Abstract
We describe the development of a selectable, bi-cistronic subgenomic replicon for bovine viral diarrhea virus (BVDV) in Huh-7 cells, similar to that established for hepatitis C virus (HCV). The selection marker and reporter (Luc-Ubi-Neo) in the BVDV replicon was fused with the amino-terminal protease Npro, and expression of the nonstructural proteins (NS3 to NS5B) was driven by an encephalomyocarditis virus internal ribosome entry site. This BVDV replicon allows us to compare RNA replication of these two related viruses in a similar cellular background and to identify antiviral molecules specific for HCV RNA replication. The BVDV replicon showed similar sensitivity as the HCV replicon to interferons (alpha, beta, and gamma) and 2′-β-C-methyl ribonucleoside inhibitors. Known nonnucleoside inhibitor molecules specific for either HCV or BVDV can be easily distinguished by using the parallel replicon systems. The HCV replicon has been shown to block, via the NS3/4A serine protease, Sendai virus-induced activation of interferon regulatory factor 3 (IRF-3), a key antiviral signaling molecule. Similar suppression of IRF-3-mediated responses was also observed with the Huh-7-BVDV replicon but was independent of NS3/4A protease activity. Instead, the amino-terminal cysteine protease Npro of BVDV appears to be, at least partly, responsible for suppressing IRF-3 activation induced by Sendai virus infection. This result suggests that different viruses, including those closely related, may have developed unique mechanisms for evading host antiviral responses. The parallel BVDV and HCV replicon systems provide robust counterscreens to distinguish viral specificity of small-molecule inhibitors of viral replication and to study the interactions of the viral replication machinery with the host cell innate immune system.
Hepatitis C virus (HCV) and bovine viral diarrhea virus (BVDV) are both members of the Flaviviridae family and share many molecular and virological similarities (20, 27). They both have single-strand RNA genomes that replicate via negative-strand intermediates and produce a single polyprotein that is cleaved into individual proteins by a combination of host and viral proteases. Although HCV is a hepacivirus and BVDV is a pestivirus, there is a low degree of sequence homology between their respective nonstructural proteins and both RNA-dependent RNA polymerases are structurally very similar (9).
HCV is a major etiological agent for viral hepatitis. The World Health Organization estimates that 170 million people are chronically infected with HCV worldwide, and of those, 4 million are in the United States. Within 10 to 20 years of infection, 20 to 30% of chronic carriers develop cirrhosis, making HCV infection one of the major reasons for liver transplantation. Current therapies are limited to interferon treatment, either alone or in combination with ribavirin (23, 38), but patient response for certain genotypes is still unsatisfactory. This unmet medical need has created an urgent demand for the development of new drugs to treat chronic hepatitis C. However, the lack of an in vitro infection system has hampered HCV drug development. For this reason, BVDV has sometimes been used as a surrogate infectivity model in vitro.
BVDV infection represents an economically important disease of cattle. Infection of cattle with BVDV leads to a variety of disorders ranging in severity from subclinical or mild to fatal. The virus is classified into two biotypes, cytopathic (cpBVDV) and noncytopathic (ncpBVDV), based on their effects in tissue culture. cpBVDV induces a type I interferon response in bovine macrophages (1) and leads to apoptotic cell death in cultured cells (14). However, ncpBVDV-infected macrophages do not produce type I interferon, and infected cells do not respond to double-stranded RNA treatment (26, 31). Indeed, the activity of the double-stranded RNA analog, poly(I) · poly(C), against vesicular stomatitis virus is inhibited by ncpBVDV coinfection (28). Infection of cells with ncpBVDV can enhance the replication of other viruses by blocking the production of interferon (15). Production of NS3 generally correlates with cytopathogenicity (24). In ncpBVDV, cleavage at the NS2/3 site does not occur and only uncleaved NS2/3 is observed. In the case of cpBVDV biotypes, both NS3 and NS2/3 products are found in virus-infected cells.
The development of a bi-cistronic subgenomic replicon system for HCV has significantly advanced the pace of HCV drug discovery (5, 22). It facilitates the rapid screening of large compound libraries against multiple viral targets at a high throughput capacity. Additionally, the HCV replicon has been used to characterize drug resistance by either selecting for drug-resistant replicons in vitro (35) or by engineering known drug-resistant mutations into the replicon (39).
Despite these advances, there is a significant drawback in using HCV replicon cell culture for antiviral screening, due to its dependence on cell cycle progression and thus its high sensitivity to cytotoxic or cytostatic agents. In other words, compounds with subtle toxic effects may still cause a reduction in replicon RNA levels by affecting certain aspects of the cell cycle and be identified as active antiviral hits (false positives). Clearly, such compounds should not be pursued for further characterization, as they are not direct antivirals and are likely replicon artifacts. In this case, the preferred compounds should possess potent activity against viral targets but have minimal effect on cellular functions. In search of HCV-specific, non-nucleoside-based antivirals, it is reasonable to assume that compounds highly active against HCV but with little or no activity against a closely related virus (e.g., BVDV) are likely to be HCV specific and less likely to have undesirable cellular toxicity. Currently, BVDV testing is typically performed with live viruses in a bovine cell line, such as Madin-Darby bovine kidney cells. The drastic difference in cellular background between Madin-Darby cells and Huh-7 cells, in addition to the difference in viral RNA replication dynamics between a live virus infection, which is much less sensitive to the cytotoxicity induced by the test compounds, and replicon RNA synthesis in a stable cell culture system further compromises the validity of using a BVDV infectivity assay to determine the selectivity for potential HCV inhibitors. To address this issue, we developed a bi-cistronic, subgenomic replicon for BVDV that replicates efficiently in Huh-7 cells. This enables us to compare RNA replication of HCV and BVDV in similar cellular backgrounds and to identify antiviral compounds specific for HCV in the absence of significant cytotoxicity. We demonstrate the utility of this approach with interferon and other known antiviral molecules. We also compare how the two replicons are able to evade the host immune system.
MATERIALS AND METHODS
Cell lines and culture conditions.
Huh-7 cell lines were grown as described previously (8). Neomycin-resistant lines were grown in the presence of G418 at 0.25 to 0.5 mg/ml.
Construction of subgenomic BVDV reporter replicons.
Bi-cistronic reporter replicons were constructed by replacement of the genes that are not required for replication with marker-reporter genes and the encephalomyocarditis virus (EMCV) internal ribosome entry site (IRES) (16). The segment of the BVDV genome (strain NADL, cytopathic biotype) encoding the portion of the polyprotein comprising C, Erns, E1, E2, P7, and NS2 was replaced with two elements in tandem: (i) a firefly luciferase reporter and neomycin phosphotransferase selection cassette, inserted in frame with the Npro coding region, and (ii) the IRES element from EMCV, inserted such that translation started at a methionine codon added upstream of glycine 1801, at the N terminus of NS3 (11, 34). The luciferase reporter and the neomycin selection marker were separated by an in-frame 76-codon sequence encoding monomeric human ubiquitin (33). These sequences were inserted into the C-NS2 deletion window of the infectious clone (pNADLp15), giving rise to the reporter replicon termed pNS3-5B (also called pLN-BR) (2, 36, 37). DNA segments were joined by overlap extension PCR and ligated into the pNADLp15 plasmid using standard methods to create pLN-BR. This plasmid was further modified to create pN-BR by subcloning a fragment from an XhoI site in the 5′ untranslated region to a NotI site at the 3′ end of the neomycin phosphotransferase gene. QuikChange XL reactions were performed to remove the luciferase and ubiquitin regions of the construct. The new XhoI-NotI fragment was sequenced and reinserted into pLN-BR, giving rise to pN-BR, a selectable BVDV replicon. The construction and characterization of the HCV replicon have been described elsewhere (22).
Sequence analysis.
Sequencing was performed with the Big Dye terminator cycle sequencing kit with AmpliTaq (Applied Biosystems) and analyzed with an Applied Biosystems model 373A sequencer.
In vitro transcription.
SacII-linearized plasmids encoding BVDV replicons were in vitro transcribed by using T7 RNA polymerase with an Ambion Megascript kit under nuclease-free conditions following the manufacturer's instructions. The reaction was terminated by incubation with DNase I and precipitation with LiCl according to the manufacturer's instructions. RNA was resuspended in nuclease-free water, quantified by absorbance at 260 nm, immediately frozen in dry ice in 10-μg aliquots, and stored at −80°C.
Transfection of HCV and BVDV replicon RNA and selection of colonies.
Subconfluent Huh-7 cells were washed in flasks with 1× Earl's salt and then trypsinized. The cells were washed with ice-cold phosphate-buffered saline, resuspended at 107 cells/ml in Cytomix (120 mM KCl, 0.15 mM CaCl2, 10 mM K2HPO4/KH2PO4, 25 mM HEPES, 2 mM EGTA, 5 mM MgCl2; pH 7.6) containing 2 mM ATP plus 5 mM glutathione and 1.25% dimethyl sulfoxide and kept on ice. Then, 0.4-ml aliquots (4 × 106 cells) were subjected to electroporation with 1 μg of in vitro-transcribed RNA and 9 μg of carrier RNA (total yeast RNA) by pulsing at 270 V and 975 μF with a Bio-Rad Genepulser II. Electroporated cells were diluted with 10 ml of growth medium and plated evenly onto three 10-cm dishes. After 24 h cells were washed twice with phosphate-buffered saline and new growth medium was added. After 72 h medium was replaced with selection medium (G418) at concentrations ranging between 0.250 and 1 mg/ml. G418-resistant cells were picked from the cultures with 0.250 mg of G418/ml and expanded by growth in individual plates.
Northern blotting.
For Northern blotting, 10 μg of total RNA extracted from Huh-7-derived cells was subjected to electrophoresis on a 1% agarose-formaldehyde gel, blotted onto Ambion BrightStar-Plus membranes, and hybridized to a replicon RNA probe. Electrophoresis, blotting, and hybridization procedures were performed by following the Northern Max instruction manual (Ambion).
TaqMan quantification of replicon RNA.
Replicon RNA was quantified by a real-time, 5′-exonuclease PCR (TaqMan) assay with a primer-probe set that recognized a portion of the neomycin gene. The primers were selected with the Primer Express software (PE Applied Biosystems). TaqMan analysis was performed with the neomycin primer set neo-15F (TGGATTGCACGCAGGTTCT) and neo-76R (GTGCCCAGTCATAGCCGAAT) and probe neo-35T (6-carboxyfluorescein-CGGCCGCTTGGGTGGAGAGG-N,N,N′,N′-tetramethyl-6-carboxyrhodamine). The primers were used at 375 nM, and the probe was used at 312.5 nM in a 20-μl reaction volume. The reactions were performed with a TaqMan Gold reverse transcriptase PCR (RT-PCR) kit (PE Applied Biosystems) and included a 30-min reverse transcription step at 48°C, followed by 10 min at 95°C and 40 cycles of amplification under the universal TaqMan standardized conditions (15 s of denaturation at 95°C followed by a 1-min annealing-extension step at 60°C). Human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA, quantified with a specific primer set (PE Applied Biosystems), was used as an endogenous reference.
Compounds and reagents.
Alpha interferon 2b (IFN-α2b; intron A; Schering-Plough), IFN-β (BioSource), and IFN-γ (InterMune) were purchased from commercial sources. The interferon sensitive response element (ISRE) reporter plasmid was a kind gift from Nancy Reich, SUNY—Stony Brook. The ISG56, RANTES, and IFN-β reporter plasmids were kind gifts from Michael Gale, Jr., University of Texas Southwestern Medical Center.
Luciferase assays.
BVDV/Huh-7 or HCV/Huh-7 cells expressing luciferase were plated in white 96-well plates at a density of 6,000 cells/well in 90 μl of medium. The cells were allowed to attach overnight (∼18 h) before treatment (typically 10 μl of compound). Following a further incubation of 6 to 48 h, the plates were allowed to cool to room temperature and an equal volume of Bright-Glo reagent (Promega) was added, and the plates were read in luminescent mode on an LJL Analyst plate reader (Molecular Devices).
DNA transfections.
Transfection of plasmid DNA was carried out with FuGENE 6 transfection reagent (Roche Molecular Biochemicals). For each luciferase reporter experiment, we transfected cells in multiple wells of 24-well plates. Each well contained 4 × 104 cells in 0.5 ml of medium and was transfected with a master mixture containing pCMV Renilla-luc (Promega) to monitor transfection efficiency and the indicated interferon regulatory factor 3 (IRF-3)-dependent promoter-firefly luciferase reporter plasmid. The dual-luciferase reporter assay system (Promega) was used to quantify luciferase activity. The assays were carried out with 10 μl of cell lysate and 50 μl of assay reagent. Luciferase activity was measured with a TR717 dual-injector microplate luminometer (Applied Biosystems) over two 5-s intervals, each preceded by a 2-s delay after the addition of the assay reagent to the cell lysate. All analyses were conducted on cells or cell extracts 48 to 72 h posttransfection.
Protease cloning and mutagenesis.
PCR products generated from replicon plasmids with primers designed to amplify either the HCV NS3/4A, BVDV NS3/4A, or BVDV Npro regions were cloned into pcDNA3.1/Hygro(-) using engineered XhoI and HindIII restriction sites.
Residues essential for Npro protease activity, glutamic acid 22 (Glu22) and histidine 49 (His49) (30), were both mutated to alanine in the BVDV Npro expression plasmid by using the QuikChange XLII site-directed mutagenesis kit (Invitrogen).
RESULTS
Construction of BVDV subgenomic replicons.
BVDV replicons were constructed from the cytopathic NADL strain (Fig. 1A) as described in Materials and Methods. Briefly, viral genes encoding C, Erns, E1, E2, P7, and NS2 were replaced with a luciferase-ubiquitin-neomycin (Luc-Ubi-Neo) reporter-selection cassette and the IRES element from EMCV (pLN-BD) (Fig. 1B). Translation of the second cistron is driven by the EMCV IRES, leading to the production of BVDV nonstructural proteins required for replicon RNA replication. A second replicon, pN-BR (Fig. 1C), was also produced. The luciferase and ubiquitin regions of pLN-BD were removed, leaving the Npro gene fused directly to the neomycin phosphotransferase gene. These constructs correspond to the HCV constructs I389luc-ubi-neo/NS3-3′/wt/5.1 (17) and I389neo/NS3-3′/wt/5-15 (22), respectively. They also retain the complete coding sequence of the Npro cysteine protease, an autoprotease used to release the downstream capsid protein via proteolytic cleavage. A similar construct lacking Npro was found unable to replicate in Huh-7 cells previously, suggesting that the inclusion of Npro may help establish stable replication of the BVDV replicon in the human cell line (data not shown).
FIG. 1.
Schematic representation of the BVDV genome and subgenomic replicons. (A) Genome organization of BVDV. (B) Structural genes C, Erns, E1, E2, P7, and NS2 were replaced with a luciferase-ubiquitin-neomycin phosphotransferase cassette (Luc-Ubi-Neo) to create pLN-BR. (C) Further deletion of the luciferase and ubiquitin genes produced pN-BD.
The BVDV replicon replicates in Huh-7 cells.
In vitro transcripts derived from pLN-BR and PN-BR constructs, as well as from HCV NK5.1 replicon construct (17), were electroporated into Huh-7 cells. Transfected cells were grown for 2 to 3 weeks under G418 selection, and G418-resistant colonies were formed. No significant difference in the rate of colony formation between these constructs was observed (Fig. 2A). A number of well-isolated colonies were then picked, expanded, and tested for the presence of BVDV replicon RNA by quantitative RT-PCR (Fig. 2B) and Northern blot analysis (Fig. 2C). As shown, these analyses revealed that high levels of BVDV replicon RNA, comparable to those of HCV replicon, were present in the BVDV replicon cells, confirming that the subgenomic BVDV RNA was capable of replicating efficiently and establishing persistence in Huh-7 cells.
FIG. 2.
Establishment of the BVDV replicon in Huh-7 cells. (A) Colony-forming abilities of RNA transcribed from, left to right, pLN-BR, pN-BR, and HCV replicon I389luc-ubi-neo/NS3-3′/wt/5.1. (B) TaqMan quantitative PCR analysis of RNA samples isolated from the indicated cell clones. A specific probe for the neomycin phosphotransferase sequence (neo) was used to quantitate replicon RNA, and a GAPDH probe was used to quantitate the host gene. Higher Ct values indicate lower RNA abundance. Respective replicon RNA was detected in BVDV and HCV cell lines but not in parental Huh-7 cells. Similar levels of GAPDH mRNA were present in all samples. (C) Northern blot analysis of total RNA isolated from the replicon cells. A radiolabeled probe corresponding to a portion of the neomycin phosphotransferase gene was used to detect the replicon RNA, and a GAPDH probe was used as RNA control. One cell clone from pLN-BR (BVDV Luc-Ubi-Neo) (lane 1), two from pN-BR (BVDV Neo1 and Neo2) (lanes 2 and 3), and two from HCV NK5.1 (HCV Neo1 and Neo2) (lanes 4 and 5) were analyzed. 5-15 is a previously established HCV replicon cell line.
No adaptive mutations in BVDV replicon RNA were isolated from Huh-7 cells.
Accumulation of adaptive mutations is essential for efficient replication of HCV replicon RNA in Huh-7 cells (17, 21). To determine whether similar adaptive mutations are also needed for replication of the BVDV replicon, total cellular RNA was isolated from several independent Huh-7-BVDV replicon cell lines, and viral genes were amplified by RT-PCR followed by sequencing analysis. Surprisingly, the complete sequence of the recovered BVDV genome did not reveal any consensus adaptive mutations (data not shown), indicating that the original BVDV replicon RNA was capable of replicating in Huh-7 cells.
The BVDV replicon in Huh-7 cells is sensitive to interferon treatment.
Current treatment for HCV infection is (pegylated) IFN-α either alone or in combination with ribavirin (23). The HCV replicon has been shown to be sensitive to IFN-α, -β, and -γ (7), and BVDV is known to be interferon sensitive (6, 32). We compared the sensitivity of the Huh-7-BVDV and Huh-7-HCV replicons against IFN-α, -β, and -γ (Fig. 3). The replicon cells were incubated with 0 to 10 IU of IFN-α, -β, or -γ/ml for 48 h, and inhibition of replicon RNA synthesis was determined. As shown in Fig. 3, 50% effective concentration (EC50) values for the interferons tested were comparable in both replicon systems. In Huh-7-BVDV and Huh-7-HCV cells treated with IFN-α, the EC50 values were 0.2 and 0.4 IU/ml, respectively (Fig. 3A). IFN-β was slightly less effective, with an EC50 value of 2.6 IU/ml in both replicon cell lines (Fig. 3B), while IFN-γ had EC50 values of 0.3 and 0.6 IU/ml for Huh-7-HCV and Huh-7-BVDV replicons, respectively (Fig. 3C).
FIG. 3.
Effects of interferons on BVDV and HCV replicon RNA replication in Huh-7 cells. Luciferase-expressing BVDV/Huh7 or HCV/Huh7 cells in 96-well plates were treated with the indicated amounts of IFN-α (A), IFN-β (B), or IFN-γ (C) for 48 h. Luciferase signal detected in the absence of interferons was defined as 100% for each data set, and the remaining data points were expressed as a percentage of the response in untreated cells.
Parallel replicon assays for identification of virus-specific inhibitors.
Although BVDV and HCV are closely related, there is low sequence similarity or identity between the corresponding proteins. The high degree of structural similarity between the polymerase motifs of BVDV and HCV NS5B (9, 18) suggests that compounds that inhibit viral replication by targeting the catalytic site of the polymerase (e.g., nucleoside- or nucleotide-based inhibitors) will likely be inhibitory in both systems. However, compounds that are inhibitory but target less-conserved regions of the polymerase enzyme may not be cross-active against both viruses. This should be the case for most, if not all, non-nucleoside-based inhibitors. By utilizing the parallel Huh-7-HCV and Huh-7-BVDV replicon systems, it is possible to quickly identify inhibitors specifically inhibiting HCV RNA replication and eliminate those affecting replicon RNA synthesis nonspecifically by inhibiting a host function or via general cytotoxicity.
To validate such an approach, we tested the Huh-7-HCV and Huh-7-BVDV replicons in parallel against a number of inhibitor compounds with known specificities (Fig. 4). 2′-β-C-methyl ribonucleosides are known to inhibit HCV polymerase by incorporation into viral RNA and causing chain termination (12). As substrate-based inhibitors targeting the catalytic center of the polymerase enzyme, this class of compounds would be predicted to be active against BVDV as well. As expected, 2′-β-C-methyl adenosine (Fig. 4A) and 2′-β-C-methyl cytosine (Fig. 4B) showed comparable EC50 values in the parallel replicon assays. For 2′-β-C-methyl adenosine, the EC50 values against Huh-7-BVDV and Huh-7-HCV were 0.4 and 1.2 μM, respectively, while for 2′-β-C-methyl cytosine those values were 0.3 and 3.0 μM, respectively. In contrast, when two nonnucleoside polymerase inhibitors were tested, a greater difference in virus specificity was observed. VP32947 is a triazinoindole derivative that is a potent inhibitor of BVDV polymerase which acts by targeting a region near the amino terminus and away from the active site (3); GSK compound 4 (GSK-4) is a benzothiadiazine derivative that shows specific activity against the HCV polymerase (25). As shown in Fig. 4C, VP32947 showed potent activity against Huh-7-BVDV, with an EC50 of <0.14 μM, but was not active against Huh-7-HCV. The EC50 of VP32947 against BVDV-Huh-7 was subsequently determined to be 25 nM, giving a selectivity index (SI) of 12,000 (data not shown). Conversely, for GSK-4 the EC50 values against Huh-7-BVDV and Huh-7-HCV were 25 and 0.4 μM, respectively, which resulted in an SI of 63 (Fig. 4D). The relatively low SI for GSK-4 may be due to the intrinsic cytotoxicity associated with the compound, which affected both replicons. Taken together, these results illustrated the utility of the parallel replicon assays in quickly separating antiviral activity from cytotoxicity and the identification of compounds with high specificity towards HCV.
FIG. 4.
Demonstration of viral specificity with known inhibitor compounds. BVDV/Huh7 or HCV/Huh7 cells expressing luciferase were plated in white 96-well plates and allowed to attach overnight (∼18 h) before treatment with known small-molecule inhibitors for a further 48 h. (A and B) 2′-β-C-methyl adenosine (A) and 2′-β-C-methyl cytosine (B) showed comparable EC50 values in the parallel replicon assays. (C) VP32947, a triazinoindole derivative, showed potent activity against BVDV/Huh-7, with an EC50 of <0.14 μM, but was not active against HCV/Huh-7. (D) GSK-4, a benzothiadiazine derivative, showed specific activity against HCV/Huh7 but much lower activity against BVDV/Huh7.
BVDV replicon blocks SV-induced IRF-3 activation.
It has been reported that the HCV replicon blocks ISRE-mediated transcription by preventing the phosphorylation and subsequent nuclear translocation of IRF-3 (13). In addition, BVDV has also been shown to block IFN-β transcription in bovine cells (4). Given the similarity between HCV and BVDV, we investigated the effect of the BVDV replicon RNA in Huh-7 cells on ISRE-dependent transcription upon SV infection, which is a strong inducer of IRF-3 activation. Briefly, control Huh-7 cells and several Huh-7-BVDV or Huh-7-HCV replicon cell lines were transfected with a firefly luciferase reporter plasmid under the control of an ISRE. These cells were then infected with SV 24 h after transfection. Following an 18-h incubation, the cells were lysed and the production of luciferase was quantified. Luciferase signals were normalized for transfection efficiency against a constitutively active Renilla luciferase reporter cotransfected into the cells, and the data are expressed as the fold increase before and after SV infection in firefly luciferase signal relative to that in uninfected Huh-7 cells. As shown in Fig. 5A, similar to Huh-7-HCV replicon cell lines (5-15 and HCV Neo1, -2, and -6), all of the Huh-7-BVDV cell lines tested (BVDV Neo1, -2, -3, and -5), but not the parental Huh-7 cells, showed a remarkable suppression of ISRE-mediated transcription. Furthermore, up-regulation of transcription from other IRF-3-responsive promoters, ISG56, RANTES, and IFN-β, were all similarly blocked (Fig. 5B to D). This result demonstrated that similar to HCV, BVDV also develops a mechanism to suppress SV-induced IRF-3 activation in the Huh-7 replicon cells.
FIG. 5.
BVDV replicon blocks IRF-3-dependent transcription after SV infection. Parental Huh-7 cells and several BVDV/Huh7 or HCV/Huh7 cell lines (40,000 cells/well in a 24-well plate) were transfected with a firefly luciferase reporter plasmid under the control of an IRF-3-responsive promoter. Cells were infected with SV 24 h after transfection. After an 18-h incubation postinfection, the cells were lysed and the production of luciferase was quantified. Luciferase signals were normalized for transfection efficiency against a constitutively active Renilla luciferase reporter cotransfected into the cells, and the data are expressed as the fold increase before and after SV infection in firefly luciferase signal relative to that in uninfected Huh-7 cells. Both BVDV/Huh-7 and HCV/Huh-7 blocked SV-induced transcription from ISRE (A), ISG56 (B), RANTES (C), and IFN-β (D) promoters. Three independent HCV replicon clones (HCV Neo1, Neo2, and Neo6) and four independent BVDV replicon clones (BVDV Neo1, Neo2, Neo3, and Neo5) were tested in addition to parental Huh-7 and 5-15 cells. Error bars represent the standard deviations.
Suppression of SV-induced activation of IRF-3 is dependent on the presence of BVDV RNA.
It is clear that the SV-induced activation of IRF-3 and subsequent IRF-3-dependent transcription were strongly suppressed in the Huh-7-BVDV replicon cell lines. However, these cell lines were generated through long-term drug selection with G418, and it is conceivable that excessive exposure to G418 might have caused significant changes in the cells that rendered them defective in activating IRF-3 upon SV infection. To address this possibility, we took advantage of the specific inhibitor compounds VP32947 and GSK-4 (Fig. 4C and D) to pretreat the replicon cells in order to eliminate viral RNA before subjecting them to SV infection and IRF-3 activation analysis. VP32947 is a BVDV-specific inhibitor with an EC50 of about 25 nM against the Huh-7-BVDV replicon, whereas GSK-4 is specific for HCV with an EC50 of 0.4 μM against the Huh-7-HCV replicon (Fig. 4). Control Huh-7 and replicon-containing cells were either mock treated, treated with 0.25 μM (∼10 times the EC50) of VP32947, or 4 μM (∼10 times the EC50) of GSK-4 for 2 weeks in the absence of G418. The treated cells were then infected with SV and assayed for IRF-3-dependent transcription using the ISRE reporter plasmid. Levels of replicon RNA in the treated cells were also analyzed by using real-time quantitative PCR as described in Materials and Methods.
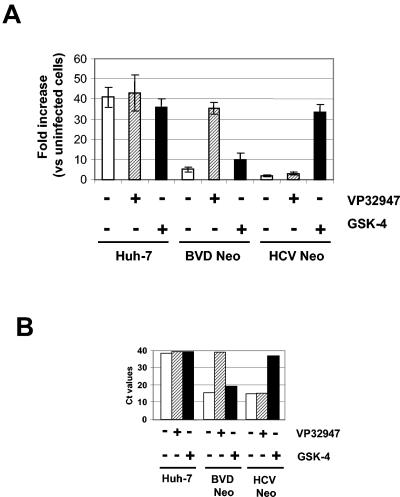
As shown in Fig. 6A, the BVDV replicon cells (BVD Neo) restored SV-induced, ISRE-mediated transcription only after treatment with the BVDV-specific inhibitor VP32947, but not with the HCV-specific inhibitor GSK-4 or in cells that were mock treated. Contrarily, the HCV replicon cells (HCV Neo) restored the ISRE-mediated transcription only after treatment with GSK-4, but not with VP32947. As expected, ISRE-mediated transcription was unaffected in the control Huh-7 cells with either compound treatment. Real-time quantitative RT-PCR analysis confirmed that viral RNA in the replicon cells was drastically reduced to below the detection limit (based on increases in Ct values of >10) after treatment with virus-specific inhibitors (i.e., VP32947 for BVD Neo and GSK-4 for HCV Neo) (Fig. 6B). These results together demonstrate a clear correlation between the presence of the viral RNA and suppression of SV-induced IRF-3 activation in the replicon cells. They are consistent with the data reported previously by other groups (13). Our results with the BVDV replicon support the notion that the suppression of IRF-3 activation observed in the replicon cells is a viral RNA-dependent event rather than due to certain defects in the replicon cells caused during the drug selection process.
FIG. 6.
Suppression of IRF-3-dependent transcription after SV infection requires the presence of replicon RNA. Control Huh-7 cells and replicon-containing cells (BVD Neo and HCV Neo) were treated with either VP32947 (0.25 μM) or GSK-4 (4 μM) or mock treated for 2 weeks in the absence of G418 selection. Medium was replaced every 3 to 4 days with freshly added inhibitor compound, and cells were split before reaching confluence. At the end of treatment, cells were assayed for SV-induced IRF-3 activation by using an ISRE reporter plasmid (see the legend to Fig. 5). Equal amounts of total RNA isolated from the untreated and treated cells were analyzed for levels of replicon RNA by using sequence-specific (neo) real-time quantitative RT-PCR analysis (TaqMan) as described in Materials and Methods. (A) Fold induction of ISRE-mediated luciferase signal after SV infection. The cell origin and inhibitor used are indicated at the bottom of the graph. Luciferase signals were normalized for transfection efficiency against a constitutively active Renilla luciferase reporter cotransfected into the cells, and the data are expressed as the fold increase before and after SV infection in firefly luciferase signal relative to that in the uninfected Huh-7 cells. (B) Relative levels of replicon RNA after inhibitor treatments, expressed as Ct values. Higher Ct values indicate lower RNA abundance. Cell origin and the inhibitor used are indicated below.
BVDV Npro autoprotease, but not NS3/4A serine protease, is partly responsible for blocking ISRE-mediated transcription.
The BVDV subgenomic replicon in this study contains two protease genes, Npro cysteine protease and NS3/4A serine protease. This N-terminal cysteine protease (Npro) is unique to pestiviruses and cleaves at its C terminus to release the N terminus of the capsid protein (C). Autoproteolysis is so far the only known function of Npro.
Since HCV NS3/4A serine protease has been shown to be responsible for the blockade of SV-induced activation of IRF-3 in HCV replicon cells (13), we wanted to determine whether a BVDV protease could have the same effect. We cotransfected plasmids expressing either HCV NS3/4A, BVDV NS3/4A, or BVDV Npro, and an ISRE-luciferase reporter plasmid into Huh-7 cells. These cells were then infected with SV as described in the previous section, and ISRE-dependent transcription was determined. As shown in Fig. 7A, expression of HCV NS3/4A serine protease blocked the up-regulation of ISRE-mediated transcription in a dose-dependent manner. Surprisingly, no inhibitory effect was observed when BVDV NS3/4A protease was produced. Instead, expression of Npro protease showed a dose-dependent suppression, at least partially, of ISRE-directed transcription. This result suggests that BVDV may have developed a different mechanism than HCV to prevent virus-induced IRF-3 activation. It will be very interesting to understand the molecular mechanism of this Npro-mediated suppression of the cellular antiviral response.
FIG. 7.
BVDV Npro autoprotease partially blocks SV-induced IRF-3 activation. (A) Effect of HCV NS3/4A, BVDV NS3/4A, or BVDV Npro on IRF-3-dependent transcription in Huh-7 cells infected with SV. Expression of HCV NS3/4A serine protease, but not BVDV NS3/4A, blocked the up-regulation of ISRE-mediated transcription in a dose-dependent manner. Expression of Npro autoprotease also showed a dose-dependent suppression of ISRE-directed transcription. (B) Npro-mediated inhibition of SV-induced IRF-3 activation in Huh-7 cells was confirmed by mutating two of its catalytic residues (Glu22 and His49) and creating an inactive form of Npro (mtNpro). Expression of the inactive form of Npro did not suppress the ISRE-mediated transcription induced by SV. Data were analyzed with GraphPad Prism software (GraphPad Software) using a paired t test to determine the significance of observed differences between control groups and the indicated treatment groups. **, P < 0.005; ***, P < 0.0001. Error bars represent the standard deviations.
We further confirmed that this inhibition of SV-induced IRF-3 activation in Huh-7 cells was indeed due to the proteolytic activity of Npro by mutating two of its catalytic residues (Glu22 and His49) and creating an inactive form of Npro (mtNpro) (30). As expected, the inactive form of Npro lost the ability to suppress the ISRE-mediated transcription (Fig. 7B), indicating that the proteolytic activity of Npro is necessary for suppressing IRF-3 activation.
DISCUSSION
We describe here the development of a BVDV subgenomic replicon system in Huh-7 cells that is structurally similar to the HCV replicon system. Both systems are bi-cistronic, use the EMCV IRES to drive production of nonstructural proteins, and replicate in similar cellular background. We were able to establish two versions of the BVDV replicon, with or without a luciferase reporter, which replicate in Huh-7 cells at a similar efficiency as that of the HCV replicon NK5.1 (17). The success of replicating BVDV RNA in Huh-7 cells indicates that cells of human origin may contain all cellular factors required for BVDV replication. Interestingly, no adaptive mutations in the BVDV replicon RNA are needed for establishing persistent replication in the human cell line. This represents a significant difference from the HCV replicons.
The effects of exogenous interferons on HCV replication in vivo and in vitro have been well characterized (7, 19, 23). BVDV has long been known to block the activity of endogenous interferon (10), but exogenously added interferons have been shown to inhibit the replication of vesicular stomatitis virus even when the cells have been coinfected with ncpBVDV (31). Human interferons, not surprisingly, have demonstrated poor activity against BVDV in bovine cells (32). We examined the effect of various human interferons on BVDV and HCV RNA replication in the same human cellular background and found that both replicons had similar susceptibilities to the interferons.
The HCV replicon has been shown, via its NS3/4A serine protease, to block SV-induced activation of IRF-3, a key antiviral signaling molecule. This inhibition has been linked to a blockade in the phosphorylation of IRF-3 and its subsequent dimerization and nuclear translocation (13). In BVDV/Huh-7 replicon cells, we observed a similar blockade on IRF-3-dependent transcription in Huh-7 cells upon SV infection. However, the viral protein(s) responsible for this inhibition appears to be different. Unlike that of the HCV replicon, the NS3/4A protease of BVDV was unable to suppress IRF-3 activation and transcription from IRF-3-responsive promoters. Instead, another protease in the BVDV genome, the Npro autoprotease, appears to be, at least partially, responsible for suppressing SV-induced IRF-3 activation. Npro protease is unique to pestiviruses and is not found in any other members of the Flaviviridae family. It is a nonstructural protein whose only known catalytic function is to cleave between itself and the capsid protein. Interestingly, a third BVDV replicon construct, similar to the two described here but lacking the Npro gene, failed to establish stable replication in Huh-7 cells (data not shown), supporting the importance of the Npro protein in establishing efficient BVDV RNA replication in this system.
It is reasonable to assume that pestiviruses may have adopted a different strategy from that of HCV in utilizing Npro protease to evade cellular antiviral defenses. This hypothesis is consistent with the observation that the presence of Npro in classical swine fever virus, another pestivirus, correlates with its interference with cellular antiviral defense [i.e., resistance to poly(I/C)-induced apoptosis in porcine kidney SK-6 cells and inhibition of poly(I/C)-induced type I IFN production in porcine monocytic cells] (29). Both BVDV and classical swine fever virus are capable of preventing poly(I/C)-mediated induction of cellular antiviral activities. It was also shown recently that ncpBVDV induced translocation of IRF-3 into the nucleus without subsequent binding to DNA. Furthermore, ncpBVDV was able to block Semliki Forest virus-induced IFN-β production through a block in the formation of IRF-3-DNA complexes (4). Whether Npro protease is involved in this process remains to be investigated. Although the cellular pathway with which Npro might interact remains to be identified, our observation with the BVDV replicon system, together with previously published information by other groups, supports a novel function for Npro of pestiviruses in counteraction of the cellular antiviral response.
By using the parallel BVDV and HCV replicon systems, we were able to distinguish viral specificity of small-molecule inhibitors and easily separate direct antiviral activity from cytotoxicity-induced effects on replicon RNA synthesis. This is particularly important in screening for non-nucleoside-based direct antivirals, since such compounds are unlikely to be cross-active against both viruses due to low sequence similarities between them. We are applying the parallel replicon systems to our nonnucleoside compound collections and identifying those with superior selectivity against HCV RNA replication. The screening process is significantly shortened by using this approach, due to early identification and elimination of nonspecific and toxic compounds.
Acknowledgments
This work was supported by Valeant Pharmaceuticals International and supported in part by grants 97-35204-5068 and 2002-35204-11619 from the NRI program of the U.S. Department of Agriculture to R.O.D.
We are extremely grateful to Michael Gale, Jr., Nancy Reich, and Sven-Erik Behrens for providing important reagents.
REFERENCES
- 1.Adler, B., H. Adler, H. Pfister, T. W. Jungi, and E. Peterhans. 1997. Macrophages infected with cytopathic bovine viral diarrhea virus release a factor(s) capable of priming uninfected macrophages for activation-induced apoptosis. J. Virol. 71:3255-3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ansari, I. H., L. Chen, D. Liang, L. H. Gil, W. Zhong, and R. O. Donis. 2004. Involvement of a bovine viral diarrhea virus NS5B locus in virion assembly. J. Virol. 78:9612-9623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baginski, S. G., D. C. Pevear, M. Seipel, S. C. Sun, C. A. Benetatos, S. K. Chunduru, C. M. Rice, and M. S. Collett. 2000. Mechanism of action of a pestivirus antiviral compound. Proc. Natl. Acad. Sci. USA 97:7981-7986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baigent, S. J., G. Zhang, M. D. Fray, H. Flick-Smith, S. Goodbourn, and J. W. McCauley. 2002. Inhibition of beta interferon transcription by noncytopathogenic bovine viral diarrhea virus is through an interferon regulatory factor 3-dependent mechanism. J. Virol. 76:8979-8988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartenschlager, R. 2002. Hepatitis C virus replicons: potential role for drug development. Nat. Rev. Drug Discov. 1:911-916. [DOI] [PubMed] [Google Scholar]
- 6.Bielefeldt Ohmann, H., and L. A. Babiuk. 1988. Influence of interferons alpha I1 and gamma and of tumour necrosis factor on persistent infection with bovine viral diarrhoea virus in vitro. J. Gen. Virol. 69:1399-1403. [DOI] [PubMed] [Google Scholar]
- 7.Cheney, I. W., V. C. Lai, W. Zhong, T. Brodhag, S. Dempsey, C. Lim, Z. Hong, J. Y. Lau, and R. C. Tam. 2002. Comparative analysis of anti-hepatitis C virus activity and gene expression mediated by alpha, beta, and gamma interferons. J. Virol. 76:11148-11154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheney, I. W., S. Naim, V. C. Lai, S. Dempsey, D. Bellows, M. P. Walker, J. H. Shim, N. Horscroft, Z. Hong, and W. Zhong. 2002. Mutations in NS5B polymerase of hepatitis C virus: impacts on in vitro enzymatic activity and viral RNA replication in the subgenomic replicon cell culture. Virology 297:298-306. [DOI] [PubMed] [Google Scholar]
- 9.Choi, K. H., J. M. Groarke, D. C. Young, R. J. Kuhn, J. L. Smith, D. C. Pevear, and M. G. Rossmann. 2004. The structure of the RNA-dependent RNA polymerase from bovine viral diarrhea virus establishes the role of GTP in de novo initiation. Proc. Natl. Acad. Sci. USA 101:4425-4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diderholm, H., and Z. Dinter. 1966. Interference between strains of bovine virus diarrhea virus and their capacity to suppress interferon of a heterologous virus. Proc. Soc. Exp. Biol. Med. 121:976-980. [DOI] [PubMed] [Google Scholar]
- 11.Duke, G. M., M. A. Hoffman, and A. C. Palmenberg. 1992. Sequence and structural elements that contribute to efficient encephalomyocarditis virus RNA translation. J. Virol. 66:1602-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eldrup, A. B., C. R. Allerson, C. F. Bennett, S. Bera, B. Bhat, N. Bhat, M. R. Bosserman, J. Brooks, C. Burlein, S. S. Carroll, P. D. Cook, K. L. Getty, M. MacCoss, D. R. McMasters, D. B. Olsen, T. P. Prakash, M. Prhavc, Q. Song, J. E. Tomassini, and J. Xia. 2004. Structure-activity relationship of purine ribonucleosides for inhibition of hepatitis C virus RNA-dependent RNA polymerase. J. Med. Chem. 47:2283-2295. [DOI] [PubMed] [Google Scholar]
- 13.Foy, E., K. Li, C. Wang, R. Sumpter, Jr., M. Ikeda, S. M. Lemon, and M. Gale, Jr. 2003. Regulation of interferon regulatory factor-3 by the hepatitis C virus serine protease. Science 300:1145-1148. [DOI] [PubMed] [Google Scholar]
- 14.Hoff, H. S., and R. O. Donis. 1997. Induction of apoptosis and cleavage of poly(ADP-ribose) polymerase by cytopathic bovine viral diarrhea virus infection. Virus Res. 49:101-113. [DOI] [PubMed] [Google Scholar]
- 15.Inaba, Y., Y. Tanaka, T. Kumagai, T. Omori, and H. Ito. 1968. Bovine diarrhea virus. II. END phenomenon: exaltation of Newcastle disease virus in bovine cells infected with bovine diarrhea virus. Jpn. J. Microbiol. 12:35-49. [DOI] [PubMed] [Google Scholar]
- 16.Jang, S. K., M. V. Davies, R. J. Kaufman, and E. Wimmer. 1989. Initiation of protein synthesis by internal entry of ribosomes into the 5′ nontranslated region of encephalomyocarditis virus RNA in vivo. J. Virol. 63:1651-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krieger, N., V. Lohmann, and R. Bartenschlager. 2001. Enhancement of hepatitis C virus RNA replication by cell culture-adaptive mutations. J. Virol. 75:4614-4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lai, V. C., C. C. Kao, E. Ferrari, J. Park, A. S. Uss, J. Wright-Minogue, Z. Hong, and J. Y. Lau. 1999. Mutational analysis of bovine viral diarrhea virus RNA-dependent RNA polymerase. J. Virol. 73:10129-10136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larkin, J., L. Jin, M. Farmen, D. Venable, Y. Huang, S. L. Tan, and J. I. Glass. 2003. Synergistic antiviral activity of human interferon combinations in the hepatitis C virus replicon system. J. Interferon Cytokine Res. 23:247-257. [DOI] [PubMed] [Google Scholar]
- 20.Lindenbach, B. D., and C. M. Rice. 2003. Molecular biology of flaviviruses. Adv. Virus Res. 59:23-61. [DOI] [PubMed] [Google Scholar]
- 21.Lohmann, V., F. Korner, A. Dobierzewska, and R. Bartenschlager. 2001. Mutations in hepatitis C virus RNAs conferring cell culture adaptation. J. Virol. 75:1437-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lohmann, V., F. Korner, J. Koch, U. Herian, L. Theilmann, and R. Bartenschlager. 1999. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science 285:110-113. [DOI] [PubMed] [Google Scholar]
- 23.Manns, M. P., J. G. McHutchison, S. C. Gordon, V. K. Rustgi, M. Shiffman, R. Reindollar, Z. D. Goodman, K. Koury, M. Ling, and J. K. Albrecht. 2001. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 358:958-965. [DOI] [PubMed] [Google Scholar]
- 24.Meyers, G., and H. J. Thiel. 1996. Molecular characterization of pestiviruses. Adv. Virus Res. 47:53-118. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen, T. T., A. T. Gates, L. L. Gutshall, V. K. Johnston, B. Gu, K. J. Duffy, and R. T. Sarisky. 2003. Resistance profile of a hepatitis C virus RNA-dependent RNA polymerase benzothiadiazine inhibitor. Antimicrob. Agents Chemother. 47:3525-3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peterhans, E., T. W. Jungi, and M. Schweizer. 2003. BVDV and innate immunity. Biologicals 31:107-112. [DOI] [PubMed] [Google Scholar]
- 27.Rice, C. M. 1996. Flaviviridae: the viruses and their replication, p. 931-959. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Virology. Lippincott, Philadelphia, Pa.
- 28.Rossi, C. R., and G. K. Kiesel. 1983. Characteristics of the polyriboinosinic acid:polyribocytidylic acid assay for noncytopathogenic bovine viral diarrhea virus. Am. J. Vet. Res. 44:1916-1919. [PubMed] [Google Scholar]
- 29.Ruggli, N., J. D. Tratschin, M. Schweizer, K. C. McCullough, M. A. Hofmann, and A. Summerfield. 2003. Classical swine fever virus interferes with cellular antiviral defense: evidence for a novel function of Npro. J. Virol. 77:7645-7654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rumenapf, T., R. Stark, M. Heimann, and H. J. Thiel. 1998. N-terminal protease of pestiviruses: identification of putative catalytic residues by site-directed mutagenesis. J. Virol. 72:2544-2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schweizer, M., and E. Peterhans. 2001. Noncytopathic bovine viral diarrhea virus inhibits double-stranded RNA-induced apoptosis and interferon synthesis. J. Virol. 75:4692-4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sentsui, H., R. Takami, T. Nishimori, K. Murakami, T. Yokoyama, and Y. Yokomizo. 1998. Anti-viral effect of interferon-alpha on bovine viral diarrhea virus. J. Vet. Med. Sci. 60:1329-1333. [DOI] [PubMed] [Google Scholar]
- 33.Sharp, P. M., and W. H. Li. 1987. Ubiquitin genes as a paradigm of concerted evolution of tandem repeats. J. Mol. Evol. 25:58-64. [DOI] [PubMed] [Google Scholar]
- 34.Tautz, N., K. Elbers, D. Stoll, G. Meyers, and H. J. Thiel. 1997. Serine protease of pestiviruses: determination of cleavage sites. J. Virol. 71:5415-5422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tomei, L., S. Altamura, L. Bartholomew, M. Bisbocci, C. Bailey, M. Bosserman, A. Cellucci, E. Forte, I. Incitti, L. Orsatti, U. Koch, R. De Francesco, D. B. Olsen, S. S. Carroll, and G. Migliaccio. 2004. Characterization of the inhibition of hepatitis C virus RNA replication by nonnucleosides. J. Virol. 78:938-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vassilev, V. B., M. S. Collett, and R. O. Donis. 1997. Authentic and chimeric full-length genomic cDNA clones of bovine viral diarrhea virus that yield infectious transcripts. J. Virol. 71:471-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vassilev, V. B., and R. O. Donis. 2000. Bovine viral diarrhea virus induced apoptosis correlates with increased intracellular viral RNA accumulation. Virus Res. 69:95-107. [DOI] [PubMed] [Google Scholar]
- 38.Walker, M. P., T. C. Appleby, W. Zhong, J. Y. Lau, and Z. Hong. 2003. Hepatitis C virus therapies: current treatments, targets and future perspectives. Antivir. Chem. Chemother. 14:1-21. [DOI] [PubMed] [Google Scholar]
- 39.Young, K. C., K. L. Lindsay, K. J. Lee, W. C. Liu, J. W. He, S. L. Milstein, and M. M. Lai. 2003. Identification of a ribavirin-resistant NS5B mutation of hepatitis C virus during ribavirin monotherapy. Hepatology 38:869-878. [DOI] [PubMed] [Google Scholar]