Abstract
The function of the hepatitis B virus (HBV) precore or HBeAg is largely unknown because it is not required for viral assembly, infection, or replication. However, the HBeAg does appear to play a role in viral persistence. It has been suggested that the HBeAg may promote HBV chronicity by functioning as an immunoregulatory protein. As a model of chronic HBeAg exposure and to examine the tolerogenic potential of the HBV precore and core (HBcAg) proteins, HBc/HBeAg-transgenic (Tg) mice crossed with T cell receptor (TCR)-Tg mice expressing receptors for the HBc/HBeAgs (i.e., TCR-antigen double-Tg pairs) were produced. This study revealed three phenotypes of HBe/HBcAg-specific T-cell tolerance: (i) profound T-cell tolerance most likely mediated by clonal deletion, (ii) T-cell clonal ignorance, and (iii) nondeletional T-cell tolerance mediated by clonal anergy and dependent on the structure, location, and concentration of the tolerogen. The secreted HBeAg is significantly more efficient than the intracellular HBcAg at eliciting T-cell tolerance. The split T-cell tolerance between the HBeAg and the HBcAg and the clonal heterogeneity of HBc/HBeAg-specific T-cell tolerance may have significant implications for natural HBV infection and especially for precore-negative chronic hepatitis.
The nucleoprotein of the hepatitis B virus (HBV) exists in two structural forms. The nucleocapsid, designated the hepatitis core antigen (HBcAg), is an intracellular 21-kDa protein that self-assembles into particles that encapsidate the viral genome and polymerase and is essential to the function and maturation of the virion. A unique feature of the HBV is the production of a secreted, nonparticulate second form of the nucleoprotein designated the precore or hepatitis B e antigen (HBeAg). The precore and core proteins are translated from 2 distinct RNA species that have different 5′ initiation sites (14). The precore mRNA encodes a hydrophobic signal sequence that directs the precore protein to the endoplasmic reticulum, where it undergoes N- and C-terminal cleavage within the secretory pathway and is secreted as an 18-kDa monomeric protein (26, 38, 42, 53). The HBcAg and HBeAg are distinctly recognized by antibodies (22) but, due to their extensive amino acid homology, are highly cross-reactive at the T-cell level (2, 36, 50). In contrast to the HBcAg, the function of secretory HBeAg in the viral life cycle is unknown inasmuch as it is not required for assembly, infection, or replication (6, 10, 44). It has been proposed that the HBeAg may have an immunoregulatory function in promoting viral persistence. For example, HBeAg-specific Th2 cells may cross-regulate HBcAg-specific Th1 cells or the secreted HBeAg may preferentially behave as a tolerogen and inactivate HBcAg-specific T cells through deletion or clonal anergy in the periphery (35, 36).
In the most successful chronic infections, viral antigens either evade the host immune response or tolerize the host immune system. During chronic viral infections such as HBV it may be useful to consider the viral antigens as neoself-antigens due to their long-term coexistence with the host immune system. Immune tolerance to self- or neoself-antigens can be mediated by a variety of mechanisms. For example, strongly stimulating self-antigens can delete autoreactive T cells during development in the thymus or in peripheral lymphoid tissues (15, 23). Conversely, antigen receptor stimulation in the absence of costimulatory molecules or stimulation with weak antigens can elicit a nondeletional program of anergy (47). T-cell anergy is a tolerance mechanism in which the T cell is functionally inactivated following an initial antigen encounter but remains alive in a hypoactivated state. Two broad categories of T-cell anergy have been defined as clonal anergy, principally a growth arrest state, and adaptive tolerance, or in vivo anergy, which represents a more generalized inhibition of proliferation and effector functions such as cytokine production. Aspects of both types of anergy are found in regulatory T cells (47).
In this report we have compared the tolerogenic potential of the HBeAg and HBcAg in mice transgenic (Tg) for the two proteins and in HBe/HBcAg-Tg mice bred to T-cell receptor (TCR)-Tg mice expressing receptors specific for the HBe/HBcAgs (i.e., TCR-antigen [Ag] double-Tg pairs). This study revealed three phenotypes of HBe/HBcAg-specific T-cell tolerance: (i) profound T-cell tolerance most likely mediated by clonal deletion, (ii) nondeletional T-cell tolerance dependent on the structure, location, and/or concentration of the tolerogen mediated by in vivo anergy, and (iii) T-cell clonal ignorance. Importantly, the HBeAg appears more efficient at eliciting T-cell tolerance than the HBcAg, and this split immune tolerance may have significant implications during a natural HBV infection.
MATERIALS AND METHODS
Mice.
C57BL/10 (B10) (H-2b), H-2 congenic B10.S (H-2s), and B10.S MRL-Faslpr mice were obtained from the breeding colony of the Vaccine Research Institute of San Diego. The HBV-Tg lineages used in this study expressed HBV proteins of the ayw subtype, were produced as described previously, and are listed in Table 1 (16-18, 31, 33). For convenience, we designate the HBeAg-Tgs as HBe(lo) and HBe(hi) and the HBcAg-Tgs as HBc(lo) and HBc(hi) based on protein expression levels. The entire HBV-Tg represents the 1.3.32 lineage that contains a greater-than-genome-length copy of the HBV (ayw) genome and replicates HBV at high levels as described previously (17). The TCR-Tg lineages used in this study are listed in Table 2. The TCR α-chain VJ fragments and β-chain V(D)J fragments were derived from HBeAg-specific T-cell hybridomas, and characterization of Vβ, Vα, Jβ, and Jα usage and the V(D)J junctional region sequence of β chains and VJ junctional regions of α chains from the 2B2, 4E4, and 1E9 T-cell hybridomas have been published previously (8). Modified α-chain VJ fragments were inserted into an Xho-NotI-excised TCR α-chain shuttle vector which contains a rearranged TCR α-chain genomic DNA and the endogenous TCR α enhancer. Modified β-chain V(D)J fragments were inserted into a ClaI-NotI-excised TCR β-chain shuttle vector that contains a rearranged TCR β-chain genomic DNA and the endogenous β enhancer as described previously (8). The shuttle vectors (20) were kindly provided by M. M. Davis, Stanford University. The 15.4-kb α-chain and 19.8-kb β-chain TCR DNA fragments were comicroinjected into fertilized mouse (C57BL/10, B10 or B10.s) embryos. Progeny mice were screened for the presence of the TCR transgenes in peripheral blood lymphocytes by PCR analysis with primers located on the V and the CDR3 region for each TCR transgene. The expression of the TCR transgenes in peripheral blood lymphocytes and lymphoid tissues was confirmed by immunofluorescence and reverse transcription-PCR by using monoclonal antibodies (MAbs) and oligonucleotide primers specific for the transgene TCRs. Note that the 8/12-2 TCR-Tg lineage expresses the Vβ4 transgene only (Table 2). The TCR-Tg lineages were produced in cooperation with The Scripps Research Institute Transgenic Research Facility. All animal care was according to National Institutes of Health Standards as set forth in the Guide for the Care and Use of Laboratory Animals.
TABLE 1.
Summary of HBV-Tg mice used in this study
| Transgene | Promotera | Expression ofb:
|
Reference | |
|---|---|---|---|---|
| HBeAg | HBcAg | |||
| HBeAg | MT | Low (10-20 ng/ml) | − | 31 |
| HBcAg | MT | − | Low (0.25 ng/mg) | 33 |
| HBeAg | MUP | High (4-10 μg/ml) | − | 18 |
| HBcAg | MUP | − | High (1-2 μg/mg) | 16 |
| Entire HBV | HBV | Intermediate (50 ng/ml) | + | 17 |
MT, metallothionein I; MUP, major urinary protein.
+, present; −, undetectable.
TABLE 2.
Summary of HBc/HBeAg-specific TCR-Tg lineages used in this study
| Lineage | Source | T-cell hybridomaa | TCR (Vα/Vβ) | Specificity | TCR+ frequency (splenic CD4+) (%) | Avidity | Toleranceb (dbl-Tgs) |
|---|---|---|---|---|---|---|---|
| 11/4-12 | B10/eAg-Tg | 2B2 | 11.1/4 | 129-140/Ab | 67 | Low | Ignorance |
| 7/16-5 | B10/+ | 4E4 | 5/11 | 129-140/Ab | 53 | Intermediate | Anergy |
| 8/12-2 | B10.S/+ | 1E9 | −/4 | 120-131/As | 37 | High | Deletion (?) |
See reference 8 for details of these hybridomas.
Determined by breeding TCR-Tg mice with HBV-Tg lineages which express either high or low levels of HBcAg or HBeAg.
Recombinant proteins and synthetic peptides.
Recombinant HBcAg of the ayw subtype was produced in Escherichia coli and purified as described previously (45). A recombinant HBeAg corresponding in sequence to serum-derived HBeAg encompassing the 10 precore amino acids remaining after cleavage of the precursor and residues 1 to 149 of HBcAg was produced as described previously (46). The presence of the 10 precore amino acids prevents particle assembly, and HBeAg is recognized efficiently by HBeAg-specific MAbs but displays little HBc antigenicity. The following HBcAg-derived synthetic peptides representing Th cell recognition sites were used and designated by amino acid position from the N terminus of HBcAg ayw: 120 to 131 (IAs), VSFGVWIRTPPA; 129 to 140 (IAb), PPAYRPPNAPIL; and 120 to 140, a 21-mer comprising both T-cell sites. Peptides were synthesized by the simultaneous peptide synthesis method as previously described (43).
Serology.
HBeAg was measured in diluted Tg mouse sera by a commercial enzyme-linked immunosorbent assay (ELISA), and recombinant (rHBeAg) was used as a standard. Anti-HBc and anti-HBe immunoglobulin G (IgG) antibodies were measured in murine sera by an indirect solid-phase ELISA by using rHBcAg or rHBeAg as the solid-phase ligand as described previously (29). Serial dilutions of both test sera and preimmunization sera were made, and the data are expressed as antibody titers representing the reciprocals of the highest dilutions of sera required to yield an optical density at 492 nm (OD492) three times an equal dilution of preimmunization sera. IgG isotype-specific ELISAs were performed with IgG1-, IgG2a-, IgG2b-, and IgG3-specific secondary antibodies (Southern Biotechnology, Birmingham, Ala.).
In vitro cytokine analysis.
Spleen cells from either unprimed or primed TCR-Tg, TCR-Ag double- or triple-Tg, or wild-type mice were cultured (5 × 106/ml) with various concentrations of a series of antigens. Culture supernatants were harvested at 48 h for interleukin-2 (IL-2) determination and at 96 h for gamma interferon (IFN-γ) determinations. Cytokines were measured by two-site ELISA with pairs of cytokine-specific MAbs. One unlabeled MAb was adsorbed to the microtiter plate well and used as a capture antibody, and the other labeled MAb served as the probe. To determine T-cell proliferation, TCR-Tg spleen T cells were labeled in vitro with the intracellular dye carboxy-fluorescein diacetate succinimidyl ester (CFSE) by using the Vybrant CFSE SE tracer kit (Molecular Probes, Eugene, Oreg.), CFSE (0.5 mM) was added to the cell suspensions, and the mixture was incubated for 10 min at 37°C. The labeling reaction was stopped by repetitive washing with ice-cold RPMI medium-10% fetal calf serum. Labeled cells (5 × 106/ml) were cultured with various concentrations of antigen for 4 or 7 days. Cultured cells were harvested, washed, and labeled with anti-TCR Vβ11 antibodies. The number of cell divisions are determined by dilution of the intracellular dye CFSE in TCR Vβ11+ gated T cells by flow cytometry.
Flow cytometry.
Single-cell suspensions of thymus or spleen were prepared. Before staining, cells were incubated with an anti-Fc MAb (2.4G2) to block nonspecific Fc receptor uptake. For staining with directly labeled antibodies, 106 cells were incubated with antibodies at 4°C for 15 min. Cells were washed three times and analyzed in a FACScan (Becton Dickinson). Gates were set only on viable cells, and usually >104 cells were analyzed with LYSIS II (Becton Dickinson). The murine antibodies used for two- and three-color staining were as follows: anti-TCR Vβ4 (KT4), anti-TCR Vβ11 (RR3-15), anti-TCR Vα11 (RR8-1), anti-CD4 (H129.19), and anti-CD8a (53-6.7) (BD Bioscience, Palo Alto, Calif.).
Liver injury model.
7/16-5 TCR single-Tg, TCR × HBc/HBe double-Tg, or TCR × HBc × HBe triple-Tg mice were injected with the 7/16-5 TCR-specific peptide 129 to 140 (50 μg) emulsified in complete Freund's adjuvant (CFA). At multiple time points, the extent of hepatocellular injury was monitored by measuring serum alanine aminotransferase (sALT) activity. sALT activity was measured in a Kodak Etachem DTSC II chemistry analyzer (Ortho Clinical Diagnostics, Raritan, N.J.). Normal sALT levels in mice in our colony range from 20 to 40 U/liter; therefore, we designated sALT levels greater than 100 U/liter elevated.
Adoptive transfer.
Donor spleen cells (30 × 106) derived from 7/16-5 TCR-Tg mice were injected intravenously into CD4/CD8-depleted HBcAg or HBeAg single-Tg recipient mice. The spleen cell inocula contained ∼3 × 106 TCR+ CD4+ T cells. Whole-spleen inocula were used for convenience and yielded similar results to transferred, purified CD4+-T-cell populations.
RESULTS
Differing phenotypes of HBc/HBeAg-specific T-cell tolerance.
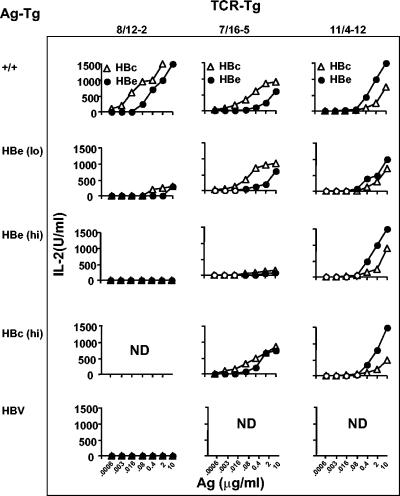
Three TCR-Tg lineages (Table 2) were selected that represent three phenotypes of antigen responsiveness but more importantly of tolerance induction by the HBc/HBeAgs in TCR/Ag double-Tg pairs. Naive splenic T cells from 8/12-2 single TCR-Tg mice are efficiently activated by culture with relatively low concentrations of the HBc/HBeAgs as measured by production of IL-2 (Fig. 1, top row), IFN-γ, and proliferation (data not shown). This is especially noteworthy because the frequency of HBc/HBeAg-specific T cells is quite low (<5%) among Vβ4+ cells due to pairing with endogenous Vα chains in this Vβ4+-only TCR-Tg lineage. The estimate of <5% is based on the percentage of CFSE-labeled, 8/12-2 Vβ4+ T cells that undergo cell division in the presence of the HBcAg (data not shown). Naive splenic T cells of 7/16-5 single TCR-Tg mice demonstrate intermediate HBc/HBeAg-specific IL-2 production in terms of levels of IL-2 produced and minimum antigen dose required compared to the 8/12-2 TCR-Tg lineage. We have previously classified 11/4-12 TCR-Tg T cells as low avidity, consistent with the right-shifted dose-response curve (8). It is also noteworthy that 11/4-12 TCR-Tg splenic T cells preferentially recognize the HBeAg compared to the particulate HBcAg, which is a very unusual pattern (Fig. 1, top row).
FIG. 1.
Summary of HBc/HBeAg-specific T-cell activation (IL-2 production) in TCR single-Tg and TCR × HBc or HBe double-Tg mice reveals the heterogeneity of HBeAg-specific T-cell tolerance. Unprimed, naive spleen cells from three TCR-Tg lineages (row 1) were cultured for 48 h with various concentrations of rHBcAg (HBc) and rHBeAg (HBe), and T-cell activation was determined by measuring IL-2 production. Rows 2 to 5 represent in vitro IL-2 production of naive T cells derived from TCR × Ag double-Tg pairs. The TCR-Tg (top) and the Ag-Tg (left side) lineages that comprise the double-Tg mice are depicted. The graphs represent the T-cell responses of single mice but are representative of the results from a minimum of six animals. ND, not determined; +/+, wild type.
The three TCR-Tg lineages representing relatively high, intermediate, and low avidity for the HBc/HBeAgs were bred with antigen-Tg mice (Table 1) expressing either high (hi) or relatively low (lo) levels of the HBc/HBeAgs. As shown in Fig. 1 (second row), IL-2 production by naive splenic T cells of 8/12-2 × HBe(lo) double-Tg mice is significantly reduced (≤300 U/ml) compared to 8/12-2 single TCR-Tg mice at all in vitro HBc/HBeAg concentrations. Predictably, naive splenic T cells of 8/12-2 TCR-Tg mice bred with high-serum HBeAg expressers (4 to 10 μg/ml) (Fig. 1, third row) produced absolutely no IL-2 upon culture with the HBc/HBeAgs. An intermediate HBeAg serum concentration of 50 ng/ml, as present in HBV-Tg mice, also eliminated in vitro IL-2 production in 8/12-2 × HBV double-Tg mice (Fig. 1, row 5). This result indicated the tolerogenic potential of serum HBeAg in the context of the other viral proteins and in an in vivo model of HBV replication.
A completely different phenotype is represented by 11/4-12 × HBc/HBeAg double-Tg mice. Regardless of the level of HBc/HBeAg expression in these double-Tg mice, 11/4-12 TCR-Tg T cells were unaffected and produced equivalent levels of HBc/HBeAg-specific IL-2 in vitro, as did single TCR-Tg 11/4-12 T cells (Fig. 1, column 3). We have previously reported that 11/4-12 TCR-Tg T cells are not tolerized in HBe(lo) double-Tg mice (8); however, the observation that 11/4-12 TCR-Tg T cells also ignore the HBc/HBeAgs in high-expresser double-Tg mice is interesting because the serum concentration of HBeAg in the high-expresser Tg mouse (4 to 10 μg/ml) is 10-fold higher than the minimal HBeAg concentration required to activate the 11/4-12 T cells in vitro (0.4 μg/ml).
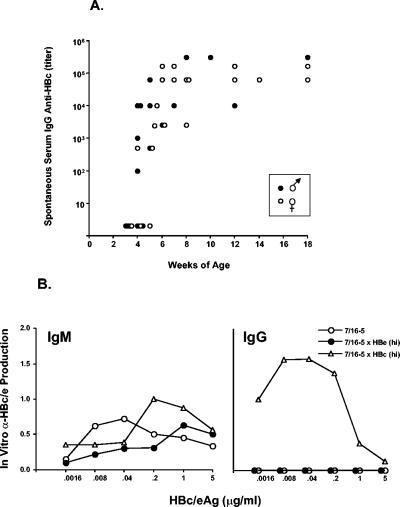
A more complex phenotype is illustrated by 7/16-5 TCR-Tg T cells. The 7/16-5 T cells appear to ignore low in vivo concentrations of HBeAg (and HBcAg) (data not shown) in terms of in vitro IL-2 production by 7/16-5 × HBe(lo) double-Tg T cells, which is equivalent to IL-2 production in single 7/16-5 TCR-Tg T cells. However, at the higher in vivo HBeAg concentration present in 7/16-5 × HBe(hi) double-Tg mice, naive T cell HBc/HBeAg-specific in vitro IL-2 production is significantly reduced compared to 7/16-5 single TCR-Tg mice. It is notable that the tolerance exhibited in 7/16-5 × HBe(hi) double-Tg mice is not due to clonal deletion of 7/16-5 TCR-Tg T cells either in the thymus or in the spleen, as determined by fluorescence-activated cell sorter (FACS) analysis (data not shown). It is also of interest that 7/16-5 TCR-Tg T cells are not significantly tolerized in 7/16-5 × HBc(hi) double-Tg mice, as demonstrated by relatively efficient HBc/HBeAg-specific in vitro IL-2 production. In fact, 7/16-5 × HBc(hi) double-Tg mice undergo spontaneous anti-HBc seroconversion between 4 and 6 weeks of age and remain anti-HBc antibody positive (Fig. 2A). Male 7/16-5 × HBc(hi) double-Tg mice spontaneously seroconvert to anti-HBc positivity somewhat earlier than females, which is consistent with higher HBcAg expression in the livers of male mice (data not shown). In contrast, 7/16-5 × HBe(hi) double-Tg mice do not spontaneously produce anti-HBe antibody in vivo. Whereas spleen cells of all 7/16-5 TCR-Tg mice produce IgM anti-HBc antibody in vitro upon culture with the HBcAg, only 7/16-5 × HBc(hi) double-Tg spleen cells produce IgG anti-HBc antibody in vitro (Fig. 2B). The lack of IgG anti-HBe antibody production in vitro by 7/16-5 × HBe(hi) double-Tg spleen cells indicates that the failure to detect anti-HBe in vivo was not due to masking or immune complexing with excess serum HBeAg. Therefore, in vivo tolerance induction of 7/16-5 TCR-Tg T cells in double-Tg mice is nondeletional, HBeAg concentration dependent, and preferentially induced in HBeAg double-Tg but not HBcAg double-Tg mice (i.e., T-cell tolerance is split between the HBeAg and the HBcAg).
FIG. 2.
Spontaneous anti-HBc seroconversion in 7/16-5 × HBc(hi) double-Tg mice (A) and comparative in vitro anti-HBc IgM and IgG antibody production in 7/16-5 TCR single-Tg and double-Tg mice (B). Individual double-Tg 7/16-5 × HBc(hi) female and male mice were bled beginning at 3 weeks of age and at intervals thereafter to determine the onset of anti-HBc IgG seroconversion and the persistence of anti-HBc antibodies (A). Serum IgG anti-HBc antibody titer (1/dilution) was determined by ELISA. Single HBc(hi)-Tg or single 7/16-5 TCR-Tg mice did not spontaneously produce anti-HBc antibody. (B) Unprimed naive spleen cells (5 × 105/ml) from adult mice of the indicated 7/16-5 TCR single-Tg and 7/16-5 × HBe(hi) or 7/16-5 × HBc(hi) double-Tg strains were cultured with various concentrations of the rHBc/HBeAgs for 5 days. The 5-day supernatants were collected and assayed for IgM and IgG anti-HBc or anti-HBe antibodies by ELISA. The level of in vitro antibody production is expressed as an OD492 reading of undiluted supernatant in the antigen-specific ELISA. This experiment was performed on at least three separate occasions and is representative.
Mechanisms of tolerance in double-Tg mice. (i) 8/12-2 × HBeAg double-Tg mice.
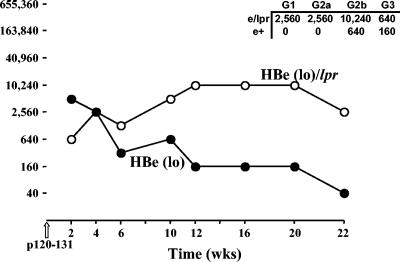
The fact that 8/12-2 TCR-Tg T cells are of relatively high avidity and are very sensitive to tolerance induction by even low concentrations of serum HBeAg predicts that clonal deletion is a likely mechanism. However, because the frequency of Vβ4+ TCR-Tg T cells specific for the HBc/HBeAgs is so low (<5%) in this Vβ-only TCR-Tg lineage, it is not possible to obtain conclusive evidence of deletion by FACS analysis. Although a decrease in Vβ4+ T-cell frequency is observed in the thymuses and spleens of 8/12-2 × HBe(lo) double-Tg mice compared to 8/12-2 single TCR-Tg mice, the differences are inconclusive. Low serum HBeAg concentrations are sufficient to elicit fatty acid synthase (FAS)-mediated apoptosis in the periphery as shown by comparing B10.S HBe(lo)-Tg mice with FAS-deficient (lpr/lpr) HBe(lo)-Tg mice in terms of T-cell peptide 120 to 131-induced anti-HBe autoantibody production (Fig. 3). The HBc/HBeAg 120 to 131 sequence is the recognition site for 8/12-2 TCR-Tg T cells and also the predominant T-cell recognition site in wild-type B10.S (H-2s) mice. For example, neonatal tolerance induction with the 120 to 131 peptide renders B10.S mice nonresponsive to the entire HBc/HBeAg at the T-cell level (30). Although B10.S/HBe(lo)-Tg mice are highly tolerant to the HBeAg, injection with p120-131 elicits low levels and transient anti-HBe IgG antibody production of primarily the IgG2b isotype. This indicates that at least low-level residual T helper cell function persists in B10.S/HBe(lo)-Tg mice, perhaps reflecting T cells that avoided deletion in the thymus (Fig. 3). B10.S HBe(lo)-Tg mice on a homozygous lpr background (e/lpr) produce significantly more anti-HBe antibody with a wider IgG isotype distribution, indicating that FAS-mediated apoptosis in the periphery may at least contribute to maintaining T-cell tolerance to the HBeAg in the B10.S strain. We suspect that both thymic (central) and peripheral deletion mechanisms play a role in HBeAg-specific tolerance among high-avidity T cells, as illustrated by the 8/12-2 TCR-Tg lineage and polyclonal HBeAg-specific T cells in B10.S HBeAg-Tg mice.
FIG. 3.
Comparative anti-HBe antibody production in B10.S HBe(lo) versus HBe(lo)/lpr single-Tg mice. Groups of five B10.S HBe(lo)-Tg or HBe(lo)/lpr-Tg mice were injected with the synthetic T-cell site p120-131 (50 μg) (incomplete Freund adjuvant). Mice were bleed at 2-week intervals, sera were pooled from five mice per group, and anti-HBe IgG antibody endpoint titers were determined by ELISA. Titers are expressed as the reciprocal of the highest dilution of sera to yield an OD492 value three times that of pre-p120-131 injection sera. wks, weeks.
(ii) 11/4-12 × HBc/HBeAg double-Tg mice.
We have previously shown that 11/4-12 TCR-Tg T cells are not deleted in the thymus or spleen of double-Tg mice expressing low levels of the HBc/HBeAgs (8). Further, FACS analysis of high-expresser HBc/HBeAg × 11/4-12 double-Tg mice also revealed no clonal deletion in the thymus or the spleen (data not shown). Furthermore, the function of 11/4-12 TCR-Tg T cells in terms of in vitro cytokine production is not altered by the coexpression of high levels of the HBc/HBeAgs in double-Tg mice. Therefore, low-avidity 11/4-12 TCR-Tg T cells are neither tolerized nor activated by coexpression of even high levels of the HBc/HBeAgs and coexist with these viral antigens in vivo by virtue of clonal ignorance.
(iii) 7/16-5 × HBc/HBeAg double-Tg mice.
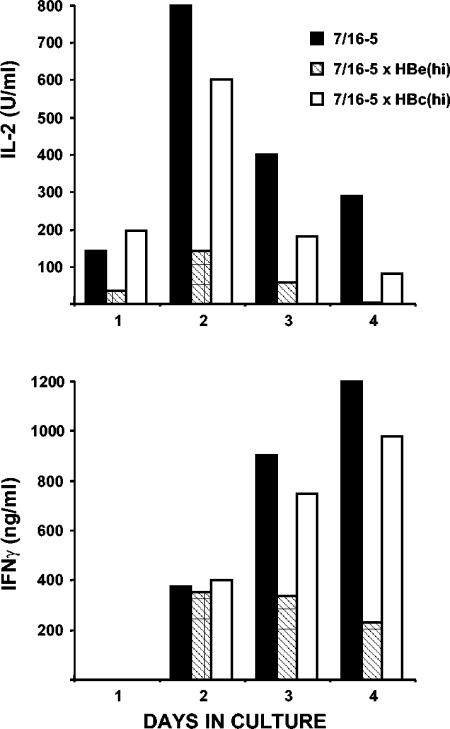
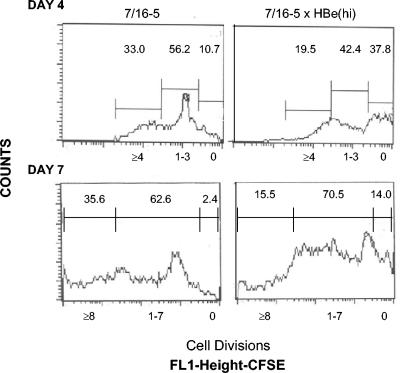
FACS analysis of 7/16-5 × HBc and 7/16-5 × HBe double-Tg mice expressing low and even high levels of the HBc/HBeAgs revealed no clonal deletion in the thymus or the spleen of 7/16-5 TCR-Tg T cells (data not shown). However, functional analysis of 7/16-5 T cells derived from HBc/HBeAg-expressing double-Tg mice revealed apparent clonal ignorance in low-HBc/HBeAg-expressing environments, whereas high serum concentrations of HBeAg in vivo significantly altered 7/16-5 T-cell function (Fig. 1). The tolerogenic effects of secreted HBeAg observed in 7/16-5 × HBe(hi) double-Tg mice were not observed for the HBcAg in 7/16-5 × HBc(hi) double-Tg mice. For example, comparison of the kinetics of HBc/HBeAg-specific in vitro IL-2 and IFN-γ production by naive splenic T cells revealed low-level and transient IL-2 and IFN-γ production by 7/16-5 T cells derived from 7/16-5 × HBe(hi) double-Tg mice compared to 7/16-5 single TCR-Tg and 7/16-5 × HBc(hi) double-Tg mice (Fig. 4). Note that IL-2 production peaked in all cultures at day 2; however, it was significantly lower at day 2 in the 7/16-5 × HBe(hi) T-cell culture and was undetectable by day 4. Similarly, IFN-γ levels were rising in the 7/16-5 and 7/16-5 × HBc(hi) T-cell cultures at day 4 but were declining in the 7/16-5 × HBe(hi) T-cell culture. The in vitro proliferative capacities of 7/16-5 T cells derived from 7/16-5 single TCR-Tg mice and 7/16-5 × HBe(hi) double-Tg mice were also compared. Naive splenic T cells were labeled with the intracellular fluorescent dye CFSE and cultured with p120-140 (0.5 μg/ml) for 4 or 7 days, and the harvested Vβ11+ T cells were analyzed by FACS (Fig. 5). Note that 7/16-5 TCR-Tg T cells derived from 7/16-5 × HBe(hi) double-Tg mice were able to proliferate (as demonstrated by dilution of the CFSE dye) upon p120-140 peptide stimulation, which indicates an absence of a state of profound or absolute anergy in these T cells. Rather, the 7/16-5, Vβ11+ T cells derived from 7/16-5 × HBe(hi) double-Tg mice were substantially slower to enter cell division than 7/16-5 single TCR-Tg T cells after 4 days in culture (i.e., 37.8% nondivided cells compared to 10.7%, respectively). Furthermore, after 7 days in culture, 14.0% of 7/16-5 × HBe(hi)-derived Vβ11+ T cells failed to divide compared to only 2.4% of 7/16-5-derived T cells. Similarly, T cells derived from 7/16-5 × HBe(hi) double-Tg mice undergo fewer total cell divisions than single TCR-Tg T cells (compare the ≥4 and ≥8 cell division windows at days 4 and 7, respectively) (Fig. 5). Therefore, 7/16-5 TCR-Tg T cells are not deleted but are rendered anergic in the presence of high serum levels of HBeAg, as determined by reduced proliferation and cytokine production upon antigen-specific recall in vitro. Two broad categories of T-cell anergy designated either clonal anergy or adaptive tolerance (in vivo anergy) have been described previously (47). The 7/16-5 TCR-Tg T cells in HBe(hi) double-Tg mice appear to exemplify adaptive tolerance because IFN-γ, as well as IL-2, production is inhibited (Fig. 4) and not reversed by exogenous IL-2 (data not shown) and antigen persistence is required to maintain the anergic state in vivo (9). In adaptive tolerance model systems, a phase of clonal activation and expansion often occurs and the surviving mature T cells are anergic to further activation (47). The low-level cytokine production and lower frequency of proliferating cells observed in T cells derived from 7/16-5 × HBe(hi) double-Tg mice (Fig. 4 and 5) may be due to recent thymic emigrant T cells in the activation phase, and the mature surviving T cells may actually be profoundly anergic. To confirm this hypothesis, adult thymectomy experiments will be necessary.
FIG. 4.
Comparative kinetics of antigen-specific in vitro IL-2 and IFN-γ production by unprimed splenic T cells derived from 7/16-5 TCR single-Tg and 7/16-5 × HBe(hi) or 7/16-5 × HBc(hi) double-Tg mice. Unprimed spleen cells (5 × 105/ml) from the indicated single- or double-Tg mice were cultured with the HBc/HBeAg-specific T-cell peptide p120-140 (0.5 μg/ml) for 4 days. Each day, culture supernatant was collected and analyzed for IL-2 or IFN-γ by ELISA. The data are representative of experiments performed a minimum of three times.
FIG. 5.
Comparative proliferative responses of 7/16-5 T cells derived from TCR single-Tg and 7/16-5 × HBe(hi) double-Tg mice. Unprimed spleen cells from 7/16-5 single-Tg or 7/16-5 × HBe(hi) double-Tg mice were labeled with the intracellular dye CFSE (0.5 mM) and cultured with 0.5 μg of the HBc/HBeAg-specific p120-140 for 4 or 7 days. Cultured cells were harvested at 4 or 7 days and labeled with anti-TCR Vβ11 antibodies. The number of cell divisions is measured by the dilution of the CFSE dye, which is reduced by 50% at each cell division, in TCR Vβ11+-gated T cells by flow cytometry. The numbers above the windows represent the percentage of Vβ11+ T cells that have undergone the indicated number of cell divisions. The data are representative of the results from two separate experiments.
Implications of HBeAg-specific T-cell tolerance for natural HBV infection. (i) Secreted HBeAg is tolerogenic for a polyclonal T-cell repertoire.
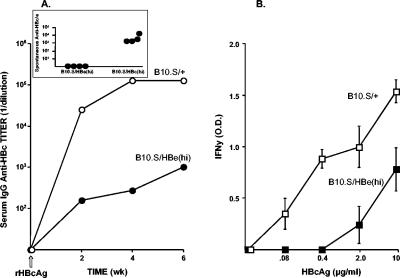
The 8/12-2 TCR-Tg lineage, which is highly sensitive to HBeAg-induced tolerance, was derived from a T-cell hybridoma produced in the B10.S strain. It was of interest to determine whether the tolerogenic potential of the HBeAg is relevant to the polyclonal HBeAg-specific T-cell repertoire in B10.S mice. For this purpose B10.S/+ wild-type and HBe(hi)-Tg [B10.S/HBe(hi)] mice were immunized with rHBcAg. The HBcAg was used as an immunogen because serum HBeAg present in B10.S/HBe(hi)-Tg mice will not mask or immune complex with anti-HBc antibody to prevent its detection because the two antigens are not cross-reactive at the B-cell level. Further, it was of interest to determine whether potential tolerance to the HBeAg could affect the immune response to the HBcAg, since the two antigens are cross-reactive at the T-cell level. As shown in Fig. 6A, IgG anti-HBc antibody production is profoundly reduced in B10.S/HBe(hi)-Tg mice compared to B10.S/+ mice after HBcAg immunization. Furthermore, the T-cell response to HBcAg, as measured by splenic IFN-γ production recalled by the HBcAg in vitro, was severely reduced in B10.S/HBe(hi)-Tg mice compared to B10.S/+ mice immunized with the HBcAg (Fig. 6B). This indicates that the polyclonal HBc/HBeAg-specific T-cell repertoire in B10.S mice behaves similarly to the 8/12-2 TCR-Tg lineage in terms of its sensitivity to tolerance induction by circulating HBeAg and that tolerance elicited by the HBeAg affects the HBcAg-specific immune response. This is relevant because both forms of the antigen are present during chronic HBV infection and tolerance split between the HBeAg and the HBcAg is likely. For example, B10.S/HBe(hi)-Tg mice do not spontaneously produce anti-HBe in vivo and B10.S/HBc(hi)-Tg mice do spontaneously seroconvert to anti-HBc (Fig. 6A, inset), suggesting that the HBcAg is less tolerogenic in this polyclonal T-cell model, as previously indicated in TCR × HBc double-Tg mice (Fig. 2).
FIG. 6.
Effect of serum HBeAg on the humoral and T-cell response to immunization with rHBcAg. (A) Groups of three adult B10.S wild-type (B10.S/+) and B10.S HBe(hi)-Tg mice were immunized with rHBcAg (10 μg) (incomplete Freund adjuvant), and IgG anti-HBc antibody was measured in pooled sera by ELISA at 2-week intervals. Anti-HBc antibody is expressed as an endpoint titer (1/dilution). (B) Spleen cells from the mice depicted in Fig. 6A were harvested 6 weeks after rHBcAg immunization and cultured (5 × 106/ml) with various concentrations of rHBcAg for 4 days. Supernatants were collected and analyzed for IFN-γ. Comparative IFN-γ levels were expressed as an OD492 reading of the undiluted supernatants in the IFN-γ-specific ELISA. Data represent means ± standard deviations of the results for 3 mice per group. The experiment shown is representative of three performed. wk, week.
(ii) Perinatal exposure to HBeAg is tolerogenic.
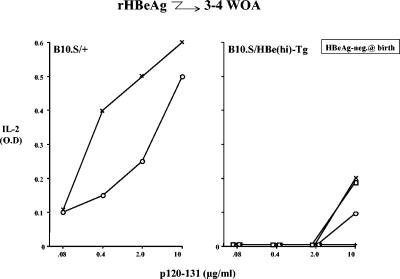
A number of studies indicate that the HBeAg, either free or complexed to immunoglobulin, can cross the human placenta (1, 21, 24, 51, 54). Previous studies suggested that in utero exposure to HBeAg through placental transfer may result in T-cell tolerance and explain the greater than 90% chronicity rates of infants born to HBV-infected, HBeAg-positive mothers (31). Because the ability of the HBeAg to cross the murine placenta has been questioned (40), we performed an experiment to determine whether early postnatal exposure to the HBeAg may also be tolerogenic. For this purpose, B10.S/HBe(hi)-Tg mice were used because the developmentally regulated major urinary protein promoter is not active until after birth and B10.S/HBe(hi)-Tg mice are HBeAg negative at birth (18; data not shown) but serum HBeAg becomes detectable by postnatal day 7 and reaches a stable plateau by 4 weeks of age (18). Therefore, young (3 to 4 weeks of age) B10.S/+ and B10.S/HBe(hi)-Tg mice were immunized with rHBeAg, and splenic IL-2 production recalled by the dominant HBeAg-specific T-cell site (p120-131) in H-2s mice was measured. As shown in Fig. 7, B10.S/HBe(hi)-Tg mice, although negative for HBeAg at birth, were nonetheless tolerant after immunization with rHBeAg at 3 to 4 weeks of age in terms of in vitro, antigen-specific, T-cell IL-2 production. Therefore, exposure to serum HBeAg after birth is capable of inducing T-cell tolerance during the perinatal period. This has implications for perinatal HBV infection and indicates that tolerance to HBeAg is not only acquired in utero but can occur postnatally as well.
FIG. 7.
Perinatal exposure to the HBeAg is tolerogenic. Young (3 to 4 weeks of age [WOA]) B10.S wild-type (B10.S/+) or B10.S HBe(hi)-Tg mice, which were HBeAg negative at birth, were immunized with rHBeAg (10 μg) (incomplete Freund adjuvant). Ten days later, spleen cells were harvested and cultured with various concentrations of the dominant HBc/HBeAg-specific T-cell site in B10.S mice, p120-131. After 2 days of spleen culture, supernatants were collected and analyzed for IL-2 by ELISA. Comparative IL-2 levels were expressed as OD492 readings of undiluted supernatants in the IL-2-specific ELISA. The symbols represent individual mice. This experiment was performed on two occasions, and the results are representative.
(iii) Immunomodulatory role for HBeAg.
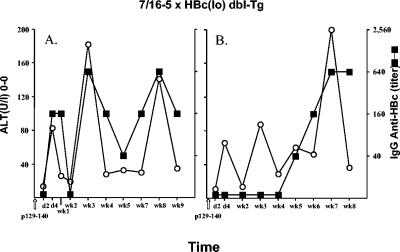
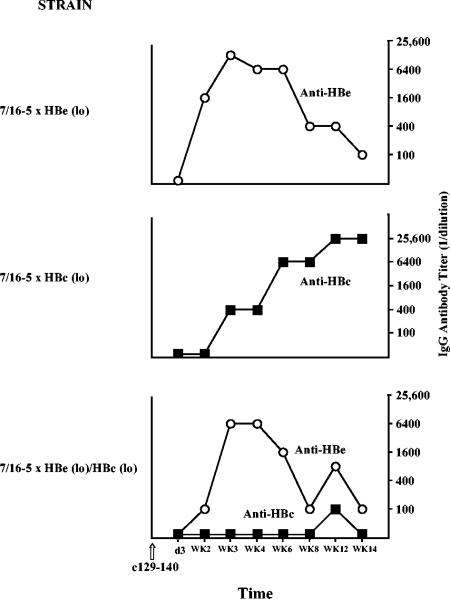
Because 7/16-5 × HBc(lo) or 7/16-5 × HBe(lo) double-Tg mice do not appear to be tolerant to the HBc/HBeAgs (Fig. 1), these mice provide a model of T cells of intermediate avidity coexisting with at least low-level expression of their specific antigen. These models provide an opportunity to determine the consequences of activating these apparently quiescent T cells in vivo by injecting the TCR-specific p129-140 peptide. Injection of the TCR-specific peptide activates HBc/HBeAg-specific T cells but not B cells. Anti-HBc/HBeAg-specific antibody production is dependent on B-cell recognition of the endogenous transgene-encoded HBc/HBeAgs. A single dose of p129-140 activates TCR+ T cells in 7/16-5 × HBc(lo) double-Tg mice and results in episodes of sporadic chronic liver injury (sALT elevations) of relatively low magnitude (Fig. 8). Single 7/16-5 TCR-Tg or single HBc(lo)-Tg mice injected with p129-140 do not demonstrate liver injury (data not shown). Note that anti-HBc antibody production correlates with periods of liver injury most likely due to the release of intracellular HBcAg by injured hepatocytes. A similar injection of p129-140 into 7/16-5 × HBe(lo) double-Tg mice results in anti-HBe seroconversion (Fig. 9) but no or only limited liver injury (Fig. 10). Also note that the anti-HBe antibody elicited by p129-140 injection in 7/16-5 × HBe(lo) double-Tg mice is transient, whereas the anti-HBc antibody elicited by p129-140 injection in 7/16-5 × HBc(lo) double-Tg mice is long lived (Fig. 9), suggesting an immunoregulatory function for the HBeAg.
FIG. 8.
Induction of low-level liver injury in 7/16-5 × HBc(lo) double-Tg mice. Two 7/16-5 × HBc(lo) double-Tg mice (A and B) were injected with the 7/16-5 TCR-specific peptide 129-140 (100 μg) (CFA), and at multiple intervals, sera were collected and analyzed for sALT and IgG anti-HBc antibodies. No liver injury occurred in 7/16-5 TCR single-Tg or HBcAg single-Tg mice injected with p129-140 (data not shown). d, day; WK, week.
FIG. 9.
Induced seroconversion in 7/16-5 TCR × HBc and HBeAg double-Tg and triple-Tg mice. Groups of three mice each of the indicated double- and triple-Tg strains were injected with the 7/16-5 TCR-specific peptide 129-140 (100 μg) (CFA), and sera were collected at multiple intervals, pooled, and analyzed for IgG anti-HBe and IgG anti-HBc antibodies by ELISA. Antibody titer is defined as the endpoint serum dilution to yield three times the OD492 value of equally diluted preimmunization sera. The results are representative of experiments performed on two separate occasions. d, day; WK, week.
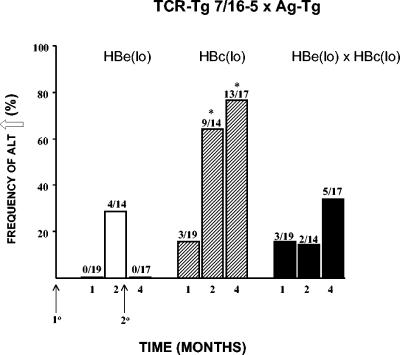
FIG. 10.
Liver injury induced in 7/16-5 × HBc(lo) double-Tg mice is moderated by serum HBeAg. Groups of six 7/16-5 × HBe(lo) double-Tg, 7/16-5 × HBc(lo) double-Tg, and 7/16-5 × HBe(lo) × HBc(lo) triple-Tg mice were injected at time zero with p129-140 (100 μg) (CFA) and again at 2 months (50 μg) (incomplete Freund adjuvant). At multiple intervals, 2 to 3 mice from each group were bled and individual sera were analyzed for ALT elevation. sALT was considered elevated above 100 U/liter. Frequencies of sALT elevations in each group during months 1, 2, and 4 are shown. The numbers above the bars depict the absolute number of sera demonstrating heightened ALT values per total number of sera tested in a given time frame. *, P < 0.01 versus 7/16-5 × HBe(lo) and 7/16-5 × HBe(lo) × HBc(lo) groups (unpaired t test).
Interestingly, p129-140 injection in 7/16-5 × HBc(lo) × HBe(lo) triple-Tg mice elicited anti-HBe antibodies but not anti-HBc antibodies even though anti-HBc was produced in 7/16-5 × HBc(lo) double-Tg mice (Fig. 9). This suggests that the presence of HBeAg in the serum can down-regulate antibody production to another viral protein, the HBcAg. Because the HBeAg and HBcAgs are cross-reactive at the level of T-cell recognition, the regulation by serum HBeAg is most likely mediated at the T-cell level. This was confirmed by examining liver injury in double- and triple-Tg mice (Fig. 10). Injection of 2 doses of p129-140 resulted in a relatively high frequency of ALT elevations in a group of six 7/16-5 × HBc(lo) double-Tg mice and a low frequency of liver injury in a group of six 7/16-5 × HBe(lo) double-Tg mice. Because of the sporadic and unpredictable nature of liver injury and the fact that each mouse could not be bled each week, 2 to 3 mice per group were bled on alternate weeks and the data are presented as the frequency of sALT elevations (ALT ≥ 100 U/liter) observed in the combined bleeds in each group during months 1, 2, and 4. As shown in Fig. 10, p129-140 injection in 7/16-5 × HBc(lo) × HBe(lo) triple-Tg mice resulted in a relatively low frequency of sALT elevations, indicating that p129-140-induced liver injury was inhibited by the presence of HBeAg in the serum. These data suggest that the HBeAg may serve an immunomodulatory role in natural HBV infection and that secreted HBeAg can down-modulate the immune response to the HBcAg. Because the HBcAg-specific T-cell response is predominantly Th1-like (34), inhibition of this response may cause predisposition to chronicity.
(iv) T-cell tolerance split between HBeAg and HBcAg.
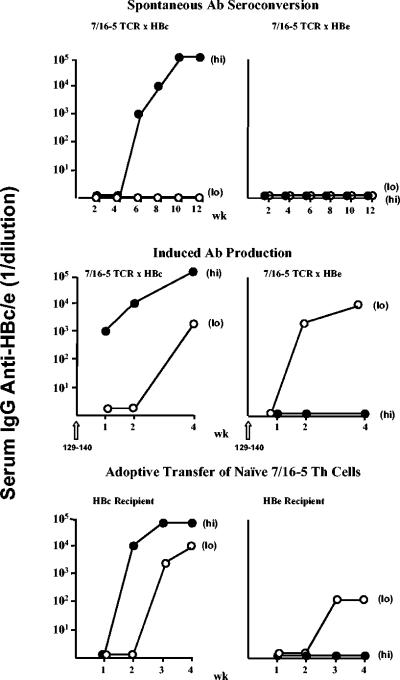
A number of experiments described herein suggest that secreted HBeAg is a superior T-cell tolerogen compared to the intracellular HBcAg, even though the two forms of the antigen are cross-reactive at the T-cell level. T-cell tolerance split between the HBc/HBeAgs is best exemplified in studies of 7/16-5 × HBc or HBe double-Tg mice expressing high and low levels of the HBc/HBeAgs. Figure 11 represents a summary of studies comparing 7/16-5 × HBc and 7/16-5 × HBe double-Tg mice in terms of spontaneous anti-HBc/HBe seroconversion, induced anti-HBc/HBe antibody production in vivo, and the ability of naive 7/16-5 TCR-Tg T cells to elicit antibody production after adoptive transfer into HBcAg- or HBeAg-Tg recipients. As shown in Fig. 2A, 7/16-5 × HBc(hi) double-Tg mice spontaneously seroconvert to anti-HBc positivity between 4 and 6 weeks of age and 7/16-5 TCR+ double-Tg mice expressing a lower level of HBcAg do not. Therefore, the low HBcAg level appears limiting for immunogenicity, and the high level of HBcAg is immunogenic rather than tolerogenic. In contrast, 7/16-5 TCR+ double-Tg mice expressing either high or low levels of the secreted HBeAg do not spontaneously produce anti-HBe (Fig. 11, top panel). Induced anti-HBc/HBe antibody production in TCR/Ag double-Tg mice was examined by injecting the 7/16-5 TCR-specific peptide p129-140. High- and low-HBcAg-expressing double-Tg mice could be induced to produce anti-HBc and high HBcAg expression correlated with higher titer. In contrast, only low expressers of the HBeAg could be induced to produce anti-HBe antibody, consistent with the concentration dependence of T-cell tolerance to the secreted HBeAg (Fig. 11, middle panel). The ability to induce anti-HBe antibodies by p129-140 injection in 7/16-5 × HBe(lo) double-Tg mice indicates that even this low concentration of serum HBeAg (10 to 20 ng/ml) is not limiting as an immunogen and further indicates that the lack of anti-HBe production at the higher level of HBeAg is due to tolerance mechanisms as opposed to limiting amounts of antigen. Lastly, adoptive transfer of naive 7/16-5 TCR-Tg spleen cells into HBcAg-Tg recipients elicits efficient anti-HBc antibody production in high-HBcAg-expressing recipients and lower levels of anti-HBc antibody in low-HBcAg-expressing recipients. In contrast, adoptive transfer of the same number of 7/16-5 TCR-Tg spleen cells into high-HBeAg-expressing Tg recipients elicited no anti-HBe antibody production and only very low anti-HBe antibody production in HBeAg(lo) expresser Tg recipients (Fig. 11, bottom panel). This indicates that serum HBeAg need not be present at birth or even perinatally to function as a tolerogen. Rather, exposure of adult, naive 7/16-5 TCR-Tg T cells to circulating HBeAg for a relatively brief period of time in the HBeAg-Tg recipients is sufficient to render the T cells tolerant or at least incapable of mediating anti-HBe antibody production. The absence of serum anti-HBe antibodies was not the result of masking by an excess of serum HBeAg because no IgG anti-HBe antibody production occurred in cultures of the HBeAg-Tg recipient spleen (data not shown). Although relative comparisons of the concentration of serum HBeAg versus cellular HBcAg are not possible, the positive correlation between in vivo HBcAg concentration and immunogenicity and conversely the inverse correlation between HBeAg serum concentration and immunogenicity is noteworthy. Taken together with the low levels of cytokine production and proliferative T-cell responses observed in 7/16-5 × HBe(hi) double-Tg mice, the failure to produce either spontaneous or induced anti-HBe antibodies indicates that serum concentrations of HBeAg of 4 to 10 μg/ml are highly tolerogenic for 7/16-5 TCR-Tg T cells. No similar tolerance induction in the same 7/16-5 TCR-Tg T cells was observed in response to the HBcAg regardless of the level of antigen expression. This apparent split tolerance between the HBeAg and HBcAg is likely due to the efficient secretion of monomeric HBeAg as opposed to the intracellular location of the particulate HBcAg. Secreted HBeAg has access to the thymus and the secondary lymphoid system, whereas only very small amounts of the HBcAg exit the liver. Monomeric HBeAg is significantly less immunogenic than particulate HBcAg at the T- and B-cell levels even when formulated in adjuvants (29). Furthermore, monomeric secreted antigens tend to be more tolerogenic than immunogenic in general (41, 52).
FIG. 11.
Summary of spontaneous anti-HBc/HBe antibody (Ab) seroconversion and induced antibody production in 7/16-5 × HBc and 7/16-5 × HBe double-Tg mice and antibody production after adoptive transfer of 7/16-5 T cells reveals the tolerance split between the HBeAg and the HBcAg. Spontaneous antibody seroconversion (top row) was monitored by bimonthly blood samples and measurement of IgG anti-HBc and anti-HBe antibodies by ELISA. “(Hi)” and “(Lo)” represent the antigen expression levels of HBc-Tg (left panels) and HBe-Tg (right panels) mice. Induced IgG antibody production in the indicated 7/16-5 double-Tg mice (middle row) was accomplished by injecting p129-140 (50 to 100 μg) (incomplete Freund adjuvant) at time zero and measuring anti-HBc or anti-HBe antibody production at 1, 2, and 4 weeks postinjection. In adoptive transfer experiments (bottom row), unprimed, naive spleen cells derived from 7/16-5 TCR-Tg mice were transferred (30 × 106 cells, intravenously) into CD4/CD8-depleted HBc-Tg or HBe-Tg recipients. Recipient sera were collected at weekly intervals and analyzed for anti-HBc and anti-HBe IgG antibodies by ELISA. The symbols in this figure represent individual mice; however, the results are representative of experiments performed on multiple occasions with a minimum of 6 mice per group. wk, week.
DISCUSSION
The purpose of this study was to gain insight into the condition of the HBc/HBeAg-specific CD4+-T-cell repertoire during long-term exposure to the secreted and intracellular forms of the HBV nucleocapsid antigens, as occurs in chronically infected patients. As a model of chronic HBc/HBeAg exposure, we have used TCR × HBc or HBeAg double-Tg mice. The function of the secreted HBeAg has been the subject of much speculation, since it is not required for viral assembly, infection, or replication. We have previously suggested that the HBeAg acts as an immunomodulatory protein via the induction of tolerance and Th1/Th2 cross-regulation (35, 36). In natural HBV infection, a dichotomy exists between the apparent tolerogenic and immunogenic functions of the HBeAg (28). In this present study, several mechanisms are described which may help to explain this dichotomy.
Serum HBeAg was shown to be tolerogenic for HBc/HBeAg-specific TCR-Tg T cells, and three distinct phenotypes of tolerance were observed in double-Tg mice. One phenotype, illustrated by the 8/12-2 TCR-Tg lineage, was profound tolerance induction by the HBeAg even at very low serum concentrations (10 ng/ml), most likely mediated by clonal deletion and FAS-mediated apoptosis in the periphery. A second phenotype, represented by the 11/4-12 TCR-Tg lineage, is one of clonal ignorance in which low-avidity HBc/HBeAg-specific T cells are neither tolerized nor activated by coexistence with even high concentrations of HBc/HBeAg and remain quiescent in vivo. However, as shown previously, 11/4-12 TCR-Tg T cells can be activated by antigen in vitro and can mediate liver injury upon adoptive transfer into HBc/HBeAg-Tg recipients (8). The third tolerance phenotype, illustrated by the 7/16-5 TCR-Tg lineage, is nondeletional and HBeAg concentration dependent and is best described as adaptive tolerance or in vivo anergy. Exposure of 7/16-5 T cells to high concentrations of serum HBeAg in vivo does not result in clonal deletion in the thymus or the periphery, but instead, the ability of the T cells to produce cytokines or proliferate to recall antigens in vitro and to provide T-cell help for in vivo anti-HBe antibody production is severely compromised.
Therefore, tolerance to the HBeAg is clonal, and HBc/HBeAg-specific T-cell clones can be quite heterogeneous in terms of sensitivity to tolerance induction. What explains multiple HBeAg-specific T-cell tolerance phenotypes? The immunogenicity and tolerogenicity of a T-cell epitope are likely to be proportional at least over a large range of TCR-peptide-major histocompatibility protein (TCR-pMHC) avidities. For example, a high-avidity TCR-pMHC interaction is likely to result in strong immunogenicity for a given T-cell epitope unless that epitope is a self-epitope, in which case T-cell tolerance is the likely outcome. An important contributor to overall TCR-pMHC avidity is the affinity of the peptide-MHC interaction. In previous studies, we have demonstrated that the immunodominant HBeAg-specific 120-131/I-As T-cell epitope represents a high-affinity pMHC interaction, promotes a Th1-like phenotype, and is highly tolerogenic in a neonatal mouse model compared to the immunodominant HBeAg 129-140/I-Ab epitope, which represents a relatively low-affinity pMHC interaction, promotes a Th2-like phenotype, and is poorly tolerogenic in a neonatal mouse model (30). Furthermore, the same HBeAg transgene results in profound T-cell tolerance when expressed in B10.S (H-2s) mice and significantly less T-cell tolerance in B10 (H-2b) mice (32). Therefore, it may not be surprising that 120-131/I-As-specific 8/12-2 TCR-Tg mice are highly sensitive to HBeAg-induced T-cell tolerance and the 129-140/I-Ab-specific 7/16-5 and 11/4-12 TCR-Tg lineages are less sensitive and demonstrate different tolerance phenotypes. We have functionally classified the 8/12-2, 7/16-5, and 11/4-12 TCRs as high, intermediate, and low avidity, respectively, based on parameters of T-cell activation, and this hierarchy also appears to correlate well with sensitivity to T-cell tolerance.
In the context of chronic HBV (CHB) infection, a multiplicity of HBeAg-specific T-cell clones are likely to coexist even within the same patient, and the balance between activation or tolerance status among the heterogeneous HBeAg-specific T-cell repertoire may influence degrees of liver injury and viral clearance. The question of whether the HBeAg acts as a tolerogen or an immunogen during HBV infection must be addressed at the clonal level. In the so-called tolerance phase of CHB infection, the majority of HBeAg-specific T-cell clones would be expected to be tolerant, although the mechanism of tolerance may vary (i.e., deletion, anergy, or ignorance). The status quo of the various clonal tolerance phenotypes would likely be maintained as long as the HBeAg concentration and/or the noninflammatory hepatic environment remains unchanged. However, a nonspecific increase in hepatic inflammation or a decrease in HBeAg serum concentration, perhaps due to the emergence of core promoter region (4) or precore region (37) mutants, may allow activation of low-avidity ignorant HBeAg-specific T cells and/or reverse the anergic state of others, respectively. Such a shift from HBeAg-specific T-cell tolerance to T-cell activation may precipitate the so-called clearance phase of CHB infection. As previously discussed, high-avidity HBeAg-specific T-cell clones are likely to be physically or functionally deleted and not available to participate in antiviral clearance mechanisms after a chronic infection has become established. Intermediate- or low-avidity HBeAg-specific T-cell clones that are not physically deleted at least have the potential to be activated either through the reversal of adaptive tolerance (anergy) or the primary activation of ignorant HBeAg-specific T cells. Therefore, treatment modalities for chronic HBV infection should be directed at activating the relatively low-avidity HBeAg-specific T cells. Given the efficacy of the present antiviral drugs in terms of reducing HBV and antigen load, reversing HBeAg-specific adaptive tolerance by reducing or eliminating circulating levels of the HBeAg may be an achievable goal and may in fact explain viral clearance in a percentage of chronic patients on long-term antiviral therapy. As demonstrated by 7/16-5 TCR-Tg T cells, the total elimination of the tolerogen (HBeAg) may not be required, since maintenance of HBeAg-specific T-cell tolerance for these T cells requires relatively high concentrations of HBeAg. Furthermore, if circulating HBeAg can be eliminated for a sufficient period of time, even deleted high-avidity HBeAg-specific T-cell clones may repopulate the peripheral T-cell repertoire, possibly depending on the age of the patient. Because antiviral therapy reduces the immunogenic stimulus as well as the tolerogenic stimulus, a dual antiviral/vaccine strategy may be necessary in the treatment of chronic HBV. Such a treatment strategy has shown some efficacy in a woodchuck model of chronic infection (27).
The clonal heterogeneity of HBeAg-specific T-cell tolerance may also explain how a primarily tolerogenic protein can exert pressure on the immune response to select an HBeAg-negative mutant. For example, high-avidity HBeAg-specific T-cell clones may be tolerized and simultaneously lower-avidity T-cell clones may be activated and involved in selecting HBeAg-negative mutants in the same patient.
Why is the reversal of T-cell tolerance to the secreted, nonstructural HBeAg an important goal in terms of treating chronic HBV infection? The HBeAg and HBcAg are cross-reactive at the level of T-cell recognition, and T-cell tolerance to the HBeAg would be expected to pertain to the HBcAg as well. However, the present studies demonstrate a split in T-cell tolerance between the HBeAg and the HBcAg. The secreted HBeAg appears significantly more efficient at eliciting T-cell tolerance than the HBcAg. If T-cell tolerance towards the nucleocapsid of HBV is an important determinant of chronicity, a secreted tolerogenic form of the antigen may be necessary to insure viral persistence and may actually be the primary function of the HBeAg in the virus life cycle. During a chronic infection with wild-type HBV, both immunogenic (HBcAg) and tolerogenic (HBeAg) forms of the same antigen coexist. As shown herein, the presence of serum HBeAg can inhibit anti-HBc antibody production in B10.S/HBe(hi)-Tg mice immunized with rHBcAg (Fig. 6) and in 7/16-5 × HBc(lo) × HBe(lo) triple-Tg mice (Fig. 9). Furthermore, the presence of serum HBeAg reduced the frequency of HBc/HBeAg-specific liver injury in triple-Tg mice (Fig. 10) as well as down-regulated anti-HBc antibody production. We suggest that the HBeAg functions as an immunoregulatory protein by virtue of eliciting tolerance (i.e., deletion and/or anergy) in CD4+ T cells specific for both the HBc/HBeAgs in a manner which is not likely to occur to the HBcAg alone. The effects of HBeAg-specific T-cell tolerance may be apparent in utero (31), during the perinatal period (Fig. 7), or during adult immunization and/or infection, as demonstrated by the absence of anti-HBe antibody production in HBeAg(hi)-Tg recipients of 7/16-5 TCR-Tg T cells (Fig. 11, bottom panel).
A number of clinical observations suggest that the HBeAg plays a role in HBV chronicity (19, 28). For example, neonatal infection with an HBeAg-negative mutant virus often results in acute fulminant rather than chronic HBV infection (7, 49). Similarly, infection of young woodchucks with a WHeAg-negative mutant of woodchuck hepatitis virus results in a low rate of chronic infection in contrast to the high rate of chronicity observed in young woodchucks infected with wild-type woodchuck hepatitis virus (12). Furthermore, adult infection with an HBeAg-negative mutant is often associated with a fulminant course of infection rather than the relatively benign acute course which characterizes most adult-onset infections with wild-type HBV (5, 13, 25, 39, 48). In addition, emergence of an HBeAg-negative mutant during a chronic active HBV infection, especially in the presence of a high viral load, can correlate with an exacerbation of liver injury and a worse prognosis (3, 11). A number of relatively common mutations in the precore and core promoter regions inhibit HBeAg production to varying degrees (4, 37). The finding that serum HBeAg can act as an efficient T-cell tolerogen and HBeAg-specific T-cell anergy can be HBeAg concentration dependent and reversible in the absence of HBeAg (i.e., 7/16-5 T cells) may explain the correlation between precore and core promoter mutations and severe liver injury (3, 5, 7, 11, 13, 25, 39, 48, 49). Taken together, the clinical observations and murine experimental studies suggest that the HBeAg is important in biasing the virus-host interaction toward chronicity and accomplishes this by down-regulating the host T-cell response to the nucleocapsid antigens via a variety of tolerance-inducing mechanisms.
Acknowledgments
This work was supported by National Institutes of Health grants 5 R01 AI20720-21, 5 R01 AI49730-03, and 5 R01-CA40489 and grants from the Swedish Cancer Foundation and the Swedish Science Council.
We thank F. Schödel and D. Peterson for providing recombinant HBc/HBeAgs and Rene Lang for editorial assistance.
REFERENCES
- 1.Arakawa, K., F. Tsuda, K. Takahashi, I. Ise, S. Naito, E. Kosugi, Y. Miyakawa, and M. Mayumi. 1982. Maternofetal transmission of IgG-bound hepatitis B e antigen. Pediatr. Res. 16:247-250. [DOI] [PubMed] [Google Scholar]
- 2.Bertoletti, A., C. Ferrari, F. Fiaccadori, A. Penna, R. Margolskee, H. J. Schlicht, P. Fowler, and F. V. Chisari. 1991. HLA class I-restricted human cytotoxic T cells recognize endogenously synthesized hepatitis B virus nucleocapsid antigen. Proc. Natl. Acad. Sci. USA 88:10445-10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunetto, M. R., M. M. Giarin, F. Oliveri, E. Chiaberge, M. Baldi, A. Alfarano, A. Serra, G. Saracco, G. Verme, H. Will, and F. Bonino. 1991. Wild-type and e antigen-minus hepatitis B viruses and course of chronic hepatitis. Proc. Natl. Acad. Sci. USA 88:4186-4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carman, W. F., M. R. Jacyna, S. Hadziyannis, P. Karayiannis, M. J. McGarvey, A. Makris, and H. C. Thomas. 1989. Mutation preventing formation of hepatitis B e antigen in patients with chronic hepatitis B infection. Lancet ii:588-590. [DOI] [PubMed] [Google Scholar]
- 5.Carman, W. F., E. A. Fagan, S. Hadziyannis, P. Karayiannis, N. C. Tassopoulos, R. Williams, and H. C. Thomas. 1991. Association of a precore genomic variant of hepatitis B virus with fulminant hepatitis. Hepatology 14:219-222. [PubMed] [Google Scholar]
- 6.Chang, C., G. Enders, R. Sprengel, N. Peters, H. E. Varmus, and D. Ganem. 1987. Expression of the precore region of an avian hepatitis B virus is not required for viral replication. J. Virol. 61:3322-3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, H.-L., C.-J. Chang, M.-S. Kong, F.-C. Huang, H.-C. Lee, C.-C. Lin, C.-C. Liu, I.-H. Lee, T.-C. Wu, and S.-F. We. 2004. Pediatric fulminant hepatic failure in endemic areas of hepatitis B infection: 15 years after universal hepatitis B vaccination. Hepatology 39:58-63. [DOI] [PubMed] [Google Scholar]
- 8.Chen, M., M. Sällberg, S. N. Thung, J. Hughes, J. Jones, and D. R. Milich. 2000. Nondeletional T-cell receptor transgenic mice: model for the CD4+ T-cell repertoire in chronic hepatitis B virus infection. J. Virol. 74:7587-7599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, M. T., J.-N Billaud, M. Sällberg, L. G. Guidotti, F. V. Chisari, J. Jones, J. Hughes, and D. R. Milich. A function of the hepatitis B virus precore protein is to regulate the immune response to the core antigen. Proc. Natl. Acad. Sci. USA 101:14913-14918. [DOI] [PMC free article] [PubMed]
- 10.Chen, Y., M. C. Kew, W. E. Hornbuckle, B. C. Tennant, P. J. Cote, J. L. Gerin, R. H. Purcell, and R. H. Miller. 1992. The precore gene of the woodchuck hepatitis virus genome is not essential for viral replication in the natural host. J. Virol. 66:5682-5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chu, C. M., C. T. Yeh, C. S. Lee, I. S. Sheen, and Y. F. Liaw. 2002. Precore stop mutant in HBeAg-positive patients with chronic hepatitis B: clinical characteristics and correlation with the course of HBeAg-to-anti-HBe seroconversion. J. Clin. Microbiol. 40:16-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cote, P. J., B. E. Korba, R. H. Miller, J. R. Jacob, B. H. Baldwin, W. E. Hornbuckle, R. H. Purcell, and J. L. Gerin. 2000. Effects of age and viral determinants on chronicity as an outcome of experimental woodchuck hepatitis virus infection. Hepatology 31:190-200. [DOI] [PubMed] [Google Scholar]
- 13.Fagan, E. A., P. M. Smith, F. Davison, and R. Williams. 1986. Fulminant hepatitis B in successive female sexual partners of two anti-HBe-positive males. Lancet ii:538-540. [DOI] [PubMed]
- 14.Ganem, D., and R. J. Schneider. 2001. Hepadnaviridae and their replication, p. 2923-2970. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, B. Roizman, M. A. Martin, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 15.Goodnow, C. C., R. Glynne, S. Akkaraju, J. Rayner, D. Mack, J. I. Healy, S. Chaudhry, L. Miosge, L. Wilson, P. Papathanasiou, and A. Loy. 2001. Autoimmunity, self-tolerance and immune homeostasis: from whole animal phenotypes to molecular pathways. Adv. Exp. Med. Biol. 490:33-40. [DOI] [PubMed] [Google Scholar]
- 16.Guidotti, L. G., V. Martinez, Y.-T. Loh, C. E. Rogler, and F. V. Chisari. 1994. Hepatitis B virus nucleocapsid particles do not cross the hepatocyte nuclear membrane in transgenic mice. J. Virol. 68:5469-5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guidotti, L. G., B. Matzke, H. Schaller, and F. V. Chisari. 1995. High-level hepatitis B virus replication in transgenic mice. J. Virol. 69:6158-6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guidotti, L. G., B. Matzke, C. Pasquinelli, J. M. Shoenberer, C. E. Rogler, and F. V. Chisari. 1996. The hepatitis B virus (HBV) precore protein inhibits HBV replication in transgenic mice. J. Virol. 70:7056-7061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hilleman, M. R. 2003. Critical review and outlook: pathogenesis, prevention, and treatment of hepatitis and hepatocarcinoma caused by hepatitis B virus. Vaccine 21:4626-4649. [DOI] [PubMed] [Google Scholar]
- 20.Ho, W. Y., M. P. Cooke, C. C. Goodnow, and M. M. Davis. 1994. Resting and anergic B cells are defective in CD28-dependent costimulation of naive CDR+ T cells. J. Exp. Med. 179:1539-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsu, H. Y., M. H. Chang, K. H. Hsieh, C. Y. Lee, H. H. Lin, L. H. Hwang, P. J. Chen, and D. S. Chen. 1992. Cellular immune response to HBcAg in mother-to-infant transmission of hepatitis B virus. Hepatology 15:770-776. [DOI] [PubMed] [Google Scholar]
- 22.Imai, M., M. Nomura, T. Gotanda, T. Sano, K. Tachibana, H. Miyamoto, K. Takahashi, S. Toyama, Y. Miyakawa, and M. Mayumi. 1982. Demonstration of two distinct antigenic determinants on hepatitis B e antigen by monoclonal antibodies. J. Immunol. 128:69-72. [PubMed] [Google Scholar]
- 23.Kearney, E. R., K. A. Pape, D. Y. Loh, and M. K. Jenkins. 1994. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity 1:327-339. [DOI] [PubMed] [Google Scholar]
- 24.Lee, S. D., K. J. Lo, J. C. Wu, Y. T. Tsai, J. Y. Wang, L. P. Ting, and M. J. Tong. 1986. Prevention of maternal-infant hepatitis B virus transmission by immunization: the role of serum hepatitis B virus DNA. Hepatology 6:369-373. [DOI] [PubMed] [Google Scholar]
- 25.Liang, T. J., K. Hasegawa, N. Rimon, J. R. Wands, and E. Ben-Porath. 1991. A hepatitis B virus mutant associated with an epidemic of fulminant hepatitis B. N. Engl. J. Med. 324:1705-1709. [DOI] [PubMed] [Google Scholar]
- 26.McLachlan, A., D. R. Milich, A. K. Raney, M. G. Rigs, J. L. Hughes, J. Sorge, and F. V. Chisari. 1987. Expression of hepatitis B virus surface and core antigens: influences of pre-S and precore sequences. J. Virol. 61:683-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Menne, S., C. A. Roneker, B. D. Tennant, B. E. Korba, J. L. Gerin, and P. J. Cote. 2002. Immunogenic effects of woodchuck hepatitis virus surface antigen vaccine in combination with antiviral therapy: breaking of humoral and cellular immune tolerance in chronic woodchuck hepatitis virus infection. Intervirology 45:237-250. [DOI] [PubMed] [Google Scholar]
- 28.Milich, D., and T. J. Liang. 2003. Exploring the biological basis of hepatitis B e antigen in hepatitis B virus infection. Hepatology 38:1075-1081. [DOI] [PubMed] [Google Scholar]
- 29.Milich, D. R., A. McLachlan, S. Stahl, P. Wingfield, G. B. Thornton, J. L. Hughes, and J. E. Jones. 1988. Comparative immunogenicity of hepatitis B virus core and E antigens. J. Immunol. 141:3617-3624. [PubMed] [Google Scholar]
- 30.Milich, D. R., J. E. Jones, A. McLachlan, R. Houghten, G. B. Thornton, and J. L. Hughes. 1989. Distinction between immunogenicity and tolerogenicity among HBcAg T cell determinants. Influence of peptide-MHC interactions. J. Immunol. 143:3148-3156. [PubMed] [Google Scholar]
- 31.Milich, D. R., J. E. Jones, J. L. Hughes, J. Price, A. K. Raney, and A. McLachlan. 1990. Is a function of the secreted hepatitis B e antigen to induce immunologic tolerance in utero? Proc. Natl. Acad. Sci. USA 87:6599-6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Milich, D. R., A. McLachlan, A. K. Raney, R. Houghten, G. B. Thornton, T. Maruyama, J. L. Hughes, and J. E. Jones. 1991. Autoantibody production in hepatitis B e antigen transgenic mice elicited with a self T-cell peptide and inhibited with nonself peptides. Proc. Natl. Acad. Sci. USA 88:4348-4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Milich, D. R., J. E. Jones, J. L. Hughes, T. Maruyama, J. Price, I. Melhado, and F. Jirik. 1994. Extrathymic expression of the intracellular hepatitis B core antigen results in T cell tolerance in transgenic mice. J. Immunol. 152:455-466. [PubMed] [Google Scholar]
- 34.Milich, D. R., F. Schödel, J. Hughes, J. E. Jones, and D. L. Peterson. 1997. The hepatitis B virus core and e antigen elicit different Th cell subsets: antigen structure can affect Th cell phenotype. J. Virol. 71:2192-2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Milich, D. R. 1997. Influence of T-helper cell subsets and crossregulation in hepatitis B virus infection. J. Viral. Hep. 4:48-59. [DOI] [PubMed] [Google Scholar]
- 36.Milich, D. R., M. K. Chen, J. L. Hughes, and J. E. Jones. 1998. The secreted hepatitis B precore antigen can modulate the immune response to the nucleocapsid: a mechanism for persistence. J. Immunol. 160:2013-2021. [PubMed] [Google Scholar]
- 37.Okamoto, H., F. Tsuda, Y. Akahane, Y. Sugai, M. Yoshiba, K. Moriyama, T. Tanaka, Y. Miyakawa, and M. Mayumi. 1994. Hepatitis B virus with mutations in the core promoter for an e antigen-negative phenotype in carriers with antibody to e antigen. J. Virol. 68:8102-8110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ou, J.-H., O. Laub, and W. J. Rutter. 1986. Hepatitis B gene function: the precore region targets the core antigen to cellular membranes and causes the secretion of e-antigen. Proc. Natl. Acad. Sci. USA 83:1578-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petrosillo, N., G. Ippolito, L. Solforosi, P. E. Varaldo, M. Clementi, and A. Manzin. 2000. Molecular epidemiology of an outbreak of fulminant hepatitis B. J. Clin. Microbiol. 38:2975-2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reifenberg, K., T. Deutschle, J. Wild, R. Hanano, I. Gastrock-Balitsch, R. Schirmbeck, and H. J. Schlicht. 1998. The hepatitis B virus e antigen cannot pass the murine placenta efficiently and does not induce CTL immune tolerance in H-2b mice in utero. Virology 243:45-53. [DOI] [PubMed] [Google Scholar]
- 41.Romball, C. G., and W. O. Weigle. 1993. In vivo induction of tolerance in murine CD4+ cell subsets. J. Exp. Med. 178:1637-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roossinck, M. J., S. Jameel, S. H. Loukin, and A. Siddiqui. 1986. Expression of hepatitis B viral core region in mammalian cells. Mol. Cell. Biol. 6:1393-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sällberg, M., U. Ruden, L. O. Magnius, E. Norrby, and B. Wahren. 1991. Rapid “tea-bag” peptide synthesis using 9-fluorenylmethoxycarbonyl (Fmoc) protected amino acids applied for antigenic mapping of viral proteins. Immunol. Lett. 30:59-68. [DOI] [PubMed] [Google Scholar]
- 44.Schlicht, H. J., J. Salfeld, and H. Schaller. 1987. The duck hepatitis B virus pre-C region encodes a signal sequence which is essential for synthesis and secretion of processed core proteins but not for virus formation. J. Virol. 61:3701-3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schödel, F., A. M. Moriarty, D. L. Peterson, J. Zheng, J. L. Hughes, H. Will, D. J. Leturcq, J. S. McGee, and D. R. Milich. 1992. The position of heterologous epitopes inserted in hepatitis B virus core particles determines their immunogenicity. J. Virol. 66:106-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schödel, F., D. Peterson, J. Zheng, J. E. Jones, J. L. Hughes, and D. R. Milich. 1993. Structure of hepatitis B virus core and e-antigen: a single precore amino acid prevents nucleocapsid assembly. J. Biol. Chem. 268:1332-1337. [PubMed] [Google Scholar]
- 47.Schwartz, R. H. 2003. T cell anergy. Annu. Rev. Immunol. 21:305-334. [DOI] [PubMed] [Google Scholar]
- 48.Tanaka, S., M. Yoshiba, S. Iino, M. Fukuda, H. Nakao, F. Tsuda, H. Okamoto, Y. Miyakawa, and M. Mayumi. 1995. A common-source outbreak of fulminant hepatitis B in hemodialysis patients induced by precore mutant. Kidney Int. 48:1972-1978. [DOI] [PubMed] [Google Scholar]
- 49.Terazawa, S., M. Kojima, T. Yamanaka, S. Yotsumoto, H. Okamoto, F. Tsuda, Y. Miyakawa, and M. Mayumi. 1991. Hepatitis B virus mutants with precore-region defects in two babies with fulminant hepatitis and their mothers positive for antibody to hepatitis B e antigen. Pediatr. Res. 29:5-9. [DOI] [PubMed] [Google Scholar]
- 50.Townsend, K., M. Sällberg, J. O'Dea, T. Banks, J. Hughes, D. R. Milich, D. J. Jolly, S. M. Chang, and W. T. Lee. 1997. Characterization of CD8+ cytotoxic T-lymphocyte responses after genetic immunization with retrovirus vectors expressing different forms of the hepatitis B virus core and e antigens. J. Virol. 71:3365-3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang, J. S., and Q. R. Zhu. 2000. Infection of the fetus with hepatitis B e antigen via the placenta. Lancet 355:909-910. [DOI] [PubMed] [Google Scholar]
- 52.Weigle, W. O. 1980. Analysis of autoimmunity through experimental models of thyroiditis and allergic encephalomyelitis. Adv. Immunol. 30:159-273. [DOI] [PubMed] [Google Scholar]
- 53.Weimber, T., J. Salfeld, and H. Will. 1987. Expression of the hepatitis B virus core gene in vitro and in vivo. J. Virol. 61:3109-3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhanhui, W., J. Zhang, H. Yang, L. Xiuhu, W. Shujuan, G. Yabing, J. Sun, and J. Hou. 2003. Quantitative analysis of HBV DNA level and HBeAg titer in hepatitis B surface antigen positive mothers and their babies: HBeAg passage through the placenta and the rate of decay in babies. J. Med. Virol. 71:360-366. [DOI] [PubMed] [Google Scholar]