Abstract
Background
This study investigated the mechanism underlying the activating mutation of SHP-2 in promoting malignant biological behaviors of glioma cells.
Material/Methods
The SHP-2 empty plasmid pcDNA3.1 and SHP-2 activating mutation plasmid pcDNA3.1 SHP-2 D61G mutant eukaryotic expression vectors were designed; stable SHP-2-expressing cells transfected with pcDNA3.1 SHP-2 D61G mutant were set as the mutation group; cells transfected with pcDNA3.1 were set as the empty vector group; and cells without transfection were set as the control group. The cell reproductive capacity in each group was measured by MTT method. The invasion ability of cells in vitro was detected by Transwell chamber assay, the cell apoptosis in each group was detected by Annexin-V/PE dual-staining method, and the clone formation ability of cells in vitro was detected by Tablet clone-forming assay. The activation of ERK1/2, ARK, and p38MAPK signal pathways in each group was determined by Western blot.
Results
After transfection, the expression of SHP-2 protein in the mutant group was significantly higher than that in the control group and empty vector group. The proliferation ability of transfected cells, the apoptosis rate, the invasion ability, and the expression levels of phosphorylated ERK1/2, AKT, and p38 in the mutation group was significantly higher than in the empty vector group and the control group (P<0.05). Moreover, the cell clone formation ability of the mutation group was obviously enhanced (P<0.05).
Conclusions
The activating mutation of SHP-2 can lead to malignant changes in biological behaviors of glioma cells, and the specific mechanism may be related to the activation of ERK1/2, AKT, and p38 signal pathway. SHP-2 protein may become a new target for anti-malignant transformation of glioma.
MeSH Keywords: Behavior, Addictive; Glioma; Protein Tyrosine Phosphatase, Non-Receptor Type 11
Background
The malignant degree of glioma is high, and the proliferation ability, migration, and invasion of malignant tumor cells are strong, with refractoriness to treatment [1]. Studies have shown that [2], with current therapeutic regimens, the median survival time of patients is no more than 18 months, and the 5-year survival rate is no more than 5%. Therefore, it has become a clinical focus of attention to find new therapeutic methods or targets for glioma. Previous studies have confirmed that the malignant biological behaviors of glioma and other tumors may be related to gene mutation or deletion [3]. SH2 domain-containing protein tyrosine phosphatase-2 (SHP-2) is one of the members of protein tyrosine kinase (PTK) family [4]. With relatively conserved molecular evolution, SHP-2 is widely expressed in human tissues, organs, and cells, participating in embryonic development and regulating cell proliferation, differentiation, adhesion, migration, and other biological behaviors [5]. The literature has shown [6] that SHP-2 activating mutation is closely related to the occurrence and progression of malignant tumors. Li et al. [7] found that the expression level of Tim-4 is closely associated with glioma, and Shang et al. [8] showed that miR-338-3p acted as a tumor-suppressing gene whose silencing can inhibit malignant biological behaviors of glioma cells. Research has shown that GOLPH3 and NDRG1 both play an important role in glioma etiology [9]. However, there are few studies reporting the correlation between SHP-2 and the malignant behaviors of glioma cells. Based on this and the related biological experiments, this study aimed to explore the possible mechanism of SHP-2 involvement in the malignant biological behaviors of glioma cells through transfecting human glioma cell line U251 with SHP-2 eukaryotic expression vector, in order to provide a new reference for the treatment of malignant glioma in clinic.
Material and Methods
Reagents and instruments
Human brain glioma U251 cell line was purchased from Wuhan Punuosai Life Science & Technology Co., Ltd, SHP-2HRP antibody was purchased from Abcam Company (USA), and monoclonal antibodies of rabbit anti-human phospho-AKT, phospho-ERK1/2, and phospho-p38 were purchased from Sigma Company (USA). The flow cytometer was the product of Beckman Company (USA), the SM600 multimode reader was purchased from Shanghai Yongchuang Medical Devices Co., Ltd, the BCA protein quantitative detection kit and Annexin-V apoptosis detection kit were purchased from Invitrogen Company (USA), and the Western blot specific kit was purchased from Guangzhou Saicheng Biotechnology Co. Ltd.
General methods
Construction of SHP-2 siRNA empty plasmid
According to the sequence and structure of the encoding gene of the target protein published on the website of http://www.ncbi.nih.gov/, http://www.hprd.org/ FOXO3a, SHP-2 empty plasmid was designed using FastPCR, Primer 5.0, and other software. The design is as follows: the upstream primer sequence was: 5′-CCGCTCGAGATGACATCGCGGAG-3′, the downstream primer sequence was 5′-CGCGGATCCTGTCTGAAACTCTT-3′. The primers were synthesized by Guangzhou Ribo Biotechnology Co. Ltd, and were sequenced after confirming with double-enzyme digestion. The primers of the high expression plasmid pcDNA3.1SHP-2 were designed according to the cDNA encoding sequence of empty plasmid SHP-2, the design is as follows: the upstream primer sequence was 5′-CCGCTCGAGATGACATCGCGGAGATGG-3′, the downstream primer sequence was 5′-CGCGGATCCCGTCTGAAACTTTTCTGCT-3′, and the product size was 1782bp. The reaction parameters were as follows: pre-denaturing at 95°C for 5 min, 95°C for 1 min, 55°C for 1 min, and 73°C for 2 min. After a total of 30 cycles, followed by product extension at 72°C for 10 min, the template of high expression plasmid pcDNA3.1 SHP-2 was obtained, and based on this, SHP-2D61G mutation protein (mutant) was obtained by PCR amplification (T>G). It was sequenced by Shanghai Bohao Biotechnology Co., Ltd after confirming with dual-enzyme digestion.
Establishment of U251 cell transfection and stable cell line
The U521 cells were cultured until adherent, and inoculated into 24-well plates. When the cell confluence achieved 80%, G418 at 0, 400, 500, 600, 700, 800, and 900 g/mL were placed into the wells. The optimal screening concentration of G418 was determined by selecting the lowest working concentration which could cause all cell death. When cells were cultivated with the density of no less than 90%, and the cell viability was no less than 95% in trypan blue assay, the recombinant pcDNA3.1 SHP-2D61G mutant plasmid was transfected into the U251 cells to establish the mutation group. Cells transfected with empty pcDNA plasmid were set as the empty vector group, and cells without transfection were set as the control group. For each of the above described groups, 3 replicate wells were set. Cells were kept in culture continually and were passed at the ratio of 1: 6. The resistant clones were obtained after 15~21 days of screening. The individual clones were picked up with the sterile tips of a pipette and kept at expansion for 21~30 days to obtain the optimal G418 resistant stable U521 cell lines.
Determination of SHP-2 protein expression by Western blot (the mRNA expression had been determined during the designing of SHP-2 expression vector, so mRNA expression requires no further quantification)
Cells of the mutation group were washed with PBS at 4°C 3 times, 1 min/time, then 400 μL lysing buffer containing PMSF was dropped into the cells and maintained on ice for 30 min for cell lysis. The cells were then scraped and transferred into a 1.5-m LEP tube, and spun at 12 000 rpm at 4°C for 30 min. The protein in the supernatant was quantified by BCA assay according to the instructions of the kit. Thirty μg of loading proteins were boiled for 5 min in hot water, loaded for electrophoresis, and then transferred onto the nitrocellulose membrane. The expression of SHP-2 protein was determined by Western blot following the instructions of the kit. β-actin expression was set as the internal control, the gray-scale value was analyzed, and the ratio of the gray-scale value in each group was calculated.
Determination of effect of SHP-2 activating mutation on the biological behaviors of U251 cells
Detection of Cell proliferation by MTT method
Cells were cultured for 0 h, 24 h, and 48 h in each group, and the proliferation of the selected 3 wells of cells in each group was detected by MTT method. About 20 μl of 5 mg/mL MTT were dropped into each well for culturing in the dark at 37°C for 4 h. The supernatant was then discarded, and about 120~150 μl of DMSO liquid was dropped into each well; the culture plate was oscillated until all the crystals were fully dissolved. The absorbance at OD570nm of each well was detected by the reader, with 3 measurements to obtain an average value.
Determination of cell apoptosis by Annexin-V/PE dual-staining method
After all the cells of the 4 groups were treated by 4 μg/μL cisplatin for 48 h, pancreatin digestion and collecting cells, and the cell density was adjusted to5×105/mL. Then, the cell apoptosis was determined by Annexin-V/PE dual-staining in accordance with the instructions of the kit. The apoptosis rate was detected by flow cytometry.
Determination of cell invasion in vitro by Transwell chamber
After 48-h culturing, 200 μL of DMEM cell suspensions containing 0.5% FBS were dropped into the upper chamber of the Transwell migration system, and 600 μL of DMEM containing 2% FBS were dropped into the lower chamber of the Transwell migration system, then they were incubated at 37°C with 5% CO2 for 12 h. The cells randomly moved into the lower chamber and were counted with the cell counter. The non-migrated cells in the upper chamber were cleared away. Cells which had moved through the cup hole to the bottom of the cup were fixed with methanol. After staining, cells containing polycarbonate membrane were cleared off, and its reverse side was placed upward. The phase-contrast microscope was used for observing, photo-taking, and counting. Five visual fields were taken to calculate the average value. The operation was repeated 3 times.
Detection of U251 cell clone formation ability
Flat plate clone formation test was used to detect the two-dimensional clone formation ability of the cells. The number of formed clones was counted, with the measuring standard as follows: the number of cells was no less than 50. The clone formation rate (%) is = the number of clones/the number of inoculated cells×100%, the detection was performed 3 times to obtain an average value.
Detection of expressions of phosphorylated ERK1/2, AKT, and p38 signal pathways by Western blot
The extraction and quantification of proteins were the same as described in 1.2.3. The expressions of phosphorylated ERK1/2, AKT, and p38 signal pathway was detected by Western blot. Five μl primary antibody-rabbit anti-human phospho-AKT, phospho-ERK1/2 and phospho-p38 monoclonal antibodies (diluted with TBST liquid at the ratio of 1: 400) [13] were dropped, respectively, and then 5 μl alkaline phosphatase-labeled second antibody was added. The rest of the procedures were performed in accordance with the instructions of the kit, with β-actin as the internal control. The gray-scale value was analyzed and the signal protein expression in each group was calculated.
Statistics processing
The experimental data were processed by SPSS 12.0 software, and the t test and variance analysis were used as the statistical methods for analysis of significant differences, SNK-q test was used for multiple comparison between groups, and P<0.05 represents a statistically significant difference.
Results
Detection of protein expression level of U251 cells after transfection SHP-2
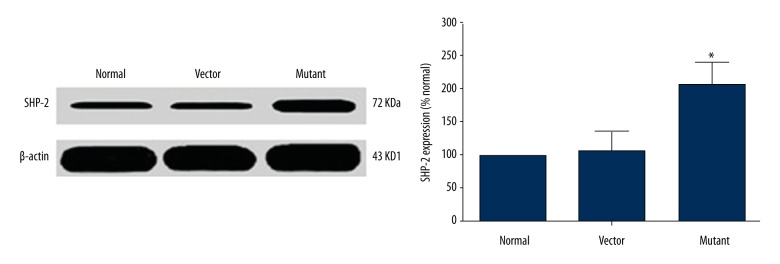
To determine SHP-2 protein expression level in U251 cells after transfection, Western blot analysis showed that, compared with empty vector group, the intracellular protein expression level of SHP-2 of the mutation group was increased significantly after transfection, and the difference was statistically significant (n=3, P<0.05). As shown in Figure 1, this indicates that SHP-2 is abnormally activated and results in mutations after transfection of U251 cells.
Figure 1.
The SHP-2 protein expression level of SHP-2 ofU251 cells after transfection.
Detection of the proliferation of glioma cells by the MTT method
To determine the proliferative activity of glioma cells after transfection, OD values at 570 nm of cells in the control group, empty vector group, and mutation group were detected after cultivated for 0 h, 24 h, and 48 h by the MTT method. At 0 h, there was no significant difference in the proliferation rats of the cells in each group (n=3, P>0.05), At 24 h and 48 h, the proliferation rate of the cells in the mutation group was significantly higher than in the control group and the empty vector group, and the difference was statistically significant (n=3, P<0.05). As shown in Figure 2, this indicates that the proliferative activity of glioma cells was significantly enhanced after SHP-2 transfection at 24 h.
Figure 2.
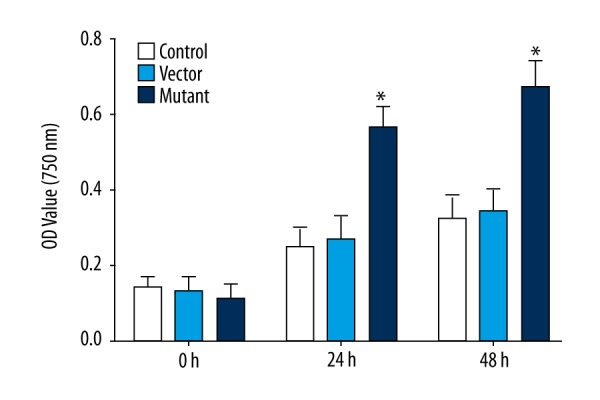
Comparison of OD value at 570 nm at different time points of different groups (* P<0.05).
Detection of glioma cell proliferation activity by plate cloning formation test
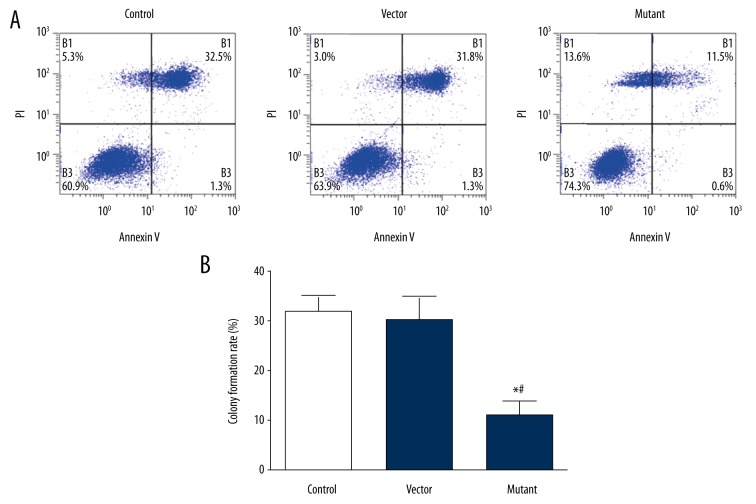
Clonal formation ability is also one of the criteria to evaluate the activity of tumor cell proliferation, so we used the plate clone formation test to further test the value of cell proliferation activity. The results showed that there were cell clones in each group, but the clone formation rate in the mutant group was significantly higher than that in the control group and the empty vector group (t=17.295, P<0.05), as shown in Figure 3. This indicated that the clonogenic ability of the mutant group was significantly enhanced, which was consistent with the results of the previous MTT assay, further demonstrating that the proliferative activity of the glioma cells after SHP-2 transfection was significantly enhanced.
Figure 3.
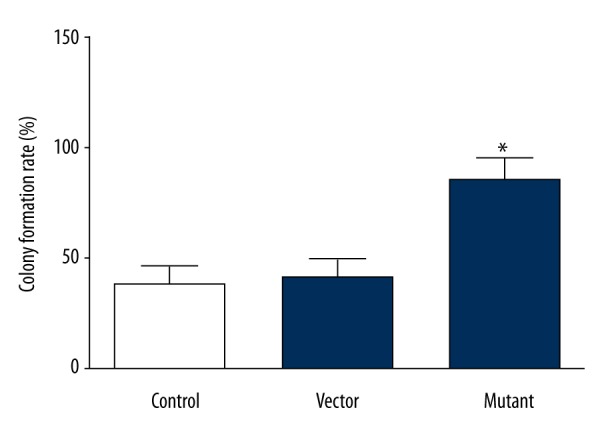
Comparison of clone-forming ability of cells in each group (* P<0.001).
Detection of apoptosis of glioma cells by Annexin-V/PE single-staining method
Apoptosis is one of the criteria for the detection of tumor cells, to determine the apoptosis of glioma cells after transfection, the number of cells in each group was detected by Annexin-V/PE monoclonal assay. The results revealed there were more apoptotic cells in the empty vector group and the control group, and the rate of apoptotic cells was significantly higher than that of the mutation group (P<0.05), as shown in Figure 4.
Figure 4.
Comparison of the anti-apoptotic ability of the cells in each group. (A) Flow cytometric detection of apoptosis. (B) The anti-apoptotic ability of each group cells (*# P<0.05).
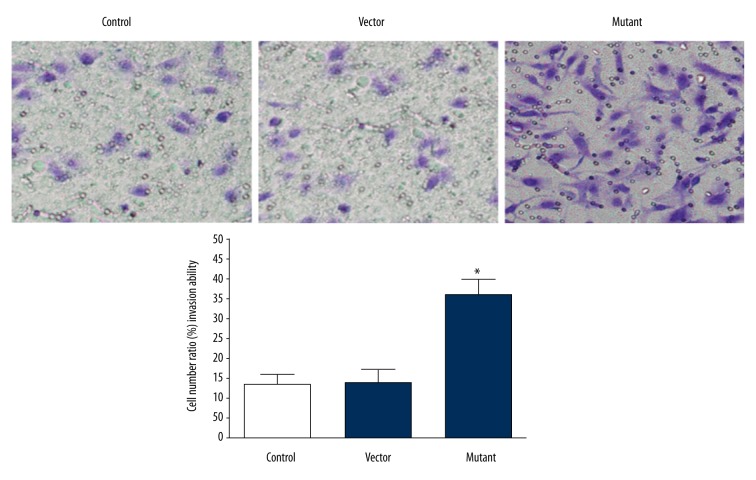
Determination of invasion ability of glioma cells in vitro by Transwell chamber assay
In vitro invasion ability is one of the important criteria for evaluating tumor cells. To determine the invasion ability of glioma cells transfected with SHP-2 in vitro, Transwell chamber method was used to determine the number of cells migrating into the lower chamber. The results showed that few cells moved into the lower chamber of the control group and the empty vector group, and there was no significant difference (N=3, P<0.05), and the number of cells that moved into the lower chamber of the mutant group was significantly higher than that of the control group and empty vector group (P<0.05), which indicated that the invasion ability of the control group and empty vector group was significantly lower than in the mutant group, as shown in Figure 5.
Figure 5.
Comparison of the invasive movement ability of cells in vitro in each group.
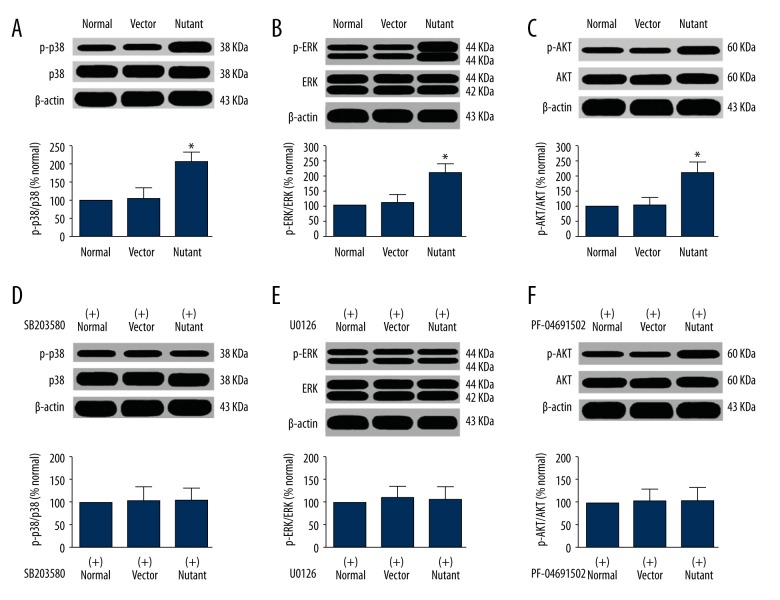
Possible mechanisms of SHP-2 mutation affect the biological behavior of glioma cells
The above experimental results show that the mutation induced by SHP-2 transfection enhanced the viability of glioma cells, including increased proliferation, decreased apoptosis, and increased invasive capacity. To briefly explore the possible mechanisms of SHP-2 mutations in glioma cells, the activation of p38, ERK1/2, and AKT signal pathways in each group were detected by Western blot. The results revealed that the expressions of p38, ERK1/2, and AKT in the mutation group were significantly higher than those in the empty vector group and the control group (P<0.05), as shown in Figure 6A–6C. If the specific antagonists SB203580, U0126, and PF-04691502 of p38, ERK1/2, and AKT were added to SHP-2 transfected cells, the expression of phosphorylated p38, ERK1/2, and AKT in mutant group has no significant difference with the control group and the empty vector group (P>0.05), as shown in Figure 6D–6F. This suggests that the activation of enzymes such as p38, ERK1/2, and AKT are involved in SHP-2 mutations and affect the biological behavior of glioma cells, suggesting that SHP-2 mutations may be mediated by p38, ERK1/2, and AKT and other signal pathways to affect the biological behavior of glioma cells.
Figure 6.
The expressing quantity of pro-apoptotic signal pathway protein by phosphorylation in the cells of each group. (A) The expression of protein p-p38; (B) The expression of protein p-ERK; (C) The expression of protein p-AKT; (D) The expression of p-p38 protein after added antagonists SB203580; (E) The expression of p-ERK protein after added the antagonist U0126; (F) The expression of p-AKT protein after added the antagonist PF-04691502.
Discussion
SHP-2, as one of the subsets of protein tyrosine phosphatase family [10,11], plays an important role in cellular proliferation, apoptosis, differentiation, migration, and other processes, and is also the downstream signal molecule of a variety of cytokines, gene antigens, and extracellular matrix. The literature showed that [12] there is a DNA substrate homology domain within the molecular structure of associated adapter protein 2 (Gab2). Since multiple proline sequences and tyrosine residues are contained in this region, it can be phosphorylated by a variety of cytokines or growth factors, thereby promoting the signal transduction through the recruitment of downstream signal molecules. Furthermore, after producing signal pathway through interaction of SHP-2 and Gab2, SHP-2 can be catalyzed by phosphatase, which can positively regulate the PTKs receptor signal transduction pathway. Conversely, in certain circumstances, SHP-2 may also have a negative regulation effect on cell signal transduction [13]. This makes SHP-2 a bidirectional regulator for cytokines and growth-factors-mediated signal transduction. A previous study has shown [14] that abnormal expression of SHP-2 can promote the initiation, progression, and metastasis of breast cancer, gastric cancer, liver cancer, lung cancer, and other cancers. Therefore, there is great significance in prevention and treatment of glioma through studying the role and mechanism of SHP-2 in tumors.
A previous experiment proved that [15] the activating mutation points of SHP-2 in cancer patients mainly exist in the N terminal SH2 domain or PTP domain. When the activating mutation occurs in these domains, it can lead to the partial or complete dissociation of the autologous binding of N-SH2 and PTP domains, resulting in gene mutation in PTP domain, which can induce the upregulation of the phosphatase activity of SHP-2 tyrosine, and leads to the aggregation of mutation-related binding regions. Furthermore, it can induce the activity of SHP-2 from non to high through the amino acids replacement [16], as a result, the Gain-of-Function effects (GOF) were produced. In the present study, the pcDNA3.1 (empty SHP-2 particles) and pcDNA3.1SHP-2D61Gmutant (mutant SHP-2 particles) were transfected into glioma U251 cells by our experimental team, and clonal cells with the highest expression of SHP-2 protein were obtained after Western blot detection and screening, and were used in the subsequent tests for detecting cell malignant biological behaviors. Rapid cell proliferation and slow apoptosis are the typical characteristics of malignant tumor cells. The result of MTT assay revealed that the proliferation rate of cells from the mutation group was significantly higher than that of the control and empty vector groups (P<0.05), which revealed that SHP-2 transfection could promote the rapid proliferation of glioma cells. A previous study [17] has shown that the factors that affected the expression of SHP2 in breast cancer cells can alter the expression of AKT, so as to regulate the drug resistance of cancer cells to cisplatin, and indirectly interfere with apoptosis. In this study, cisplatin induced cell apoptosis, as detected by Annexin-V/PE dual-staining, revealed that the cell apoptosis rate in the mutation group was significantly lower than that in the control and empty vector groups (P<0.05), suggesting that SHP-2 activating mutations can also affect the cisplatin drug sensitivity of glioma cells through inhibiting apoptosis.
Cell clone-forming ability can directly affect the population dependence and proliferation of tumor cells. The stronger the cloning ability is, the stronger tumorigenicity of tumor cells becomes, and the higher degree of malignancy is [18]. In the present study, the tablet cloning experiments showed that the cell clone formation number and colony formation rate from the mutation group were both significantly higher than those of the empty vector and control groups (P<0.05). This result demonstrates that SHP-2 has turned into a proto-oncogene after its activating mutation. It mediates the malignant proliferation signaling pathway in place of the normal cell growth signaling pathway, thus increasing the degree of malignancy and the invasion ability of glioma cells. The invasion and metastasis of tumors are mainly due to the high adhesion and invasion ability of tumor cells. In the present study, the experimental results revealed that the migration, adhesion, and invasion abilities of the cells in the mutation group were significantly higher than those of the empty vector and control groups (P<0.05), proving that SHP-2 plays an important role in the processes of promoting proliferation and invasion of glioma cells. Data in the literature [19] show that the possible mechanism may be that the SHP-2 mutation increases the expression of matrix metalloproteinase, and decreases the expression of E-cadherin in glioma cells, thereby increasing the fluidity of cells, and cells could become easy to invade and metastasize to distant tissues or organs.
The mitogen-activated protein kinase (MAPK) signal transduction pathway is one of the main signal transduction pathways of eukaryotic cells [20], and the activated MAPK can phosphorylate nuclear transcription factors and other protein kinases, trigger the relevant gene transcription machinery, regulate cell growth, proliferation, and apoptosis, and other physiological processes. ERK and P38 are members of the MAPK family. Under certain conditions, activation of the ERK signaling pathway can transfer a stimulus signal from outside into cells [21], thereby affecting cell growth, proliferation, and differentiation, and promoting cell abnormity, malignant transformation, and tumor formation. As one of the important pathways of intracellular and extracellular membrane receptor signal transduction, phosphatidyl inositol kinase-3/Serine (threonine) protein kinase (PI3K/AKT) can also regulate cell proliferation, differentiation, survival, and migration [22]. In the present study, effects of SHP-2 activating mutation on the activation of P38, ERK1/2, and AKT pathways were detected through Western blot. The results revealed that P38, ERK1/2, and AKT signal transduction pathways were highly activated, and the protein expressions were upregulated in the SHP-2 activating mutant glioma cells, suggesting that effects of SHP-2 activating mutation and overexpression on the biological phenotype of glioma cells are related to the activation of RAS-RAF-MAPK and PI3K-AKT pathways. In addition, the pharmacological mechanism of cisplatin is to transfer the damage message by coupling with cell DNA, and inhibit the activation of PI3K/AKT, MAPK, and P38 pathways to induce cell apoptosis. However, SHP-2 can activate the high expressions of PI3K/AKT, MAPK, and P38, and increase the apoptosis threshold [23], which results in tolerance to apoptosis induced by cisplatin. It thus can be seen that the underlying mechanism of changes of malignant biological behaviors of glioma cells induced by SHP-2 activating mutation may be related to the activation of P38, ERK1/2, and AKT signaling pathways.
Conclusions
SHP-2 activating mutation can promote glioma cells to exhibit malignant biological behaviors of apoptosis, proliferation, and invasion, and its mechanism may be related to the activation of P38, ERK1/2, and AKT signaling pathways. SHP-2 can thus function as a potential anti-cancer target, so as to improve the prevention and treatment of glioma.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Tokunbo W, Leyte Y. Celecoxib and LLW-3-6 reduce survival of human glioma cells independently and synergistically with sulfasalazine. Anticancer Res. 2015;35:6419–24. [PMC free article] [PubMed] [Google Scholar]
- 2.Sainan L, Yubo C, Shangchen L, et al. Cystoid angiocentric glioma: A case report and literature review. J Radiol Case Rep. 2015;9:1–9. doi: 10.3941/jrcr.v9i7.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Houlston B, Kinnersley J, Mitchell K, et al. Quantifying the heritability of glioma using genome-wide complex trait analysis. Sci Rep. 2015;5:17267. doi: 10.1038/srep17267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaiyasate A, Boyer A, Krishnan L, et al. Prenatal diagnosis of nasal glioma associated with metopic craniosynostosis: Case report and review of the literature. J Radiol Case Rep. 2015;9:1–8. doi: 10.3941/jrcr.v9i4.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang Z, Yi X, Shuyuan W, et al. Low MiR-149 expression is associated with unfavorable prognosis and enhanced Akt/mTOR signaling in glioma. Int J Clin Exp Pathol. 2015;8:11178–84. [PMC free article] [PubMed] [Google Scholar]
- 6.Hanna M, Krotz F. SHP-2 regulates growth factor dependent vascular signalling and function. Mini Rev Med Chem. 2014;14:471–83. doi: 10.2174/1389557514999140506094738. [DOI] [PubMed] [Google Scholar]
- 7.Li W, Li X, Xu S, et al. Expression of Tim4 in glioma and its regulatory role in LN-18 glioma cells. Med Sci Monit. 2016;22:77–82. doi: 10.12659/MSM.894963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shang C, Hong Y, Guo Y, et al. Mir-338-3p inhibits malignant biological behaviors of glioma cells by targeting MACC1 gene. Med Sci Monit. 2016;22:710–16. doi: 10.12659/MSM.897055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li X, Li M, Tian X, et al. Golgi phosphoprotein 3 inhibits the apoptosis of human glioma cells in part by downregulating N-myc downstream regulated gene 1. Med Sci Monit. 2016;22:3535–43. doi: 10.12659/MSM.900349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirito T, Mitsumori Y, Nozaki I, et al. Hypoxia inhibits JAK2V617F activation via suppression of SHP-2 function in myeloproliferative neoplasm cells. Exp Hematol. 2014;42:783–92. doi: 10.1016/j.exphem.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Long D, Qianxue W, Zhibiao C, et al. EFEMP2 is upregulated in gliomas and promotes glioma cell proliferation and invasion. Int J Clin Exp Pathol. 2015;8:10385–93. [PMC free article] [PubMed] [Google Scholar]
- 12.Yongxin P, Ling L, Shuyan Z, et al. MicroRNA-126 acts as a tumor suppressor in glioma cells by targeting insulin receptor substrate 1 (IRS-1) Int J Clin Exp Pathol. 2015;8:10345–54. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Ren J, Gao JY, Zhang YH, et al. Decreased expression of SOX9 indicates a better prognosis and inhibits the growth of glioma cells by inducing cell cycle arrest. Int J Clin Exp Pathol. 2015;8:10130–38. [PMC free article] [PubMed] [Google Scholar]
- 14.Ishikawa S, Motegi Y, Yokoyama S, et al. Pathogenesis of multiple lentigines in LEOPARD syndrome with PTPN11 gene mutation. Acta Derm Venereol. 2015;95:978–84. doi: 10.2340/00015555-2123. [DOI] [PubMed] [Google Scholar]
- 15.Zihou X, Shixin D, Hao M, et al. Tyrosine phosphatase SHP-2 mediates C-type lectin receptor-induced activation of the kinase Syk and anti-fungal TH17 responses. Nat Immunol. 2015;16:642–52. doi: 10.1038/ni.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duyster S, Gorantla K, Zirlik A, et al. F604S exchange in FIP1L1-PDGFRA enhances FIP1L1-PDGFRA protein stability via SHP-2 and SRC: A novel mode of kinase inhibitor resistance. Leukemia. 2015;29:1763–70. doi: 10.1038/leu.2015.70. [DOI] [PubMed] [Google Scholar]
- 17.Huang LK, Iwai L, Payne M, et al. Phosphoproteomics of collagen receptor networks reveals SHP-2 phosphorylation downstream of wild-type DDR2 and its lung cancer mutants. Biochem J. 2013;454:501–13. doi: 10.1042/BJ20121750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fülep A, Page C, Fortin H, et al. Downregulation of inhibitory SRC homology 2 domain-containing phosphatase-1 (SHP-1) leads to recovery of T cell responses in elderly. Cell Commun Signal. 2014;12:2. doi: 10.1186/1478-811X-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wardega P, Heldin C, Lennartsson J. Mutation of tyrosine residue 857 in the PDGF β-receptor affects cell proliferation but not migration. Cell Signal. 2010;22:1363–68. doi: 10.1016/j.cellsig.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Ashley Z, Vinicio A, Perez J, et al. Oxido-reductive regulation of vascular remodeling by receptor tyrosine kinase ROS1. J Clin Invest. 2014;124:5159–74. doi: 10.1172/JCI77484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi HJ, Park KJ, Ahn JH, et al. Protein-protein interaction between caveolin-1 and SHP-2 is dependent on the N-SH2 domain of SHP-2. BMB Rep. 2015;48:184–89. doi: 10.5483/BMBRep.2015.48.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gu PF, Cai WJ, Guo HQ, et al. Expression and clinical significance of tyrosine phosphatase SHP-2 in colon cancer. Biomed Pharmacother. 2014;68:285–90. doi: 10.1016/j.biopha.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 23.Wang YX, Zhang J, Soto K, et al. Nuclear receptor SHP, a death receptor that targets mitochondria, induces apoptosis and inhibits tumor growth. Mol Cell Biol. 2010;30:1341–56. doi: 10.1128/MCB.01076-09. [DOI] [PMC free article] [PubMed] [Google Scholar]