Abstract
Background
This research focused on detecting the expressions of RhoA and cyclooxygenase-2 (COX-2) proteins in early gastric cancer tissues and to explore their role in the development of gastric cancer.
Material/Methods
Surgically resected gastric cancer tissues and the paired normal paracancerous tissues were collected from 26 patients with early gastric cancer from January 2015 to November 2015. The expressions of RhoA and COX-2 proteins were detected by using RT-PCR and immunohistochemistry techniques, respectively. Cell proliferation and migration experiments were conducted on the RhoA-silenced A6-B9 cells and COX-2-silenced D7-B8 cells so as to discuss their role in the development of gastric cancer.
Results
Relative mRNA expressions of RhoA and COX-2 in the cancer tissues were 0.823±0.021 and 0.892±0.103, respectively, which showed significant differences compared to the normal cancerous tissues (0.295±0.014 and 0.129±0.037) (p<0.05). Immunohistochemical staining indicated that the expressions of RhoA and COX-2 proteins in tumor tissues were significantly upregulated as compared to normal cancerous tissues (p<0.05). Cell cloning and streaking assays showed that silencing of RhoA and COX-2 gene caused a considerable decline in the proliferation and migration capacities of the gastric cancer cells, respectively (p<0.05).
Conclusions
RhoA and COX-2 were upregulated in early gastric cancer tissues, which facilitated the proliferation and migration of gastric cancer cells. Both proteins may be used as potential markers for the diagnosis of early gastric cancer.
MeSH Keywords: Cyclooxygenase 2, Immunohistochemistry, rhoA GTP-Binding Protein
Background
Gastric cancer is among the most common cancers of the digestive tract throughout the world. In China, there are about 300,000 newly diagnosed cases of gastric cancer every year, accounting for a third of the world’s total newly diagnosed cases of gastric cancer [1]. It is reported that the 5-year survival can reach up to 90% for early gastric cancer and only 16.6% for advanced gastric cancer [2]. Early diagnosis and treatment are important to securing good outcomes. In spite of advances in endoscopy and improved rates of early diagnosis of gastric cancer, only 10% of patients with early gastric cancer are accurately diagnosed in China [3]. There is an urgent need to identify molecular markers closely associated with the occurrence and development of early gastric cancer in order to improve the diagnosis of early gastric cancer.
RhoA is a member of the Ras homology family of small GTPases and considered to be closely associated with cancers. RhoA protein is upregulated in a variety of tumor tissues [4]. Cyclooxygenase-2 (COX-2) is a key enzyme in prostaglandin synthesis and its expression can be induced by many growth factors and proinflammatory cytokines; moreover, COX-2 is found to be upregulated in many cancers [5]. Research [4–6] shows that RhoA and COX-2 proteins are upregulated in many cancers and closely associated with the development of cancers, but the knowledge is limited as to their role in early gastric cancer. This study detected the RhoA and COX-2 expressions in surgically resected gastric cancer tissues and the paired normal paracancerous tissues from 26 patients with early gastric cancer. Their role in the development of gastric cancer was tested via different cell experiments.
Material and Methods
Specimens
Surgically resected gastric cancer tissues were collected from 26 patients with early gastric cancer (infiltration of gastric mucosa confined to the mucosal layer and/or submucosal layer) from January 2015 to November 2015. As a control, the normal gastric mucosa (5 cm from the paracancerous tissues) was collected from 26 cases. All of the cases, including 16 males and 10 females (aged from 34 to 72 years, with a mean of 51.3±10.2 years), had not received radiotherapy or chemotherapy before operation. All gastric cancer patients were diagnosed with stage T1, including seven cases of T1a stage and 19 cases of T1b stage.
Reagents
Gastric cancer cell line MGC-803 was purchased from American Type Culture Collection (ATCC). RhoA-silenced A6-B9 cells and COX-2-silenced D7-B8 were prepared by ourselves. Tissue RNA extraction kit was purchased from Tiangen Biotech (Beijing) Co., Ltd. Reverse transcription kit was purchased from Fermentas. Semi-quantitative PCR kit was purchased from ThermoFisher. Rabbit polyclonal antibodies against RhoA and COX-2 were purchased from Santa Cruz. Goat anti-rabbit polyclonal antibodies were purchased from Shanghai Pufei Biotechnology Co., Ltd. Rabbit immunohistochemistry staining kit was purchased from Shanghai Yanji Biotechnology Co., Ltd.
RT-PCR detection of mRNA expressions of RhoA and COX-2
Total RNA was extracted from heart tissue using the tissue RNA extraction kit and the RNA concentration was determined. Then 1,000 ng of cDNA was synthesized from the extracted RNA using the reverse transcription kit (20 uL system), and 20 uL of MilliQ water was added. Semi-quantitative RT-PCR was performed using the synthesized cDNA as template. PCR primers for RhoA and COX-2 were designed using the NCBI tool Primer-BLAST, as shown in Table 1. Next 10 uL of PCR product was analyzed by 1.5% RNA-PAGE (12 V, 15 minutes). The gel was imaged using the Bio-Rad imager. The data were analyzed using the Quantity One software, and the relative expression levels of the target genes were calculated with respect to the β-actin gene.
Table 1.
RT-PCR primer design. Primer sequences of gene RhoA, COX-2 and β-actin were designed.
| Gene | Primer sequence (5′–3′) | Length (bp) |
|---|---|---|
| RhoA | F: CAAGCATTTCTGTCCCAACG | 276 |
| R: CCCACGGAACAGAACACTTT | ||
| COX-2 | F: GCATCTCAGACGCTCAGGAA | 294 |
| R: TACGAATGGGGGCTTCAACC | ||
| β-actin | F: AAGTACTCCGTGTGGATCGG | 615 |
| R: TCAAGTTGGGGGACAAAAAG |
Immunohistochemistry detection of RhoA and COX-2 proteins
Immunohistochemical staining was performed by adding the primary antibodies according to the instruction of immunohistochemical staining kit. For negative control, PBS was added instead. Ten fields of view were randomly selected for each slice under 400x magnification. The positive areas of RhoA and COX-2 (S value) and the mean optical density (OD) values were calculated.
Cell cloning assay
Into the 6-well plate 2×103 cells/mL were inoculated with 2 mL in each well. The culture medium was replaced every three days for about three weeks. The culture was terminated when visible clones appeared in the wells, and then the supernatant was collected. The cells were washed with PBS twice and fixed in 4% formaldehyde for 15 minutes, and then the supernatant was collected. Staining with 0.25% crystal violet was performed for 25 minutes and the cells were slowly washed with sterilized water. After that, the cells were dried on the sterile ultra-clean bench, photographed and counted.
Streaking assay
Into the 6-well plate, 2×103 cells/mL were inoculated with 2 mL in each well. The plate was streaked on the next day using the pipette perpendicularly. The cells were washed with PBS three times to remove the streaked cells, and serum-free medium was added. The plate was then placed into a 5% CO2 incubator at 37°C for 24 hours. The colonies were photographed.
Statistical method
Statistical analyses were performed using SPSS 19.0 software. The mRNA and protein expressions of RhoA and COX-2 in the cancer tissues and paracancerous tissues were compared using the paired t-test; p<0.05 indicated significant difference.
Results
mRNA expressions of RhoA and COX-2
The mRNA expressions of RhoA and COX-2 were significantly higher in gastric cancer tissues than in normal paracancerous tissues (p<0.05) (Table 2, Figure 1).
Table 2.
The mRNA rxpression of RhoA and COX-2 in different tissues. Both RhoA and COX-2 have significantly different (p<0.05).
| Group | N/case | RhoA | COX-2 |
|---|---|---|---|
| Tumor tissue | 26 | 0.815±0.189 | 0.901±0.0121 |
| Normal tissue | 26 | 0.308±0.175 | 0.129±0.0109 |
| t | --- | 227.673 | 560.897 |
| P | --- | <0.05 | <0.05 |
Figure 1.
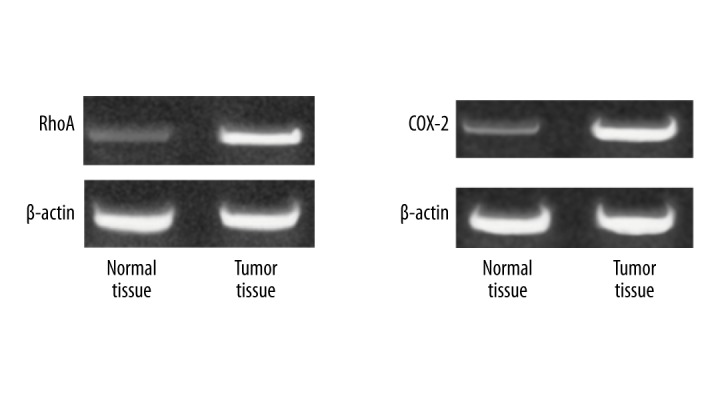
The mRNA expression of RhoA and COX-2 in different tissues. The normal tissues 5 cm from the paracancerous tissues and tumor tissues from early gastric cancer patients were separately detected the mRNA expression.
Protein expressions of RhoA and COX-2
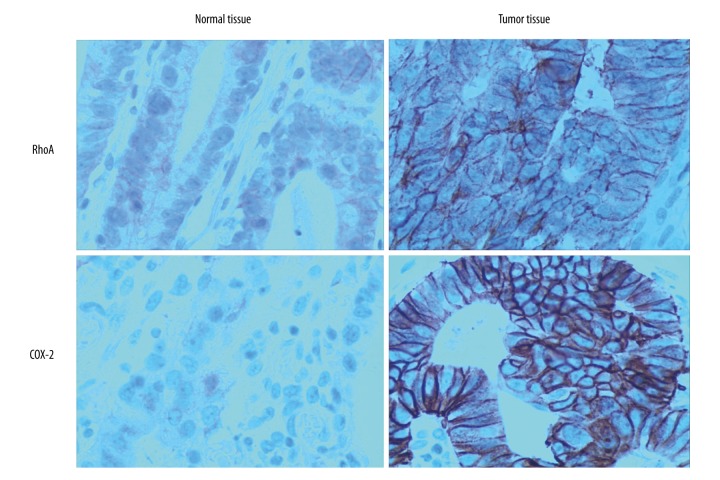
The positive areas (S value) and average OD values were calculated for RhoA and COX-2 within 10 randomly selected fields of view for each slice (400× magnification). It was found that the S values and average OD values of gastric cancer tissues were significantly higher than those in the normal paracancerous tissues (p<0.05) (Table 3, Figure 2).
Table 3.
S and OD value of RhoA and COX-2 in different tissues with immunohistochemistry. There are statistical significances between normal tissue and tumor tissue (p<0.05).
| Group | N/case | RhoA | COX-2 | ||
|---|---|---|---|---|---|
| S value | OD value | S value | OD value | ||
| Tumor tissue | 26 | 2.424±0.0471 | 1.434±0.0459 | 2.367±0.0921 | 1.173±0.0772 |
| Normal tissue | 26 | 1.521±0.0421 | 0.429±0.0320 | 1.358±0.0187 | 0.254±0.0730 |
| t | 306.709 | 168.206 | 271.145 | 109.174 | |
| P | <0.05 | <0.05 | <0.05 | <0.05 | |
Figure 2.
Immunohistochemistry of RhoA and COX-2 in different tissues.
Effects of RhoA and COX2 on the proliferation and migration capacities of the gastric cancer cells
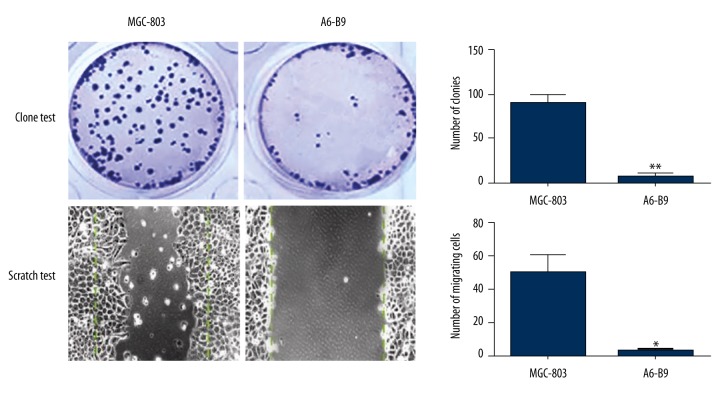
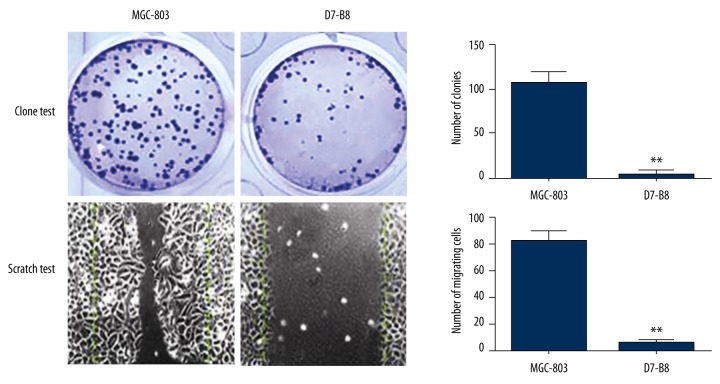
According to the results of cell cloning assay and streaking assay on the MGC-803 cells, RhoA-silenced A6-B9 cells and COX-2-silenced D7-B8 cells, the number of clones of A6-B9 cells and D7-B8 cells was considerably lower than that of MGC-803 cells (p<0.05). Moreover, the migration capacity of A6-B9 cells and D7-B8 cells was greatly reduced as compared with the MGC-803 cells (p<0.05) (Figures 3, 4).
Figure 3.
Effect of RhoA expression on colonization and migration of gastric cancer cells. Cell cloning assay and streaking assay on the MGC-803 cells, RhoA-silenced A6-B9 cells the number of clones of A6-B9 cells was considerably lower than that of MGC-803 cells (p<0.05). Moreover, the migration capacity of A6-B9 cells was greatly reduced as compared with the MGC-803 cells (p<0.05).
Figure 4.
Effect of COX-2 expression on colonization and migration of gastric cancer cells. Cell cloning assay and streaking assay on the MGC-803 cells, COX-2-silenced D7-B8 cells the number of clones of D7-B8 cells was considerably lower than that of MGC-803 cells (p<0.05). Moreover, the migration capacity of D7-B8 cells was greatly reduced as compared with the MGC-803 cells (p<0.05).
Discussion
RhoA encodes for a small GTPase protein and acts as a cancer-promoting gene by regulating the intracellular signal transduction. It has been established [7,8] that RhoA upregulation is closely related to the progression, prognosis, and treatment of many cancers. One recent study indicated [9] that RhoA was upregulated in 87 cases with diffuse gastric cancer and non-synonymous mutation of RhoA was detected in 25.3% of cases (22/87), from which the increased proliferation and invasion capacities of cancer cells were derived. According to Xu et al., [10] RhoA/ROCK signaling pathway plays an important role in the apoptosis of gastric cancer cells, and inhibiting this pathway can promote the apoptosis of cancer cells. Therefore, RhoA/ROCK signaling pathway may serve as the potential target of gastric cancer treatment [11,12]. The aforementioned facts all imply the close connection between RhoA upregulation and the development of gastric cancer. Among 26 cases with early gastric cancer in our study, RhoA mRNA expressions, S values, and OD values in immunohistochemical staining were significantly higher in the gastric cancer tissues than in the normal paracancerous tissues (p<0.05). Luo et al. [13] detected RhoA expression in the gastric cancer tissues based on TNM classification and found that the positive expression rate of RhoA was 66.4%.
COX protein, also known as prostaglandin-endoperoxide synthase, has double functions, namely, cyclooxygenase and catalase activity. It is the key enzyme in the catalytic conversion of arachidonic acid into prostaglandin. There are two isoenzymes of COX, COX-1, and COX-2, and the former is the structural isoenzyme. COX-1 is mainly found in the stomach and kidney and involved in the regulation of vasomotion, gastric mucosal blood flow and kidney functions. It can help protect the gastrointestinal mucosa and regulate platelet aggregation, peripheral vessel resistance, and kidney blood flow distribution [14]. COX-2 is the induced isoenzyme. Chemical, physical, and biological injuries can activate the hydrolysis of membrane phospholipids by phospholipase A2, producing arachidonic acid, which is then catalyzed into prostaglandin. COX-2 is proved crucial to the inflammatory response [15–17]. We found that the S values and OD values of COX-2 mRNA in early gastric cancer tissues were significantly higher compared to the normal paracancerous tissues (p < 0.05). Under normal circumstances, COX-2 is not expressed, but only expressed under the stimuli from oncogenes, cancer-promoting genes, and cancer-promoting factors. Excess upregulation of COX-2 can inhibit the apoptosis of cells and facilitate the formation of cancer cells [18]. Furthermore, COX-2 upregulation can lead to increased activity of free radicals in cells, thus increasing the risk of gene mutations and canceration. Mishra et al. [19] confirmed that COX-2 was significantly differentially upregulated in gastric cancer tissues with different differentiation degree. This means COX-2 may be a potential marker for the diagnosis and classification of gastric cancer. RhoA and COX-2 may also be significant by increased metastatic lymph node ratio [20,21].
Further assays indicated that silencing of RhoA and COX-2 caused a significant decline in the proliferation and migration capacities of the gastric cancer cells, respectively (p<0.05). Invasion and migration are among the main features of cancer cells and also the root causes of failed treatment or death of cancer patients [22]. ROCK protein is one of the most important effectors of RhoA proteins and can regulate actin, a major component of the cytoskeleton. This is the mechanism of ROCK protein regulating cell morphology, G1/S transition and cell migration. RhoA can also regulate cell migration through another downstream effector, mDia. Activated mDia can cause the actin monomers to orient with their cleft towards the same end of the filament. Activated mDia can induce the elongation of actin filaments and facilitate cell migration by inhibiting the binding to the actin capping proteins. As analyzed above, COX-2 upregulation can inhibit cell apoptosis and increase the probability of normal cells turning into cancer cells through mutation. For this reason, COX-2 upregulation is favorable for the proliferation and migration of gastric cancer cells.
Conclusions
Both RhoA and COX-2 are upregulated in early gastric cancer tissues, which facilitates the proliferation and migration of gastric cancer cells. RhoA and COX-2 may be used as potential markers for the diagnosis of early gastric cancer.
Footnotes
Disclosure of interest
None.
Source of support: Departmental sources
References
- 1.Liu Z, Liu S, Zheng G, et al. Clinicopathological features and prognosis of coexistence of gastric gastrointestinal stromal tumor and gastric cancer. Medicine (Baltimore) 2016;95(45):e5373. doi: 10.1097/MD.0000000000005373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Q, Chen ZY, Chen CD, et al. Training in early gastric cancer diagnosis improves the detection rate of early gastric cancer: An observational study in China. Medicine. 2015;94:0000000000000384. doi: 10.1097/MD.0000000000000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kosaka T, Endo M, Toya Y, et al. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: A single-center retrospective study. Dig Endosc. 2014;26:183–91. doi: 10.1111/den.12099. [DOI] [PubMed] [Google Scholar]
- 4.Huang KH, Lan YT, Chen MH, et al. The correlation between RhoA expression and clinicopathological characteristics in gastric cancer patients after curative surgery. World J Surg. 2015;39:2289–99. doi: 10.1007/s00268-015-3095-4. [DOI] [PubMed] [Google Scholar]
- 5.Tseng YC, Tsai YH, Tseng MJ, et al. Notch2-induced COX-2 expression enhancing gastric cancer progression. Mol Carcinog. 2012;51:939–51. doi: 10.1002/mc.20865. [DOI] [PubMed] [Google Scholar]
- 6.Li Y, Tan BB, Fan LQ, et al. Heterogeneity of COX-2 and multidrug resistance between primary tumors and regional lymph node metastases of gastric cancer. Tumori. 2012;98:516–22. doi: 10.1177/030089161209800418. [DOI] [PubMed] [Google Scholar]
- 7.Karlsson R, Pedersen ED, Wang Z, et al. Rho GTPase function in tumorigenesis. Biochim Biophys Acta. 2009;2:91–98. doi: 10.1016/j.bbcan.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–9. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kakiuchi M, Nishizawa T, Ueda H, et al. Recurrent gain-of-function mutations of RHOA in diffuse-type gastric carcinoma. Nat Genet. 2014;46:583–87. doi: 10.1038/ng.2984. [DOI] [PubMed] [Google Scholar]
- 10.Xu XT, Song QB, Yao Y, et al. Inhibition of RhoA/ROCK signaling pathway promotes the apoptosis of gastric cancer cells. Hepatogastroenterology. 2012;59:2523–26. doi: 10.5754/hge12147. [DOI] [PubMed] [Google Scholar]
- 11.Zhang C, Wang HJ, Bao QC, et al. NRF2 promotes breast cancer cell proliferation and metastasis by increasing RhoA/ROCK pathway signal transduction. Oncotarget. 2016;4:12435. doi: 10.18632/oncotarget.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin MT, Lin BR, Chang CC, et al. IL-6 induces AGS gastric cancer cell invasion via activation of the c-Src/RhoA/ROCK signaling pathway. Int J Cancer. 2007;120:2600–8. doi: 10.1002/ijc.22599. [DOI] [PubMed] [Google Scholar]
- 13.Chang HR, Nam S, Lee J, et al. Systematic approach identifies RHOA as a potential biomarker therapeutic target for Asian gastric cancer. Oncotarget. 2016;28:12963. doi: 10.18632/oncotarget.12963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Z, Wang ZC, Yan XQ, et al. Design, synthesis, biological evaluation and molecular modeling of dihydropyrazole sulfonamide derivatives as potential COX-1/COX-2 inhibitors. Bioorg Med Chem Lett. 2015;25:1947–51. doi: 10.1016/j.bmcl.2015.03.022. [DOI] [PubMed] [Google Scholar]
- 15.Mestre F, Gutierrez A, Rodriguez J, et al. Radiation therapy overcomes adverse prognostic role of cyclooxygenase-2 expression on Reed-Sternberg cells in early Hodgkin lymphoma. Int J Radiat Oncol Biol Phys. 2015;92:84–90. doi: 10.1016/j.ijrobp.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Roseweir AK, Powell AG, Bennett L, et al. Relationship between tumour PTEN/Akt/COX-2 expression, inflammatory response and survival in patients with colorectal cancer. Oncotarget. 2016;20:12134. doi: 10.18632/oncotarget.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu LQ, Li Y, Li YY, et al. Antinociceptive effects of prim-O-glucosylcimifugin in inflammatory nociception via reducing spinal COX-2. Biomol Ther. 2016;24:418–25. doi: 10.4062/biomolther.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park EJ, Cheenpracha S, Chang LC, et al. Suppression of cyclooxygenase-2 and inducible nitric oxide synthase expression by epimuqubilin A via IKK/IkappaB/NF-kappaB pathways in lipopolysaccharide-stimulated RAW 264.7 cells. Phytochem Lett. 2011;4:426–31. doi: 10.1016/j.phytol.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ben Ayed-Guerfali D, Benhaj K, Khabir A, et al. Hypermethylation of tumor-related genes in Tunisian patients with gastric carcinoma: Clinical and biological significance. J Surg Oncol. 2011;103:687–94. doi: 10.1002/jso.21875. [DOI] [PubMed] [Google Scholar]
- 20.Isik A, Peker K, Firat D, et al. Importance of metastatic lymph node ratio in non-metastatic, lymph node-invaded colon cancer: A clinical trial. Med Sci Monit. 2014;20:1369–75. doi: 10.12659/MSM.890804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Isik A, Okan I, Firat D, et al. A new prognostic strategy for gastric carcinoma: Albumin level and metastatic lymph node ratio. Minerva Chir. 2014;69:147–53. [PubMed] [Google Scholar]
- 22.Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–92. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]