformation of the solid matrix, i.e., the nonporous solid backbone. To quantify for this effect, Ravikovitch and Neimark29 introduced the volumetric adsorption stress σa defined as the derivative of the grand thermodynamic potential of adsorbed fluid, Ωa, with respect to the pore volume, Vp, at given temperature T and adsorbate chemical potential μ:
| 1 |
The grand thermodynamic potential of the adsorbed fluid, Ωa(μ,Vp,T), is related to the amount adsorbed Na(μ,Vp,T) by the Gibbs equation,68
| 2 |
Here μr is the chemical potential of the reference state that defines relative strains and respective stresses. The standard reference states are the dry state at μr → −∞ and wet or saturated state at μr = 0; the chemical potential is reckoned from the state of liquid–vapor equilibrium at given temperature. Assuming that the bulk adsorbate behaves like an ideal gas at experimental conditions μ = RgT ln(p/p0) holds with Rg the gas constant, p the gas pressure of the adsorbate, and p0 the respective saturation pressure.
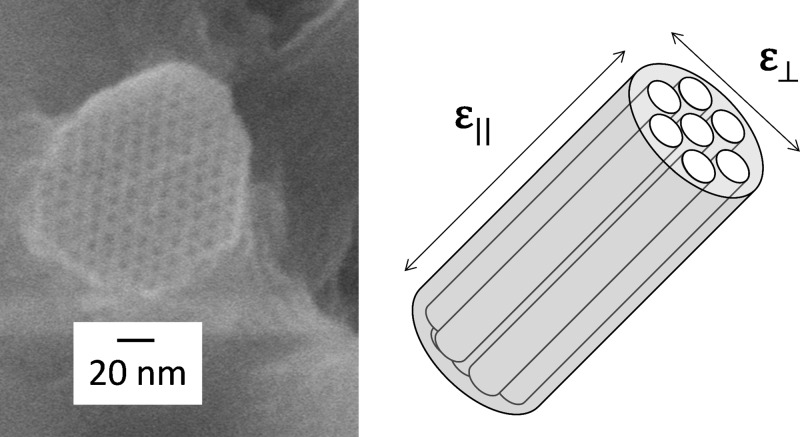
In this work, we consider adsorption-induced deformation of materials with geometrically well-defined and ordered cylindrical pores. The cylindrical shape implies inherent anisotropy of the stress tensor, and in order to account for this anisotropy, we have to differentiate between the radial (normal) σa,⊥ and axial (tangential) σa,∥ components of the adsorption stress tensor, which for the cylindrical pore of radius R, length L, and consequently volume Vp = πR2L, are defined as
| 3 |
| 4 |
While the adsorption isotherm and, respectively, the grand potential depend nontrivially on the pore radius, the length of the pore, which is assumed to be significantly larger than the pore radius (L/R ≫ 1), does not affect the fluid density, so that Ωa(μ,Vp,T), is proportional to L. As such, eq 4 can be transformed into
| 5 |
Equation 5 implies that the pore wall deformation in axial direction does not affect the adsorption potential and, respectively, the density of adsorbed fluid. For a discussion of the limitations of this assumption see a recent publication.49
In order to explicitly calculate the adsorption stress, one needs to know the adsorption isotherm for a pore of given geometry, which can be determined by various means: by theoretical models such as Langmuir,69 Dubinin,70 or Derjaguin–Broekhoff–de Boer (DBdB)58−60 equations as well as by molecular simulations based on density functional theory (e.g., refs (29, 43)) or Monte Carlo methods (e.g., (27, 34, 39)). Following our earlier work,38 here we apply the DBdB theory of capillary condensation, since it offers an analytical solution for the entire adsorption process in mesopores including the transitions between film and filled pore state, i.e., capillary condensation and evaporation, respectively. According to DBdB theory, adsorption in a cylindrical mesopore is described by the equivalence of the chemical potential of adsorbed and gaseous phase for given temperature and relative gas pressure p/p0:
| 6 |
Here VL is the molar volume of the adsorbate in liquid form, h is the film thickness of the adsorbed phase, γlv is the liquid–vapor surface energy, and Π(h) is the disjoining pressure of the adsorbed film, which is usually determined on a macroporous reference material.
The critical film thickness hc at which capillary condensation occurs is given by
| 7 |
The film thickness for equilibrium capillary evaporation he is given by the Derjaguin equation:
| 8 |
Here pe is the gas pressure corresponding to he according to eq 6.
The molar amount adsorbed Na in film and filled pore regime, respectively, is given for a single cylindrical pore by
| 9a |
| 9b |
where the correlation of h and p/p0 follows again from eq 6.
As was shown in ref (38), the combination of eq 3 with the framework of the DBdB theory leads to the following expressions for the radial stress σa,⊥ in the film and filled pore regime of a cylindrical mesopore, respectively:
| 10a |
| 10b |
Here γs is the surface energy of the dry solid under vacuum conditions, γsl the surface energy of the wet solid in contact with liquid, and γsv the surface energy of the solid covered by an adsorption film of thickness h. The reduction of the solid surface energy by the adsorption process
| 11 |
applied in eq 10a follows from the Gibbs adsorption equation for a flat surface38 and (γs – γsv(h))/R is the corresponding stress due to Bangham’s law(38,71) causing monotonic expansion with progressing adsorption. Counteracting the Bangham stress is the Laplace pressure γlv(1/(R – h) – 1/R) resulting from the curved liquid vapor interface, which is numerically smaller than the Bangham stress in most cases. Additionally, in the filled pore state (eq 10b) the pore is subjected to RgT/VL ln(p/p0), i.e., the classical capillary pressure pcap. For the second equality in eq 10b, the Frumkin–Derjaguin (FD) equation (see, e.g., refs (72) and (73)) for the cylindrical pore geometry is applied to correlate γs and γsl (for details, see the Supporting Information (SI)):
| 12 |
In direct analogy to eq 10, the combination of eq 5 and DBdB theory yields the axial (tangential) stress σa,∥ inside the cylindrical mesopore (for details, see the SI):
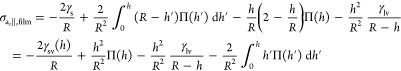 |
13a |
| 13b |
The quantitatively dominant term in eq 13a is 2γsv(h)/R corresponding to an axial Bangham stress twice as large as in radial direction (eq 10a). Notably the factor of 2 between Bangham stress in axial and radial direction is intrinsic to the cylindrical geometry and was already reported in previous works, e.g., ref (71). In the filled pore regime both stresses σa,⊥,filled and σa,∥,filled (eq 10b and 13b, respectively) are found to be directly proportional to pcap fitting the concept of an isostatic capillary pressure. However, axial and radial stress in the filled pore regime are shifted relative to one another by a constant offset γsl/R, which again is a result of the cylindrical geometry of the pore. It is important to note that the equality of eq 13a and b describes the equilibrium of grand potential in film and filled pore state (see eq 5) and therefore corresponds to the condition of capillary evaporation.58 If the surface energy difference γs – γsl between dry and wet solid is taken from the modified FD equation (eq 12), the equality of eq 13a and b results in the Derjaguin equation (eq 8), which implies p0 ≪ pcap(pe/p0).
3.2. Mechanical Models for Porous Struts with Cylindrical Pores
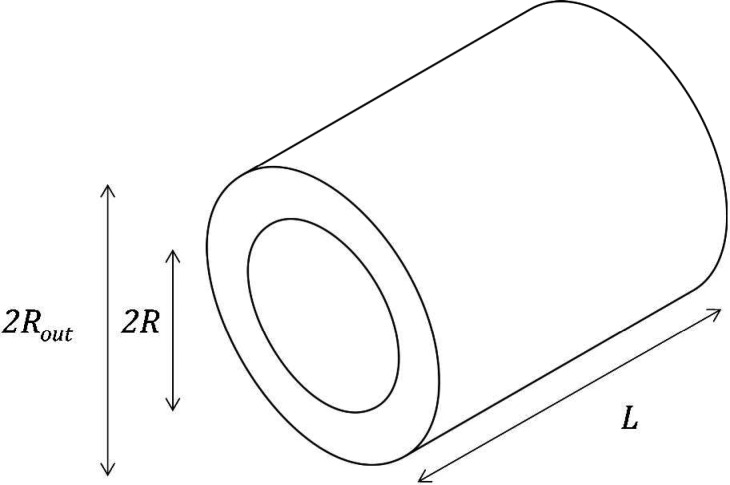
As the simplest model describing the deformation of a porous strut with aligned cylindrical mesopores (Figure 1), we consider a cylindrical tube of inner radius R, outer radius Rout and therefore porosity ϕ = R2/Rout2 (Figure 2). This model simplistically represents a unit cell in the strut’s pore arrangement shown in Figure 1.
Figure 2.
Schematic of a single cylindrical tube of inner radius R, outer radius Rout , and length L.
For comparison with experimental data the potentially relevant deformations of the cylindrical tube are the axial strain, i.e. the relative elongation of the tube εa,∥ = δL/L, the circumferential strain, i.e. the relative change of the outer radius εa,⊥ = δRout/Rout , and the volumetric strain, i.e., the relative change εa,vol of volume occupied by tube and pore. The sought strains follow from the solution of the Lamé problem with boundary conditions provided by the radial (normal) σa,⊥ and axial (tangential) σa,∥ components of the adsorption stress (for details, see the SI):74,75
| 14 |
| 15 |
| 16 |
Here E and ν are the Young’s modulus and the Poisson’s ratio of the nonporous solid forming the cylindrical tube, respectively. Notably, above equations imply an isotropic and elastic solid, i.e., E and ν are directional and strain independent constants. For eq 16 it was furthermore assumed that axial and circumferential strains are significantly smaller than 1, which is a reasonable approximation for most adsorbate–adsorbent combinations.
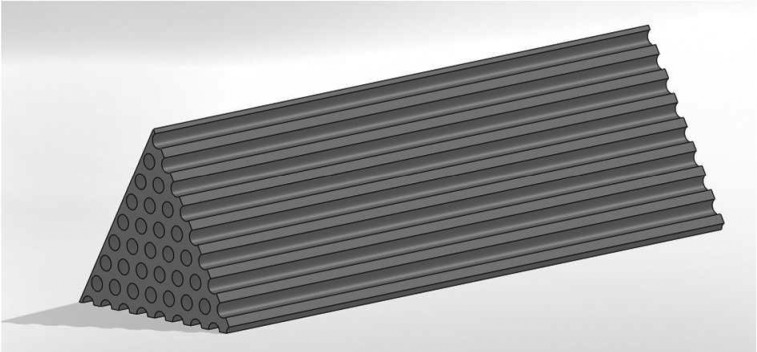
A more complex description of the sample structure is a hexagonal lattice of cylindrical pores forming a strut. A respective three-dimensional structure, whose mechanical response to adsorption-induced stress in the mesopores was investigated by the finite element method (FEM),76 is shown in Figure 3. To reduce the calculation time we exploited the symmetry of the hexagonal strut modeling only a sixth of it and reducing the strut length compared to the real counterpart by a factor of approximately 20; the latter modification was checked to have no impact on the results of the FEM calculations. In analogy to the cylindrical tube the structural parameters of the FEM model are the length of the strut L and the pore radius R complemented by the lattice parameter dl, i.e., the distance between adjacent pore centers, which is related to the pore radius by the porosity of the strut ϕstrut via dl = R(2π/(ϕstrut√3))1/2.
Figure 3.
Three-dimensional model used for FEM calculations representing a sixth of a hexagonal strut with cylindrical mesopores arranged on a hexagonal lattice. The reduction to a sixth of the strut follows from the symmetry conditions of the system; the full strut exhibits 217 pores.
For the FEM model, the stresses were applied the same way as for the single cylindrical tube, i.e. the radial stress (eq 10) is acting normal to the surface area of the mesopores, while the average axial stress (eq 13 and eq S9) is applied to the solid on the front side of the strut. Furthermore, the strut was assumed to be free-standing without any confinement whatsoever. As for the cylindrical tube mechanical properties of the nonporous solid phase, E and ν, are assumed to be constant and isotropic. The FEM calculations were performed with the commercial software SOLIDWORKS 2011. The results evaluated from the output of the FEM calculations are the average strain of the strut length δL/L corresponding to εa,∥ (eq 15) and the average strain of the lattice parameter δdl/dl corresponding to εa,⊥ (eq 14). Notably, to minimize boundary effects we evaluated δdl/dl only for pores exhibiting six neighboring pores. Furthermore, we investigated the dependence of the strain δdl/dl on the number of pores within the strut: in the range of 127 to 217 pores the relative deviations of δdl/dl were below 0.2%. The applied model is therefore considered sufficiently large to effectively exclude boundary effects.
4. Results and Discussion
In this section, we apply the theoretical framework presented in section 3 to the N2 (77 K) adsorption and strain isotherms determined by the in situ dilatometry experiment on the hierarchical structured porous silica (section 2). Both experimental data sets are presented in Figure 4. Following the IUPAC recommendations for physisorption of gases77 the adsorption isotherm of the sample investigated is of type IV(a); its shape including the adsorption hysteresis is characteristic for mesoporous materials. Also the experimental strain isotherm shows similar trends as reported by previous experimental studies on the deformation of mesoporous materials (see, e.g., refs6−8,14), though the strain hysteresis accompanying the adsorption hysteresis and the net strain at saturation pressure are rather small.
Figure 4.
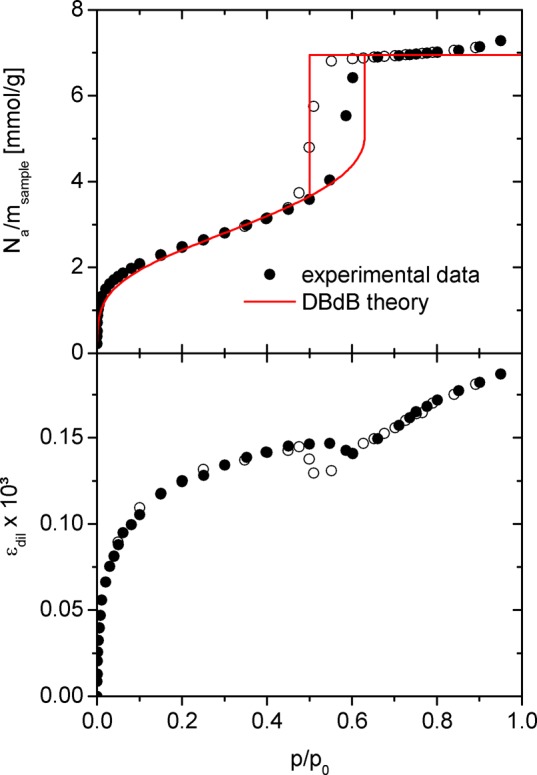
Upper panel: N2 adsorption isotherm of the sample investigated and respective modeling by DBdB theory (eqs 6–9). Full symbols denote adsorption, and open symbols desorption. Lower panel: corresponding strain isotherm determined by the in situ dilatometry experiment.
For the analysis of the experimental results we start with the modeling of the N2 adsorption isotherm of the sample by the DBdB theory for a cylindrical mesopore (eqs 6–9). Since experimental adsorption isotherms are typically given in terms of specific molar amount adsorbed, we normalize the results from eq 9 to the sample mass msample, i.e., adsorption isotherms are given as Na/msample. An input parameter for the modeling is the disjoining pressure Π(h), which we determined independently from the N2 adsorption isotherm of a purely macroporous reference sample (see the SI).
The first step of the modeling was to determine the pore radius R via eq 8 from the experimental relative pressure of capillary evaporation leading to a pore diameter of dDBdB = 2R = 4.7 nm. Second, based on eq 9b we adapted the specific surface area of the sample to SDBdB = 2πRL/msample = 205 m2/g to reproduce the experimental amount adsorbed at the plateau in the filled pore regime. As a last step, inserting R and SDBdB into eqs 6, 7, and 9b yielded predictions for the adsorption in the film regime as well as the point of capillary condensation. The resulting theoretical adsorption isotherm is shown in Figure 4 along with its experimental counterpart. The relative pressure of capillary condensation for the theoretical adsorption isotherm is slightly higher than in the experiment, but apart from this the model gives an overall reasonable description of the data. At the same time the numerical values for dDBdB and SDBdB are in good agreement with d̅meso and SBET, respectively (compare Table 1). In conclusion, the modeling of the experimental adsorption isotherm by DBdB theory supports that the sample investigated exhibits indeed well-defined cylindrical mesopores and essentially no microporosity.
On a side note, it is generally possible to include a distribution of pore sizes into the modeling process in order to achieve better agreement of experimental and theoretical adsorption isotherms. However, the sample’s pore size distribution would also introduce additional (potentially arbitrary) model parameters. Moreover, for the sample investigated, a more accurate description of the adsorption isotherm’s hysteresis loop is unlikely, since the pore diameter of dDBdB = 4.7 nm is slightly below the commonly accepted application limit of DBdB theory (compare ref (78)). As a consequence, we refrained from considering a pore size distribution.
Based on the disjoining pressure Π(h) and the modeling parameter R, we determined the stresses σa,⊥ and σa,∥ according to eqs 10 and 13 assuming a common reference point at the evacuated state (Figure 5). For these calculations, we furthermore applied the surface energy γlv = 8.88 × 10–3 J/m2 and molar volume VL = 34.66 mol/cm3 of liquid N2 at T = 77.4 K.38 The constant strain difference in film and filled pore regime was obtained via the FD equation (eq 12), yielding γs – γsl = 50.6 × 10–3 J/m2.
Figure 5.
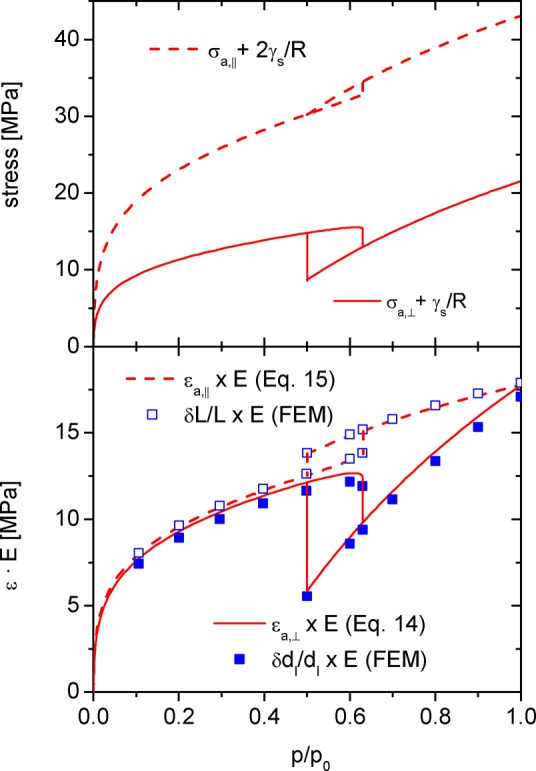
Upper panel: axial and radial stresses derived by eqs 10 and 13 and set to zero stress at vacuum conditions. Lower panel: axial and radial strains derived from the stresses in the upper panel for the single cylindrical tube (eqs 14 and 15) and the FEM model (Figure 3) each multiplied by the Young’s modulus E. For both models, the Poisson’s ratio of the nonporous solid backbone was set to ν = 0.2.
As can be seen from Figure 5, the predicted radial and axial stresses in the pore vary significantly over the whole relative pressure range. For a detailed comparison, we divide the stress isotherms into three relative pressure segments: the film region (0 ≤ p/p0 ≤ 0.5), the hysteresis region (0.5 ≤ p/p0 ≤ 0.65) and the filled pore region (0.65 ≤ p/p0 ≤ 1). In the film region the axial and radial stress (eqs 10a and 10b) differ by a factor of 2 to 3. As described in section 3.1, the numerical dominant Bangham stress in axial direction is twice as large as the Bangham stress in the radial plane of the cylindrical pore for just geometrical reasons; this effect on its own would lead to σa,∥ = 2σa,⊥. On top of that, σa,⊥ is reduced by the Laplace pressure arising from the curved liquid–vapor interface increasing the difference between σa,∥ and σa,⊥ even further.
On the contrary, in the filled pore regime axial and radial stresses are just shifted relative to one another by a constant value, since σa,⊥,filled and σa,∥,filled (eqs 10b and 13b) both depend only on the isostatic capillary pressure pcap within the pore. The constant shift between σa,⊥,filled and σa,∥,filled results again from the cylindrical geometry of the pore and the corresponding difference of the Bangham stress.
For the intermediate hysteresis regime, characteristic hysteresis loops for axial and radial stress are predicted. The hysteresis shape of the radial stress component corresponds to experimental strain data commonly reported for mesoporous materials, i.e., the stress in the filled state is lower than that for the film covered pore (for details, see ref (38)), while the reversed hysteresis is obtained for the axial stress. The result for the hysteresis of the axial stress follows from eq 5, i.e., the axial stress is directly proportional to the negative grand potential of the adsorbate and the filled pore state is energetically lower than the adsorbate film on the pore walls. However, the process of capillary evaporation is a thermodynamic equilibrium transition and consequently there the grand potentials and axial stresses of film and filled pore state are equal.
Applying the derived axial and radial stresses shown in the upper panel of Figure 5 to the mechanical models described in section 3.2, we can predict the axial and circumferential strains of a mesoporous solid, i.e. εa,⊥and εa,∥ for the cylindrical tube (eqs 14 and 15) as well as δdl/dl and δL/L for the hexagonal lattice of cylindrical mesopores (see lower panel of Figure 5). In both models R = 2.35 nm was taken from the modeling of the adsorption isotherm by DBdB theory (Figure 4); furthermore, for the cylindrical tube ϕ was set to ϕstrut = 0.34, while for the lattice model dl = 7.7 nm was calculated from the results of the sample characterization (compare Table 1).
The mechanical properties E and ν of the nonporous solid backbone of our sample are a priori unknown, but—as said before—are expected to be constant and isotropic. Since this makes the Young’s modulus E a simple scaling factor for the strain isotherms, in Figure 5 strain times Young’s modulus is plotted. The impact of the Poisson’s ratio on the strain isotherms is generally more complex, however, for silica based materials we may estimate ν = 0.20 ± 0.05 based on values reported in literature.75 As can be seen from Figure S4 for this limited range of ν the variations of εa,⊥and εa,∥ are rather small making ν = 0.2 a reasonable estimate for considerations preliminary to the actual modeling of experimental data.
The comparison of the strain isotherms (Figure 5, lower panel) shows that axial and circumferential strains derived for the cylindrical tube and the hexagonal lattice, respectively, are very similar. The axial strains of both models are essentially identical, while the circumferential strain in the hexagonal lattice is slightly lower than for the single tube. The latter is the result of the slight systematic deviation between the thickness of the pore walls applied in both models, which in turn follows from the different cross sections of the cylindrical tube and the hexagonal lattice.9 However, since the numerical deviations between the strains predicted by the different mechanical models are minor we conclude that the cylindrical tube is a reasonable approximation of the more complex lattice arrangement making it the preferable model for the evaluation of experimental data due to its simple analytical nature.
Comparing axial and circumferential strains in general, we find the strains to be nearly identical in the film regime, while in the filled pore regime the slope of the circumferential strain isotherm is approximately 3 times larger than the slope of the axial strain isotherm, which is a consequence of the applied Poisson’s ratio of ν = 0.2. With respect to the stress isotherms (Figure 5, upper panel) and their previously discussed differences, the similarity between axial and radial strain may appear counterintuitive, but is a simple consequence of the cylindrical pore geometry and its anisotropic response to axial and radial stress (compare eqs 14 and 15).
Finally, we compare the strain data from the in situ dilatometry experiment with the theoretical strains predicted for the cylindrical tube. Based on the network structure of the sample investigated (Figure 1) we assume that the macroscopically measured strain εdil is a superposition of theoretical axial and circumferential strains of the cylindrical tube
| 17 |
where x is the relative contribution of the circumferential strain to the macroscopic strain and (1 – x) the respective contribution of the axial strain. This approach corresponds to a simplification of the macroporous strut network to two perpendicularly arranged struts as shown in Figure 6, where the circumferential and axial deformation of the individual struts is given by the cylindrical tube model. On a side note, x = 2/3 represents the situation, where the volumetric strain of the monolith is equal to the volumetric strain of the cylindrical tube (eq 16), i.e., εa,vol = 3εdil.
Figure 6.
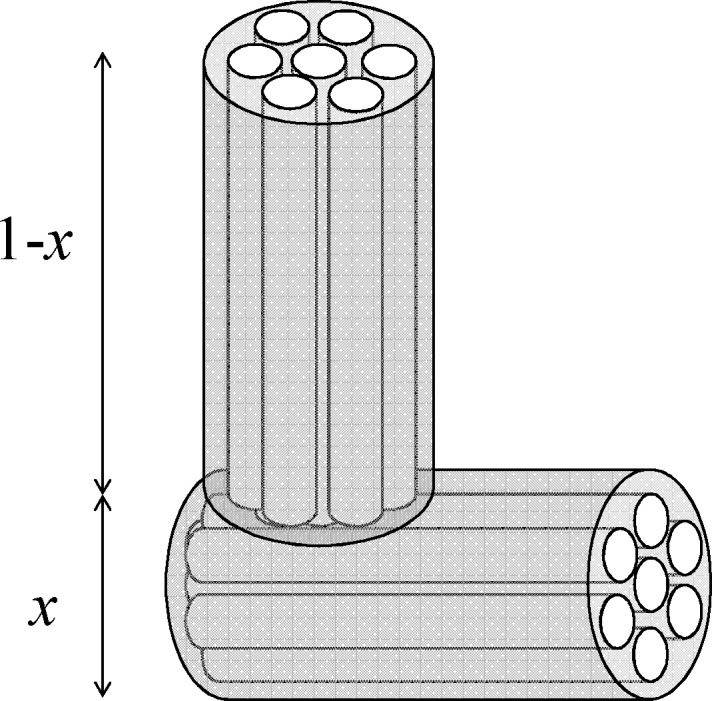
Model of two perpendicularly arranged struts as a representation of the whole strut network. x and 1 – x denote the relative contributions of axial and radial tube strain, respectively, to the effective strain that would be measured in vertical direction.
For the quantitative analysis of the experimental strain data, we start from the filled pore state, where axial and circumferential strains are directly proportional to capillary pressure (compare eqs 10b and 13b). The proportionality of the dilatometric strain in the filled pore regime and capillary pressure is demonstrated in Figure 7. Based on slope and intercept of the linear fit shown in Figure 7, dεdil/dpcap and εdil(p0), respectively, the combination of eq 17 with eqs 10 and 14 as well as eqs13 and 15 yields two correlations for the model parameters:
| 18 |
| 19 |
For eq 18, it was assumed that the impact of the saturation pressure p0 on the theoretical strains is quantitatively negligible resulting in the equality of εa,⊥ and εa,∥ at saturation; this is supported by the strain isotherms shown in Figures 5 and S4, where p0 was included in the calculations. Noteworthy, the reciprocal value of dεdil/dpcap was denoted as pore load modulus by the some of the authors in previous studies;8 here its value is nearly 200 GPa. As shown in refs (8) and (9) and illustrated by eq 19, the pore load modulus is an effective modulus, which depends on various structural and mechanical parameters of the material investigated and should by no means be confused with the Young’s modulus of the nonporous backbone E.
Figure 7.
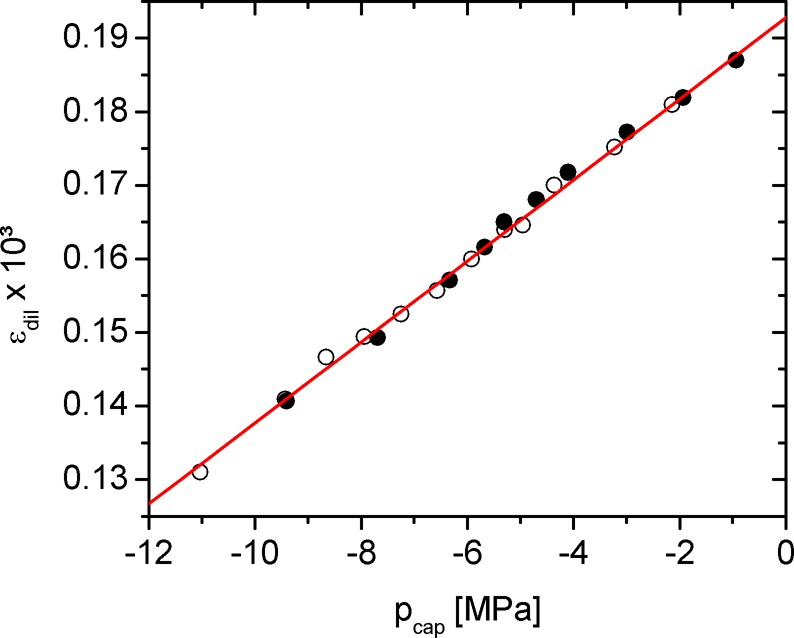
Dilatometric strain εdil for the sample investigated obtained during N2 adsorption at 77 K (compare Figure 4) plotted as a function of the capillary pressure pcap. Note that the zero of the ordinate axis is suppressed.
Inserting eqs 14, 15, 18, and 19 into eq 17 gives the dilatometric strain as a function of the parameters dεdil/dpcap and k, which is another effective mechanical modulus of the sample investigated:
| 20a |
| 20b |
While dεdil/dpcap and εdil(p0) follow from the fit of the dilatometry signal in the filled pore regime shown in Figure 7, γs – γsl and R were already determined from modeling of the experimental adsorption isotherm and the FD equation. Thus, the modeling of the experimental adsorption isotherm and the linear fit of the strain in the filled pore regime are sufficient to predict k and therefore the dilatometric strain isotherm over the whole relative pressure range. Figure 8 shows the dilatometric strain as determined in the experiment and the respective prediction by eq 20 as a function of relative pressure and the specific amount adsorbed assuming the stresses σa,⊥ and σa,∥ shown in Figure 5. Since the application of eq 18 and 19 ensures good agreement between experimental and theoretical strain in the filled pore regime, the quality of the prediction has to be evaluated from film and hysteresis regime. Here we see that the model generally underestimates the dilatometric strain. However, while the discrepancy between theory and experiment is particularly pronounced in the low relative pressure regime for p/p0 < 0.25, it continuously recedes for increasing relative pressure and eventually vanishes at the point of capillary condensation. As a consequence, the hysteresis loop of the strain isotherm is correctly predicted on the qualitative level, i.e., in the hysteresis regime the strain in the filled pore state is lower than in the film state.
Figure 8.
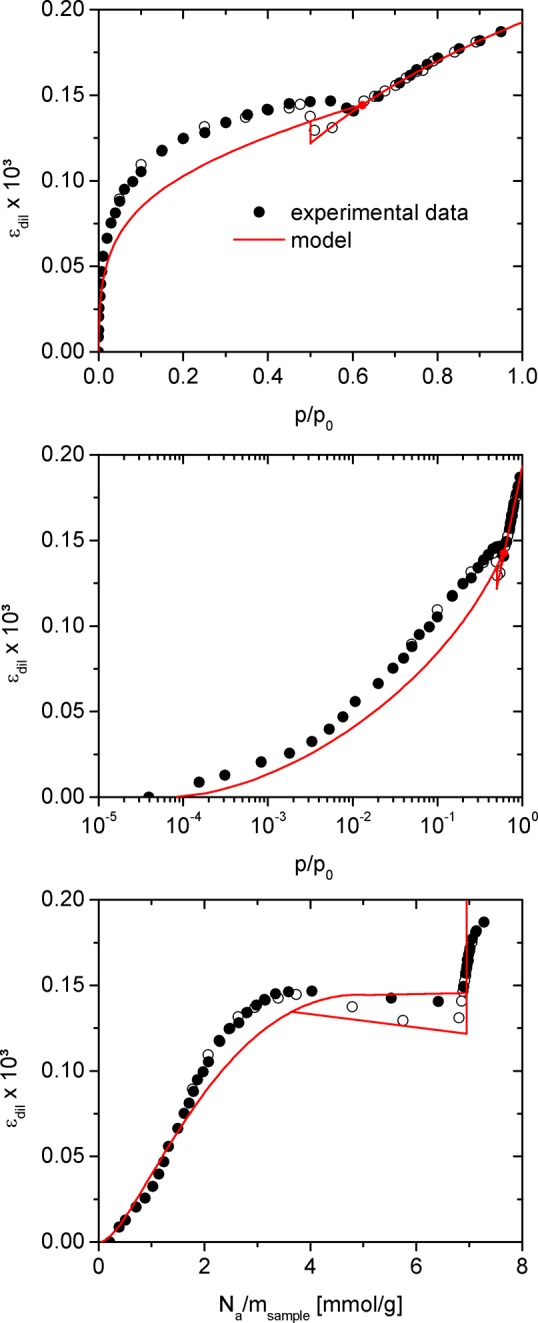
Dilatometric strain εdil for the sample investigated obtained during N2 adsorption at 77 K and the respective prediction from eq 20. The upper panel shows the strains as a function of relative pressure on linear scale, the middle panel as a function of relative pressure on logarithmic scale and the lower panel as a function of specific amount adsorbed. The red dot in upper and middle panel indicates the point of capillary condensation as predicted by the model.
Assuming ν = 0.20 ± 0.05, eqs 18 and 19 yield the parameters E = (92 ± 6) GPa and x = 0.33 ± 0.02 for the sample investigated. Based on the structure shown in Figure 6 the ratio (1 – x)/x should correspond to the average ratio of strut length to strut diameter within our sample, which can be estimated from SEM images (Figure 1) in the range of 2–5 resulting in x ≈ 0.26 ± 0.06. Therefore, the numerical value of x obtained from our model is approximately in line with expectations. Furthermore, x < 0.5 emphasizes that the dilatometric strain predicted by eq 20 is dominated by the axial strain of the mesoporous struts as would be intuitively assumed. The value for E obtained from our model is of a reasonable order of magnitude but appears rather high when e.g. compared to fused silica exhibiting E = 73 GPa.79 At this point, it should be noted, that modeling of the experimental strain isotherm is also possible for a given value of x such as x = 2/3 corresponding to εdil = εa,vol/3 or x = 1 corresponding to εdil = εa,⊥; in this case eqs 18 and 19, directly yield the mechanical parameters E and ν. However, for increasing x and consequently decreasing contribution of axial strain to the dilatometric strain E and ν increase beyond above given values into a range, which appears highly unreasonable for a silica based material. We therefore conclude that consideration of the axial strain εa,∥ is crucial to understand the adsorption-induced deformation of the hierarchically structured porous silica as seen by dilatometry.
Regarding the origin of the quantitative deviations between model and experiment, in particular with respect to the Young’s modulus E, two major explanatory approaches come to mind:
(i) The simplistic model of the strut network shown in Figure 6 neglects potential bending and rearrangement of struts within the network. If the struts bend during the adsorption process, some of their strain is redirected into the macropore volume and consequently the actual strain of the struts is larger than the strain of the network monitored by in situ dilatometry. The Young’s modulus evaluated by our model would thus increase beyond its actual value. The origin of strut bending could be mechanical confinement imposed on the struts by the knots within the network or slight inherent distortions of the cylindrical mesopores within struts. Also the extent of strut bending may increase with the adsorption-induced strain inside the strut.
(ii) The mechanical parameters of the nonporous backbone forming the mesoporous walls are assumed to be isotropic and independent of adsorption as well as strain. While effect (i) potentially causes apparent changes of the mechanical parameters, there may also be actual changes of the stiffness of the nonporous backbone resulting from the strain of the nonporous phase or the adsorption process (see, e.g., refs (80) and (81)).
5. Conclusions
We extended the adsorption stress model for cylindrical mesopores to account for the inherent anisotropy of the cylindrical geometry. This extension leads to the conclusion that adsorbates in cylindrical mesopores exhibit qualitatively and quantitatively different stress components in radial and axial direction of the pore. Explicit expressions for the different stress components were obtained within the theoretical framework proposed by Derjaguin, Broekhoff, and de Boer providing clear analytical solutions. Applying the predicted adsorption stress tensor components to the mechanical models of an individual cylindrical tube and the hexagonal array of cylindrical pores revealed that the tube model is a sufficient approximation of the more complex lattice arrangement. The connection of the extended adsorption stress model, the theory of Derjaguin, Broekhoff, and de Boer and the cylindrical tube model resulted in an analytical description of anisotropic deformation of materials with cylindrical mesopores. Notably, the applied approach could easily be transferred to other pore geometries, in particular, the commonly used slit-shaped pore model.
Applying the proposed theoretical approach to experimental adsorption and strain isotherms measured on a sample of hierarchically structured porous silica, we obtained qualitatively and partially quantitatively consistent descriptions of the experimental data. The analysis clearly suggests that the strain of hierarchically structured silica is dominated by the stress in the axial direction of cylindrical pores, which was previously disregarded. The structural and mechanical parameters obtained from our model are in reasonable agreement with expectations derived from independent measurements or literature. Although there are factors discussed above that may be responsible for the quantitative discrepancies between experimental and predicted strain (in particular the simplification of the macropore structure), the major mechanisms of the sample’s adsorption-induced deformation are apparently covered by the proposed model.
Following our previous work,26 strain isotherms obtained from in situ scattering techniques would be a valuable set of data complementing the presented dilatometric measurement. The advantage of in situ scattering techniques is their capability of directly probing the radial strain on the pore level,8,22,26 i.e., εa,⊥ within our model (Figure S5). Unfortunately, the in situ scattering setup available to us works at ambient conditions only and thus requires adsorbates such as water or pentane, which are significantly more challenging with respect to the modeling of the adsorption process compared to nitrogen. Furthermore, the resolution of adsorption-induced strain as seen by in situ scattering techniques is about 10–4, corresponding to half of the maximum strain detected for the investigated sample by in situ dilatometry (Figure 8). Therefore, even when available, in situ scattering experiments performed for N2 adsorption at 77 K would probably not be able to provide experimental data of sufficient accuracy to validate the results presented in this work. Future work will therefore focus on material–adsorbate combinations exhibiting larger adsorption-induced strains, which can be investigated by in situ dilatometry and in situ scattering techniques, while still allowing for the application of the presented theoretical framework.
Acknowledgments
We are grateful to Lena Weigold and Cornelia Stark (both Bavarian Center for Applied Energy Research) for their support in performing the FEM modeling. We also thank Roland Morak, Lukas Ludescher (both Montanuniversitaet Leoben), and Michael Elsaesser (Paris Lodron University Salzburg) for valuable discussions. All authors except N.B. acknowledge financial support from an international program funded by the Austrian Science Foundation FWF (Project I 1605-N20) and the German Science Foundation DFG (Project RE 1148/10-1) within the framework of the DACH agreement. AVN acknowledges partial support from the NSF Rutgers ERC on structured organic composite systems. AVN thanks George Scherer (Princeton University) for valuable discussions. The work of N.B. was supported by Office of Naval Research through the U.S. Naval Research Laboratory's basic research program.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.langmuir.7b00468.
Derivation of the Frumkin–Derjaguin equation for cylindrical pores; derivation of the axial stress in the cylindrical pore according to DBdB theory; solution of the Lamé problem for the cylindrical tube; determination of the reference isotherm for DBdB theory; dependence of axial and circumferential strain on the Poisson’s ratio; prediction of strain isotherm from in-situ scattering (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Gor G. Y.; Huber P.; Bernstein N. Adsorption-Induced Deformation of Nanoporous Materials - a Review. Appl. Phys. Rev. 2017, 4, 011303. 10.1063/1.4975001. [DOI] [Google Scholar]
- Whipple F. J. W. The Theory of the Hair Hygrometer. Proc. Phys. Soc. London 1921, 34, i–v. 10.1088/1478-7814/34/1/312. [DOI] [Google Scholar]
- Meehan F. T. The Expansion of Charcoal on Sorption of Carbon Dioxide. Proc. R. Soc. London, Ser. A 1927, 115, 199–207. 10.1098/rspa.1927.0085. [DOI] [Google Scholar]
- Balzer C.; Cimino R. T.; Gor G. Y.; Neimark A. V.; Reichenauer G. Deformation of Microporous Carbons during N2, Ar, and CO2 Adsorption: Insight from the Density Functional Theory. Langmuir 2016, 32, 8265–8274. 10.1021/acs.langmuir.6b02036. [DOI] [PubMed] [Google Scholar]
- Kowalczyk P.; Balzer C.; Reichenauer G.; Terzyk A. P.; Gauden P. A.; Neimark A. V. Using in-situ adsorption dilatometry for assessment of micropore size distribution in monolithic carbons. Carbon 2016, 103, 263–272. 10.1016/j.carbon.2016.02.080. [DOI] [Google Scholar]
- Reichenauer G.; Scherer G. W. Nitrogen Adsorption in Compliant Materials. J. Non-Cryst. Solids 2000, 277, 162–172. 10.1016/S0022-3093(00)00304-5. [DOI] [Google Scholar]
- Mogilnikov K. P.; Baklanov M. R. Determination of Young’s modulus of porous low-k films by ellipsometric porosimetry. Electrochem. Solid-State Lett. 2002, 5, F29–F31. 10.1149/1.1517771. [DOI] [Google Scholar]
- Prass J.; Muter D.; Fratzl P.; Paris O. Capillarity-driven deformation of ordered nanoporous silica. Appl. Phys. Lett. 2009, 95, 083121. 10.1063/1.3213564. [DOI] [Google Scholar]
- Gor G. Y.; Bertinetti L.; Bernstein N.; Hofmann T.; Fratzl P.; Huber P. Elastic response of mesoporous silicon to capillary pressures in the pores. Appl. Phys. Lett. 2015, 106, 261901. 10.1063/1.4923240. [DOI] [Google Scholar]
- Van Opdenbosch D.; Fritz-Popovski G.; Wagermaier W.; Paris O.; Zollfrank C. Moisture-Driven Ceramic Bilayer Actuators from a Biotemplating Approach. Adv. Mater. 2016, 28, 5235–5240. 10.1002/adma.201600117. [DOI] [PubMed] [Google Scholar]
- Boudot M.; Elettro H.; Grosso D. Converting Water Adsorption and Capillary Condensation in Usable Forces with Simple Porous Inorganic Thin Films. ACS Nano 2016, 10, 10031–10040. 10.1021/acsnano.6b04648. [DOI] [PubMed] [Google Scholar]
- Bangham D. H.; Fakhoury N. The Swelling of Charcoal. Part I - Preliminary Experiments with Water Vapour, Carbon Dioxide, Ammonia, and Sulphur Dioxide. Proc. R. Soc. London, Ser. A 1930, 130, 81–89. 10.1098/rspa.1930.0189. [DOI] [Google Scholar]
- McIntosh R.; Haines R. S.; Benson G. C. The Effect of Physical Adsorption on the Electrical Resistance of Activated Carbon. J. Chem. Phys. 1947, 15, 17–27. 10.1063/1.1746280. [DOI] [Google Scholar]
- Amberg C. H.; McIntosh R. A Study of Adsorption Hysteresis by Means of Length Changes of a Rod of Porous Glass. Can. J. Chem. 1952, 30, 1012–1032. 10.1139/v52-121. [DOI] [Google Scholar]
- Yates D. J. C. The Expansion of Porous Silica Glass Produced by the Adsorption of Non-Polar Gases at Liquid Air Temperatures. Trans. Br. Ceram. Soc. 1955, 54, 272–299. [Google Scholar]
- Lakhanpal M. L.; Flood E. A. Stresses and Strains in Adsorbate-Adsorbent Systems IV. Contractions of Activated Carbon on Adsorption of Gases and Vapors at Low Initial Pressures. Can. J. Chem. 1957, 35, 887–899. 10.1139/v57-121. [DOI] [Google Scholar]
- Bering B. P.; Krasil'nikova O. K.; Sarakhov A. I.; Serpinskii V. V.; Dubinin M. M. Alteration of Zeolite Granule Dimensions under Krypton Adsorption. Bull. Acad. Sci. USSR, Div. Chem. Sci. 1977, 26, 2258–2261. 10.1007/BF00958705. [DOI] [Google Scholar]
- Tvardovski A. V.; Fomkin A. A.; Tarasevich Y. I.; Polyakova I. G.; Serpinski V. V.; Guseva I. M. Investigation of Cation-Substituted Vermiculite Deformation Upon Water Vapor Sorption. J. Colloid Interface Sci. 1994, 164, 114–118. 10.1006/jcis.1994.1149. [DOI] [Google Scholar]
- Yakovlev V. Y.; Fomkin A. A.; Tvardovski A. V. Adsorption and deformation phenomena at the interaction of CO2 and a microporous carbon adsorbent. J. Colloid Interface Sci. 2003, 268, 33–36. 10.1016/S0021-9797(03)00696-9. [DOI] [PubMed] [Google Scholar]
- Balzer C.; Braxmeier S.; Neimark A. V.; Reichenauer G. Deformation of Microporous Carbon during Adsorption of Nitrogen, Argon, Carbon Dioxide, and Water Studied by in Situ Dilatometry. Langmuir 2015, 31, 12512–12519. 10.1021/acs.langmuir.5b03184. [DOI] [PubMed] [Google Scholar]
- Dendooven J.; Devloo-Casier K.; Levrau E.; Van Hove R.; Pulinthanathu Sree S.; Baklanov M. R.; Martens J. A.; Detavernier C. In Situ Monitoring of Atomic Layer Deposition in Nanoporous Thin Films Using Ellipsometric Porosimetry. Langmuir 2012, 28, 3852–3859. 10.1021/la300045z. [DOI] [PubMed] [Google Scholar]
- Günther G.; Prass J.; Paris O.; Schoen M. Novel Insights into Nanopore Deformation Caused by Capillary Condensation. Phys. Rev. Lett. 2008, 101, 086104. 10.1103/PhysRevLett.101.086104. [DOI] [PubMed] [Google Scholar]
- Dolino G.; Bellet D.; Faivre C. Adsorption strains in porous silicon. Phys. Rev. B: Condens. Matter Mater. Phys. 1996, 54, 17919–17929. 10.1103/PhysRevB.54.17919. [DOI] [PubMed] [Google Scholar]
- Reichenauer G.; Wiener M.; Brandt A.; Wallacher D.. In-situ monitoring of the deformation of nanopores due to capillary forces upon vapor sorption. In BENSC Experimental Reports 2008; Rödig A., Brandt A., Graf H. A., Eds.; Berlin Neutron Scattering Center, 2009; p 216. [Google Scholar]
- Shao L. H.; Jin H. J.; Viswanath R. N.; Weissmuller J. Different measures for the capillarity-driven deformation of a nanoporous metal. Europhys. Lett. 2010, 89, 66001. 10.1209/0295-5075/89/66001. [DOI] [Google Scholar]
- Balzer C.; Morak R.; Erko M.; Triantafillidis C.; Hüsing N.; Reichenauer G.; Paris O. Relationship Between Pore Structure and Sorption-Induced Deformation in Hierarchical Silica-Based Monoliths. Z. Phys. Chem. 2015, 229, 1189–1209. 10.1515/zpch-2014-0542. [DOI] [Google Scholar]
- Grosman A.; Puibasset J.; Rolley E. Adsorption-induced strain of a nanoscale silicon honeycomb. EPL 2015, 109, 56002. 10.1209/0295-5075/109/56002. [DOI] [Google Scholar]
- Jakubov T. S.; Mainwaring D. E. Adsorption-induced dimensional changes of solids. Phys. Chem. Chem. Phys. 2002, 4, 5678–5682. 10.1039/b206883d. [DOI] [Google Scholar]
- Ravikovitch P. I.; Neimark A. V. Density functional theory model of adsorption deformation. Langmuir 2006, 22, 10864–10868. 10.1021/la061092u. [DOI] [PubMed] [Google Scholar]
- Ustinov E. A.; Do D. D. Effect of adsorption deformation on thermodynamic characteristics of a fluid in slit pores at sub-critical conditions. Carbon 2006, 44, 2652–2663. 10.1016/j.carbon.2006.04.015. [DOI] [Google Scholar]
- Rusanov A. I.; Kuni F. M. On the theory of the mechanochemical sorption-striction phenomenon in nanoporous bodies with dispersion forces. Russ. J. Gen. Chem. 2007, 77, 371–392. 10.1134/S1070363207030097. [DOI] [Google Scholar]
- Do D. D.; Nicholson D.; Do H. D. Effects of Adsorbent Deformation on the Adsorption of Gases in Slitlike Graphitic Pores: A Computer Simulation Study. J. Phys. Chem. C 2008, 112, 14075–14089. 10.1021/jp8032269. [DOI] [Google Scholar]
- Grosman A.; Ortega C. Influence of elastic deformation of porous materials in adsorption-desorption process: A thermodynamic approach. Phys. Rev. B: Condens. Matter Mater. Phys. 2008, 78, 085433. 10.1103/PhysRevB.78.085433. [DOI] [Google Scholar]
- Kowalczyk P.; Ciach A.; Neimark A. V. Adsorption-Induced Deformation of Microporous Carbons: Pore Size Distribution Effect. Langmuir 2008, 24, 6603–6608. 10.1021/la800406c. [DOI] [PubMed] [Google Scholar]
- Gunther G.; Schoen M. Sorption strain as a packing phenomenon. Phys. Chem. Chem. Phys. 2009, 11, 9082–9092. 10.1039/b903514a. [DOI] [PubMed] [Google Scholar]
- Gunther G.; Schoen M. Sorption strains and their consequences for capillary condensation in nanoconfinement. Mol. Simul. 2009, 35, 138–150. 10.1080/08927020802412370. [DOI] [Google Scholar]
- Mushrif S. H.; Rey A. D. An integrated model for adsorption-induced strain in microporous solids. Chem. Eng. Sci. 2009, 64, 4744–4753. 10.1016/j.ces.2009.04.014. [DOI] [Google Scholar]
- Gor G. Y.; Neimark A. V. Adsorption-Induced Deformation of Mesoporous Solids. Langmuir 2010, 26, 13021–13027. 10.1021/la1019247. [DOI] [PubMed] [Google Scholar]
- Kowalczyk P.; Furmaniak S.; Gauden P. A.; Terzyk A. P. Carbon Dioxide Adsorption-induced Deformation of Microporous Carbons. J. Phys. Chem. C 2010, 114, 5126–5133. 10.1021/jp911996h. [DOI] [Google Scholar]
- Neimark A. V.; Coudert F. X.; Boutin A.; Fuchs A. H. Stress-Based Model for the Breathing of Metal-Organic Frameworks. J. Phys. Chem. Lett. 2010, 1, 445–449. 10.1021/jz9003087. [DOI] [PubMed] [Google Scholar]
- Vandamme M.; Brochard L.; Lecampion B.; Coussy O. Adsorption and strain: The CO2-induced swelling of coal. J. Mech. Phys. Solids 2010, 58, 1489–1505. 10.1016/j.jmps.2010.07.014. [DOI] [Google Scholar]
- Weissmueller J.; Duan H. L.; Farkas D. Deformation of solids with nanoscale pores by the action of capillary forces. Acta Mater. 2010, 58, 1–13. 10.1016/j.actamat.2009.08.008. [DOI] [Google Scholar]
- Gor G. Y.; Neimark A. V. Adsorption-Induced Deformation of Mesoporous Solids: Macroscopic Approach and Density Functional Theory. Langmuir 2011, 27, 6926–6931. 10.1021/la201271p. [DOI] [PubMed] [Google Scholar]
- Schoen M.; Gunther G. Capillary condensation in deformable mesopores: wetting versus nanomechanics. Mol. Phys. 2011, 109, 83–95. 10.1080/00268976.2010.513346. [DOI] [Google Scholar]
- Brochard L.; Vandamme M.; Pellenq R. J. M.; Fen-Chong T. Adsorption-Induced Deformation of Microporous Materials: Coal Swelling Induced by CO2-CH4 Competitive Adsorption. Langmuir 2012, 28, 2659–2670. 10.1021/la204072d. [DOI] [PubMed] [Google Scholar]
- Brochard L.; Vandamme M.; Pellenq R. J. M. Poromechanics of microporous media. J. Mech. Phys. Solids 2012, 60, 606–622. 10.1016/j.jmps.2012.01.001. [DOI] [Google Scholar]
- Kowalczyk P.; Furmaniak S.; Gauden P. A.; Terzyk A. P. Methane-Induced Deformation of Porous Carbons: From Normal to High-Pressure Operating Conditions. J. Phys. Chem. C 2012, 116, 1740–1747. 10.1021/jp209364x. [DOI] [Google Scholar]
- Diao R.; Fan C.; Do D. D.; Nicholson D. Monte Carlo Simulation of Adsorption-Induced Deformation in Finite Graphitic Slit Pores. J. Phys. Chem. C 2016, 120, 29272–29282. 10.1021/acs.jpcc.6b10135. [DOI] [Google Scholar]
- Gor G. Y.; Bernstein N. Revisiting Bangham’s Law of Adsorption-Induced Deformation: Changes of Surface Energy and Surface Stress. Phys. Chem. Chem. Phys. 2016, 18, 9788–9798. 10.1039/C6CP00051G. [DOI] [PubMed] [Google Scholar]
- Bakhshian S.; Sahimi M. Adsorption-induced swelling of porous media. Int. J. Greenhouse Gas Control 2017, 57, 1–13. 10.1016/j.ijggc.2016.12.011. [DOI] [Google Scholar]
- Bangham D. H.; Fakhoury N. The Translational Motion of Molecules in the Adsorbed Phase on Solids. J. Chem. Soc. 1931, 0, 1324–1333. 10.1039/JR9310001324. [DOI] [Google Scholar]
- Eriksson J. C. Thermodynamics of surface phase systems: V. Contribution to the thermodynamics of the solid-gas interface. Surf. Sci. 1969, 14, 221–246. 10.1016/0039-6028(69)90056-9. [DOI] [Google Scholar]
- Coasne B.; Long Y.; Gubbins K. E. Pressure effects in confined nanophases. Mol. Simul. 2014, 40, 721–730. 10.1080/08927022.2013.829227. [DOI] [Google Scholar]
- Long Y.; Sliwinska-Bartkowiak M.; Drozdowski H.; Kempinski M.; Phillips K. A.; Palmer J. C.; Gubbins K. E. High pressure effect in nanoporous carbon materials: Effects of pore geometry. Colloids Surf., A 2013, 437, 33–41. 10.1016/j.colsurfa.2012.11.024. [DOI] [Google Scholar]
- Long Y.; Palmer J. C.; Coasne B.; Sliwinska-Bartkowiak M.; Jackson G.; Muller E. A.; Gubbins K. E. On the molecular origin of high-pressure effects in nanoconfinement: The role of surface chemistry and roughness. J. Chem. Phys. 2013, 139, 144701. 10.1063/1.4824125. [DOI] [PubMed] [Google Scholar]
- Long Y.; Palmer J. C.; Coasne B.; Sliwinska-Bartkowiak M.; Gubbins K. E. Under pressure: Quasi-high pressure effects in nanopores. Microporous Mesoporous Mater. 2012, 154, 19–23. 10.1016/j.micromeso.2011.07.017. [DOI] [Google Scholar]
- Long Y.; Palmer J. C.; Coasne B.; Sliwinska-Bartkowiak M.; Gubbins K. E. Pressure enhancement in carbon nanopores: a major confinement effect. Phys. Chem. Chem. Phys. 2011, 13, 17163–17170. 10.1039/c1cp21407a. [DOI] [PubMed] [Google Scholar]
- Derjaguin B. V. A theory of capillary condensation in the pores of sorbents and of other capillary phenomena taking into account the disjoining action of polymolecular liquid films. Acta Physicochim. URSS 1940, 12, 181. [Google Scholar]
- Broekhoff J. C. P.; De Boer J. H. Studies on Pore Systems in Catalysts. X. Calculations of Pore Distributions from Adsorption Branch of Nitrogen Sorption Isotherms in Case of Open Cylindrical Pores. B. Applications. J. Catal. 1967, 9, 15–27. 10.1016/0021-9517(67)90175-3. [DOI] [Google Scholar]
- Broekhoff J. C. P.; De Boer J. H. Studies on Pore Systems in Catalysts. IX. Calculation of Pore Distributions from Adsorption Branch of Nitrogen Sorption Isotherms in Case of Open Cylindrical Pores. A. Fundamental Equations. J. Catal. 1967, 9, 8–14. 10.1016/0021-9517(67)90174-1. [DOI] [Google Scholar]
- Kresge C. T.; Leonowicz M. E.; Roth W. J.; Vartuli J. C.; Beck J. S. Ordered Mesoporous Molecular-Sieves Synthesized by a Liquid-Crystal Template Mechanism. Nature 1992, 359, 710–712. 10.1038/359710a0. [DOI] [Google Scholar]
- Zhao D. Y.; Feng J. L.; Huo Q. S.; Melosh N.; Fredrickson G. H.; Chmelka B. F.; Stucky G. D. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548–552. 10.1126/science.279.5350.548. [DOI] [PubMed] [Google Scholar]
- Brandhuber D.; Torma V.; Raab C.; Peterlik H.; Kulak A.; Husing N. Glycol-modified silanes in the synthesis of mesoscopically organized silica monoliths with hierarchical porosity. Chem. Mater. 2005, 17, 4262–4271. 10.1021/cm048483j. [DOI] [Google Scholar]
- Brandhuber D.; Huesing N.; Raab C. K.; Torma V.; Peterlik H. Cellular mesoscopically organized silica monoliths with tailored surface chemistry by one-step drying/extraction/surface modification processes. J. Mater. Chem. 2005, 15, 1801–1806. 10.1039/b417675h. [DOI] [Google Scholar]
- Brunauer S.; Emmett P. H.; Teller E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. 10.1021/ja01269a023. [DOI] [Google Scholar]
- Gurvich L. G. Physio-Chemical Attractive Force. J. Russ. Phys.-Chem. Soc. 1915, 47, 805–827. [Google Scholar]
- Woignier T.; Phalippou J. Skeletal Density of Silica Aerogels. J. Non-Cryst. Solids 1987, 93, 17–21. 10.1016/S0022-3093(87)80024-8. [DOI] [Google Scholar]
- Hill T. L. Theory of Physical Adsorption. Adv. Catal. 1952, 4, 211–258. 10.1016/S0360-0564(08)60615-X. [DOI] [Google Scholar]
- Langmuir I. The adsorption of gases an plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. 10.1021/ja02242a004. [DOI] [Google Scholar]
- Dubinin M. M.; Zaverina E. D.; Radushkevich L. V.; Sorbtsiya I. Struktura Aktivnykh Uglei.1. Issledovanie Adsorbtsii Organicheskikh Parov. Zh. Fiz. Khim. 1947, 21, 1351–1362. [Google Scholar]
- Scherer G. W. Dilatation of Porous Glass. J. Am. Ceram. Soc. 1986, 69, 473–480. 10.1111/j.1151-2916.1986.tb07448.x. [DOI] [Google Scholar]
- Churaev N. V. Wetting Films and Wetting. Rev. Phys. Appl. 1988, 23, 975–987. 10.1051/rphysap:01988002306097500. [DOI] [Google Scholar]
- Derjaguin B. V.; Churaev N. V.; Muller E. A.. Surface Forces; Springer Science+Business Media: New York, 1987. [Google Scholar]
- Timoshenko S.; Goodier J. N.. Theory of Elasticity; McGraw-Hill Book Company: New York, 1951. [Google Scholar]
- Atanackovic T. M.; Guran A.. Theory Of Elasticity For Scientists and Engineers; Birkhäuser: Boston, 2000. [Google Scholar]
- Cook R. D.Finite Element Modeling For Stress Analysis; John Wiley & Sons: New York, 1995. [Google Scholar]
- Thommes M.; Kaneko K.; Neimark A. V.; Olivier J. P.; Rodriguez-Reinoso F.; Rouquerol J.; Sing K. S. W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. 10.1515/pac-2014-1117. [DOI] [Google Scholar]
- Ravikovitch P.; Neimark A. Calculations of Pore Size Distributions in Nanoporous Materials from Adsorption and Desorption Isotherms. Stud. Surf. Sci. Catal. 2000, 129, 597–606. 10.1016/S0167-2991(00)80262-1. [DOI] [Google Scholar]
- Haynes W. M.CRC Handbook of Chemistry and Physics, 96th ed.; Taylor & Francis: London, 2015. [Google Scholar]
- Mouhat F.; Bousquet D.; Boutin A.; Bouessel du Bourg L.; Coudert F. X.; Fuchs A. H. Softening upon Adsorption in Microporous Materials: A Counterintuitive Mechanical Response. J. Phys. Chem. Lett. 2015, 6, 4265–9. 10.1021/acs.jpclett.5b01965. [DOI] [PubMed] [Google Scholar]
- Coasne B.; Haines J.; Levelut C.; Cambon O.; Santoro M.; Gorelli F.; Garbarino G. Enhanced mechanical strength of zeolites by adsorption of guest molecules. Phys. Chem. Chem. Phys. 2011, 13, 20096–20099. 10.1039/c1cp22477h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.