Abstract
The host immune response plays a key role in breast cancer progression and response to therapy. However, relative to primary invasive breast cancers, the immune milieu of breast ductal carcinoma in situ is less understood .Here , we profile tumor infiltrating lymphocytes and expression of the immune checkpoint ligand PD-L1 in 27 cases of ductal carcinoma in situ (DCIS) with known estrogen receptor, progesterone receptor and human epidermal growth factor-2 expression using tissue microarrays. Twenty-four cases were pure DCIS, and 3 had associated invasive ductal carcinoma. Tumors were stained by immunohistochemistry for PD-L1, as well as the lymphocyte markers CD3, CD4,CD8, FoxP3 , and CD20. The expression of PD-L1 by DCIS carcinoma cells and tumor infiltrating lymphocytes was determined, and the average tumor infiltrating lymphocytes per high power field was manually scored. None of the DCIS cells expressed PD-L1, but 81% of DCIS lesions contained PD-L1+ tumor infiltrating lymphocytes. DCIS with moderate-diffuse tumor infiltrating lymphocytes were more likely to have PD-L1+ tumor infiltrating lymphocytes (p=0.004) . Tumor infiltrating lymphocytes with high levels of PD-L1 expression (>50% cells) were seen only in triple negative DCIS (p=0.0008), and PD-L1-tumor infiltrating lymphocytes were seen only in estrogen receptor+/human epidermal growth factor-2 - DCIS (p=0.12). The presence of PD-L1+ tumor infiltrating lymphocytes was associated with a younger mean patient age (p=0.01). Further characterization of the DCIS immune microenvironment may identify useful targets for immune-based therapy and breast cancer prevention.
Keywords: breast cancer, ductal carcinoma in situ, PD-L1 (B7-H1), tumor infiltrating lymphocytes
Introduction
Data from both laboratory studies and clinical trials support an important role for the host immune response in breast cancer progression and in patient response to therapy( 1, 2). Tumor infiltrating lymphocytes , both within tumor cell nests and in tumor-associated stroma, are associated with improved patient outcomes . In treatment naïve triple-negative breast carcinomas, tumor infiltrating lymphocytes are an independent prognostic factor for improved survival (3, 4), decreased distant recurrence (4, 5), and increased time to metastasis (6). The presence of tumor infiltrating lymphocytes in triple-negative breast carcinomas post-neoadjuvant therapy is also prognostic, predicting longer metastasis-free and overall survival( 3). In addition, the presence of tumor infiltrating lymphocyte spredicts response to therapy, as larger numbers of tumor infiltrating lymphocytes correlate with better responses to chemotherapy in triple-negative breast carcinomas and estrogen receptor(ER) negative breast cancer( 1, 2), and to trastuzumab-based chemotherapy in human epidermal growth factor receptor-2 (HER-2) positive breast carcinomas (5). Multiple tumor infiltrating lymphocyte subsets, including CD8+ cytotoxic T cells, CD4 + helper T cells, and CD20+ B cells , predict patient survival across the breast carcinoma subtypes (1, 2). In contrast, high numbers of FoxP3+ regulatory T cells (Tregs) relative to CD8+ T cells predicts decreased progression free survival and overall survival( 7) in breast carcinoma patients.
Gene expression profiling defined five major molecular subtypes of invasive primary breast carcinomas with unique clinicopathologic characteristics and outcomes:luminal A, luminal B, HER-2-enriched (HER-2+), basal-like, and normal breast-like( 8). These molecular subtypes have immunohistochemical correlates, where luminal A cancers are ER + progesterone receptor (PR)+HER-2− Ki67low, luminal B cancers are ER+PR+HER-2− Ki67highor ER +PR+HER-2+, HER-2+ cancers are ER− PR− HER-2+, and basal-like carcinomas are typically ER− PR− HER-2− (triple negative breast carcinoma)with cytokeratin (CK)5/6 or epidermal growth factor (EGFR) expression (9). The luminal Aphenotype has an improved prognosis relative to luminal B, HER-2+ or basal-like carcinomas (9). These same gene expression profiles and immunohistochemical phenotypes are observed in ductal carcinoma in situ (DCIS), the only established precursor to invasive primary breast carcinomas (10).
While HER -2+ breast cancers and triple negative breast carcinomas have a poor prognosis relative to other subtypes, they typically have more vigorous tumor lymphocyte infiltrates . Larger numbers of tumor infiltrating lymphocytes are seen in ER − relative to ER+ carcinomas (11). In addition, genetic correlates of the immune response predict better survival in ER− and HER-2+ carcinomas, but not ER + carcinomas (12). While robust immune infiltrates are associated with ER− and HER -2+ carcinomas, evidence suggests functional skewing toward a pro -tumorigenic phenotype. High levels of Treg are more common in ER− carcinomas (13), and predicts horter progression free and overall survival( 7). In addition, relative to luminal A cancers, triple negative breast carcinomas tend to harbor tumor infiltrating lymphocytes with the T helper type 2 phenotype thought to promote tumor growth (14).
Growing evidence supports a major role for immune checkpoint pathways, such as those mediated by interactions between programmed cell death protein 1 (PD-1) and programmed death ligand 1 (PD-L1), in regulating anti-tumor responses( 1, 2). PD-1 (B7-1)is upregulated following the activation of lymphocytes and belongs to the B7-CD28 family. Its primary ligands are PD-L1 and PD-L2, which are inducible by IFN -γ and other inflammatory cytokines on the surface of tumor cells and tumor infiltrating T cells, B cells, macrophages and dendritic cells (15). The interaction of PD-1 with PD -L1/2 inhibits T cell activation and proliferation (16). PD-L1 may also be constitutively expressed on tumor cells as a result of oncogenic signaling or epithelial to mesenchymal transition (17, 18). Tumor cells may also counter an active anti-tumor immuneresponse by upregulating PD -L1 expression through a process known as adaptive immune resistance (19). PD-L1 is expressed on the tumor cells of invasive breast carcinomas and their associated tumor infiltrating lymphocytes( 17, 18, 20)and correlates with lack of ER expression, increased numbers of tumor infiltrating lymphocytes, response to chemotherapy, and the triple negative phenotype ( 21). The role of PD-L1 expression as a prognostic factor in breast carcinoma remains unclear. In one study, PD-L1 expression was associated with improved clinical outcomes( 11), however a separate study reported an association with negative clinical outcomes( 22).
We previously reported differential patterns of tumor infiltrating lymphocytes across matched primary and metastatic breast carcinomas (23), and in invasive primary breast carcinomas with associated DCIS( 24). Our data suggest that tumor immunobiology evolves as tumorsprogress . Little is known about the immune milieu of DCIS or how the antitumor immune response evolves as breast tumors progress from in situ to invasive and then metastatic lesions. Here we profile the composition of tumor infiltrating lymphocytes and PD -L1 expression in 27 cases of DCIS with known ER, PR, and HER-2 expression.
Methods
Case selection and tissue microarray construction
This study was approved by the Institutional Review Board of the Johns Hopkins Medical Institutions. We evaluated tissue microarrays previously constructed from archived, paraffin-embedded blocks of primary DCIS (25, 26), with 27 cases evaluable. Each DCIS case was sampled with 2–5 cores/tumor, with each core measuring 1.4 mm in diameter; 1 core/case sampled benign breast lobules. Clinicopathologic characteristics were recorded, including patient age, gender, race, tumor size, presence or absence of associated invasive carcinoma, DCIS nuclear grade, presence or absence of necrosis, ER, PR and H ER-2 status, and patient outcome (local recurrence, metastasis and survival). Carcinoma recurrence was defined as any in situ or invasive carcinoma recurrence in the ipsilateral breast, axilla or chest wall. Younger women were defined as <40 years old, and older women as >40 years old.
Immunohistochemistry and tumor infiltrating lymphocyte quantification
To characterize the DCIS subtype, tissue microarrays were stained by immunohistochemistry for ER(clone 6F11 ;Leica Microsystems, Bannockburn, IL), PR(clone 16 ;Leica Microsystems, Bannockburn, IL), and HER-2 (Ventana 4B5; Ventana Medical Systems Inc, Tucson, AZ) using standard automated methods. Tumors were classified as Luminal A (ER +/PR+/HER-2− ), Luminal B(E R+/PR+/HER-2+), HER-2+(ER − /PR− /HER-2+) and triple negative (ER− /PR− /HER-2− ). HER-2 positivity was defined as >10% complete strong membranous DCIS cell labeling as per the 2012 ASCO/CAP guidelines for HER-2 immunohistochemistry.
Each case was assigned a qualitative stromal tumor infiltrating lymphocyte density score on the hematoxylin and eosin (H&E) stained sections: 0 (no tumor infiltrating lymphocytes), 1 (mild, <5% tumor area with tumor infiltrating lymphocytes), 2 (moderate, 5–50% tumor area with tumor infiltrating lymphocytes), and 3 (diffuse/marked, >50% infiltration) (19, 24, 27).Tumor infiltrating lmphocytes were scored by two pathologists blinded to clinicopathologic characteristics (ET and ACM). Tissue microarrays were stained for CD20 (monoclonal, clone MS/L26, catalogue no. 760–2531, Ventana Medical Systems Inc, Tucson, AZ), CD3 (mouse monoclonal, clone PS1, catalogue no. ORG-8982, Leica Microsystems; Bannockburn, IL), CD4 (rabbit monoclonal, clone SP35, catalogue no. 790-4423, Ventana Medical Systems Inc, Tucson, AZ), CD8 (mouse monoclonal, clone C8/C8144B, catalogue no. 760-4250, Cell Marque; Rockin, CA) and FoxP3 (mouse monoclonal, clone 236A/E7, catalogue no. 14-4777-80, dilution 1:50, eBioscience; San Diego, CA)to characterize tumor infiltrating lymphocytes as previously described (23). CD20, CD3, CD4 and CD8 expression were defined by membranous lymphocyte labeling, and FoxP3 expression was defined by nuclear labeling in lymphocytes. The total number of tumor infiltrating lymphocytes/high power field was manually counted in one high power field/tissue microarray tumor core, and averaged across the case to give the mean number of tumor infiltrating lymphocytes/high power field. Each high power field was chosen as representative of the overall tumor lymphocytic infiltration in each tumor core.
Tissue microarrays were stained for PD-L1 (B7-H1) using the murine anti-human PD-L1 antibody, clone 5H1 (2 μg/ml) with a paired isotype murine IgG1 control as previously described (19). PD-L1 staining was scored by two pathologists (ET and ACM) blinded to patient clinicopathologic characteristics; discrepancies were adjudicated by a third pathologist (JT). PD-L1 staining on carcinoma cells and tumor infiltrating lymphocytes was scored by percent membranous staining. PD-L1 positivity by DCIS carcinoma cells was defined as >5% membranous staining. PD-L1 positivity in tumor infiltrating lymphocytes was scored as none (0), focal (1+; <5%), moderate (2+; 5–50%), or marked (3+; 51–100%)percentage of tumor infiltrating lymphocytes expressing PD-L1. PD-L1 expression was also scored as low (<50% tumor infiltrating lymphocytes) or high (>50% of tumor infiltrating lymphocytes).
Statistical analysis
Statistical analysis was performed using Fisher’s Exact Test and paired, two -tailed Student’s T-test.
Results
Clinicopathologic features of DCIS cases
The clinicopathologic features of 27 evaluable patients with DCIS are detailed in Table 1. The median patient age was 38 years (mean 41 years, range 18–74), with 52% white, 26% black, 7% Asian and 4% Hispanic patients. The DCIS phenotype of 26 evaluable patients included 62% luminal A, 15% luminal B, 12% HER-2+ and 12% triple negative, for a total of 77% ER+ and 23% ER− cases. The ER/PR/HER-2 status of one case could not be determined, and the case was not included in analyses that subdivided by DCIS phenotype. Most cases were nuclear grade 2 (52%) or grade 3 (44%). The average tumor size was 2.4 cm; 37% were multifocal. Twenty-four cases were pure DCIS (pathologic stage pTis), without any associated invasive carcinoma. Three cases had associated infiltrating ductal carcinoma, which were also present on the tissue microarray cores . Two of these cases were triple negative DCIS and one was luminal ADCIS . The mean and median follow-up time were 82 months and 80 months (approximately 7 years), respectively. Two (7%) DCIS patients had an ipsilateral recurrence, both of which were nuclear grade 3; one patient with triple negative DCIS and concurrent infiltrating ductal carcinoma developed recurrent infiltrating ductal carcinoma in the ipsilateral chest wall one year after initial diagnosis, and one patient with HER-2+ DCIS experienced recur rent ipsilateral DCIS three years after initial diagnosis. No patients received neoadjuvant therapy. All patients received adjuvant therapy for pure DCIS or infiltrating carcinomaas per the standard of care.
Table 1.
Clinicopathologic Characteristics of Ductal Carcinoma in situ Cases.
| DCIS (all cases) | Phenotype1 | ||||
|---|---|---|---|---|---|
|
| |||||
| Luminal A | Luminal B | HER-2 | Triple negative | ||
|
| |||||
| Total Number | n=27 | 16 (62%) | 4 (15%) | 3 (12%) | 3 (12%) |
|
| |||||
| Age (years) | |||||
| median (range) | 38 (18–74) | 45 | 37 | 33 | 37 |
| <=40 | 19 (70%) | 10 (62%) | 4 (100%) | 2 (67%) | 2 (67%) |
| >40 | 8 (30%) | 6 (38%) | 0 | 1 (33%) | 1 (33%) |
|
| |||||
| Race | |||||
| White | 14 (52%) | 9 (57%) | 2 (50%) | 2 (50%) | 1 (25%) |
| Black | 7 (26%) | 4 (25%) | 0 (0%) | 0 (0%) | 2 (50%) |
| Asian | 2 (7%) | 1 (6%) | 1 (25%) | 0 (0%) | 0 (0%) |
| Hispanic | 1 (4%) | 1 (6%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Other | 3 (11%) | 1 (6%) | 1 (25%) | 1 (25%) | 0 (0%) |
|
| |||||
| Nuclear Grade | |||||
| 1 | 1 (4%) | 1 (6%) | 0 (0%) | 0 (0%) | 0 (0%) |
| 2 | 14 (52%) | 11 (69%) | 2 (50%) | 0 (0%) | 0 (0%) |
| 3 | 12 (44%) | 4 (25%) | 2 (50%) | 3 (100%) | 3 (100%) |
|
| |||||
| Necrosis | |||||
| no | 6 (22%) | 5 (31%) | 0 (0%) | 0 (0%) | 0 (0%) |
| yes | 21 (78%) | 11 (69%) | 4 (100%) | 3 (100%) | 3 (100%) |
|
| |||||
| Multifocality | |||||
| no | 17 (63%) | 11 (69%) | 2 (50%) | 2 (67%) | 2 (67%) |
| yes | 10 (37%) | 5 (31%) | 2 (50%) | 1 (33%) | 1 (33%) |
|
| |||||
| Size (cm) | |||||
| mean | 2.4 | 1.7 | 4.2 | 4.8 | 2.8 |
| <1 | 7 (26%) | 6 (38%) | 1 (25%) | 0 (0%) | 0 (0%) |
| >1 | 9 (34%) | 7 (44%) | 0 (0%) | 0 (0%) | 1 (33%) |
| >2 | 2 (7%) | 1 (6%) | 0 (0%) | 1 (33%) | 0 (0%) |
| >3 | 2 (7%) | 1 (6%) | 0 (0%) | 0 (0%) | 1 (33%) |
| >4 | 5 (19%) | 1 (6%) | 3 (75%) | 1 (33%) | 0 (0%) |
| NR | 2 (7%) | 0 (0%) | 0 (0%) | 1 (33%) | 1 (33%) |
|
| |||||
| Recurrence | |||||
| no | 25 (93%) | 16 (100%) | 0 (0%) | 2 (67%) | 2 (67%) |
| yes | 2 (7%) | 0 (0%) | 0 (0%) | 1 (33%) | 1 (33%) |
|
| |||||
| Associated Infiltrating Carcinoma | |||||
| no | 24 (89%) | 15 (94%) | 0 (0%) | 0 (0%) | 1 (33%) |
| yes | 3 (11%) | 1 (6%) | 0 (0%) | 0 (0%) | 2 (67%) |
(Abbreviations: cm=centimeter; DCIS=ductal carcinoma in situ; HER-2=human epidermal growth factor 2; n=number; NR=not reported;
The phenotype for one case was unable to be determined.
Quantification of tumor infiltrating lymphocytesin DCIS
We first evaluated tumor infiltrating lymphocytes associated with DCIS by histopathologic density scoring on a scale from 0–3. All cases had tumor infiltrating lymphocytes, with 78% showing either moderate (score 2) or diffuse (score 3) tumor infiltrating lymphocyte density (Table 2). Only 2 cases (7%) contained diffuse tumor infiltrating lymphocytes. ER+ DCIS and DCIS in older patients tended to have lower tumor infiltrating lymphocyte density scores than ER− DCIS o r DCIS in younger patients(p = 0. 28 and 0.32, respectively). There was only one case of grade 1 DCIS, precluding group wide comparison, and there was no discernible difference in tumor lymphocytic infiltration between the grade 2 DCIS and grade 3 DCIS.
Table 2.
Immune Parameters of Ductal Carcinoma in situ.
| Phenotype | p- value1 | Age (years) | p- value | Concurrent IDC | p-value | Recurrence | p-value | TIL PD-L1 Status | p-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||
| Immune parameter | All cases | ER positive | ER negative | 0.28 | <=40 | >40 | 0.32 | No | Yes | 1 | No | Yes | 1 | Negative | Positive | 0.004* |
|
|
|
|
|
|
||||||||||||
| n= 27 | n=20 | n=6 | 19 (70%) | 8 (30%) | 24 (89%) | 3 (11%) | 25 (93%) | 2 (7%) | 5 (19%) | 22 (81%) | ||||||
|
|
|
|
|
|
||||||||||||
| Tumor infiltrating lymphocyte density2 | ||||||||||||||||
| None: 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| Focal: 1 | 6 (22%) | 6 (30%) | 0 | 3 (16%) | 3 (38%) | 6 (25%) | 0 | 6 (24%) | 0 | 4 (80%) | 2 (9%) | |||||
| Moderate: 2 | 19 (70%) | 13 (65%) | 5 (83%) | 15 (19%) | 4 (50%) | 18 (75%) | 1 (33%) | 19 (76%) | 1 (50%) | 1 (20%) | 18 (82%) | |||||
| Diffuse: 3 | 2 (7%) | 1 (5%) | 1 (17%) | 1 (5%) | 1 (13%) | 0 | 2 (67%) | 0 | 1 (50%) | 0 | 2 (9%) | |||||
| Moderate or diffuse: 2–3 | 21 (78%) | 14 (70%) | 6 (100%) | 16 (84%) | 5 (62%) | 18 (75%) | 3 (100%) | 19 (76%) | 2 (100%) | 1 (20%) | 20 (91%) | |||||
|
| ||||||||||||||||
| CD3/hpf | 41.5 | 37.3 | 58.2 | 0.29 | 43.6 | 36.9 | 0.71 | 43.4 | 27.3 | 0.53 | 39.7 | 59.9 | 0.77 | 18.6 | 47.3 | 0.15 |
|
| ||||||||||||||||
| CD4/hpf | 19.3 | 17 | 30.2 | 0.22 | 21.7 | 13.7 | 0.41 | 19.4 | 18.4 | 0.94 | 18.1 | 33.8 | 0.87 | 6.7 | 22.2 | 0.18 |
|
| ||||||||||||||||
| CD8/hpf | 16.8 | 14.3 | 25.8 | 0.12 | 19.6 | 10.3 | 0.16 | 15.5 | 27.3 | 0.23 | 15.4 | 34.5 | 0.34 | 7.4 | 18.4 | 0.1 |
|
| ||||||||||||||||
| Foxp3/hpf | 8.3 | 6.3 | 15.3 | 0.06 | 8.9 | 6.9 | 0.66 | 8.4 | 7 | 0.13 | 7.5 | 17.3 | 0.72 | 1.46 | 10 | 0.3 |
|
| ||||||||||||||||
| CD20/hpf | 12.8 | 11.2 | 19.9 | 0.49 | 13.8 | 10.3 | 0.76 | 12.1 | 18.3 | 0.7 | 10.5 | 41.5 | 0.45 | 0.67 | 15.5 | 0.1 |
|
| ||||||||||||||||
| CD8/FoxP33 | n=25 | n=19 | n=6 | n=17 | n=8 | n=22 | n=3 | n=23 | n=2 | n=5 | n=20 | |||||
| <1 | 5 (20%) | 3 (18%) | 2 (33%) | 0.56 | 2 (12%) | 3 (38%) | 0.28 | 5 (23%) | 0 | 1 | 4 (17%) | 1 (50%) | 0.37 | 1 (20%) | 4 (20%) | 1 |
| >1 | 20 (80%) | 16 (84%) | 4 (67%) | 15 (88%) | 5 (62%) | 17 (77%) | 3 (100%) | 19 (83%) | 1 (50%) | 4 (80%) | 16 (80%) | |||||
| <4 | 15 (60%) | 10 (53%) | 5 (83%) | 0.34 | 9 (53%) | 6 (75%) | 0.40 | 14 (64%) | 1 (33%) | 0.54 | 13 (57%) | 2 (100%) | 0.50 | 3 (60%) | 12 (60%) | 1 |
| >4 | 10 (40%) | 9 (47%) | 1 (17%) | 8 (47%) | 2 (25%) | 8 (36%) | 2 (67%) | 10 (43%) | 0 | 2 (40%) | 8 (40%) | |||||
|
| ||||||||||||||||
| CD20/hpf | ||||||||||||||||
| <5 | 16 (59%) | 13 (65%) | 2 (33%) | 0.35 | 10 (52%) | 6 (75%) | 0.40 | 14 (58%) | 2 (67%) | 1 | 15 (63%) | 0 | 0.118 | 5 (100%) | 11 (50%) | 0.06 |
| >5 | 11 (41%) | 7 (35%) | 4 (67%) | 9 (48%) | 2 (25%) | 10 (42%) | 1 (33%) | 10 (40%) | 2(100%) | 0 (0%) | 11 (50%) | |||||
|
| ||||||||||||||||
| Tumor infiltrating lymphocyte PD-L1 status | ||||||||||||||||
| PD-L1pos | 22 (81%) | 14 (70%) | 6 (100%) | 0.29 | 18 (96%) | 4 (50%) | 0.02* | 19 (79%) | 3 (100%) | 1 | 20 (80%) | 2 (100%) | 1 | |||
| PD-L1neg | 5 (19%) | 5 (25%) | 0 (0%) | 1 (5%) | 4 (50%) | 5 (21%) | 0 (0%) | 5 (20%) | 0 (0%) | |||||||
(Abbreviations: DCIS=ductal carcinoma in situ; ER=estrogen receptor; hpf=high power field; IDC=invasive ductal carcinoma; lo=low; n=number; neg=negative; pos=positive; TNBC=triple negative breast cancer.
P-values were calculated using Fisher’s exact test or two-tailed Student’s T-test. Asterisks indicate statistically significant differences of p<0.05.
The p-values for tumor infiltrating lymphocyte density are derived from comparison between the group tumor infiltrating lymphocyte density 0–1 and group tumor infiltrating lymphocyte density 2–3.
Only 25 patients had calculable CD8/FoxP3 ratios (i.e., 2 cases with unevaluable FoxP3 counts were excluded).
We quantified subsets of tumor infiltrating lymphocytes using CD3, CD4, CD8, CD20 and FoxP3 staining. CD3+ T cells predominated across all DCIS subtype sat all ages , with slightly more CD4+ T cells than CD8+T cells on average. CD20 +B cells were the next most common tumor infiltrating lymphocytes, followed by FoxP3+ Treg (Table 2). On average, ER− DCIS contained higher numbers of all tumor infiltrating lymphocyte subsets than ER +DCIS, and ER+ DCIS was more likely to have to have a high CD8/FoxP3 ratio( >4) than ER− DCIS . DCIS in young women also contained higher numbers of all tumor infiltrating lymphocyte subsets relative to older women; however, these differences were not statistically significant. There was no difference in tumor infiltrating lymphocyte subset distribution between the nuclear grade 2 DCIS and grade 3 DCIS.
Although the case numbers are small and results should be interpreted with caution , differences in tumor infiltrating lymphocytes were also seen in DCIS with concurrent infiltrating ductal carcinoma(n=3) , and in DCIS that later recurred(n=2) . The 2 cases of DCIS with diffuse tumor infiltrating lymphocytes (score 3) were DCIS with concurrent infiltrating ductal carcinoma (p=0.009). DCIS with concurrent infiltrating ductal carcinoma showed higher levels of infiltrating CD8+ T cells and CD20+ B cells than cases with only in situ disease, but this was not statistically significant. DCIS that recurred had higher numbers of all tumor infiltrating lymphocyte subsets relative to DCIS that did not recur. DCIS that recurred also had the greatest number of CD8+T cells of any subset of DCIS cases examined. However, this DCIS phenotype also showed a lower CD8/FoxP3 ratio , indicating a commensurate increase in regulatory T cells in these two cases.
PD-L1 expression in DCIS and tumor infiltrating lymphocytes
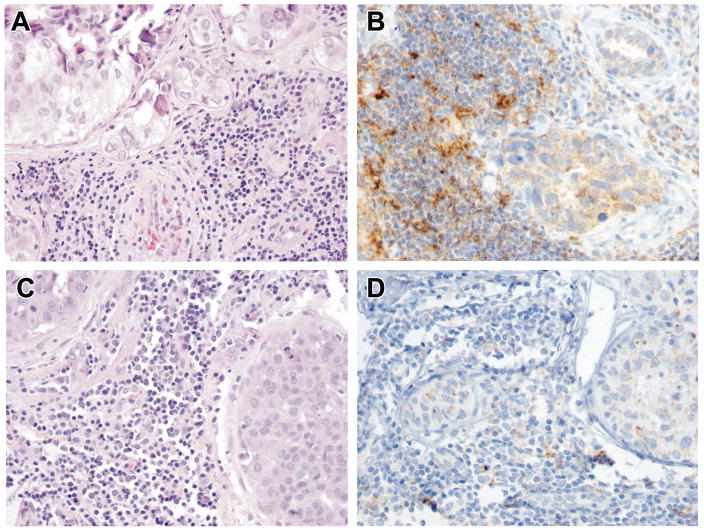
We next examined the cell surface expression of PD-L1 by DCIS tumor cells and associated tumor infiltrating lymphocytes. There was no expression of PD-L1 on DCIS tumor cells in any case, although nonspecific PD-L1 staining was seen within the necrotic debris of central comedonecrosis. In DCIS with concurrent infiltrating ductal carcinoma(n=3) , the PD-L1 status was concordant(negative) between in situ and invasive components, as we have previously reported (24). While DCIS tumor cells were PD -L1− , 81% of the DCIS cases showed PD-L1 expression by tumor infiltrating lymphocytes( Figure 1A–B and Table 2).All DCIS cases with PD-L1− tumor infiltrating lymphocytes were of the ER+ luminal A phenotype (p=0.12) (Figure 1C–D), while all ER− cases had PD-L1+ tumor infiltrating lymphocytes( Table 3). PD-L1 expression was also scored as low (<50% of tumor infiltrating lymphocytes) or high (>50% of tumor infiltrating lymphocytes). All luminal A, luminal B, and HER-2+ DCIS with PD -L1+ tumor infiltrating lymphocytes showed low PD-L1 expression on tumor infiltrating lymphocytes( Table 3). In contrast, 100% of triple negative DCIS had high expression of PD -L1 on tumor infiltrating lymphocytes(p=0.0008 ).In addition, all cases with high expression of PD -L1 on tumor infiltrating lymphocytes were nuclear grade 3 DCIS compared to nuclear grade 2 (p=0.07) (Table 3). The average age of DCIS patients with PD-L1+ and PD -L1− tumor infiltrating lymphocytes was 38 and 54 years (p=0.01), respectively, with 80% of DCIS containing PD-L1− tumor infiltrating lymphocytes occurred in older patients (p=0.02) . All high risk DCIS (concurrent infiltrating ductal carcinoma and recurrent DCIS) had PD-L1+ tumor infiltrating lymphocytes, though this was not statistically significant.
Figure 1. PD-L1 staining patterns differ in triple negative and estrogen receptor (ER)+ ductal carcinoma in situ (DCIS).
All cases of triple negative DCIS (A, H&E) contain tumor infiltrating lymphocytes with high PD-L1+ expression (>50% cells) (B). ER+DCIS (C, H&E) shows low(<50% cells) or absent PD-L1 tumor infiltrating lymphocyte staining .
Table 3.
Relationship of Tumor Infiltrating Lymphocyte PD-L1 labeling to Ductal Carcinoma in situ Phenotype and Nuclear grade
| Tumor infiltrating lymphocyte PD-L1 status |
|||||
|---|---|---|---|---|---|
| PD-L1pos | PD-L1hi 1 | PD-L1lo 2 | PD-L1neg | ||
|
| |||||
| Phenotype | |||||
| Luminal A | n=16 (62%) | 11 (52%) | 0 | 11 (61%) | 5 (100%) |
| Luminal B | n=4 (15%) | 4 (19%) | 0 | 4 (22%) | 0 |
| Her-2 | n=3 (12%) | 3 (14%) | 0 | 3 (16%) | 0 |
| Triple negative | n=3 (12%) | 3 (14%) | 3 (100%) | 0 | 0 |
| Total | n=26 | n=21 | n=3 | n=18 | n=5 |
|
| |||||
| Nuclear grade | |||||
| Grade 1 | n=1 (4%) | 1 (5%) | 0 | 1 (6%) | 0 |
| Grade 2 | n=14 (52%) | 11 (52%) | 0 | 11 (61%) | 3 (60%) |
| Grade 3 | n=12 (44%) | 9 (43%) | 3 (100%) | 6 (33%) | 2 (40%) |
| Total | n=27 | n=22 | n=3 | n=18 | n=5 |
(Abbreviations: DCIS=ductal carcinoma in situ; HER-2=human epidermal growth factor 2; hi=high; lo=low; n=number; neg=negative; yr=years-old.
PD-L1hi expression was defined as >50% tumor infiltrating lymphocytes with PD-L1 labeling.
PD-L1lo expression was defined as <=50% TIL with PD-L1 labeling).
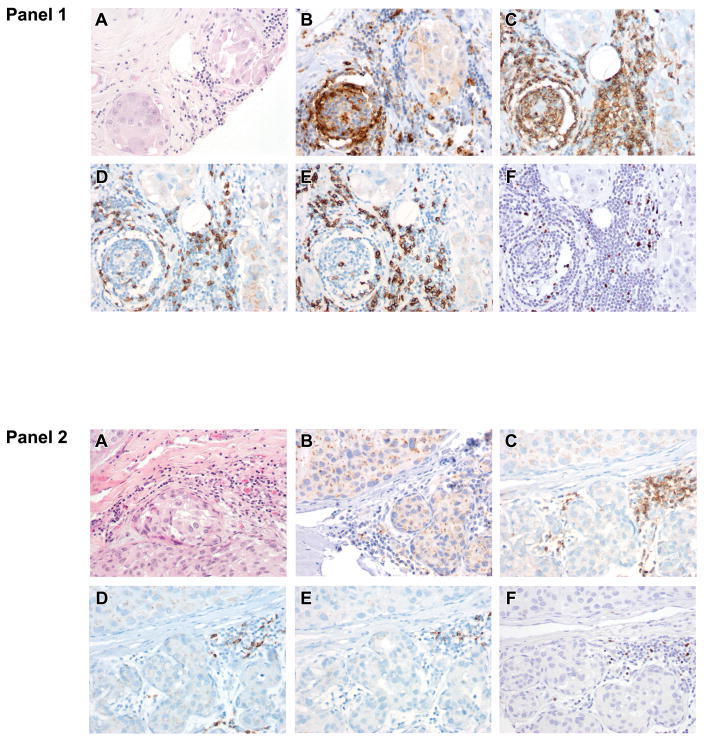
DCIS with low tumor infiltrating lymphocyte density scores (score 1) were more likely to be PD -L1− than PD -L1+ (p=0.004). In contrast, cases of DCIS with moderate or diffuse tumor infiltrating lymphocyte density scores (scores 2–3) were more likely to be PD-L1+ than PD -L1− (Figure 2 and Table 2). DCIS with PD-L1+ tumor infiltrating lymophocytes had higher numbers of tumor infiltrating lymphocytes across all subsets examined when compared to tumors with PD-L1− tumor infiltrating lymphocytes , though this was not statistically significant (Figure 2 and Table 2). Both PD-L1+ and PD -L1− DCIS had similar CD8/FoxP3 ratios.
Figure 2. Immunologic features of the ductal carcinoma in situ (DCIS) tumor microenvironment.
Most DCIS cases (81%) (Panel 1; A, H&E) display PD-L1+ tumor infiltrating lymphocytes (B). PD-L1+ tumor infiltrating lymphocytes are associated with greater numbers of all tumor infiltrating lymphocyte subsets including CD4 helper T cells (C), CD8 + cytotoxic T cells (D), CD20+ B cells (E), and FoxP3 + Tregs (F) relative to DCIS with PD-L1− tumor infiltrating lymphocytes(Panel 2). Importantly, no DCIS carcinoma cell displayed cell surface PD-L1 staining .
Discussion
Here, we evaluate tumor infiltrating lymphocyte composition and PD -L1 expression in breast DCIS. Our cohort is primarily pure DCIS of nuclear grade 2–3, and our data reveal several characteristics of this cohort. First, tumor infiltrating lymphocytes are present within all cases of DCIS examined. A trend towards different patterns of tumor infiltrating lymphocyte distribution and CD8/Foxp3 ratios are seen in ER− relative to ER +DCIS and younger women relative to older women with DCIS . Second, while DCIS carcinoma cells do not express cell surface PD-L1 in this study, the majority of DCIS -associated tumor infiltrating lymphocytes are PD -L1+. The three DCIS cases with high PD-L1 expression on tumor infiltrating lymphocytes(>50% PD -L1+ tumor infiltrating lymphocytes) are triple negative and high nuclear grade. In three cases where DCIS was associated with concurrent infiltrating ductal carcinoma, no cell surface PD-L1 expression was seen on the carcinoma cells of either the in situ or invasive component. Although the conclusions are limited by the small number of cases with concurrent invasion , this finding is in agreement with our previous study showing concordance of carcinoma cell PD-L1 status by frank infiltrating ductal carcinoma and its associated DCIS (24). Third, PD-L1 expression by tumor infiltrating lymphocytes is associated with a younger patient age. Fourth, PD-L1 expression by tumor infiltrating lymphocytes is associated with higher overall levels of tumor infiltrating lymphocytes within DCIS. Our findings suggest a n active immune response within breast DCIS and supports tumor infiltrating lymphocyte expression of PD -L1 as a marker of downregulation of the body’s immune response within DCIS. The presence of PD-L1+ tumor infiltrating lymphocytes with in situ breast carcinoma suggests that investigation of immune-based therapies may be warranted even in pre-invasive disease.
Most studies of the immunobiology of breast carcinomas have focused on frankly invasive primary breast carcinomas, with little attention to immune infiltrates associated with in situ lesions. Studies in mouse models of breast cancer have identified robust CD8+T cell responses associated with hyperplastic, pre-neoplastic lesions (28). Genomic analyses of DCIS and invasive breast cancers revealactivation of interleukin signaling pathway profiles, extracellular matrix pathways, and cell-cell adhesion pathways; activation of these pathways is absent or low in adjacent benign breast tissues. Furthermore, genomic profiling of DCIS shows patterns consistent with cytotoxic T cell signaling, expression of chemokines known to recruit T cells, IFN- γ signaling, and NOX4 activity, an oxygen-sensing NADPH oxidase related to the production of reactive oxygen species by granulocytes( 14). One study reported clusters of tumor infiltrating lymphocytes adjacent to involved ducts or in the interductal stroma, often associated with plump endothelial-lined vessels suggestive of high endothelial venule-like vasculature (29). DC-based vaccination of DCIS patients also suggest that distinct DCIS phenotypes may have different immunogenicity at baseline, as vaccination produced more complete responses in ER− DCIS than ER+ DCIS ( 30, 31).
Our findings suggest an that an active adaptive immune response may exist within DCIS. Nuclear grade 2–3 DCIS is associated with tumor infiltrating lymphocytephenotypes similar to those seen in invasive breast carcinomas . This includes higher levels of tumor infiltrating lymphocytesin ER − tumors ( 11), higher levels of FoxP3+ Tregs in ER− tumors ( 13), strong expression of PD-L1 in ER− tumors and triple negative breast carcinomas (11), and tumor infiltrating lymphocyte PD -L1 expression when higher overall levels of tumor infiltrating lymphocytes are present (21). The two cases of DCIS that eventually recurred had large numbers of tumor infiltrating lymphocytes, but high levels of FoxP3+ Treg. Although the conclusions are limited by the small number of recurrences in this series, our data are consistent with prior reports that increased numbers of FoxP3+ Tregs in DCIS are associated with increased recurrence risk( 10). These data together suggest that even very early immune responses to breast DCIS may predict future disease behavior.
Our work also demonstrates that while DCIS tumor cells lack PD-L1 expression, the majority of tumor infiltrating lymphocytes associated with DCIS do ex press PD-L1. We have previously shown that the majority of tumor infiltrating lymphocytes associated with infiltrating ductal carcioma also express PD-L1( 24), but that 21% primary infiltrating ductal carcinoma have PD -L1+ carcinoma cells. This dichotomy suggests there may be differences between the character of the immune response to in situ and invasive disease. Little is known about the evolution of the immune response and immunologic alterations within the tumor microenvironment along the continuum from in situ to invasive disease. It is not clear if DCIS eventually develops expression of PD-L1 and gives rise to PD-L1+ invasive carcinoma, or if, alternatively, the presence of an invasive PD-L1+ tumor modulates the immune response within DCIS, leading to expression of PD-L1 by the DCIS tumor cells. If the infiltrating component does not generate a robust enough immune response, insufficient IFN- γ may be generated to upregulate PD-L1 on the surface of DCIS cells. Studies are needed to elucidate the mechanisms behind the lack of PD-L1 expression by pure DCIS and the expression of PD -L1 by invasive tumors.
In addition, there is limited data on the spatial distribution of the immune response within either DCIS or invasive ductal carcinoma. In other tumor types, PD-L1 expression is often seen expressed primarily at the “leading edge” of the tumor or at the tumor-stromal interface( 19). This supports the possibility that interactions between invasive tumors and the stromal/immune environment drive PD-L1 expression on tumor cells, which could then modulate the immune milieu of associated in situ lesions. In studies of melanoma, IFN- γ and other cytokines were shown to co localize with tumor cells in PD-L1+ tumors, but not in PD-L1− tumors( 19, 32) . Such spatial distribution of inflammatory mediators could also contribute to differences in PD -L1 expression in DCIS and invasive breast carcinoma. Indeed, pure DCIS lesions are architecturally distinct from most invasive carcinomas because, while some DCIS are mass -forming lesions that spatially have a “leading edge,” the majority of DCIS spread diffusely, segmentally or irregularly through the breast ducts and lack a distinct “leading edge.”
The expression of immune checkpoint molecules in DCIS identify them as potential targets for DCIS therapy and secondary breast cancer prevention. DCIS can be responsive to immune based therapy (30, 31, 33), and PD-L1 antagonists are active in triple negative breast carcinomas (34, 35). While PD-L1 is not expressed on the surface of DCIS tumor cells in pure in situ lesions, the expression of PD-L1 by DCIS tumor infiltrating lymphocytes suggests that targeting PD-L1 is worth investigating as a treatment strategy for high risk DCIS . ER− and HER -2+ DCIS and DCIS in young women have the greatest numbers of tumor infiltrating lymphocytes. These patients may be ideal for testing immune-based breast cancer prevention strategies, and this may be particularly true for young women who have a higher DCISCD8/FoxP3 ratio.
Additionally, we examine tumor infiltrating lymphocyte subsets and PD-L1 expression in DCIS classified into subtypes by ER, PR and HER-2 status. Our study is limited by the small sample size and lack of nuclear grade 1 cases. In addition, the tissue microarray methodology limits analysis of the geography of tumor infiltrating lymphocytesand PD -L1 expression, including the “leading edge” of tumors known to be immunologically important in invasive carcinomas( 36). However, 2–5 cores per tumor were taken to mitigate sampling limitations. Moreover, DCIS also tends to be more diffuse or segmental than invasive breast cancers, which tend to be mass -forming, potentially limiting the importance of the “leading edge” in purely in situ lesions. Finally, the tissue microarray methodology also limits our ability to detect lymphoid aggregates, which have been shown to be important in invasive breast carcinomas (2).
In conclusion, we demonstrate differential patterns of tumor infiltrating lymphocyte infiltrates in distinct phenotypes of DCIS. Differences are seen relative to ER expression and patient age. Additional studies are needed to further characterize the association of immune parameters with the presence of concurrent infiltrating ductal carcinoma and eventual recurrence in DCIS. We previously reported differential patterns of tumor infiltrating lymphocytes across matched primary and metastatic breast carcinomas (23). Our findings in breast DCIS are similar to patterns of tumor infiltrating lymphocytes in primary breast carcinomas, and distinct from patterns of tumor infiltrating lymphocytes in metastatic breast carcinomas. The presence of tumor infiltrating lymphocytes in DCIS, combined with the expression of PD-L1 on tumor infiltrating lymphocytes within the DCIS microenvironment suggests the potential for immune based therapies to treat DCIS . Further characterization of the DCIS immune microenvironment may yield additional targets for immune -based therapy and prevention of recurrence, and may help elucidate the role of the immune response in the evolution of breast cancer from in situ to invasive and ultimately to recurrent or metastatic disease.
Footnotes
Conflicts of interest: Dr. Cimino-Mathews and Dr. Emens receive research funding from Genentech, Incorporated, and Roche, Incorporated, and Dr. Emens receives research funding from EMD Serono, Merck, and Astrazeneca. Dr. Taube receives research funding from and is a member of the advisory board for Bristol-Myers Squibb.
References
- 1.Emens LA. Breast cancer immunobiology driving immunotherapy: vaccines and immune checkpoint blockade. Expert Rev Anticancer Ther. 2012;12:1597–611. doi: 10.1586/era.12.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cimino-Mathews A, Foote JB, Emens LA. Immune targeting in breast cancer. Oncology (Williston Park) 2015;29:375–85. [PubMed] [Google Scholar]
- 3.Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013;31:860–7. doi: 10.1200/JCO.2011.41.0902. [DOI] [PubMed] [Google Scholar]
- 4.Adams S, Gray RJ, Demaria S, Goldstein L, Perez EA, Shulman LN, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32:2959–67. doi: 10.1200/JCO.2013.55.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loi S, Michiels S, Salgado R, Sirtaine N, Jose V, Fumagalli D, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol. 2014;25:1544–50. doi: 10.1093/annonc/mdu112. [DOI] [PubMed] [Google Scholar]
- 6.Kreike B, van Kouwenhove M, Horlings H, Weigelt B, Peterse H, Bartelink H, et al. Gene expression profiling and histopathological characterization of triple-negative/basal-like breast carcinomas. Breast Cancer Res. 2007;9:R65. doi: 10.1186/bcr1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, Harris AL, et al. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006;24:5373–80. doi: 10.1200/JCO.2006.05.9584. [DOI] [PubMed] [Google Scholar]
- 8.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 9.Norum JH, Andersen K, Sorlie T. Lessons learned from the intrinsic subtypes of breast cancer in the quest for precision therapy. Br J Surg. 2014;101:925–38. doi: 10.1002/bjs.9562. [DOI] [PubMed] [Google Scholar]
- 10.Vincent-Salomon A, Lucchesi C, Gruel N, Raynal V, Pierron G, Goudefroye R, et al. Integrated genomic and transcriptomic analysis of ductal carcinoma in situ of the breast. Clin Cancer Res. 2008;14:1956–65. doi: 10.1158/1078-0432.CCR-07-1465. [DOI] [PubMed] [Google Scholar]
- 11.Schalper KA, Velcheti V, Carvajal D, Wimberly H, Brown J, Pusztai L, et al. In situ tumor PD-L1 mRNA expression is associated with increased TILs and better outcome in breast carcinomas. Clin Cancer Res. 2014;20:2773–82. doi: 10.1158/1078-0432.CCR-13-2702. [DOI] [PubMed] [Google Scholar]
- 12.Desmedt C, Haibe-Kains B, Wirapati P, Buyse M, Larsimont D, Bontempi G, et al. Biological processes associated with breast cancer clinical outcome depend on the molecular subtypes. Clin Cancer Res. 2008;14:5158–65. doi: 10.1158/1078-0432.CCR-07-4756. [DOI] [PubMed] [Google Scholar]
- 13.Ghebeh H, Barhoush E, Tulbah A, Elkum N, Al-Tweigeri T, Dermime S. FOXP3+ Tregs and B7-H1+/PD−1+ T lymphocytes co-infiltrate the tumor tissues of high-risk breast cancer patients: Implication for immunotherapy. BMC Cancer. 2008;8:57. doi: 10.1186/1471-2407-8-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kristensen VN, Vaske CJ, Ursini-Siegel J, Van Loo P, Nordgard SH, Sachidanandam R, et al. Integrated molecular profiles of invasive breast tumors and ductal carcinoma in situ (DCIS) reveal differential vascular and interleukin signaling. Proc Natl Acad Sci U S A. 2012;109:2802–7. doi: 10.1073/pnas.1108781108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 16.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–22. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mittendorf EA, Philips AV, Meric-Bernstam F, Qiao N, Wu Y, Harrington S, et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014;2:361–70. doi: 10.1158/2326-6066.CIR-13-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gatalica Z, Snyder C, Maney T, Ghazalpour A, Holterman DA, Xiao N, et al. Programmed Cell Death 1 (PD-1) and Its Ligand (PD-L1) in Common Cancers and Their Correlation with Molecular Cancer Type. Cancer Epidemiol Biomarkers Prev. 2014;23:2965–70. doi: 10.1158/1055-9965.EPI-14-0654. [DOI] [PubMed] [Google Scholar]
- 19.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghebeh H, Mohammed S, Al-Omair A, Qattan A, Lehe C, Al-Qudaihi G, et al. The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia. 2006;8:190–8. doi: 10.1593/neo.05733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wimberly H, Brown JR, Schalper KA, Haack H, Silver MR, Nixon C, et al. PD-L1 expression correlates with tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy in breast cancer. Cancer Immunol Res. 2014 doi: 10.1158/2326-6066.CIR-14-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muenst S, Schaerli AR, Gao F, Daster S, Trella E, Droeser RA, et al. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2014;146:15–24. doi: 10.1007/s10549-014-2988-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cimino-Mathews A, Ye X, Meeker A, Argani P, Emens LA. Metastatic triple-negative breast cancers at first relapse have fewer tumor-infiltrating lymphocytes than their matched primary breast tumors: a pilot study. Hum Pathol. 2013;44:2055–63. doi: 10.1016/j.humpath.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cimino-Mathews ATE, Taube JM, Ye X, Lu Y, Meeker A, Xu H, Sharma R, Lecksell K, Cornish TC, Cuka N, Argani P, Emens LA. PD-L1 (B7-H1) Expression and the Immune Tumor Microenvironment in Human Primary and Metastatic Breast Carcinomas. Hum Pathol. 2015 Sep 21; doi: 10.1016/j.humpath.2015.09.003. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.VandenBussche CJ, Elwood H, Cimino-Mathews A, Bittar Z, Illei PB, Warzecha HN. Clinicopathologic features of ductal carcinoma in situ in young women with an emphasis on molecular subtype. Hum Pathol. 2013;44:2487–93. doi: 10.1016/j.humpath.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 26.Cimino-Mathews A, Subhawong AP, Elwood H, Warzecha HN, Sharma R, Park BH, et al. Neural crest transcription factor Sox10 is preferentially expressed in triple-negative and metaplastic breast carcinomas. Hum Pathol. 2013;44:959–65. doi: 10.1016/j.humpath.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2014 doi: 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ursini-Siegel J, Cory S, Zuo D, Hardy WR, Rexhepaj E, Lam S, et al. Receptor tyrosine kinase signaling favors a protumorigenic state in breast cancer cells by inhibiting the adaptive immune response. Cancer Res. 2010;70:7776–87. doi: 10.1158/0008-5472.CAN-10-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee AH, Happerfield LC, Bobrow LG, Millis RR. Angiogenesis and inflammation in ductal carcinoma in situ of the breast. J Pathol. 1997;181:200–6. doi: 10.1002/(SICI)1096-9896(199702)181:2<200::AID-PATH726>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 30.Fracol M, Xu S, Mick R, Fitzpatrick E, Nisenbaum H, Roses R, et al. Response to HER-2 pulsed DC1 vaccines is predicted by both HER-2 and estrogen receptor expression in DCIS. Ann Surg Oncol. 2013;20:3233–9. doi: 10.1245/s10434-013-3119-y. [DOI] [PubMed] [Google Scholar]
- 31.Sharma A, Koldovsky U, Xu S, Mick R, Roses R, Fitzpatrick E, et al. HER-2 pulsed dendritic cell vaccine can eliminate HER-2 expression and impact ductal carcinoma in situ. Cancer. 2012;118:4354–62. doi: 10.1002/cncr.26734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taube JM, Young GD, McMiller TL, Chen S, Salas JT, Pritchard TS, et al. Differential Expression of Immune-Regulatory Genes Associated with PD-L1 Display in Melanoma: Implications for PD-1 Pathway Blockade. Clin Cancer Res. 2015;21:3969–76. doi: 10.1158/1078-0432.CCR-15-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Czerniecki BJ, Roses RE, Koski GK. Development of vaccines for high-risk ductal carcinoma in situ of the breast. Cancer Res. 2007;67:6531–4. doi: 10.1158/0008-5472.CAN-07-0878. [DOI] [PubMed] [Google Scholar]
- 34.Emens LABF, Cassier P, DeLord JP, Eder JP, Shen X, Xiao Y, Wang Y, Hedge PS, Chen DC, Krop I. Inhibition of PD-L1 by MPDL3280A leads to clinical activity in patients with metastatic triple-negative breast cancer. Cancer Res. 2015;75:PD1–6. [Google Scholar]
- 35.Lipson EJ, Forde PM, Hammers HJ, Emens LA, Taube JM, Topalian SL. Antagonists of PD-1 and PD-L1 in Cancer Treatment. Semin Oncol. 2015;42:587–600. doi: 10.1053/j.seminoncol.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–37. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]