Abstract
Moringa stenopetala (Baker f.) Cufod. is a medicinal plant that has been used for the treatment of different ailments such as hypertension and diabetes in Ethiopia. This study aims to assess the diuretic activity of the aqueous crude extract and hot tea infusion of M. stenopetala leaves in saline-loaded rats. Male Wistar rats were divided into ten groups (n = 5). The control group received distilled water (5 mL/kg), whereas the reference group received Furosemide (10 mg/kg). Groups III–X orally received different doses of aqueous crude extract (62.5, 125, 250, and 500 mg/kg) and hot tea infusion (1, 2, 4, and 6 teaspoons [Tsp]) based on community use. Urine volume was recorded every hour until the end of the 5th hour, and total urine volume of each animal was calculated. The diuretic activity and diuretic action were determined based on the urine output. Additionally, concentration of urinary sodium, chloride, and potassium ions was determined. The urinary Na+/K+ ratio and carbonyl anhydrase activity (Cl−/(Na+/K+)) were also assessed. The findings verified that the aqueous crude extract as well as the hot tea infusion of the leaves of M. stenopetala possesses significant (P < 0.01) diuretic, natriuretic, and kaliuretic effects. The aqueous crude extract (125 mg/kg) and hot tea infusion (2 Tsp) displayed the highest diuretic activity (101% and 96%, respectively) comparable to the reference drug, Furosemide (10 mg/kg). They also displayed a good natriuretic activity. The aqueous crude extract and hot tea infusion revealed a significant Na+ urinary excretion (P < 0.001) and Na+/K+ ratio (P < 0.05) at all test doses. There was also a significant (P < 0.01) Cl− urinary excretion at all test doses of aqueous crude extract except 62.5 mg/kg and all test doses of hot tea infusion except higher doses (4 and 6 Tsp). Thus, the aqueous crude extract as well as the hot tea infusion of the leaves of M. stenopetala causes a plausible increase in the urine volume and concentration of urinary electrolytes in rats.
Keywords: Moringa stenopetala, urine volume, electrolyte excretion, diuretic activity, rats, dose simulation
Introduction
Moringa stenopetala, or commonly known as “cabbage tree”, is a tropical plant belonging to the family Moringaceae.1 It is a tree of 6- to 12-m height, and is domesticated in the east African lowlands and indigenous to southern Ethiopia, Kenya, and Somalia.2 Many different ecotypes and varieties of M. stenopetala are found in Ethiopia. M. stenopetala is an important indigenous vegetable in southwestern Ethiopia where it is cultivated as a food crop.1 The fresh leaves are cooked and consumed by the local communities. The roots of the plant are often used to treat malaria and other health problems.3 Various reports also show the antileshmanial, antitrypanosomal,4,5 antifertility,6 and antidiabetic activities of M. stenopetala.7,8 In particular, people with high blood pressure boil the leaves and drink the water to get relief from their aliment.9
Various reports indicate the value of the plant in the treatment of hypertension. A study on the aqueous crude leaf extract of M. stenopetala has indicated the hypotensive and antihypertensive effects of the extract and a significant drop in systolic blood pressure, diastolic blood pressure, and mean arterial blood pressure in normotensive anesthetized guinea pigs and fructose-induced rats.10,11 The extract also showed an inhibitory effect on high-K+ (80 mM)-induced contraction in isolated aorta of guinea pigs.10,12 Another similar study has reported the potential diuretic activity of the hydroalcoholic extract of the leaves of M. stenopetala.13 Accordingly, this study was designed to assess the diuretic activity of the aqueous crude extract and hot water infusion of M. stenopetala leaves to simulate community method of preparation of the remedy.
Materials and methods
Experimental animals
Male Wistar rats, aged 4–6 weeks and weighing 200–250 g, were selected for this study. All animals used for this study were obtained from the laboratory animal breeding facility of the Ethiopian Public Health Institute (EPHI). The rats were allowed to acclimatize to the laboratory conditions for a period of 1 week, and each animal was placed in a metabolic cage 16 hours before actual experiments were carried out. The animals were maintained in a constant 12-hour/12-hour light/dark cycle of laboratory conditions, and the average ambient temperature was 25°C. The animals were provided with standard laboratory animal feed and water ad libitum. Ethical approval for this study was obtained from the Scientific and Ethical Review Committee of EPHI. All animals used in this study were treated humanely throughout the study period according to the International Guidelines of Laboratory Animal Care and Use.14
Dose calculation
Based on community use, the Moringa tea was prepared by soaking 2 teaspoons (Tsp) (about 2 g) of loose tea leaves in 100 mL of water (0.68 g dried tea extract) which is to be consumed by an adult with the average weight considered to be 60 kg. Based on this previous information, the effective human dose is calculated to be 11.33 mg/kg, from which the animal dose was derived. This dose was then halved (1 Tsp), doubled (4 Tsp), and tripled (6 Tsp) to explore the diuretic activity of the leaves at different dose levels in rats. The dose calculation was based on slight modifications of the US FDA recommendations for dose extrapolation between species.15
| (1) |
The weight of tea leaves to be measured was determined based on the total weight of the respective group of animals and the final administration volume of 5 mL/kg. A total volume of 100 mL was prepared as follows: the loose tea leaves of M. stenopetala were macerated in hot distilled water (94.5°C) for 15 minutes which was filtered through cotton gauze to give clear straw-colored infusions with different depths of colors. The dose for the aqueous extract was determined based on a previous animal diuretic study that was conducted with the hydroalcoholic leaf extract of M. stenopetala.13 Accordingly, 500 mg/kg which was observed to be an optimal dose served as the maximum dose from which the other dose levels were proportionally derived (250, 125, and 62.5 mg/kg).
Grouping and dosing
The experimental animals were divided into ten groups, each group comprising five rats. Group I (negative control) received normal saline (NS) solution, whereas Group II (positive control) received the standard diuretic drug Furosemide (10 mg/kg). Groups III–X received the test substances (aqueous crude extract at test doses of 62.5, 125, 250, and 500 mg/kg and hot tea infusion at test doses of 1, 2, 4, and 6 Tsp) orally using gavage.
Drugs and chemicals
Furosemide (Sigma-Aldrich Chemie GmbH, Munich, Germany) was used as the standard drug. All the chemicals used in the present study were purchased from reliable sources and were of standard quality.
Plant material
The fresh leaves of M. stenopetala were collected from Arbaminch, a town located 437 km south of the capital. The plant material was authenticated by a taxonomist in the Directorate of Traditional and Modern Medicine Research of EPHI, and a voucher specimen with the number AL-001was deposited.
Preparation of extract
Fresh M. stenopetala leaves were shed, dried, and crushed manually using mortar and pestle. One thousand grams of the crushed leaves was macerated with distilled water for 4 hours at room temperature under a rotary shaker (DS-500 Orbital Shaker; VWR International, Radnor, PA, USA) until exhaustion. The extract was first filtered using cotton gauze and later with Whatman filter paper No. 1. The filtrate was a freeze-dried with a lyophilizer (12 L Console Freeze Dry System (7754040), 230 V, 60 Hz; Labconco, Kansas City, MO, USA). The total yield of the extract was calculated to be 17% (w/w). The dried extract was kept in a silicon desiccator until further use.
Preparation of hot tea infusion
The shed and dried leaves of M. stenopetala were crushed using mortar and pestle until a loose tea leave-like consistency was obtained. The resulting tea leaves were soaked in hot distilled water (94.5°C) for 15 minutes and filtered through cotton gauze. The filtrate was allowed to cool to body temperature before administering to night-fasted male Wistar rats.
Phytochemical screening
The aqueous extract and the hot tea infusion of M. stenopetala leaves used for the diuresis study were subjected to phytochemical screening following methods described by Trease and Evans.16 The extract and tea infusion along with negative controls were tested for the presence of alkaloids, coumarins, glycosides, phlobotannins, steroids, saponins, flavonoids, terpenoids, and tannins.
Screening for diuretic activity
The Kau method with some modification was used to screen all test substances for their diuretic activity.17 Food and water were withdrawn 18 hours prior to the experiment, and the test animals were individually placed in metabolic cages with graduated and transparent tubes to collect their urine and determine the volume every hour.
The total urine volume was measured every hour up to the end of the 5th hour for all groups. The parameters determined were cumulative urine volume and urinary ionic concentration of Na+ and K+. The volume of the urine excreted until the 5th hour of the study by each group was expressed as the percent of the liquid (NS) administered giving rise to the measure of “urinary excretion” independent of group weight. The ratio of urinary excretion in the test group to urinary excretion in the control group was considered as the measure of the diuretic index for a given dose of the drug. As the diuretic index is prone to variability, a parameter known as Lipschitz value was calculated. To obtain Lipschitz value, the diuretic index of the test substance was compared to that of the standard drug in the test group. The ratio of urinary excretion in test group and urinary excretion in the control group was denoted as “diuretic index”, which was used as the measure of degree of diuresis:18
| (2) |
where Vo is the total urinary output and Vi is the total volume of fluid administered:
| (3) |
where Vt is the mean urine volume of test group and Vc is the mean urine volume of control group:
| (4) |
where Vt is the mean urine volume of test group and Vr is the mean urine volume of reference group:
| (5) |
where Ct is the concentration of electrolyte in the urine of test group and Cc is the concentration of electrolyte in the urine of control group:
| (6) |
where Cn is the concentration of Na+ in the urine of a group and Ck is the concentration of K+ in the urine of the same group:
| (7) |
where Cl− is the urinary chloride concentration, Na+ is the urinary sodium concentration, and K+ is the urinary potassium concentration.
Determination of urinary Na+, K+, and Cl−
Urinary Na+, K+, and Cl− concentrations of the experimental, control, and standard groups were determined using Ion Selective Electrode analysis (AVL 9180 Electrolyte Analyzer; Roche, Basel, Switzerland).
Determination of urine pH
The pH of the fresh urine samples of all groups was measured with a calibrated digital pH meter (Mettler Toledo, Columbus, OH, USA).
Statistical analysis
Statistical analysis was performed using ANOVA, and a P value of less than 0.05 was considered to be statistically significant. Results are expressed as mean ± standard error of mean. SPSS version 16 was used for data analysis.
Results
The rats that received the lower doses (62.5 and 125 mg/kg) showed a significant (P < 0.05) urine excretion from the 3rd hour of administration of aqueous crude extract of M. stenopetala (ACMS). As seen in Table 1, the animals that received higher doses (250 and 500 mg/kg) revealed a significant (P < 0.05) urine excretion from the 4th hour (Figure 1).
Table 1.
Urinary output after the administration of aqueous leaves extract of Moringa stenopetala in rats
| Treatment | Urinary output (mL)
|
||||
|---|---|---|---|---|---|
| 1st hour | 2nd hour | 3rd hour | 4th hour | 5th hour | |
| Distilled water | 0.39 ± 0.63 | 0.67 ± 0.70 | 0.98 ± 0.93 | 1.04 ± 1.01 | 1.48 ± 0.77 |
| Furosemide (10 mg/kg) | 1.85 ± 0.87 | 3.64 ± 1.38* | 5.05 ± 1.13** | 6.48 ± 2.19*** | 7.05 ± 2.28*** |
| ACMS (62.5 mg/kg) | 1.46 ± 1.37 | 2.85 ± 1.45 | 4.13 ± 1.19* | 6.60 ± 1.50*** | 7.52 ± 0.99*** |
| ACMS (125 mg/kg) | 1.77 ± 1.44 | 2.82 ± 1.47 | 4.00 ± 1.95* | 5.36 ± 1.91** | 6.95 ± 1.90*** |
| ACMS (250 mg/kg) | 0.6 ± 0.57 | 2.01 ± 1.04 | 3.68 ± 1.26 | 4.8 ± 0.60* | 5.34 ± 0.45 *** |
| ACMS (500 mg/kg) | 0.85 ± 1.49 | 1.92 ± 1.59 | 3.16 ± 2.15 | 4.99 ± 2.25* | 6.01 ± 1.94** |
Notes: Values represent mean ± standard error of mean (n = 6).
P < 0.05;
P < 0.01;
P < 0.001.
Abbreviation: ACMS, aqueous crude extract of M. stenopetala.
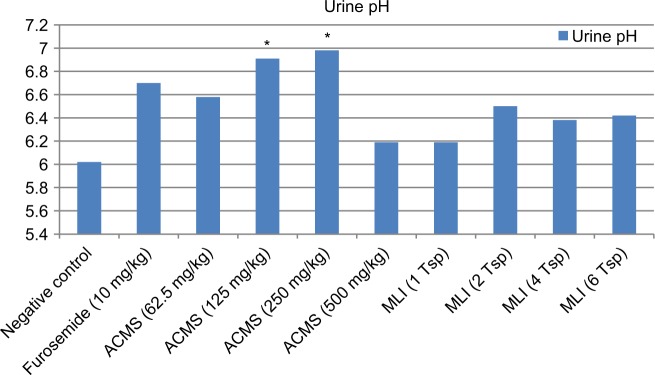
Figure 1.
The urine pH after the administration of the aqueous crude extract and hot tea infusion of Moringa stenopetala leaves in rats.
Note: *P < 0.05.
Abbreviations: ACMS, aqueous crude extract of M. stenopetala; MLI, M. stenopetala leaf infusion; Tsp, teaspoon(s).
A significant (P < 0.05) urine excretion was observed at 3rd hour from rats that received moderate doses (2 and 4 Tsp) of MLI, whereas those rats that received high dose (6 Tsp) did not show significant excretion and those that received low dose (1 Tsp) revealed a significant excretion (P < 0.01) at the last hour (5th) of MLI administration (Table 2).
Table 2.
Urinary output after the administration of hot tea infusion of Moringa stenopetala in rats
| Treatment | Urinary output (mL)
|
||||
|---|---|---|---|---|---|
| 1st hour | 2nd hour | 3rd hour | 4th hour | 5th hour | |
| Distilled water | 0.39 ± 0.63 | 0.67 ± 0.70 | 0.98 ± 0.93 | 1.04 ± 1.01 | 1.48 ± 0.77 |
| Furosemide (10 mg/kg) | 1.85 ± 0.87 | 3.64 ± 1.38 | 5.05 ± 1.13* | 6.48 ± 2.19** | 7.05 ± 2.28** |
| MLI (1 teaspoon) | 0.34 ± 0.56 | 1.3 ± 1.59 | 3.30 ± 2.08 | 5.00 ± 2.73 | 6.33 ± 2.03** |
| MLI (2 teaspoons) | 0.48 ± 0.65 | 2.72 ± 1.17 | 5.64 ± 0.77** | 6.80 ± 0.97** | 8.2 ± 0.67*** |
| MLI (4 teaspoons) | 0.44 ± 0.98 | 2.6 ± 2.70 | 5.10 ± 2.88* | 6.74 ± 2.72** | 7.7 ± 2.68** |
| MLI (6 teaspoons) | 0.36 ± 0.80 | 1.64 ± 2.18 | 2.96 ± 2.02 | 4.12 ± 2.44 | 4.88 ± 2.45 |
Notes: Values represent mean ± standard error of mean (n = 6).
P < 0.05;
P < 0.01;
P < 0.001.
Abbreviation: MLI, M. stenopetala leaf infusion.
The aqueous crude leaf extract of M. stenopetala induced a significant (P < 0.01) urine output and diuretic activity at all tested doses (Table 3). The highest diuretic activity of ACMS was observed at 125 mg/kg (101%) comparable to the standard drug, Furosemide (10 mg/kg).
Table 3.
Diuretic activity of aqueous crude extract of Moringa stenopetala leaves in rats
| Treatment | Dose (mg/kg) | Cumulative urine volume (mL) | Urinary excretion | Diuretic index | Diuretic activity |
|---|---|---|---|---|---|
| Distilled water | NA | 1.48 ± 0.77 | 0.09 | 1.00 | |
| Furosemide | 10 | 7.05 ± 2.28*** | 0.46 | 5.23 | 1.00 |
| Aqueous crude extract of M. stenopetala | 62.5 | 7.52 ± 0.99*** | 0.42 | 4.75 | 0.91 |
| 125 | 6.95 ± 1.90*** | 0.47 | 5.27 | 1.01 | |
| 250 | 6.54 ±0.45*** | 0.41 | 4.60 | 0.88 | |
| 500 | 6.01 ± 1.94** | 0.34 | 3.84 | 0.73 |
Notes: Values represent mean ± standard error of mean (n = 6).
P < 0.01;
P < 0.001.
Abbreviation: NA, not applicable.
The hot tea infusion of M. stenopetala leaves induced a significant (P < 0.01) urine output and diuretic activity at all tested doses except at 6 Tsp (Table 4). The highest diuretic activity was observed at the dose of 2 Tsp (96%).
Table 4.
Diuretic activity of hot tea infusion of Moringa stenopetala leaves in rats
| Substance administered | Amount | Cumulative urine volume (mL) | Urinary excretion | Diuretic index | Diuretic activity |
|---|---|---|---|---|---|
| Distilled water | NA | 1.48 ± 0.77 | 0.09 | 1.00 | |
| Furosemide | 10 mg/kg | 7.05 ± 2.28** | 0.46 | 5.23 | 1.00 |
| M. stenopetala leaf infusion | 1 teaspoon | 6.33 ± 2.03** | 0.39 | 4.35 | 0.83 |
| 2 teaspoons | 8.2 ± 0.67*** | 0.45 | 5.03 | 0.96 | |
| 4 teaspoons | 7.7 ± 2.68** | 0.44 | 4.91 | 0.94 | |
| 6 teaspoons | 4.88 ± 2.45 | 0.30 | 3.40 | 0.65 |
Notes: Values represent mean ± standard error of mean (n = 6).
P < 0.01;
P < 0.001.
Abbreviation: NA, not applicable.
The aqueous extract of M. stenopetala leaves induced a significant (P < 0.001) urine Na+ excretion. A significant (P < 0.01) urine Cl− excretion was observed in rats that received all test doses except 62.5 mg/kg, whereas a significant (P < 0.01) urine K+ excretion was observed only in rats that received the highest dose, 500 mg/kg. On the other hand, the extract showed a significant (P < 0.05) natriuretic effect at all tested doses (Table 5).
Table 5.
Urinary electrolyte excretion after administration of aqueous extract of Moringa stenopetala leaves in rats
| Treatment | Electrolyte excretion (mmol/L)
|
Natriuretic
|
Saliuretic
|
CAI
|
Saliuretic index
|
||||
|---|---|---|---|---|---|---|---|---|---|
| Na+ | K+ | Cl− | Na+/K+ | Na+ + Cl− | Cl−/Na+ + K+ | Na+ | K+ | Cl− | |
| Distilled water | 81.23 ± 5.83 | 52.26 ± 4.02 | 122.24 ± 7.63 | 1.56 | 203.00 | 0.92 | |||
| Furosemide (10 mg/kg) | 200.64 ± 5.34*** | 101.01 ± 3.40*** | 245.55 ± 6.0*** | 1.38 | 451.00 | 0.81 | 2.47 | 1.93 | 2.00 |
| ACMS (62.5 mg/kg) | 137.2 ± 5.44*** | 55.6 ± 2.89 | 154 ± 5.57 | 2.49*** | 0.89 | 0.80 | 1.69 | 1.07 | 1.26 |
| ACMS (125 mg/kg) | 151 ± 6.47*** | 50.4 ± 3.80 | 163.2 ± 10.63** | 3.04*** | 0.93 | 0.81 | 1.86 | 0.97 | 1.34 |
| ACMS (250 mg/kg) | 150.2 ± 4.97*** | 55 ± 3.87 | 173 ± 7.30** | 2.77*** | 0.87 | 0.84 | 1.85 | 1.06 | 1.42 |
| ACMS (500 mg/kg) | 159.6 ± 9.00*** | 77.6 ± 6.65** | 170.6 ± 12.22** | 2.10* | 0.94 | 0.72 | 1.97 | 1.49 | 1.40 |
Notes: Values represent mean ± standard error of mean (n = 6).
P < 0.05;
P < 0.01;
P < 0.001.
Abbreviations: CAI, carbonic anhydrase inhibition; ACMS, aqueous crude extract of M. stenopetala.
The hot tea infusion of M. stenopetala leaves induced a significant (P < 0.001) urine Na+ excretion. A significant (P < 0.01) K+ and Cl− excretion in urine was observed in rats that received test doses of 4 and 6 Tsp, whereas a significant (P < 0.01) natriuretic effect was observed in rats that received tested doses of 1 and 2 Tsp (Table 6).
Table 6.
Urinary electrolyte excretion of hot tea infusion of Moringa stenopetala leaves in rats
| Treatment | Electrolyte excretion (mmol/L)
|
Natriuretic
|
Saliuretic
|
CAI
|
Saliuretic index
|
||||
|---|---|---|---|---|---|---|---|---|---|
| Na+ | K+ | Cl− | Na+/K+ | Na+ + Cl− | Cl−/Na+ + K+ | Na+ | K+ | Cl− | |
| Furosemide (10 mg/kg) | 200.64 ± 5.34*** | 101.01 ± 3.40*** | 245.55 ± 6.01*** | 1.38 | 451.00 | 0.81 | 2.47 | 1.93 | 2.00 |
| MLI (1 teaspoon) | 138.8 ± 2.28*** | 61.6 ± 3.42 | 157.6 ± 2.48 | 2.28** | 0.88 | 0.79 | 1.71 | 1.18 | 1.29 |
| MLI (2 teaspoons) | 132.4 ± 7.24*** | 55.4 ± 2.22 | 152.2 ± 8.16 | 2.21*** | 0.85 | 0.80 | 1.77 | 1.40 | 1.40 |
| MLI (4 teaspoons) | 141.00 ± 6.89*** | 94.40 ± 10.22** | 195.2 ± 14.52*** | 1.62 | 0.74 | 0.84 | 1.78 | 1.79 | 1.62 |
| MLI (6 teaspoons) | 157.4 ± 7.17*** | 79.6 ± 9.65* | 184.20 ± 16.85** | 1.94 | 0.83 | 0.77 | 1.86 | 1.55 | 1.50 |
Notes: Values represent mean ± standard error of mean (n = 6).
P < 0.05;
P < 0.01;
P < 0.001.
Abbreviations: CAI, carbonic anhydrase inhibition; MLI, M. stenopetala leaf infusion.
Urine pH
Urinary pH measurement revealed that the different treatment groups that received aqueous leaf extract and hot tea infusion including the negative and positive control groups produced slightly acidic urine. The average urine pH of the normal and standard controls was determined to be 6.02 and 6.70, respectively. The pH of urine from rats treated with the aqueous extract displayed a significant (P < 0.05) increase for the two doses 125 and 250 mg/kg (6.58 and 6.91, respectively), and a slight decrease in pH (6.19) was seen for the highest dose (500 mg/kg) of the aqueous crude extract. There was no statistically significant change in urinary pH in the rats that received ascending doses of the hot tea infusion (6.19, 6.5, 6.38, and 6.42 for MLI 1, 2, 3, and 4, respectively) (Figure 1).
The diverse preliminary phytochemical screening tests carried out on the aqueous crude extracts and hot tea infusion of M. stenopetala leaves revealed the presence of different secondary metabolites (Table 7). The phytoconstituents that tested positive in both the ACMS and the MLI were tannins, flavonoids, saponins, coumarins, and alkaloids, while both ACMS and MLI tested negative for the presence of steroids, phlobotannins, and glycoside. Additionally, MLI tested positive for the presence of terpenoids unlike ACMS.
Table 7.
Phytochemical screening tests for the hot water infusion and aqueous crude extract of Moringa stenopetala leaves
| Sample | Phytoconstituents
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Tannins | Flavonoids | Terpenoids | Saponins | Steroids | Phlobotannins | Glycosides | Coumarins | Alkaloids | |
| ACMS | +++ | ++ | − | +++ | − | − | − | + | +++ |
| MLI | +++ | ++ | +++ | +++ | − | − | − | + | + |
Note: +++, high; ++, medium; +, low; −, absent.
Abbreviations: ACMS, aqueous crude extract of M. stenopetala; MLI, M. stenopetala leaf infusion.
Discussion
The utilization of herbal medicines and phytonutrients/nutraceuticals continues to rise as a result of increased acceptance and public interest in both developed and developing nations.19 Herbs and natural plant products are particularly gaining popularity for the management of cardiovascular diseases and associated disorders.20 The soaring interest in traditional medicine is attributed to the failure of modern medicine to alleviate many chronic illnesses. M. stenopetala, a plant highly venerated for its nutritional values and medicinal properties,21 is used in the management of hypertensive and kidney-related disorders. The plant in this regard can be considered as a potential nutraceutical. Such substances essentially are products which, other than nutrition, are also used as medicine.22
Hot tea infusion of the leaves of M. stenopetala has long been used by the local community and has a range of promising medicinal claims, which are yet to be supported by scientific evidence. As the diuretic activity is no exception to this, the present study set out to substantiate the folkloric use of the hot tea infusion prepared from the dried leaves for its diuretic property in contrast to the aqueous crude extract. In this study, the diuretic activity of the extract as well as the hot water infusion was compared to that of Furosemide, a potent diuretic used in clinical practice.23
Alkaloids, flavonoids, and saponins, which are present in the aqueous extract and hot water infusion, have been linked with the diuretic activity of medicinal plants.24 At this juncture, however, it is nearly impossible to pin down the specific phytoconstituents that bring about the observed diuretic activity of the plant. Nevertheless, it can be suggested that the range of polar phenolic compounds such as flavonoids and tannins in combination with alkaloids might be responsible for the apparent diuretic activity of the plant.
As revealed by the preliminary phytochemical screening, both the extract and the hot water infusion have an almost identical phytochemical profiles of the tested secondary compounds except for terpenoids (which are present in the hot water infusion and absent in the aqueous crude extract, possibly due to a higher degree of extractability brought about by the heat). There was also a slight difference in intensity in precipitation formation observed during the screening for alkaloids. Therefore, it can be inferred that the subtle difference in the diuretic potential of the two arises from the slight difference in phytoconstituent content that might have arisen from difference in the concentration of active principles or the decomposition of active principles by the gentle heat applied to prepare the hot tea infusion.
The onset of diuretic action of both the extract and hot tea infusion of the M. stenopetala mimics the trend observed with the standard diuretic drug Furosemide, which is known to have an onset of action of 1 hour after oral administration, reaching its peak effect within 2–3 hours.25 The aqueous crude extract of the leaves of M. stenopetala and its hot water infusion both displayed a significant increase in urine volume and an increase in urinary ionic concentration. The maximum and minimum diuretic index of the aqueous extract was 5.27 for the 2nd highest test dose and 3.84 for the lowest test dose. This pattern was also reflected with the hot water infusion of the leaves of M. stenopetala, which showed a diuretic index of greater than 5.03 and 3.40 for the 2nd highest test dose and the lowest test dose, respectively. The diuretic index, which indicates the diuretic potential of any substance, is considered to be good if the values are greater than 1.50, moderate if the values are between 1.00 and 1.50, mild if the values lie between 0.72 and 1.00, and nil if the value is <0.72.26 Both test substances accordingly, at all doses, have shown a good diuretic potential as evidenced by the high diuretic index values. The aqueous extract at 125 mg/kg showed a diuretic activity of 101%, and the 2nd highest concentration of the hot water infusion based on a 2-Tsp formula displayed 96% diuretic activity relative to the reference drug, Furosemide (10 mg/kg).
The retention of sodium, the main extracellular cation, and increased water volume have long been considered a key players in the pathogenesis of hypertension as well as edematous conditions such as cardiac failure and cirrhosis.27,28 There was a significantly higher sodium concentration, comparable to the standard drug, for both the hot water infusion as well as the aqueous extract. The Na+/K+ ratio is gaining acceptance as a translatable biomarker of mineralocorticoid receptor (MR) antagonism following administration of single or multiple doses of compounds as the MR blockade causes an increase in urinary Na+/K+ in rats.29 Besides serving as an indicator of a good natriuretic activity, an Na+/K+ ratio of >2 also shows the ability of the test substance to excrete a greater proportion of sodium ion in contrast to potassium ion which is a very essential quality for a good diuretic,30 as one of the most devastating side effects of diuretic drugs other than spironolactones is hypokalemia, a potentially lethal condition that results from the excessive excretion of potassium. Na+/K+ values >10 indicate a potassium-sparing activity which was not the case for both M. stenopetala and Furosemide in this study.31
The Cl−/(Na+ + K+) is indicative of carbonic anhydrase inhibitory activity, and substances resulting in ratios between 0.8 and 1.0 can be excluded from this activity.18 Accordingly, all the test substances including Furosemide at all doses, except the hot tea infusion based on a 6-Tsp formula, can be assumed to elicit their diuretic action though mechanisms independent of carbonyl anhydrase inhibition. To sum up, the increased urinary Na+ and Cl− level coupled with the increased cumulative urinary excretion is in line with the plant’s use for the management of hypertension and kidney-related disorders in folkloric Ethiopian medicine.
Conclusion
The outcome of this study implies that the aqueous crude extract and hot tea infusion of the leaves of M. stenopetala both possess a palpable diuretic activity, comparable to that of the standard loop diuretic Furosemide. These findings therefore substantiate the plant’s traditional use in the management of hypertension. Nevertheless, in-depth studies are still obligatory to elucidate the mechanism of action and the active components responsible for the perceived diuretic action for the extract as well as the hot tea infusion.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Seifu E. Actual and potential applications of Moringa stenopetala, underutilized indigenous vegetable of Southern Ethiopia: a review. Int J Agric Food Res. 2014;3(4):8–19. [Google Scholar]
- 2.Orwa CA, Mutua, Kindt R, Jamnadass R, Anthony S. Agroforestree Database: a tree reference and selection guide version 4.0.2009. [Accessed October 10, 2016]. Available from: http://www.worldagroforestry.org/search/node/moringa.
- 3.Mekonnen Y. The multi-purpose Moringa tree: Ethiopia. Addis Ababa: Institute of Pathobiology, Addis Ababa University; 2003. [Accessed August 29, 2016]. Available from: http://eco-library.theplanetfixer.org/docs/trees/moringa/the-multi-purpose-moringa-tree-in-ethiopia.pdf. [Google Scholar]
- 4.Mekonnen Y, Yardley V, Rock P, Croft S. In vitro antitrypanosomal activity of Moringa stenopetala leaves and roots. Phytother Res. 1999;13(6):538–539. doi: 10.1002/(sici)1099-1573(199909)13:6<538::aid-ptr486>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 5.Mekonnen Y. Effects of ethanol extract of Moringa stenopetala leaves on guinea pig and mouse smooth muscle. Phytother Res. 1999;13(5):442–444. doi: 10.1002/(sici)1099-1573(199908/09)13:5<442::aid-ptr476>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 6.Mekonnen Y, Gessesse A. Documentation on the uses of Moringa stenopetala and its possible antileishmanial and antifertility effects. Ethiop J Sci. 1998;21(2):287–295. [Google Scholar]
- 7.Toma A, Makonnen E, Mekonnen Y, Debella A, Addisakwattana S. Intestinal α-glucosidase and some pancreatic enzymes inhibitory effect of hydroalcholic extract of Moringa stenopetala leaves. BMC Complement Altern Med. 2014;14:180. doi: 10.1186/1472-6882-14-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toma A, Makonnen E, Mekonnen Y, Debella A, Adisakwattana S. Antidiabetic activities of aqueous ethanol and n-butanol fraction of Moringa stenopetala leaves in streptozotocin-induced diabetic rats. BMC Complement Altern Med. 2015;15:242. doi: 10.1186/s12906-015-0779-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Endeshaw HG. Promoting the “miracle tree of hope”. Ethiopian Herald. 2003 Jul 19; [Google Scholar]
- 10.Mengistu M, Abebe Y, Mekonnen Y, Tolessa T. In vivo and in vitro hypotensive effect of aqueous extract of Moringa stenopetala. Afr Health Sci. 2012;12(4):545–551. [PMC free article] [PubMed] [Google Scholar]
- 11.Geleta B, Makonnen E, Debella A, Tadele A. In vivo antihypertensive and antihyperlipidemic effects of the crude extracts and fractions of Moringa stenopetala (Baker f.) Cufod. leaves in rats. Front Pharmacol. 2016;7:97. doi: 10.3389/fphar.2016.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geleta B, Makonnen E, Debella A, Abebe A, Fekadu N. In vitro vasodilatory activity of the crude extracts and fractions of Moringa stenopetala (Baker f.) Cufod. leaves in isolated thoracic aorta of guinea pigs. J Exp Pharmacol. 2016;8:35–42. doi: 10.2147/JEP.S117545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geleta B, Eyasu M, Fekadu N, Debella A, Challa F. Evaluation of diuretic activity of hydro-ethanolic extract of Moringa stenopetala leaves in Swiss albino mice. Clin Exp Pharmacol. 2015;5:190. [Google Scholar]
- 14.Institute of Laboratory Animal Resources (ILAR), Committee on Care, Use of Laboratory Animals and National Institutes of Health (US), Division of Research Resources . Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academies; 2011. [Google Scholar]
- 15.U. S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER) Guidance for Industry: Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. Rockville, MD: Center for Drug Evaluation and Research (CDER); 2005. [Accessed October 11, 2016]. Available from: http://www.fda.gov/ohrms/dockets/98fr/02d-0492-gdl0002.pdf. [Google Scholar]
- 16.Trease GE, Evans WC. Pharmacognosy. 13th ed. London: Bailliere Tindall Ltd; 1989. [Google Scholar]
- 17.Kau ST, Keddie JR, Andrews D. A method for screening diuretic agents in the rat. J Pharmacol Methods. 1984;11(1):67–75. doi: 10.1016/0160-5402(84)90054-8. [DOI] [PubMed] [Google Scholar]
- 18.Vogel HG, Vogel WH, Scholkens BA, Sandow J, Muller G, Vogel WF. Drug Discovery and Evaluation: Pharmacological Assays. 3rd ed. Berlin: Springer; 2008. [Google Scholar]
- 19.Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol. 2014;4:177. doi: 10.3389/fphar.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suroowan S, Mahomoodally F. Common phyto-remedies used against cardiovascular diseases and their potential to induce adverse events in cardiovascular patients. Clin Phytosci. 2015;1(1):1. [Google Scholar]
- 21.Arora DS, Onsare JG, Kaur H. Bioprospecting of Moringa (Moringaceae): microbiological perspective. J Pharmacogn Phytochem. 2013;1(6) [Google Scholar]
- 22.Nasri H, Baradaran A, Shirzad H, Rafieian-Kopaei M. New concepts in neutraceuticals as alternative for pharmaceuticals. Int J Prev Med. 2014;5(12):1487–499. [PMC free article] [PubMed] [Google Scholar]
- 23.Ho KM, Power BM. Benefits and risks of furosemide in acute kidney injury. Anaesthesia. 2010;65(3):283–293. doi: 10.1111/j.1365-2044.2009.06228.x. [DOI] [PubMed] [Google Scholar]
- 24.Patel U, Kulkarni M, Undale V, Bhosale A. Evaluation of diuretic activity of aqueous and methanol extracts of Lepidium sativum garden cress (Cruciferae) in rats. Trop J Pharm Res. 2009;8(3) [Google Scholar]
- 25.Food and Drug Administration (FDA) LASIX® (furosemide) tablets 20, 40, and 80 mg. 2012. [Accessed October 5, 2016]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/016273s066lbl.pdf.
- 26.Asif M, Atif M, Malik AS, Dan ZC, Ahmad I, Ahmad A. Diuretic activity of Trianthema portulacastrum crude extract in albino rats. Trop J Pharm Res. 2013;12(6):967–972. [Google Scholar]
- 27.Schrier RW. Water and sodium retention in edematous disorders: role of vasopressin and aldosterone. Am J Med. 2006;119(7 Suppl 1):S47–S53. doi: 10.1016/j.amjmed.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 28.Adrogué HJ, Madias NE. Sodium and potassium in the pathogenesis of hypertension. N Engl J Med. 2007;356(19):1966–1978. doi: 10.1056/NEJMra064486. [DOI] [PubMed] [Google Scholar]
- 29.Eudy RJ, Sahasrabudhe V, Sweeney K, et al. The use of plasma aldosterone and urinary sodium to potassium ratio as translatable quantitative biomarkers of mineralocorticoid receptor antagonism. J Transl Med. 2011;9:180. doi: 10.1186/1479-5876-9-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar BS, Swamy BV, Behara GM, Baidya M, Bilal S, Swamy A. Diuretic activity of the root of Homonoiaretusa (GRAH. EX WT.) MUELL. Pharmacologyonline. 2010;3:276–284. [Google Scholar]
- 31.Papademetriou V. Diuretics, hypokalemia, and cardiac arrhythmia: a 20-year controversy. J Clin Hypertens (Greenwich) 2006;8(2):86–92. doi: 10.1111/j.1524-6175.2005.04722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]