Abstract
Background
Nicorandil is a nicotinamide ester commonly prescribed for treatment of patients with coronary heart disease (CHD). In the present study, we aimed to explore the cardioprotective effects of nicorandil on CHD patients undergoing elective percutaneous coronary intervention (PCI).
Material/Methods
One hundred patients with CHD undergoing PCI were randomly divided into a control group (n=48) and a nicorandil group (n=52). Patients in the control group received traditional therapy, and while patients in the nicorandil group received nicorandil before PCI in addition to the traditional therapy. After PCI, all patients underwent coronary angiogram, and TIMI frame count (TFC) was calculated. Plasma levels of cardiac troponin I (cTnI), creatine kinase-MB (CK-MB), myeloperoxidase (MPO), and malondialdehyde (MDA) were determined before and at 6, 18, and 24 h after PCI. Moreover, systolic blood pressure (SBP), mean blood pressure (DBP), heart rate (HR), and left ventricular ejection fractions (LVEF) were recorded before and 3 months after PCI.
Results
There was a significant difference in the rate of no-reflow (P=0.036) between the 2 groups. The blood frames and levels of cTnI, CK-MB, MPO, and MDA in the nicorandil group were significantly decreased compared to the control group (all P<0.05). Moreover, administration of nicorandil markedly decreased SBP, MBP, and HR, but obviously increased LVEF at 3 months after PCI (P<0.05 or P<0.01).
Conclusions
Nicorandil exerts cardioprotective effects on CHD patients undergoing elective PCI by decreasing PCI-related myocardial injury and rate of no-reflow and improvement of LVEF.
MeSH Keywords: Coronary Disease, Nicorandil, Percutaneous Coronary Intervention
Background
Coronary heart disease (CHD) is a complex disorder that frequently progresses and causes disability and premature death in both developed and developing countries worldwide. The disease is characterized by narrowed lumen of arteries and reduced blood flow to the heart [1]. The incidence has been increasing globally [2,3]. In the last decade, percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG) have been considered as efficient techniques for alleviating myocardial ischemia and preserving ventricular function, and are widely used in CHD patients with different clinical manifestations [4,5]. PCI can significantly improve the symptoms of patients with CHD compared to conservative medical treatment. However, it has been reported that PCI is associated with reperfusion injury, microvascular obstruction, and no-reflow phenomenon [6,7], especially in patients who undergo new device interventions such as stenting [8]. Currently, there are several alternative drugs that can be used to reduce the incidence of peri-procedural myocardial injury, including statins [9,10], nitrates [11], beta receptor blocker [12], and glycoprotein IIb/IIIa blocker [13].
Recently, nicorandil has received great attention. Nicorandil is a potent coronary vasodilator with both nitrate-like and ATP-sensitive potassium-channel (K+ATP) that acts on the venous and arterial beds of the systemic circulation [14]. It is commonly used in treating angina in patients with CHD [5,15,16]. In recent years, intracoronary injection of nicorandil was used to resolve severe no-reflow phenomenon occurring after PCI [17]. Moreover, nicorandil has been shown to have a myocardial protective effect on patients with unstable angina during PCI [18]. A previous study found that a single oral dose of nicorandil (10 mg or 20 mg) 2 h before PCI can decrease the incidence of peri-procedure myocardial injury and PCI-related myocardial infarction [19], but the exact mechanism is unclear.
In the present study, the cardioprotective effects of nicorandil on patients with CHD undergoing PCI were investigated, as well as the underlying mechanism. We recorded the blood frames and the numbers of no-reflow, determined the markers of myocardial injury before and at 6, 18, and 24 h after PCI, and measured the blood pressure (BP), heart rate (HR), and left ventricular ejection fractions (LVEF) before and 3 months after PCI. Our results show that nicorandil may be an alternative drug that could be used for perioperative preparation for primary PCI.
Material and Methods
Patients and treatment
This study was an open-labeled, investigator-initiated, paralleled, randomized trial. Between March 2015 and August 2016, a total of 100 patients with CHD who prepared for PCI were recruited in the study. The study was approved by the hospital Medical Ethics Committees, and written informed consent was obtained from all the participants. Patients were randomly assigned to nicorandil group (n=52) and control group (n=48). Patients without oral administration of nicorandil within 5 days prior to the study were included. Patients who are allergic to nicorandil or nitrate drug, and/or patients with glaucoma, acute multiple infarction that needs emergency PCI or coronary artery bypass grafting, cardiogenic shock, obvious respiratory tract infection, and/or dysfunction of liver and kidney were excluded. All the patients underwent traditional therapy such as subcutaneous injections of low molecular heparin, as well as oral administration of aspirin (100 mg per day) and clopidogrel (75 mg per day). In addition to the traditional therapy, patients in the nicorandil group received 10 mg 3 times daily for 3 days before PCI. Patients with CHD combining with other diseases such as hypertension, diabetes mellitus (DM), and/or hyperlipidemia were given corresponding drugs. PCI was carried out by a transradial or femoral artery approach.
Measurement of coronary blood flow
Coronary blood flow was determined by the corrected TIMI frame count (CTFC). The CTFC was measured at 90–120 min after administration of thrombolytic and evaluated by 3 independent operators. The first frame was defined by a column of contrast extending across >70% of the arterial lumen anterograde motion, and the last frame was defined as the frame when the dye first entered the distal landmark branch [20]. The left anterior descending (LAD) coronary artery was longer than the mean of the right coronary artery (RCA) and left circumflex (LCX). Thus, the longer LAD frame counts were corrected by dividing by 1.7 to derive CTFC [20].
Measurement of plasma levels
Peripheral blood samples from all the patients were collected before and at 6, 18, and 24 h after PCI. The blood was centrifuged and stored at −80°C until use. Plasma cardiac troponin I (cTnI) levels and creatine kinase-MB (CK-MB) were determined by chemiluminescence immunoassay (CLIA) using a Beckman Coulter ACCESS 2 analyzer (Beckman Coulter, Brea, CA, USA). Plasma myeloperoxidase (MPO) concentration were analyzed by enzyme-linked immunosorbent assay (ELISA) method according to the manufacture’s protocol. The content of myocardial malondialdehyde (MDA) in the plasma was measured by thiobarbituric acid (TBA) method following the instructions.
Measurement of BP, HR, and LVEF
Before and 3 months after PCI, systolic BP (SBP), mean BP (MBP), HR, and LVEF were recorded. SBP was recorded at the appearance of sounds, and MBP was the diastolic pressure plus one-third of the pulse pressure. HR was recorded using a 3-lead electrocardiograph (ECG; Hewlett-Packard, Palo Alto, CA, USA). LVEF was recorded by a 2-dimensional targeted M-mode echocardiogram using a M2540A model Philips echocardiograph (Philips Electronics, Amsterdam, The Netherlands) equipped with 30 MHz transducer, operated by an expert sonographer.
Statistical analysis
Data of normal distribution are presented as mean ± standard deviation (SD). Categorical variables are expressed as percentages, and the chi-square test was applied for statistical comparisons. The t test or one-way analysis of variance (ANOVA) was used to assess the significance of the differences between the 2 groups. All data were analyzed using SPSS 18.0 (SPSS Inc., Chicago, IL, USA). P<0.05 was considered as a significant difference between values.
Results
Clinical characteristics of participants
The clinical characteristics of all the patients in the present study are summarized in Table 1. As shown in the results, the ages of the control group and nicorandil group were 64.8±10.5 years old and 66.4±10.2 years old, and there were 26 and 29 males, respectively. The included patients in the 2 groups had hypertension, DM, and/or hyperlipidemia. No statistical difference was found in demographics and clinical variables among the 2 groups before PCI. The results were comparable and could be used for further analysis.
Table 1.
Clinical characteristics of participants.
| Items | Control (n=48) | Nicorandil (n=52) | P value |
|---|---|---|---|
| Ages (years) | 64.8±10.5 | 66.4±10.2 | 0.442 |
| Male n (%) | 26 (54.2) | 29 (55.8) | 0.873 |
| Hypertension (n,%) | 32 (66.7) | 33 (63.5) | 0.835 |
| DM (n,%) | 24 (50.0) | 22 (42.3) | 0.547 |
| Hyperlipidemia (n,%) | 41 (85.4) | 40 (76.9) | 0.317 |
| Pharmacotherapy (n,%) | – | – | – |
| Aspirin | 47 (97.9%) | 50 (96.2%) | 0.606 |
| Beta blockers | 22 (45.8) | 24 (46.2) | 0.974 |
| Clopidogrel | 45 (93.8) | 49 (94.2) | 0.919 |
| ACEI/ARB | 38 (79.2) | 34 (65.3) | 0.181 |
DM – diabetes mellitus; ACEI – angiotensin-converting enzyme inhibitors; ARB – angiotensin receptor blocker.
Comparison of rate of no-reflow and blood frames
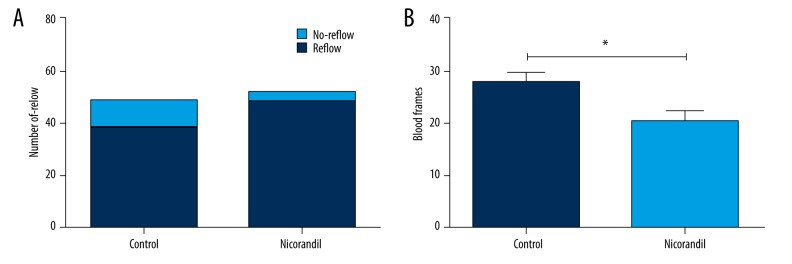
After PCI, all the patients in the 2 groups underwent a coronary angiogram. Thirty-eight patients presented reflow and 10 patients showed no-reflow in the control group. The rate of no-reflow was 20.8%. There were 49 patients with reflow and 3 patients with no-reflow in the nicorandil group, and the rate of the no-reflow was 5.8% (Figure 1A). There was a significant difference in the rate of the no-reflow (P=0.036). We observed that the blood frames were significantly decreased in the nicorandil group compared to the control group (P<0.05) (Figure 1B). The results indicate that nicorandil can improve blood flow after PCI.
Figure 1.
Comparison of the rate of no-reflow and blood frames. A total of 100 CHD patients were assigned to the control group (n=48) and nicorandil group (n=52). After PCI, all patients underwent coronary angiogram. (A) Number of patients with reflow and no-reflow in the 2 groups. (B) Blood frames in the 2 groups. CHD – coronary heart disease; PCI, percutaneous coronary intervention. * P<0.05 compared to the control group.
Comparison of levels of cTnI, CK-MB, MPO, and MDA
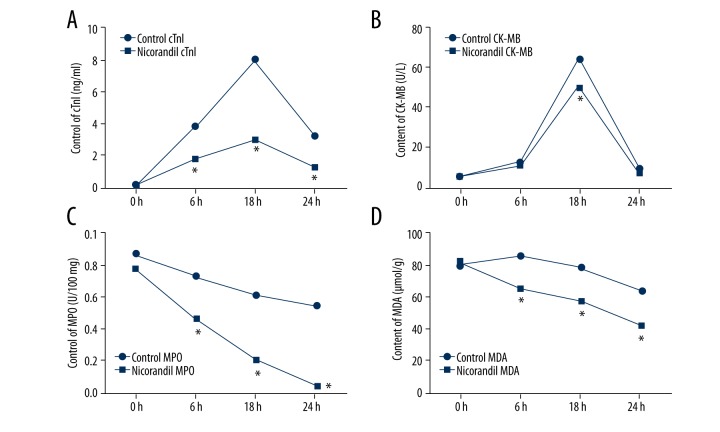
To compare the effects of nicorandil on CHD patients undergoing PCI, we analyzed the plasma levels of cTnI, CK-MB, MPO, and MDA before and at 6, 18, and 24 h after PCI. As shown in Figure 2A–2D, the results demonstrated that the although the levels of cTnI and CK-MB were increased at 6 h and 18 h after PCI, the levels were decreased at 24 h. In addition, the levels of cTnI and CK-MB in the nicorandil group were less than those in the control group. There were significant differences in the cTnI levels at 6 h, 18 h, and 24 h after PCI between the 2 groups, and at 18 h after PCI in the CK-MB (all P<0.05). Moreover, we observed that the levels of MPO and MDA were markedly reduced by nicorandil at 6 h, 18 h, and 24 h after PCI compared to the control group (all P<0.05). The results show that nicorandil protected against PCI-induced myocardial injury.
Figure 2.
Comparison of levels of cTnI, CK-MB, MPO, and MDA. The plasma levels of cTnI, CK-MB, MPO, and MDA before and at 6, 18, and 24 h after PCI in the 2 groups were analyzed. (A) Plasma levels of cTnI. (B) Plasma levels of CK-MB. (C) Plasma levels of MPO. (D) Plasma levels of MDA. cTnI – cardiac troponin I; CK-MB – creatine kinase-MB; MPO – myeloperoxidase; MDA – malondialdehyde; PCI – percutaneous coronary intervention. * P<0.05 compared to the control group.
Comparison of SBP, MBP, HR, and LVEF
We recorded the SBP, MBP, HR, and LVEF before and 3 months after PCI. As demonstrated in Figure 3A–3D, no significant differences were observed in SBP, MBP, HR, and LVEF before PCI between the 2 groups. However, SBP, MBP, and HR were dramatically decreased by administration of nicorandil 3 months after PCI compared to the control group (P<0.05 or P<0.01), and the LVEF were prominently raised by nicorandil intake (P<0.01). The results indicate that nicorandil improved myocardial contraction.
Figure 3.
Comparison of SBP, MBP, HR, and LVEF. The SBP, MBP, HR, and LVEF before and 3 months after PCI in the 2 groups were recorded. (A) SBP before and 3 months after PCI in the 2 groups. (B) MBP before and 3 months after PCI in the 2 groups. (C) HR before and 3 months after PCI in the 2 groups; (D) the LVEF before and after PCI 3 months in the 2 groups. SBP – systolic blood pressure; DBP – mean blood pressure; HR – heart rate; LVEF – left ventricular ejection fractions; PCI – percutaneous coronary intervention. * P<0.05 compared to the control group; ** P<0.01 compared to the control group.
Discussion
In the present study, we investigated the cardioprotective effects of nicorandil in patients with CHD who underwent selective PCI. The patients were administered medication 3 days before the PCI procedure. The results showed that, compared to the control group, nicorandil intake significantly decreased the rate of no-reflow and blood frames, reduced the makers of myocardial injury, and elevated LVEF. Our data suggest that nicorandil exerted cardioprotective effects on CHD patients undergoing PCI. Nicorandil might be useful as an alternative drug for perioperative preparation for primary PCI.
PCI has become an effective treatment choice for patients with acute coronary syndrome or coronary artery anomalies [21,22]. Nevertheless, no-reflow phenomenon and myocardial injury are recognized as serious complications of PCI, leading to poor prognosis [23–25]. Several drugs, such as nicorandil, have been well documented as effective for preventing these complications, Previous studies have found that intravenous and/or intracoronary injection could lessen the incidence of myocardial injury after PCI [18,26–32]. Kostic et al. observed that intracoronary nicorandil administration significantly reduced the index of microvascular resistance (IMR) after primary PCI, leading to improved coronary flow reserve (CFR) and ventricular function in patients with ST-elevation myocardial infarction (STEMI) [5]. More recently, it has been reported that oral nicorandil (5 mg orally 3 times daily) or a single oral dose of dose of nicorandil (10 mg or 20 mg) also exerted cardioprotective effects after coronary angioplasty [19,33]. Nicorandil is a nicotinamide ester with dual action, activating both ATP-sensitive potassium (K+ATP) channels and nitric oxide donor [34]. It is an important cardioprotective agent, which has been reported to dilate the coronary arteries [35], increase coronary blood flow [36], reduce cardiac preload and afterload, and decrease coronary vascular resistance [34]. However, the mechanism by which nicorandil protects against myocardial injury after PCI is not completely understood [37].
Therefore, we explored the underlying protective mechanism of nicorandil in myocardial damage. The numbers of patients with reflow and no-reflow in the 2 groups were analyzed. As indicated in our data, no-reflow phenomenon still existed after PCI, which was in line with previous studies [23,24]. However, nicorandil intake significantly decreased the rate of no-reflow compared to the patients who received conventional therapy. The blood frames were also statistically decreased by administration of nicorandil. The markers of myocardial injury before and at 6, 18, and 24 h after PCI were determined. cTnI is a cardiac-specific protein that is involved in cardiomyocyte contraction and relaxation. It is a sensitive and powerful marker of myocardial injury and contributes to cardiac disorder, and also could predict adverse cardiac events [38]. Although CK-MB is a less sensitive biomarker than cTnI for evaluating myocardial injury, measurement of peri-procedural CK-MB offers clinical and prognostic information regarding the degree of myocardial damage and risk of post-procedural morbidity and mortality [39]. MPO is a leukocyte enzyme that plays significant roles in regulating coronary inflammation and oxidative stress. It has been considered as a novel cardiac marker [40]. MDA is a well-accepted marker of lipid peroxidation and oxidative stress, and is used to indicate oxidative stress in the whole body [41]. Our results found that after PCI 6 and 12 h, the levels of cTnI and CK-MB were increased, indicating myocardial damage after PCI. However, both the levels were markedly decreased by administration of nicorandil, indicating that nicorandil alleviated myocardium damage. Moreover, the levels of MPO and MDA were also reduced by nicorandil, suggesting that nicorandil protected the myocardium by reducing oxidative stress. Further, the SBP, MBP, HR, and LVEF were recorded before and 3 months after PCI. LVEF is commonly used to assess left ventricular function, and also has prognostic significance. LVEF plays an important role in determining the therapeutic response after cardiac injury [42]. From our data, we observed that 3 months after PCI, the SBP, MBP, and HR were markedly reduced but LVEF was dramatically increased by administration of nicorandil, indicating cardiac contractility was obviously improved by nicorandil. However, there are some limitations in our study. First, our study was not a double-blind, placebo-controlled study. Second, the sample size in our study was not large enough. Third, the follow-up period was limited. Therefore, studies that are double-blind, placebo-controlled, larger scale, and longer follow-up should be performed to confirm these results.
Conclusions
Our study suggests that nicorandil intake before PCI procedure could reduce the rate of no-flow phenomenon, reduce myocardial injury, and improve myocardial contractility. Nicorandil might be an alternative treatment option for perioperative preparation for primary PCI.
Footnotes
Conflict of interest
Authors declare that they have no conflict of interest.
Source of support: Departmental sources
References
- 1.Li L, Pan Y, Dai L, et al. Association of genetic polymorphisms on vascular endothelial growth factor and its receptor genes with susceptibility to coronary heart disease. Med Sci Monit. 2016;22:31–40. doi: 10.12659/MSM.895163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaziano TA, Bitton A, Anand S, et al. Growing epidemic of coronary heart disease in low- and middle-income countries. Curr Probl Cardiol. 2010;35:72–115. doi: 10.1016/j.cpcardiol.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mendis S, Lindholm LH, Mancia G, et al. World Health Organization (WHO) and International Society of Hypertension (ISH) risk prediction charts: assessment of cardiovascular risk for prevention and control of cardiovascular disease in low and middle-income countries. J Hypertens. 2007;25:1578–82. doi: 10.1097/HJH.0b013e3282861fd3. [DOI] [PubMed] [Google Scholar]
- 4.Plotek W, Pielok J, Cybulski M, Samborska R. Emotional processes in patients undergoing coronary artery bypass graft surgeries with extracorporeal circulation in view of selected indicators of the inflammatory condition. Med Sci Monit. 2015;21:105–17. doi: 10.12659/MSM.892372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kostic J, Djordjevic-Dikic A, Dobric M, et al. The effects of nicorandil on microvascular function in patients with ST segment elevation myocardial infarction undergoing primary PCI. Cardiovasc Ultrasound. 2015;13:26. doi: 10.1186/s12947-015-0020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein LW, Kramer BL, Howard E, Lesch M. Incidence and clinical significance of transient creatine kinase elevations and the diagnosis of non-Q wave myocardial infarction associated with coronary angioplasty. J Am Coll Cardiol. 1991;17:621–26. doi: 10.1016/s0735-1097(10)80174-3. [DOI] [PubMed] [Google Scholar]
- 7.Vescovo G, Zaninotto M, Casiglia E, et al. Changes in myoglobin, creatine kinase and creatine kinase-MB after percutaneous transluminal coronary angioplasty for stable angina pectoris. Am J Cardiol. 1987;59:999–1000. doi: 10.1016/0002-9149(87)91145-3. [DOI] [PubMed] [Google Scholar]
- 8.Jaffe R, Charron T, Puley G, et al. Microvascular obstruction and the no-reflow phenomenon after percutaneous coronary intervention. Circulation. 2008;117:3152–56. doi: 10.1161/CIRCULATIONAHA.107.742312. [DOI] [PubMed] [Google Scholar]
- 9.Zemánek D, Branny M, Martinkovičová L, et al. Effect of seven-day atorvastatin pretreatment on the incidence of periprocedural myocardial infarction following percutaneous coronary intervention in patients receiving long-term statin therapy. A randomized study. Int J Cardiol. 2013;168:2494–97. doi: 10.1016/j.ijcard.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Li Q, Deng SB, Xia S, et al. Impact of intensive statin use on the level of inflammation and platelet activation in stable angina after percutaneous coronary intervention: A clinical study. Med Clin (Barc) 2013;140:532–36. doi: 10.1016/j.medcli.2012.05.042. [DOI] [PubMed] [Google Scholar]
- 11.Kurz DJ, Naegeli B, Bertel O. A double-blind, randomized study of the effect of immediate intravenous nitroglycerin on the incidence of postprocedural chest pain and minor myocardial necrosis after elective coronary stenting. Am Heart J. 2000;139:35–43. doi: 10.1016/s0002-8703(00)90306-5. [DOI] [PubMed] [Google Scholar]
- 12.Wang FW, Osman A, Otero J, et al. Distal myocardial protection during percutaneous coronary intervention with an intracoronary beta-blocker. Circulation. 2003;107:2914–19. doi: 10.1161/01.CIR.0000072787.25131.03. [DOI] [PubMed] [Google Scholar]
- 13.Tcheng JE, Harrington RA, Kottke-Marchant K, et al. Multicenter, randomized, double-blind, placebo-controlled trial of the platelet integrin glycoprotein IIb/IIIa blocker Integrelin in elective coronary intervention. IMPACT Investigators. Circulation. 1995;91:2151–57. doi: 10.1161/01.cir.91.8.2151. [DOI] [PubMed] [Google Scholar]
- 14.Markham A, Plosker GL, Goa KL. Nicorandil. An updated review of its use in ischaemic heart disease with emphasis on its cardioprotective effects. Drugs. 2000;60:955–74. doi: 10.2165/00003495-200060040-00007. [DOI] [PubMed] [Google Scholar]
- 15.IONA Study Group. Effect of nicorandil on coronary events in patients with stable angina: The Impact Of Nicorandil in Angina (IONA) randomised trial. Lancet. 2002;359:1269–75. doi: 10.1016/S0140-6736(02)08265-X. [DOI] [PubMed] [Google Scholar]
- 16.Somma SD, Liguori V, Petitto M, et al. A double-blind comparison of nicorandil and metoprolol in stable effort angina pectoris. Cardiovasc Drugs Ther. 1993;7:119–23. doi: 10.1007/BF00878320. [DOI] [PubMed] [Google Scholar]
- 17.Ono H, Osanai T, Ishizaka H, Hanada H. Nicorandil improves cardiac function and clinical outcome in patients with acute myocardial infarction undergoing primary percutaneous coronary intervention: Role of inhibitory effect on reactive oxygen species formation. Am Heart J. 2004;148:E15. doi: 10.1016/j.ahj.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 18.Kim JH, Jeong MH, Yun KH, et al. Myocardial protective effects of nicorandil during percutaneous coronary intervention in patients with unstable angina. Circ J. 2005;69:306–10. doi: 10.1253/circj.69.306. [DOI] [PubMed] [Google Scholar]
- 19.Yang J, Zhang J, Cui W, et al. Cardioprotective effects of single oral dose of nicorandil before selective percutaneous coronary intervention. Anatol J Cardiol. 2015;15:125–31. doi: 10.5152/akd.2014.5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gibson CM, Cannon CP, Daley WL, et al. TIMI frame count: A quantitative method of assessing coronary artery flow. Circulation. 1996;93:879–88. doi: 10.1161/01.cir.93.5.879. [DOI] [PubMed] [Google Scholar]
- 21.Kawecki D, Morawiec B, Dola J, et al. Comparison of first- and second-generation drug-eluting stents in an all-comer population of patients with diabetes mellitus (from Katowice-Zabrze Registry) Med Sci Monit. 2015;21:3261–69. doi: 10.12659/MSM.895095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramesh D, Setty HS, Patil V, et al. Use of CT angiogram in interventions involving coronary artery anomalies: A case series. Am J Case Rep. 2015;16:858–62. doi: 10.12659/AJCR.894107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vallejo E, Peña-Duque MA, Noroño O, et al. [The no-reflow phenomenon: its incidence and clinical characteristics in a series of cases]. Arch Inst Cardiol Mex. 1998;68(3):247–52. [in Spanish] [PubMed] [Google Scholar]
- 24.Morishima I, Sone T, Okumura K, et al. Angiographic no-reflow phenomenon as a predictor of adverse long-term outcome in patients treated with percutaneous transluminal coronary angioplasty for first acute myocardial infarction. J Am Coll Cardiol. 2000;36:1202–9. doi: 10.1016/s0735-1097(00)00865-2. [DOI] [PubMed] [Google Scholar]
- 25.Brosh D, Assali AR, Mager A, et al. Effect of no-reflow during primary percutaneous coronary intervention for acute myocardial infarction on six-month mortality. Am J Cardiol. 2007;99:442–45. doi: 10.1016/j.amjcard.2006.08.054. [DOI] [PubMed] [Google Scholar]
- 26.Sakai K, Yamagata T, Teragawa H, et al. Nicorandil enhances myocardial tolerance to ischemia without progressive collateral recruitment during coronary angioplasty. Circ J. 2002;66:317–22. doi: 10.1253/circj.66.317. [DOI] [PubMed] [Google Scholar]
- 27.Ito H, Taniyama Y, Iwakura K, et al. Intravenous nicorandil can preserve microvascular integrity and myocardial viability in patients with reperfused anterior wall myocardial infarction – its dependency on vessel size and the involvement of the ATP-sensitive potassium channels. J Am Coll Cardiol. 1999;33:654–60. doi: 10.1016/s0735-1097(98)00604-4. [DOI] [PubMed] [Google Scholar]
- 28.Ota S, Nishikawa H, Takeuchi M, et al. Impact of nicorandil to prevent reperfusion injury in patients with acute myocardial infarction: Sigmart Multicenter Angioplasty Revascularization Trial (SMART) Circ J. 2006;70:1099–104. doi: 10.1253/circj.70.1099. [DOI] [PubMed] [Google Scholar]
- 29.Ikeda N, Yasu T, Kubo N, et al. Nicorandil versus isosorbide dinitrate as adjunctive treatment to direct balloon angioplasty in acute myocardial infarction. Heart. 2004;90:181–85. doi: 10.1136/hrt.2003.013789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nameki M, Ishibashi I, Miyazaki Y, et al. Comparison between nicorandil and magnesium as an adjunct cardioprotective agent to percutaneous coronary intervention in acute anterior myocardial infarction. Circ J. 2004;68:192–97. doi: 10.1253/circj.68.192. [DOI] [PubMed] [Google Scholar]
- 31.Ueda H, Nakayama Y, Tsumura K, et al. Intravenous nicorandil can reduce the occurrence of ventricular fibrillation and QT dispersion in patients with successful coronary angioplasty in acute myocardial infarction. Can J Cardiol. 2004;20:625–29. [PubMed] [Google Scholar]
- 32.Isono T, Kamihata H, Sutani Y, et al. Nicorandil suppressed myocardial injury after percutaneous coronary intervention. Int J Cardiol. 2008;123:123–28. doi: 10.1016/j.ijcard.2006.11.219. [DOI] [PubMed] [Google Scholar]
- 33.Kato T, Kamiyama T, Maruyama Y, et al. Nicorandil, a potent cardioprotective agent, reduces QT dispersion during coronary angioplasty. Am Heart J. 2001;141:940–43. doi: 10.1067/mhj.2001.114369. [DOI] [PubMed] [Google Scholar]
- 34.Kukovetz WR, Holzmann S, Pöch G. Molecular mechanism of action of nicorandil. J Cardiovasc Pharmacol. 1992;20( Suppl 3):S1–7. doi: 10.1097/00005344-199206203-00002. [DOI] [PubMed] [Google Scholar]
- 35.Akai K, Wang Y, Sato K, et al. Vasodilatory effect of nicorandil on coronary arterial microvessels: its dependency on vessel size and the involvement of the ATP-sensitive potassium channels. J Cardiovasc Pharmacol. 1995;26:541–47. doi: 10.1097/00005344-199510000-00006. [DOI] [PubMed] [Google Scholar]
- 36.Hongo M, Takenaka H, Uchikawa S, et al. Coronary microvascular response to intracoronary administration of nicorandil. Am J Cardiol. 1995;75:246–50. doi: 10.1016/0002-9149(95)80029-r. [DOI] [PubMed] [Google Scholar]
- 37.Ito N, Nanto S, Doi Y, et al. Beneficial effects of intracoronary nicorandil on microvascular dysfunction after primary percutaneous coronary intervention: demonstration of its superiority to nitroglycerin in a cross-over study. Cardiovasc Drug Ther. 2013;27:279–87. doi: 10.1007/s10557-013-6456-y. [DOI] [PubMed] [Google Scholar]
- 38.Hammadah M, Mheid IA, Wilmot K, et al. High sensitivity cardiac troponin i is associated with mental and conventional stress induced myocardial ischemia. J Am Coll Cardiol. 2016;67:2346. [Google Scholar]
- 39.Gollop ND, Dhullipala A, Nagrath N, Myint PK. Is periprocedural CK-MB a better indicator of prognosis after emergency and elective percutaneous coronary intervention compared with post-procedural cardiac troponins? Interact Cardiovasc Thorac Surg. 2013;17:867–71. doi: 10.1093/icvts/ivt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahmed N, Carberry J, Teng V, et al. Risk assessment in patients with an acute ST-elevation myocardial infarction. J Comp Eff Res. 2016;5(6):581–93. doi: 10.2217/cer-2016-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meng G, Wang J, Xiao Y, et al. GYY4137 protects against myocardial ischemia and reperfusion injury by attenuating oxidative stress and apoptosis in rats. J Biomed Res. 2015;29:203–13. doi: 10.7555/JBR.28.20140037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Richards AM, Nicholls MG, Espiner EA, et al. B-type natriuretic peptides and ejection fraction for prognosis after myocardial infarction. Circulation. 2003;107:2786–92. doi: 10.1161/01.CIR.0000070953.76250.B9. [DOI] [PubMed] [Google Scholar]