Among the various types of oligosaccharide structures, asparagine-linked oligosaccharides (N-glycans) are the most prominent in terms of diversity and complexity. In particular, N-glycans containing sialic acid residues are involved in a variety of important physiological events, including cell–cell recognition, adhesion, signal transduction, and quality con-trol.[1] Moreover, it has long been known that the sialic acids in N-glycans on soluble proteins or peptides enhance circulatory residence,[2] that is, N-glycan-engineered erythro-poietin (EPO)[2b] and insulin[2c] exhibit a remarkably higher stability in serum, which effects their prolonged bioactivity. Antibody-dependent cellular cytotoxicity (ADCC) and/or complement-dependent cytotoxicity (CDC) have also been proposed to be modulated by the sialic acids of N-glycans in immunoglobulin G (IgG) through Siglec interactions by glycosylating or removing the sialic acids.[3] However, these important findings and previous efforts in investigating N-glycan functions have been mostly based on in vitro experiments using isolated lectins, cultured cells, and dissected tissues. Recently, interest has shifted to the dynamics of these glycans in vivo, that is, how the function and/or interaction of the individual N-glycan works synergistically through dynamic processes in the body to eventually exhibit biological phenomena. Molecular imaging[4–6] is the most promising tool to visualize the “on-time” N-glycan dynamics in vivo.
The challenge in efficient glycan imaging in living animals is to obtain the structurally pure oligosaccharides from nature or by synthetic methods.[7] In addition, the bioactivity of the oligosaccharides might be derived from the multivalency and/ or heterogeneous environment, that is, on cell surfaces that are composed of oligosaccharide clusters along with other biomolecules.[1] A single molecule of the N-glycan, obtained from either a natural or synthetic source, is readily excreted from the body.[8] Thus, efficiently mimicking and labeling such an N-glycan-involved bioenvironment, for example, by conjugating the N-glycans to the liposomes, to the clusters, or even to the surface of the cells,[9] may provide information on the “in vivo dynamics” of N-glycans. The recent successful noninvasive imaging and biodistribution study of glycans and glycoconjugates dealt with natural[10] and neo-glycoproteins, liposomes, and nanoparticles.[11]
Herein, we report the first fluorescence and positron emission tomography (PET) imaging of dendrimer-type glycoclusters using the multivalency effects of 16 molecules of N-glycans. A variety of N-glycan clusters were efficiently prepared based on the CuI-mediated “self-activating” Huisgen 1,3-dipolar cycloaddition,[12] and the remarkable dependence of the in vivo dynamics on the structure of sialic acids and organ-specific accumulation were discovered for the first time.
We designed polylysine-based dendrimer-type glycoclusters (Scheme 1); the different generations of clusters, namely those consisting of four (4-mer 1), eight (8-mer 2), and 16 (16-mer 3) molecules of N-glycan derivatives (a–e),[13] were investigated to examine cluster (multivalency) effects[1, 14] on in vivo dynamics. The clusters were designed to have a terminal lysine ε-amino group so that they could be efficiently labeled by fluorescent groups or 68Ga-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (68Ga-DOTA) as the PET radiolabel in the presence of numerous hydroxy groups through our 6π-azaelectrocyclization protocol under mild conditions.[10] The polylysine-based dendrimer core with terminal histidine and propargyl glycine residues could be synthesized by a solid-supported protocol (see the Supporting Information).
Scheme 1.

Generation and structures of glycoclusters. NBD=nitroben-zoxadiazole.
N-Glycans were subsequently introduced by “self-activating” Huisgen 1,3-dipolar cycloaddition[12] (reaction between acetylene on the polylysine template and azide on the N-glycans, Scheme 2). Examples include the terminal acetylene of the polylysine-based dendrimer (Scheme 2), which was smoothly reacted with azide partner a, bis-aNeu(2–6)Gal containing complex-type N-glycan (see the Supporting Information for preparation of azide derivatives) in the presence of equimolar amounts of copper sulfate and sodium ascorbate, and diisopropylethylamine (DIPEA; each relative to the N-glycan molecules) at room temperature for 40 min (see HPLC monitoring of the reaction in the Supporting Information). The residual copper ions were removed by chelation with DOTA and size-partitioning centrifugal filtration. Subsequent HPLC purification gave tetra-glycocluster 1a (4-mer), octa-glycocluster 2a (8-mer), and hexadeca-glycoclusters 3a– e (16-mers) with a molecular weight of circa 50 kDa in almost quantitative yields (see Schemes 1 and 2).
Scheme 2.
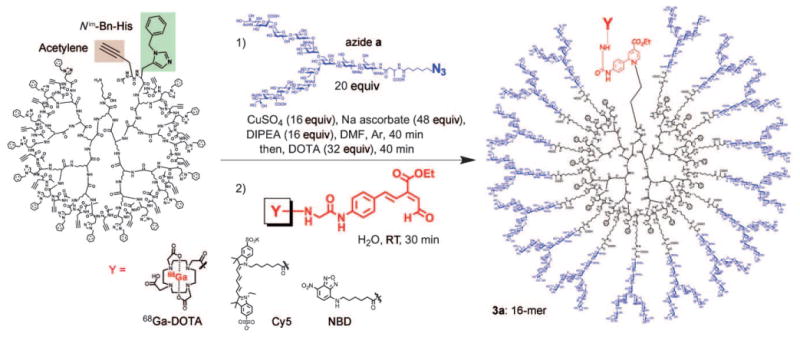
Preparation of N-glycan clusters through histidine-accelerated CuI-mediated Huisgen 1,3-dipolar cycloaddition and labeling by 6π-azaelectrocyclization. Bn=benzyl.
Detection of the mother ions by MALDI-TOFanalyses,[15] HPLC patterns of size-partitioning gel filtration, and the integration of the representative sugars, triazole, His, and Lys signals in their 1H NMR spectra all confirmed the desired clusters (see the Supporting Information). These clusters were subsequently labeled by DOTA or NBD and Cy5 fluorophores through rapid 6π-azaelectrocyclization (see Scheme 2 and the Supporting Information).[9, 10] The incorporation of 68Ga in DOTA-labeled glycoclusters 1a–3e was performed by slightly modifying the procedures previously described[16] by reaction with 68Ga in 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer (pH 3.5, 95°C, 15 min) and subsequent HPLC purification (see the Supporting Information).
Throughout the following imaging studies by both PET and fluorescence detection, glycoclusters 1a–3e (500 pmol) labeled with 68Ga-DOTA and Cy5 (excitation at 646 nm, emission at 663 nm) were administered to the tail vein of BALB/c nude mice (n=3) prior to whole-body scanning over 4 h. We initially examined the PETof glycoclusters 1–3, which consisted of bis-Neuα(2-6)Gal-containing glycan (a), asialoglycan (b), and bis-Neuα(2-3)Gal-glycan (c). Prior to injection, the radioactivity of each 68Ga-DOTA-labeled glycocluster was adjusted to 10 MBq.
First, the in vivo dynamics between the generations of clusters, namely those between the 4-mer (1a), 8-mer (2a), and 16-mer (3a), differed remarkably (Figure 1a–c). Although 4-mer 1a and 8-mer 2a were rapidly and almost completely cleared through the kidney (then to the urinary bladder) over 1 h (Figure 1a and b), the radioactivity derived from 16-mer 3a was still retained in the body after 4 h (Figure 1c), but was excreted slowly from the kidney/urinary bladder and from the gallbladder (intestinal excretion pathway). A biodistribution study of the dissected tissues after 4 h detected the 68Ga radioactivity of 3a mostly in the liver, gallbladder, and blood, then in the order of the lungs, kidney, colon, pancreas, and spleen (see the Supporting Information). These results clearly show the significant cluster and/or multivalency effects on the in vivo dynamics. Hence, the 16-mer cluster should be well suited for further biodistribution analyses by N-glycan imaging, but the 32-mer glycocluster would be unsuitable for in vivo dynamics studies because of its high molecular weight (ca. 100000), and thus was not considered for these experiments.
Figure 1.

Dynamic PET imaging of glycoclusters 1a, 2a, and 3a–c (a–e, respectively) in normal BALB/c nude mice. 68Ga-DOTA-labeled glycoclusters (10 MBq) were administered into the tail vein of the mice (n=3, 500 pmol, 100 μL/mouse) and the whole body was scanned by a small-animal PET scanner, microPET Focus 220 (Siemens Medical Solutions Inc., Knoxville, TN, USA), over 0–4 h after injection; H: heart; K: kidney; L: liver; B: urinary bladder; GB: gallbladder.
The differences in the clearance properties between the 4-mer, 8-mer, and 16-mer may be a result of their molecular size,[17] that is, the smaller 4-mer and 8-mer can be easily cleared through biofiltration in the kidney. In fact, the glomerular capillary wall in the kidney is highly permeable to water, electrolytes, and substances with relatively small molecular weights, while larger molecules such as myoglobin are filtered to a lesser degree.[17]
Alternatively, the degree of negative charge (the number of sialic acid residues) and/or degree of hydrophilicity of the clusters, that is, hydrophilic N-glycans versus the hydrophobic polylysine backbone occupying the surface of the clusters, may affect rapid clearance through the kidney. For example, the 4-mer and 8-mer, which have a more hydrophobic nature, may be trapped by scavenger receptors in the serum, similar to the degradation process for the “misfolded” hydrophobic glycoproteins inside the endoplasmic reticulum (ER; quality control of glycoproteins).[18]
We subsequently examined the in vivo dynamics and biodistribution of asialo-glycan 3b and bis-Neua(2-3)Gal glycan 3c using the 16-mer polylysine template (Scheme 1). Unlike the case of bis-Neua(2-6)Gal 3a (Figure 1c), the asialo-glycan cluster 3b rapidly cleared through the kidney to the bladder (Figure 1d), although some accumulation was observed in the liver because the asialoglycoprotein receptors are highly expressed in this organ.[2a] Dissection experiments after 4 h also found the strongest accumulation in the liver followed by the gallbladder and slight accumulation in the colon and kidney (see the Supporting Information). The results are consistent with our recent PET analyses of glycoproteins, orosomucoid, and asialoorosomucoid,[10] where the asialo congener is more rapidly excreted than orosomucoid through the kidney as well as the liver/gallbladder pathways (leading to intestinal excretion). However, the a linkage to the 3-OH of galactose in glycocluster 3c, which also contains sialic acid, was readily excreted through the kidney/urinary bladder as shown in Figure 1e.[19] These PET results on the 16-mer glycoclusters 3a–c suggest that the specific sialoside linkage to galactose, that is, the Neua(2-6)Gal linkage, in N-glycan structures plays an important role in the circulatory residence of N-glycans, which in turn results in uptake of 3a in the liver (see Figure 1c and the Supporting Information). In addition, this specific sialoside linkage markedly differentiates the excretion mechanism from those of the asialo and Neua(2-3)Gal cases, which proceed through a biofiltration pathway in the kidney.
The prolonged in vivo lifetime of the sialic acid-containing glycoclusters agrees with the well-known hypothesis of the clearance of the asialoglycoproteins through the asialoglyco-protein receptors.[2a,20] However, the notable difference in the serum stability as a result of the sialoside bond linkages to the galactose, that is, the α(2-6) versus α(2-3) linkages, is an intriguing observation.[19] These dynamic PET images suggest a new receptor-mediated excretion mechanism for Neuα(2-3)Gal-containing glycans, which usually cannot be found in serum. Alternatively, the “excretion-escaping” mechanism, by stimulating the immunosuppressive signals through the immunoreceptor tyrosine-based inhibitory motif (ITIM) molecules through Siglec families,[21] may account for the higher stability of Neuα(2-6)Gal-glycan (see below). The combination of high valency and long circulatory half-life (reduced clearance) of Neuα(2-6)Gal-glycan most likely leads to the uptake of 3a by hepatocytes through the Gal/GalNAc lectin receptor.[22, 23] The “polar transport mechanism”,[24] which “tags” the fucose to specific N-glycans in the liver, could also explain the slow clearance of bis-Neuα(2-6)Gal cluster 3a through the gallbladder.
In light of the strikingly different in vivo dynamics and excretion pathways between the N-glycan clusters 3a–c, a biodistribution study of the glycoclusters 3a–e was performed in more detail by using fluorescence imaging (Figure 2). Clusters 3d and 3e, which contain mixed Neuα(2-6)Gal and Neuα(2-3)Gal moieties, were particularly interesting because the circulatory stability was notably enhanced by Neuα(2-6)Gal, but not by Neuα(2-3)Gal, disaccharides. Thus, as visualized by fluorescence imaging (Figure 2), glycoclusters 3a, 3d, and 3e, which contain at least one Neuα(2-6)Gal nonreducing end motif, showed higher stabilities in vivo than asialo and Neuα(2-3)Gal congeners 3b and 3c. The Cy5 fluorescence derived from clusters 3a, 3d, and 3e was eventually accumulated mostly in the liver, presumably because of the interaction with asialoglycoprotein receptor,[22] but was also observed in the spleen after 4 h (Figure 2c–e). Out of the three glycoclusters, 3e, which contains both Neuα(2-6)Gal and Neuα(2-3)Gal nonreducing end structures (from the 6- and 3-hydroxy groups of branching mannose, respectively), showed the highest fluorescence intensity in the spleen (Figure 2e).
Figure 2.
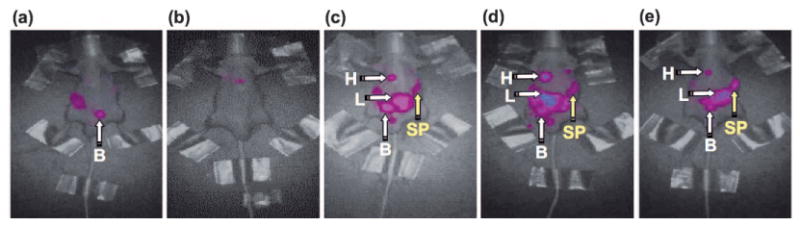
a–e) Dynamic fluorescence imaging of glycoclusters 3a–e in BALB/c nude mice: a) 3b; b) 3c; c) 3a; d) 3d; e) 3e. Cy5-labeled glycoclusters 3a–e were administered into the tail vein of the mice (n=3, 500 pmol, 100 μL/mouse) and whole-body scans were performed from the front side by eXplore Optix, GE Healthcare (excitation 646 nm, emission 663 nm) 4 h after injection. Data were normalized. H: heart; L: liver; B: urinary bladder; SP: spleen.
The spleen is an organ located in the abdomen, and it functions in the destruction of old, aged red blood cells as well as holding a reservoir of blood. Moreover, it can function as part of the immune system, such as the reticuloendothelial system (RES), or in the production of antigen-specific antibodies by interacting T cells with mature B cells. Because Siglec 2 (CD22),[21, 25] which is a Neuα(2-6)Gal-specific lectin, is overexpressed in mature B cells and is responsible for immune-negative regulations, we examined the possibility of an interaction with our clusters 3a–e (see the Supporting Information). The A20 B-cell line (expressed murine CD22), and Daudi B-cell line (expressed human CD22)[26] only detected a weak interaction with these clusters; cluster 3d exhibited the weakest interaction. Therefore, Neuα(2-6)Gal-containing clusters 3a, 3d, and 3e are most likely captured by the RES, which consists of phagocytic cells such as monocytes and macrophages that traffic to the spleen and liver as the reticular connective tissues. Yet during capture by the RES, stimulation by an “immune-suppressive signal”, through Neua(2-6)Gal–Siglec interactions on the phagocytic cells,[21] cannot be ruled out to explain the serum stability of these clusters. Overall, these in vivo images clearly visualize the importance of at least one Neuα(2-6)Gal moiety in the circulatory residence of the N-glycans and the precise regulation of the bio-distribution by a combination of the α(2-6)-and α(2-3)-sialoside linkages.
Additionally, an examination of glyco-clusters 3a–e in a cancer model was performed (see the Supporting Information). Although these clusters did not target the tumor tissue (DLD-1 implanted to the left femoral regions), markedly different in vivo dynamics were observed from those in normal mice. For example, excretion rates of glycoclusters 3d and 3e were accelerated in the cancer-affected mice and accumulation was not detected in the spleen, whereas the excretion rate of 3b was considerably suppressed in the cancer mice (see the Supporting Information). Although the phenomena could not be explained by the currently available data, these differences make N-glycan clusters applicable to a new class of diagnostic probes.
This study has, for the first time, demonstrated a marked difference in the in vivo dynamics and biodistributions between α(2-6) and α(2-3)sialosides, through a multivalent effect proven to allow high selectivity and affinity in ligand– protein interactions.[14,27] Totally different dynamics of the N-glycans between the normal and tumor models were also discovered by the present investigation. These results indicate the importance and validity of in vivo molecular imaging in living animals. Research directed towards targeting cancer, inflammation, and immune-related organs by using the developed glycoclusters is currently under way.
Experimental Section
General preparation procedure of glycoclusters (preparation of 3α): CuSO4 (64 μg, 3.2 × 10−4 mmol), sodium L-ascorbate (238 μg, 1.2 × 10−3 mmol), and DIPEA (74 nL) were added to a solution of acetylene-containing polylysine (16-mer, 158 μg, 2.0 × 10−5 mmol) and azide a (1.0 mg, 4.0 × 10−4 mmol) in DMF (50 μL) and H2O (50 μL) at room temperature. After the mixture had been stirred for 40 min at this temperature, DOTA (647 μg, 1.56 × 10−3 mmol) was added and the resulting solution was stirred for another 40 min. Low-molecular-weight compounds were removed by filtration using a Microcon centrifugal filter (YM-10, 10000 cut, Millipore), and the resulting aqueous solution was lyophilized to give glycocluster 3a as an amorphous solid (960 μg, quant.). Reverse-phase HPLC, size-partitioning gel filtration analysis, 1H NMR spectroscopy, and MALDI-TOF mass spectrometry data are shown in the Supporting Information.
Supplementary Material
Footnotes
We thank Kazuhiro Fukae, Azusa Hashimoto, and Jun Igarishi, Otsuka Chemical Co., Ltd., for supplying N-glycans. This work was supported in part by Grants-in-Aid for Scientific Research Nos. 19681024 and 19651095 from the Japan Society for the Promotion of Science, Collaborative Development of Innovative Seeds from the Japan Science and Technology Agency (JST), New Energy and Industrial Technology Development Organization (NEDO, project ID: 07A01014a), research grants from Yamada Science Foundation as well as the Molecular Imaging Research Program, and Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan.
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.201000892.
Contributor Information
Dr. Katsunori Tanaka, Department of Chemistry, Graduate School of Science, Osaka University, 1-1 Machikaneyama-cho, Toyonaka-shi, Osaka 560-0043 (Japan), Fax: (+81)6-6850-5419, ktzenori@chem.sci.osaka-u.ac.jp, koichi@chem.sci.osaka-u.ac.jp.
Dr. Eric R. O. Siwu, Department of Chemistry, Graduate School of Science, Osaka University, 1-1 Machikaneyama-cho, Toyonaka-shi, Osaka 560-0043 (Japan), Fax: (+81)6-6850-5419, ktzenori@chem.sci.osaka-u.ac.jp, koichi@chem.sci.osaka-u.ac.jp.
Kaori Minami, Department of Chemistry, Graduate School of Science, Osaka University, 1-1 Machikaneyama-cho, Toyonaka-shi, Osaka 560-0043 (Japan), Fax: (+81)6-6850-5419, ktzenori@chem.sci.osaka-u.ac.jp, koichi@chem.sci.osaka-u.ac.jp.
Dr. Koki Hasegawa, RIKEN Center for Molecular Imaging Science, 6-7-3 Minatojima, Chuo-ku, Kobe-shi, Hyogo 650-0047 (Japan)
Dr. Satoshi Nozaki, RIKEN Center for Molecular Imaging Science, 6-7-3 Minatojima, Chuo-ku, Kobe-shi, Hyogo 650-0047 (Japan)
Dr. Yousuke Kanayama, RIKEN Center for Molecular Imaging Science, 6-7-3 Minatojima, Chuo-ku, Kobe-shi, Hyogo 650-0047 (Japan)
Koichi Koyama, Kishida Chemical Co., Ltd., 14-10 Technopark, Sanda-shi, Hyogo 669-1339 (Japan).
Dr. Weihsu C. Chen, Department of Chemical Physiology, Joint Department of Molecular Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, MEM-L71, La Jolla, CA 92037 (USA)
Prof. James C. Paulson, Department of Chemical Physiology, Joint Department of Molecular Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, MEM-L71, La Jolla, CA 92037 (USA)
Prof., Dr. Yasuyoshi Watanabe, RIKEN Center for Molecular Imaging Science, 6-7-3 Minatojima, Chuo-ku, Kobe-shi, Hyogo 650-0047 (Japan)
Prof., Dr. Koichi Fukase, Department of Chemistry, Graduate School of Science, Osaka University, 1-1 Machikaneyama-cho, Toyonaka-shi, Osaka 560-0043 (Japan), Fax: (+81)6-6850-5419, ktzenori@chem.sci.osaka-u.ac.jp, koichi@chem.sci.osaka-u.ac.jp
References
- 1.Kamerling JP, Boons GJ, Lee YC, Suzuki A, Taniguchi N, Voragen AGJ, editors. Analysis of Glycans, Polysaccharide Functional Properties & Biochemistry of Glycoconjugate Glycans, Carbohydrate-Mediated Interactions: Comprehensive Glycoscience: From Chemistry to Systems Biology. II & III. Elsevier; 2007. [Google Scholar]
- 2.a) Morell AG, Irvine RA, Sternlieb I, Scheinberg IH, Ashwell G. J Biol Chem. 1968;243:155–159. [PubMed] [Google Scholar]; b) Elliott S, Lorenzini T, Asher S, Aoki K, Brankow D, Buck L, Busse L, Chang D, Fuller J, Grant J, Hernday N, Hokum M, Hu S, Knudten A, Levin N, Komorowski R, Martin F, Navarro R, Osslund T, Rogers G, Rogers N, Trail G, Egrie J. Nat Biotechnol. 2003;21:414–421. doi: 10.1038/nbt799. [DOI] [PubMed] [Google Scholar]; c) Sato M, Furuike T, Sadamoto R, Fujitani N, Nakahara T, Niikura K, Monde K, Kondo H, Nishimura SI. J Am Chem Soc. 2004;126:14013–14022. doi: 10.1021/ja046426l. [DOI] [PubMed] [Google Scholar]
- 3.Kaneko Y, Nimmerjahn F, Ravetch JV. Science. 2006;313:670–673. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- 4.a) Suzuki M, Noyori R, Langstom B, Watanabe Y. Bull Chem Soc Jpn. 2000;73:1053–1070. [Google Scholar]; b) Hosoya T, Sumi K, Doi H, Wakao M, Suzuki M. Org Biomol Chem. 2006;4:410–415. doi: 10.1039/b515215a. [DOI] [PubMed] [Google Scholar]
- 5.a) Tanaka K, Fukase K. Org Biomol Chem. 2008;6:815–828. doi: 10.1039/b718157b. [DOI] [PubMed] [Google Scholar]; b) Tanaka K, Fukase K. Mini-Rev Org Chem. 2008;5:153–162. [Google Scholar]
- 6.a) Ido T, Wan CN, Cassela V, Fowler JS, Wolf AP. J Labelled Compd Radiopharm. 1978;14:175–183. [Google Scholar]; b) Gambhir SS. Nat Rev Cancer. 2002;2:683–693. doi: 10.1038/nrc882. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka K, Fujii Y, Tokimoto H, Mori Y, Tanaka S, Bao Gm, Siwu ERO, Nakayabu A, Fukase K. Chem Asian J. 2009;4:574–580. doi: 10.1002/asia.200800411. and references therein. [DOI] [PubMed] [Google Scholar]
- 8.a) Vyas SP, Singh A, Sihorkar V. Crit Rev Ther Drug Carrier Syst. 2001;18:1–76. [PubMed] [Google Scholar]; b) Willis M, Forssen E. Adv Drug Delivery Rev. 1998;29:249–271. doi: 10.1016/s0169-409x(97)00083-5. [DOI] [PubMed] [Google Scholar]
- 9.a) Tanaka K, Minami K, Tahara T, Fujii Y, Siwu ERO, Nozaki S, Onoe H, Yokoi S, Koyama K, Watanabe Y, Fukase K. ChemMedChem. 2010;5:841–845. doi: 10.1002/cmdc.201000027. [DOI] [PubMed] [Google Scholar]; b) Tanaka K, Minami K, Tahara T, Siwu ERO, Koyama K, Nozaki S, Onoe H, Watanabe Y, Fukase K. J Carbohydr Chem. 2010;29:118–132. [Google Scholar]; c) Kishida Chemical Co., Ltd.; Lysine Labeling & Engineering Kit “STELLA+”. http://www.kishida.co.jp/ [Google Scholar]
- 10.Tanaka K, Masuyama T, Hasegawa K, Tahara T, Mizuma H, Wada Y, Watanabe Y, Fukase K. Angew Chem. 2008;120:108–111. doi: 10.1002/anie.200702989. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2008;47:102–105. [Google Scholar]
- 11.For representative examples of noninvasive imaging and biodistribution experiments, see; André S, Unverzagt C, Kojima S, Dong X, Fink C, Kayser K, Gabius HJ. Bioconjugate Chem. 1997;8:845–855. doi: 10.1021/bc970164d.André S, Unverzagt C, Kojima S, Frank M, Seifert J, Fink C, Kayser K, von der Lieth CW, Gabius HJ. Eur J Biochem. 2004;271:118–134. doi: 10.1046/j.1432-1033.2003.03910.x.André S, Kojima S, Prahl I, Lensch M, Unverzagt C, Gabius HJ. FEBSJ. 2005;272:1986–1998. doi: 10.1111/j.1742-4658.2005.04637.x.André S, Kojima S, Gundel G, Russwurm R, Schratt X, Unverzagt C, Gabius HJ. Biochim Biophys Acta Gen Subj. 2006;1760:768–782. doi: 10.1016/j.bbagen.2005.12.021.André S, Kozar T, Schuberth R, Unverzagt C, Kojima S, Gabius HJ. Biochemistry. 2007;46:6984–6995. doi: 10.1021/bi7000467.André S, Kozar T, Kojima S, Unverzagt C, Gabius HJ. Biol Chem. 2009;390:557–565. doi: 10.1515/BC.2009.072.Pimm MV, Perkins AC, Strohalm J, Ulbrich K, Duncan R. J Drug Targeting. 1996;3:385–390. doi: 10.3109/10611869608996829.Prata MIM, Santos AC, Torres S, André JP, Martins JA, Neves M, Garcia-Martin ML, Rodrigues TB, Lopez-Larrubia P, Cerdan S, Geraldes CFGC. Contrast Media Mol Imaging. 2006;1:246–258. doi: 10.1002/cmmi.111.Hirai M, Minematsu H, Kondo N, Oie K, Igarashi K, Yamazaki N. Biochem Biophys Res Commun. 2007;353:553–558. doi: 10.1016/j.bbrc.2006.12.060.Chen WC, Completo GC, Paulson JC. Glycobiology. 2008;18:963.Laughlin ST, Baskin JM, Amacher SL, Bertozzi CR. Science. 2008;320:664–667. doi: 10.1126/science.1155106.Serres S, Anthony DC, Jiang Y, Broom KA, Campbell SJ, Tyler DJ, van Kasteren SI, Davis BG, Sibson NR. J Neurosci. 2009;29:4820–4828. doi: 10.1523/JNEUROSCI.0406-09.2009.van Kasteren SI, Campbell SJ, Serres S, Anthony DC, Sibson NR, Davis BG. Proc Natl Acad Sci USA. 2009;106:18–23. doi: 10.1073/pnas.0806787106.
- 12.Tanaka K, Kageyama C, Fukase K. Tetrahedron Lett. 2007;48:6475–6479. [Google Scholar]
- 13.Kind gifts from Otsuka Chemical Co., Ltd., http://tansaku.otsukac.co.jp/en/oligo04.html. These structurally pure N-glycans were isolated from egg yolk according to the procedure developed by Kajihara and co-workers: Kajihara Y, Suzuki Y, Yamamoto N, Sasaki K, Sakakibara T, Juneja LR. Chem Eur J. 2004;10:971–985. doi: 10.1002/chem.200305115.
- 14.For representative reviews, see: Roy R, Beak MG. Rev Mol Biotechnol. 2002;90:291–309. doi: 10.1016/s1389-0352(01)00065-4.Leeuwenburgh MA, van der Marel GA, Overkleeft HS. Curr Opin Chem Biol. 2003;7:757–765. doi: 10.1016/j.cbpa.2003.10.009.Renaudet O. Mini-Rev Org Chem. 2008;5:274–286.Chabre YM, Roy R. Curr Top Med Chem. 2008;8:1237–1285. doi: 10.2174/156802608785848987.Chabre YM, Roy R. Advances in Carbohydrate Chemistry and Biochemistry. Vol. 63. Elsevier; UK: 2010. pp. 165–393.
- 15.As with previous reports on mass analysis of the sialosides, the molecular ions of the sialoglycocluster 3a were observed as a more broadened peak consisting of partially desialylated derivatives (see the Supporting Information): Fukuyama Y, Nakaya S, Yamazaki Y, Tanaka K. Anal Chem. 2008;80:2171–2179. doi: 10.1021/ac7021986.
- 16.Maecke HR, Hofmann M, Haberkorn U. J Nucl Med. 2005;46:172S–178S. [PubMed] [Google Scholar]
- 17.A molecular diameter of 3 nm is known to smoothly clear through the kidney. Clusters 1 (molecular weight, MW = 10000) and 3 (MW = 50000) in the unfolded conformation are estimated roughly to be 3 and 7 nm in size, respectively: Haraldsson B, Nystrom J, Deen WM. Physiol Rev. 2008;88:451–487. doi: 10.1152/physrev.00055.2006.Edwards A, Daniels BS, Deen WM. Am J Physiol Renal Physiol. 1999;276:F892–F902. doi: 10.1152/ajprenal.1999.276.6.F892.
- 18.Wyatt AR, Yerbury JJ, Poon S, Wilson MR. Curr Med Chem. 2009;16:2855–2866. doi: 10.2174/092986709788803187. [DOI] [PubMed] [Google Scholar]
- 19.The opposite serum stability of neoglycoproteins in mice, that is, α(2-3)-sialylated derivatives show longer half-lives than the α(2-6)-sialylated congeners, has been reported when glycans were attached to albumin: Unverzagt C, Andre S, Seifert J, Kojima S, Fink C, Srikrishna G, Freeze H, Kayser K, Gabius HJ. J Med Chem. 2002;45:478–491. doi: 10.1021/jm0110237.
- 20.Tozawa R, Ishibashi S, Osuga J, Yamamoto K, Yagyu H, Ohashi K, Tamura Y, Yahagi N, Iizuka Y, Okazaki H, Harada K, Gotoda T, Shimano H, Kimura S, Nagai R, Yamada N. J Biol Chem. 2001;276:12624–12628. doi: 10.1074/jbc.M011063200. [DOI] [PubMed] [Google Scholar]
- 21.a) Crocker PR, Varki A. Immunology. 2001;103:137–145. doi: 10.1046/j.0019-2805.2001.01241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Crocker PR, Varki A. Trends Immunol. 2001;22:337–342. doi: 10.1016/s1471-4906(01)01930-5. [DOI] [PubMed] [Google Scholar]; c) Angata T. Mol Diversity. 2006;10:555–566. doi: 10.1007/s11030-006-9029-1. [DOI] [PubMed] [Google Scholar]; d) Varki A, Angata T. Glycobiology. 2006;16:1R–27R. doi: 10.1093/glycob/cwj008. [DOI] [PubMed] [Google Scholar]
- 22.Park EI, Mi Y, Unverzagt C, Gabius HJ, Baenziger JU. Proc Natl Acad Sci USA. 2005;102:17125–17129. doi: 10.1073/pnas.0508537102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee YC, Townsend RR, Hardy MR, Lonngren J, Arnarp J, Haraldsson M, Lonn H. J Biol Chem. 1983;258:199–202. [PubMed] [Google Scholar]
- 24.a) Nakagawa T, Uozumi N, Nakano M, Mizuno-Horikawa Y, Okuyama N, Taguchi T, Gu J, Kondo A, Taniguchi N, Miyoshi E. J Biol Chem. 2006;281:29797–29806. doi: 10.1074/jbc.M605697200. [DOI] [PubMed] [Google Scholar]; b) Miyoshi E, Noda K, Yamaguchi Y, Inoue S, Ikeda Y, Wang W, Ko JH, Uozumi N, Li W, Taniguchi N. Biochim Biophys Acta Gen Subj. 1999;1473:9–20. doi: 10.1016/s0304-4165(99)00166-x. [DOI] [PubMed] [Google Scholar]
- 25.a) Cyster JG, Goodnow CC. Immunity. 1997;6:509–517. doi: 10.1016/s1074-7613(00)80339-8. [DOI] [PubMed] [Google Scholar]; b) Collins BE, Blixt O, DeSieno AR, Bovin N, Marth JD, Paulson JC. Proc Natl Acad Sci USA. 2004;101:6104–6109. doi: 10.1073/pnas.0400851101. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Crocker PR, Paulson JC, Varki A. Nat Rev Immunol. 2007;7:255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 26.a) Comelli EM, Head SR, Gilmartin T, Whisenant T, Haslam SM, North SJ, Wong NK, Kudo T, Narimatsu H, Esko JD, Drickamer K, Dell A, Paulson JC. Glycobiology. 2006;16:117–131. doi: 10.1093/glycob/cwj048. [DOI] [PubMed] [Google Scholar]; b) Tateno H, Li HY, Schur MJ, Bovin N, Crocker PR, Wakarchuk WW, Paulson JC. Mol Cell Biol. 2007;27:5699–5710. doi: 10.1128/MCB.00383-07. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) O'Reilly MK, Collins BE, Han S, Liao L, Rillahan C, Kitov PI, Bundle DR, Paulson JC. J Am Chem Soc. 2008;130:7736–7745. doi: 10.1021/ja802008q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.a) Manning DD, Hu X, Beck P, Kiessling LL. J Am Chem Soc. 1997;119:3161–3162. [Google Scholar]; b) Reuter JD, Myc A, Hayes MM, Gan Z, Roy R, Qin D, Yin R, Piehler LT, Esfand R, Tomalia DA, Baker JR., Jr Bioconjugate Chem. 1999;10:271–278. doi: 10.1021/bc980099n. [DOI] [PubMed] [Google Scholar]; c) Andre S, Kaltner H, Furuike T, Nishimura SI, Gabius HJ. Bioconjugate Chem. 2004;15:87–98. doi: 10.1021/bc0340666. [DOI] [PubMed] [Google Scholar]; d) Ladmiral V, Mantovani G, Clarkson GJ, Cauet S, Irwin JL, Haddleton DM. J Am Chem Soc. 2006;128:4823–4830. doi: 10.1021/ja058364k. [DOI] [PubMed] [Google Scholar]; e) Carlson CB, Mowery P, Owen RM, Dykhuizen EC, Kiessling LL. ACS Chem Biol. 2007;2:119–127. doi: 10.1021/cb6003788. [DOI] [PubMed] [Google Scholar]; f) Wolf-enden ML, Cloninger MJ. Bioconjugate Chem. 2006;17:958–966. doi: 10.1021/bc060107x. [DOI] [PubMed] [Google Scholar]; g) Kim J, Ahn Y, Park KM, Kim Y, Ko YH, Oh DH, Kim K. Angew Chem. 2007;119:7537–7539. [Google Scholar]; Angew Chem Int Ed. 2007;46:7393–7395. doi: 10.1002/anie.200702540. [DOI] [PubMed] [Google Scholar]; h) Lee MJ, Pal K, Tasaki T, Roy S, Jiang Y, An JY, Banerjee R, Kwon YT. Proc Natl Acad Sci USA. 2008;105:100–105. doi: 10.1073/pnas.0708465105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


