Abstract
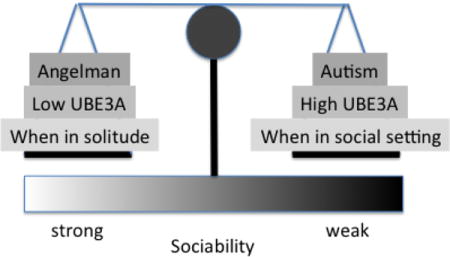
Deletions and reciprocal triplications of the human chromosomal 15q11-13 region cause two distinct neurodevelopmental disorders. Maternally-derived deletions or inactivating mutations of UBE3A, a 15q11-13 gene expressed exclusively from the maternal allele in neurons, cause Angelman syndrome, characterized by intellectual disability, motor deficits, seizures, and a characteristic increased social smiling, laughing, and eye contact. Conversely, maternally-derived triplications of 15q11-13 cause a behavioral disorder on the autism spectrum with clinical features that include decreased sociability that we recently reconstituted in mice with Ube3a alone. Based on the unique sociability features reported in Angelman syndrome and the repressed sociability observed when Ube3a gene dosage is increased, we hypothesized that mice with neuronal UBE3A loss that models Angelman syndrome would display evidence of hypersocial behavior. We report that mice with maternally-inherited Ube3a gene deletion (Ube3amKO) have a prolonged preference for, and interaction with, social stimuli in the three chamber social approach task. By contrast, interactions with a novel object are reduced. Further, ultrasonic vocalizations and physical contacts are increased in male and female Ube3amKO mice paired with an unfamiliar genotype-matched female. Single housing wild type mice increased these same social behavior parameters to levels observed in Ube3amKO mice where this effect was partially occluded. These results indicate sociability is repressed by social experience and the endogenous levels of UBE3A protein and suggest some social behavioral features observed in Angelman syndrome may reflect an increased social motivation.
Keywords: UBE3A, Angelman syndrome, autism, intellectual disability, social behavior, ultrasonic vocalizations
Graphical abstract
Introduction
Maternal deletions or loss-of-function mutations of the human gene UBE3A, located within the 15q11-13 chromosomal region, cause Angelman syndrome (AS), which is characterized by motor deficits, intellectual disability, and seizures (Matsuura T. et al., 1997; Kishino T. et al., 1997). Individuals with AS fall in the autism spectrum due to their repetitive-restrictive interests and profound language deficits (Peters S.U. et al., 2004; Peters S.U. et al., 2004; Bonati M.R. et al., 2007; Moss J. et al., 2013). However, they also display a characteristic increase of smiling and laughter, increased proactive social contacts, and an amiable and gregarious personality, suggesting potentially hypersocial behaviors, opposite to those found in autism (Williams C.M. et al., 2005; Walz N.C. et al., 2007; Pelc K. et al., 2008; Adams D. et al., 2011; Heald M. et al., 2013).
Loss of maternal UBE3A, as found in Angelman syndrome, has previously been modeled in mice (Jiang et al. 1998; Miura et al. 2002). Mice with maternal Ube3a deletions (Ube3amKO) share several phenotypic characteristics with individuals with AS including decreased motor coordination, susceptibility to audiogenic seizures, and impaired learning and memory (Jiang et al. 1998). The penetrance of these behavioral phenotypes varies with genetic background and age (Huang H.S. et al., 2013). While aspects of the motor and intellectual deficits and the epilepsy observed in AS have been modeled in Ube3amKO mice (Jiang Y.H. et al., 1998), behavioral features reflecting the distinct social phenotypes have not been reported.
Ube3a mRNA and protein are increased by neuronal activity in vitro and by exposure to environmental enrichment in vivo (Greer P.L. et al., 2010). In addition, early exposure to an enriched environment corrects several non-social behavioral phenotypes in Ube3amKO animals (Jamal I. et al., 2016). There is also evidence that prolonged single housing increases expression of Ube3a1 (but not 2 or 3) transcript with a unique 3′ UTR and encoding a truncated Ube3a protein lacking catalytic activity in rat brain (Valluy J. et al., 2015). Here we investigate the roll of Ube3a in experience-dependent regulation of social behaviors. We report that the duration of social interactions is prolonged and the paired ultrasonic vocalizations and physical contacts measured during exposure to a novel mouse are increased when the maternally-inherited Ube3a allele is deleted in mice to model the genetic defect found in Angelman syndrome. Loss of maternal Ube3a also largely blocks the suppressive effects of social experience on subsequent social behavior.
Materials and Methods
Mice
Ube3amKO mice (JAXS, B6.129S7-Ube3atm2Alb/J) were backcrossed to FVB for 10 generations. Females positive for the deletion (unaffected mothers) inherited from their male parent were bred to wild-type males to produce mice designated p-Ube3a-mKO and their wild-type littermates. Females positive for the deletion (AS affected mothers) inherited from their female parent were bred to wild-type males to produce mice designated m-Ube3a-mKO and their wild-type littermate mice. Mice were housed in same-sex groups of ≤ 5 under standard laboratory conditions. Lights were on from 7 am to 7 pm and ad libitum food and water were available. Three chamber social approach, rotorod, and novel object tests were performed between 9 am and 5 pm. Paired ultrasonic vocalizations were recorded between 9 am and 12 pm. The testing arenas were cleaned with Clidox-S spray in between each mouse. Mice were given 1 hour of acclimation to the testing environment prior to behavioral measurements. Prior to testing, mice were preconditioned to the experimenter by handling. All of the following tests were performed on breeding age female (6–13 weeks old) and male (8–13 weeks old) mice.
Testing Order
Unique cohorts of mice were measured in multiple behavioral assays in the following orders. Female p-Ube3a-mKO mice: 1. three chamber social approach, 2. female-female paired ultrasonic vocalizations, 3. novel object preference, and 4. rotorod. Female m-Ube3a-mKO mice: 1. three chamber social approach, 2. female-female paired ultrasonic vocalizations, and 3. rotorod. Male p-Ube3a-mKO mice: 1. three chamber social approach, and 2. male-female ultrasonic vocalizations. The effects of social experience on three chamber social approach and paired ultrasonic vocalizations were measured in two unique cohorts of female m-Ube3a-mKO and wild-type littermate mice. For female p-Ube3a-mKO mice, the same cohorts were used to assess the effects of social experience on three chamber social approach and then, after a one week group housed period, the effects of social experience on paired ultrasonic vocalizations were measured.
Social Approach Test
Mice were placed in a clear acrylic box (50 × 100 cm) containing dividers with small (10 × 10 cm) doors to create a three-chambered enclosure. An overhead camera recorded activity and Ethovision software (Noldus) tracked mouse movement. Two small metal enclosures (inverted pencil holders, Office Depot) were placed in opposite corners of the arena and an Erlenmeyer flask filled with water was placed upon them to prevent mice from climbing to the top of the chamber. Test mice (wild type, p-Ube3a-mKO, or m-Ube3a-mKO) naïve to the arena were allowed to explore for five minutes and then removed to a holding cage. A novel sex- and age-matched non-littermate wild-type mouse was placed in one of the two small enclosures. The location of the novel mouse was alternated to control for any innate side preference. These probe mice were habituated to the small enclosures in 1 hour sessions two a day for two days prior to testing. The test mouse was then returned to the cage and allowed to free-roam for two consecutive five minute trials while movements and the time spent in each third of the enclosure were automatically scored by Ethovision and the time sniffing the chambers was scored by a trained observer blind to genotype. The arena was evenly lit by an overhead fluorescent light source.
Rotorod
Motor function of p-Ube3a-mKO, m-Ube3a-mKO, and control wild-type littermate mice were tested using the rotarod (Ugo Basile A-Rod for mice). Each experiment was performed during the light phase inside a fume hood with an overhead fluorescent light. Mice were given two trials per day with a 60 min inter trial interval for 4 consecutive days. On each day we calculated the average time spent on the rotarod or the time until the mouse made three consecutive rotations on the rotarod. The duration of the trial was 5 minutes and the rod accelerated from 4 to 40 rpm. 10 mice per genotype were measured.
Social vocalizations
Female-Female Social Vocalization Test: Pairs of age- and genotype-matched, non-littermate, female mice were studied. Male-Female Social Vocalization Test: A wild-type or p-Ube3a-mKO male and an unfamiliar, sexually mature, wild-type female were studied. Mice were placed simultaneously (to avoid resident-intruder aggression) into a small novel clean 25.4 cm circular plastic chamber at room temperature inside an unlit sound isolation box for 5 minutes. Vocalizations were recorded with a condenser ultrasound microphone (Avisoft-Bioacoustics CM16/CMPA), an UltraSoundGate (Avisoft Bioacoustics 116Hb) and the Avisoft Recorder USGH software (Avisoft Bioacoustics). Avisoft-SASLab Pro software was used to quantify the number of vocalizations and the time spent vocalizing. The USV detection settings were: max frequency changes (3 pixels), frequency range limit (35 – 250 kHz), min whistle duration (10 ms), hold time (20 ms), min total duration (10 ms). Sampling rate is 250,000 Hz (at 976 Hz FFT size is 256 with 0% overlap). Simultaneous video recordings were made using a webcam mounted in the lid of the container. Physical interaction (direct contact) times were measured by a trained observer blinded to genotype.
Novel Object
Mice were placed in a large (50 × 50cm) open field lit from above by a fluorescent ceiling light and allowed to explore for 10 minutes. An overhead camera recorded activity and Ethovision (Noldus) was used to track mouse movement. Mobility in the open field was scored by the software. After 10 minutes a novel object (colored bottle cap) was placed into the open field near one corner and the time the mouse spend interacting with the object was scored by an observer blind to genotype for five minutes.
Statistical Analysis
Multiple groups were compared by 2-way ANOVA with the Bonferroni post-test. Three chamber data was analyzed by a repeated measure 2-way ANOVA with the Bonferroni post-test. Ultrasonic vocalization and physical interaction data were analyzed using a two-tailed unpaired Student’s t-test. Single groups were compared by 1-way ANOVA with the Bonferroni post-test.
Results
Prolonged social interest in Angelman syndrome mice in the three chamber social task
The three chamber social approach test was used to evaluate for a social preference in mice (Nadler J.J. et al., 2004). We evaluated adult female mice generated by breeding females positive for the deletion inherited from their male parent (unaffected mothers) bred to wild-type males to produce wild-type and p-Ube3a-mKO mice. A separate cohort, generated by breeding females positive for the deletion inherited from their female parent (AS mothers) to wild-type males to produce wild-type and m-Ube3a-mKO mice was also examined using this behavioral task.
There was no main effect of genotype or chamber during acclimation trials (Supplementary Fig. 1b and d). The time sniffing, measured by a blinded observer scoring time spent physically investigating the social and opposite chamber, and the time in zone, measured by automated mouse localization software, were calculated. Female wild-type, p-Ube3a-mKO, and m-Ube3a-mKO each displayed a significant social preference during the first five minutes of the social trial for both sniffing and time in zone (Fig. 1a–d) (Sniffing Times: WT, t = 4.341, p < 0.001; p-Ube3a-mKO, t = 5.161, p < 0.001; WT, t = 3.181, p < 0.01; m-Ube3a-mKO, t = 4.686, p < 0.001; Zone Times: WT, t = 3.259, p < 0.01; p-Ube3a-mKO, t = 4.466, p < 0.001; WT, t = 3.897, p < 0.001; m-Ube3a-mKO, t = 5.008, p < 0.001). During the second five minutes of the social trial, measured immediately following the first five minute trial, social preference was lost in wild-type but retained in p-Ube3a-mKO and m-Ube3a-mKO mice (Fig. 1a–d) (Sniffing Times: WT, t = 0.6654, p > 0.05; p-Ube3a-mKO, t = 4.684, p < 0.001; WT, t = 0.4430, p > 0.05; m-Ube3a-mKO, t = 3.357, p < 0.01: Zone Times: WT, t = 0.09566, p > 0.05; p-Ube3a-mKO, t = 4.407, p < 0.001; WT, t = 0.3913, p > 0.05; m-Ube3a-mKO, t = 4.279, p < 0.001).
Fig. 1.
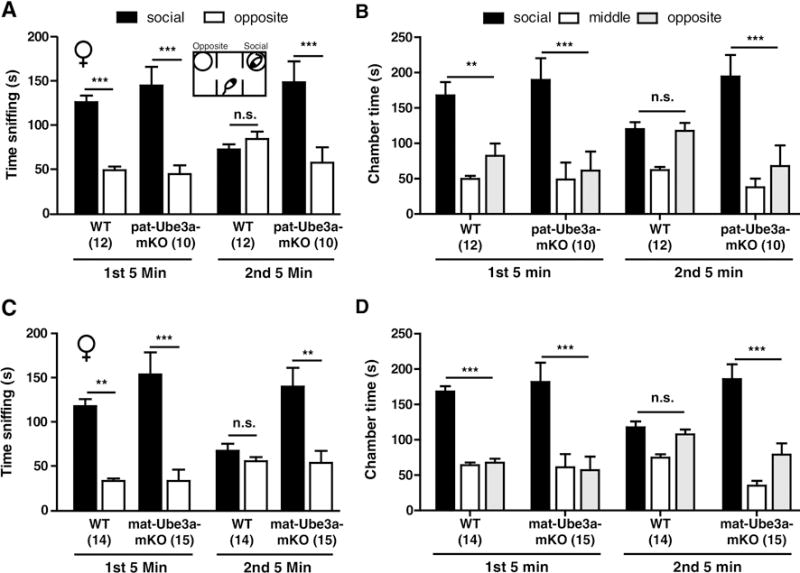
Angelman syndrome model mice are hypersocial in the three chamber test. Three chamber social preference during two consecutive five minutes trials in WT and Ube3amKO mice. (A) Time sniffing the social (novel mouse) or opposite (empty) chamber for female WT and p-Ube3a-mKO mice. (B) Time spent in the social, middle, or opposite chamber for female WT and p-Ube3a-mKO mice. (C) Time sniffing for female WT and m-Ube3a-mKO mice. (D) Time in zone for female WT and m-Ube3a-mKO mice. All values are mean ± SEM. **p < 0.01, ***p < 0.001.
There were no differences in the distance moved during the first and second five minute periods of the social trial within genotype although p-Ube3a-mKO and m-Ube3a-mKO mice were less mobile than wild type animals during each testing period (Supplementary Fig. 1a and c). Male wild type mice displayed a strong trend for sociability in the first five minutes period of the social trial that was lost during the second five minute period, while male p-Ube3a-mKO mice displayed a significant social preference during the first five minutes and a trend towards a social preference during second five minutes (Supplementary Fig. 2a–d). Prior studies of sociability in the three chamber task in Ube3amKO mice (Allensworth M. et al., 2011; Huang H.S. et al., 2013) failed to report increased sociability. These groups examined Ube3amKO mice on the C57BL/6 genetic background and reported the data as a single 10 minutes trial. Our results suggest p-Ube3a-mKO and m-Ube3a-mKO female mice have a prolonged social interest, persisting during a time when social interest is lost in wild type littermates.
Increased social vocalizations and physical contacts in Angelman syndrome mice
Female mice emit ultrasonic vocalizations (USVs) when paired with an unfamiliar female mouse (Maggio and Whitney, 1985). We have used these conspecific pairing-induced USVs as an assay to reveal impaired social vocalization in the mouse models of autism spectrum disorder with increased Ube3a gene copies (Smith et al., 2011). During a five minutes trial period, pairs of female p-Ube3a-mKO mice emitted more USVs for a longer duration (Fig. 2a,b) (t = 4.304, p < 0.001; t=4.534, p < 0.001) and spent more time physically interacting without a change in the number of contacts (Fig. 2c,d) (t=4.652, p < 0.001; t=0.2635, p < 0.05) than pairs of wild type littermates. Similarly, m-Ube3a-mKO mice emitted more USVs for a longer duration (Fig. 2e,f) (t=3.780, p < 0.01; t=4.021, p < 0.01) and spent more time physically interacting without a change in the number of contacts (Fig. 2g,h) (t=4.087, p < 0.01; t=0.0976, p < 0.05) than pairs of wild type mice.
Fig. 2.
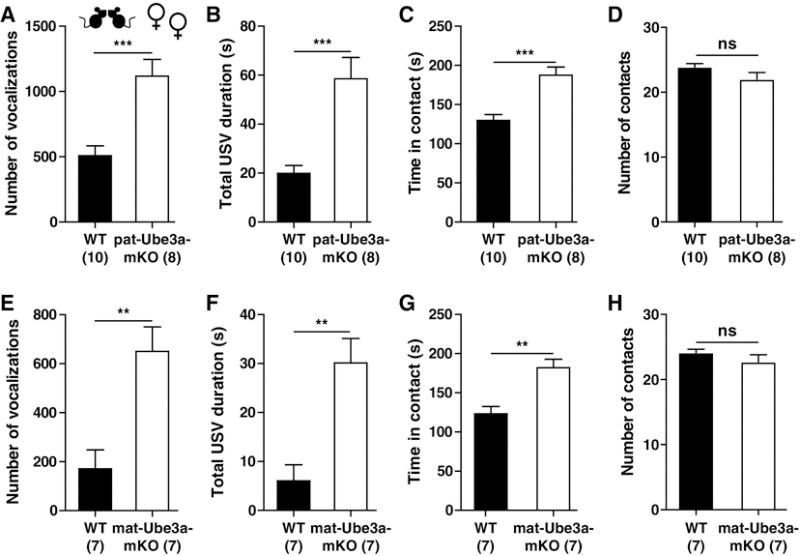
Female Angelman syndrome model mice generated increased ultrasonic vocalizations and physical contacts when paired. Female-female paired ultrasonic vocalizations [number (A) and duration (B)] and physical contacts [duration (C) and number (D)] generated by pairs of female WT or p-Ube3a-mKO mice. Female-female paired ultrasonic vocalizations [number (E) and duration (F)] and physical contacts [duration (G) and number (H)] generated by pairs of female WT or m-Ube3a-mKO mice. All values are mean ± SEM. **p < 0.01, ***p < 0.001.
Male mice emit USVs when they encounter a stranger female conspecific (Wang et al., 2008). During a five minutes trial period, male p-Ube3a-mKO mice emitted more USVs that were longer in total duration (Supplemental Fig. 2e and f) when paired with a wild-type stranger female than did littermate wild type male mice. Male m-Ube3a-mKO also displayed elevated USV output for a longer duration (Supplemental Fig. 2g and h) when paired with a wild-type stranger female than did their littermate wild-type male mice. These results suggest that Ube3a-mKO male and female mice have increased social vocal and direct physical interactions compared to wild-type littermates.
Reduced novel object exploration in Angelman syndrome mice
The increased social interactions and vocalizations found in Ube3a-mKO mice could reflect an increased response to novel stimuli whether social or non-social. To test for this possibility, we measured novel object exploration. p-Ube3a-mKO showed a longer latency to approach and a reduced time and fewer episodes investigating a novel object (Fig. 3a–c) (t = 2.318, p < 0.05; t = 3.106, p < 0.01; t = 3.434, p < 0.01). As observed in the three chamber task, p-Ube3a-mKO mice moved shorter distances during the open field trial than wild type littermates (Fig. 3d) (t = 10.55, p < 0.001). These results suggest Ube3a-mKO mice have a reduced interest in novel object exploration despite their increased interest in a novel conspecific.
Fig. 3.
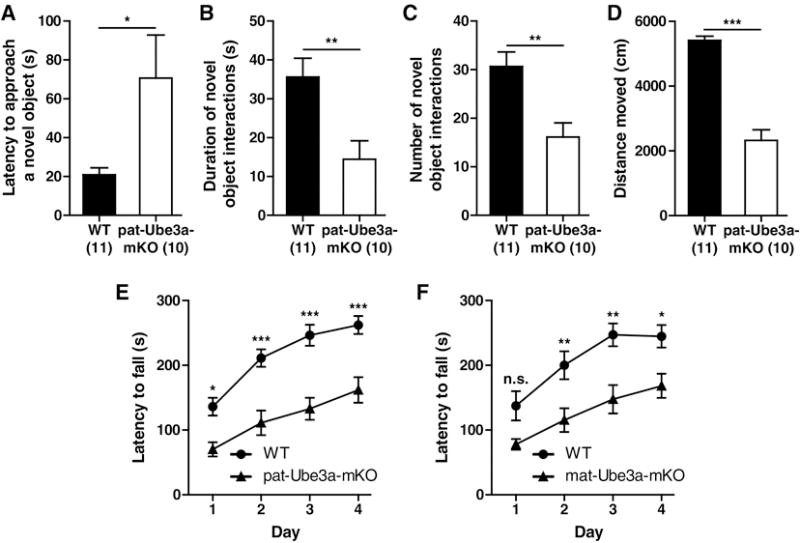
Angelman syndrome model mice display a reduced interest in a novel object and impaired rotorod performance. Latency (A), duration (B) and number (C) of novel object explorations for WT and p-Ube3a-mKO animals. (D) Distance moved in an open field in WT and p-Ube3a-mKO mice. Rotorod performance for WT and p-Ube3a-mKO (E) and WT and m-Ube3a-mKO (F) mice. All values are mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001.
Impaired performance in the rotorod task by Angelman syndrome mice
We tested the performance of the p-Ube3a-mKO and m-Ube3a-mKO mice back-crossed into FVB/NJ on the rotorod test to ensure they retain the previously described motor deficit (Jiang et al., 1998). Both p-Ube3a-mKO and m-Ube3a-mKO mouse cohorts displayed rotorod deficits with a main effect for both genotype and day (Fig. 3e,f) [p-Ube3a-mKO: Genotype, F(1, 72) = 36.42, p < 0.001, Day, F(3,72) = 11.54, p < 0.001; m-Ube3a-mKO, Genotype, F(1, 48) = 44.82, p < 0.001, Day, F(3,48) = 22.60, p < 0.001]. p-Ube3a-mKO had a strong trend for a decreased latency to fall on day one and fell faster than wild type mice on days two through four while m-Ube3a-mKO mice had a shorter latency to fall on each day (p-Ube3a-mKO; Day 1, t = 2.240, p > 0.05; Day 2, t = 3.194, p < 0.01; Day 3, t = 3.752, p < 0.01; Day 4, t = 2.885, p < 0.05; m-Ube3a-mKO; Day 1, t = 2.965, p < 0.05; Day 2, t = 4.492, p < 0.001; Day 3, t = 5.096, p < 0.001; Day 4, t = 4.507, p < 0.001). These results suggest Ube3amKO mice retain the motor deficit despite having been bred into the FVB/NJ genetic background.
Group housing decreases social interactions in the three chamber social approach task an effect blocked in Angelman syndrome mice
To directly compare these effects of neuronal UBE3A loss to the effects of single housing we measured social preference and interaction in the three chamber social approach task. All animals were group-housed five per cage from weaning. We examined the impact of single housing for 6 days. Like the effects of neuronal UBE3A loss, single housing in wild-type mice prolonged the time in the social zone during the second five minutes trial period (Fig. 4a–b) (Time in Zone: WT, t = 3.019, p < 0.05; WT, t = 2.822, p < 0.05). This effect in wild-type mice was largely occluded in p-Ube3a-mKO or m-Ube3a-mKO mice as single housing did not further increase the time these latter mice spent in the social zone (Time in Zone: p-Ube3a-mKO, t = 6.521, p < 0.001; m-Ube3a-mKO, t = 3.243, p < 0.01). Returning mice to a grouped housed setting for twenty four hours eliminated the social zone preferences during the second 5 minutes in wild-type mice, but had no significant impact in p-Ube3a-mKO or m-Ube3a-mKO mice (Fig. 4a–d) (Time in Zone: WT, t = 0.07811, p > 0.05, p-Ube3a-mKO, t = 3.598, p < 0.01; WT, t = 1.179, p > 0.05; m-Ube3a-mKO, t = 5.974, p < 0.001). These results suggest that deleting the maternal Ube3a allele blocks the group housing-induced loss of sociability in three chamber sociability during the second five minutes trial.
Fig. 4.
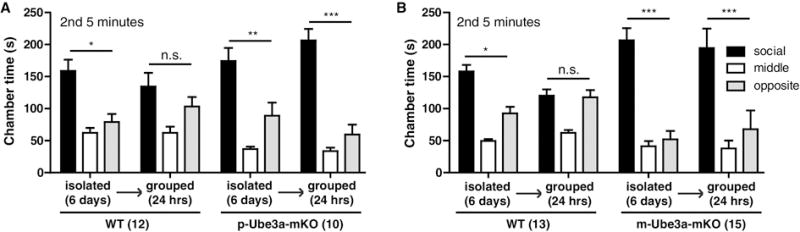
Group housing represses three chamber social preference in wild-type but not Angelman syndrome model mice. (A) Time in zone during the second five minutes trial for WT and p-Ube3a-mKO animals single housed for six days then grouped for twenty-four hours. (B) Time in zone during the second five minutes trial for WT and m-Ube3a-mKO animals single housed for six days then grouped for twenty-four hours. All values are mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001.
Group housing decreases social vocalizations an effect attenuated in Angelman syndrome mice
We next compared the effects of neuronal UBE3A loss to the effects of group vs. single housing on the ultrasonic vocalizations that occur when genotype-matched female conspecifics are paired. In this second cohort, social vocalizations were again increased in p-Ube3a-mKO and m-Ube3a-mKO female mice relative to wild-type littermates (Fig. 5a–c) (Grouped, t = 2.733, p < 0.05; Grouped, t = 3.210, p < 0.05). When these mice were single-houses for 24 hours, the social vocalizations were increased during the five minute pairing in wild-type mice and the difference across genotypes was lost (Isolated Day 2, t = 0.8267, p > 0.05; Isolated Day 2, t = 0.8780, p > 0.05). This finding suggests the social experiences of group housing strongly repress the social vocalization response in wild-type mice, whereas this effect is markedly attenuated in p-Ube3a-mKO and m-Ube3a-mKO mice. By contrast, the deprivation of social experiences through single housing strongly increased social vocalizations in wild-type mice, but only minimally increased the already high level of social vocalizations in p-Ube3a-mKO and m-Ube3a-mKO mice. The mice were then regrouped back into their original home cages with 5 mice per cage for 24 hours and retested. The 24 hours group housing social experience restored the lower social behavior in wild-types, but again minimally repressed social behavior in Ube3a-mKOs (Regrouped Day 3, t = 2.834, p < 0.05; Regrouped Day 3, t = 2.696, p < 0.05). To assess the effects of longer periods of social deprivation, p-Ube3a-mKO, m-Ube3a-mKO, and wild-type littermates were single housed for 6 days and then briefly paired to test social behavior. Wild type mice displayed a strongly increased social vocalization response (Isolated Day 9, t = 0.7564, p > 0.05; Isolated Day 9, t = 1.977, p > 0.05) while Ube3a-mKO mice showed only a modest, but non-significant increase in social vocalizations. Regrouping for 24 hours again completely suppressed the increase in social vocalizations in wild-type mice (Regrouped Day 10, t = 3.583, p < 0.05; Regrouped Day 10, t = 2.850, p < 0.05). These results suggest Ube3a may contribute to the process whereby social experiences repress social behavior.
Fig. 5.
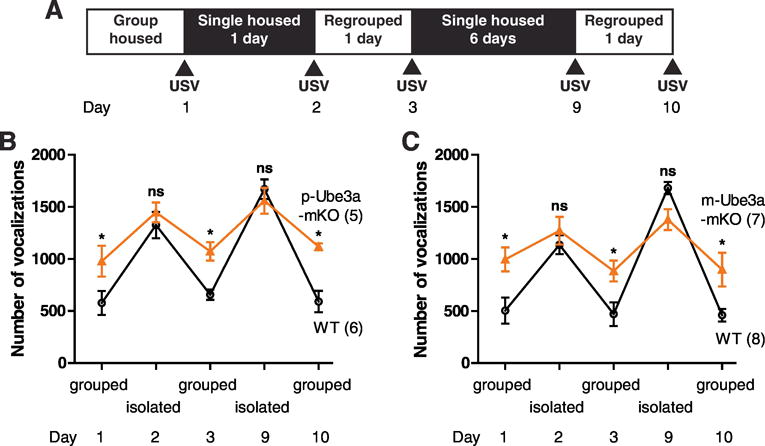
Group housing strongly represses female-female paired ultrasonic vocalizations in wild-type mice; an effect attenuated in Angelman syndrome model mice. (A) Protocol for paired ultrasonic vocalizations measurements in response to housing manipulations. (B) Female-female paired ultrasonic vocalizations were measured in WT and p-Ube3a-mKO mice before and after twenty-four hours or six days of single housing. (C) The same measurements performed in WT and m-Ube3a-mKO mice. All values are mean ± SEM. *p < 0.05.
Discussion
We report that mice with maternally-inherited deletions of Ube3a that models Angelman syndrome show an increased social preference/interaction duration in the three chamber approach task and an increased number of ultrasonic vocalizations and physical interactions when exposed to a novel female social stimulus. Yet these same mice show a reduced exploration of a novel object.
We chose to measure social behavior in Ube3amKO mice back-crossed into the FVB/NJ genetic background for several reasons. First, compared with other genetic backgrounds, FVB/NJ mice emit robust paired vocalizations when adult stranger females are paired and when a male is paired with a novel female (Smith et al., 20011). Second, the FVB/NJ has a strong social preference in the three chamber social approach test (Moy et al., 2007). Third, female FVB/NJ mice produce large average litter sizes and are good mothers. Fourth, our previous studies of the Ube3a2x mouse model of 15q11.2-13 triplication used the FVB/NJ genetic background allowing us to make a direct comparison of the effects of Ube3a loss that results in hypersociability to the effects of increased dosage of Ube3a that abolishes three chamber social approach/interaction and strongly decreases social vocalizations (Smith et al., 2011). These results suggest that reciprocal changes in Ube3a gene dosage (as in AS and 15q11-13 autism) lead to reciprocal changes in social behaviors. Several other reciprocal gene dosage disorders have been identified including MeCP2, 16p11.2, and 7q11.23-Williams Syndrome locus (Riby and Hancock, 2008; Weiss et al., 2008; Ramocki et al., 2009; Sanders et al., 2011). This pattern of bidirectional changes in gene dosage causing bidirectional changes in social behavior suggests a similar effect might be found in other behavioral domains and for other genes. The findings also suggest that achieving optimal behavioral performance may require precise control of gene expression levels. Our data supports a model where the endogenous baseline dosage of UBE3A actively represses social behavior and this effect may depend on the recent history of social experience.
Previous studies of Ube3amKO mice examined social behavior in the C57BL/6 genetic background and concluded that three chamber social approach is unaltered. These studies reported three chamber social approach during a single ten minutes trial. By recording two consecutive five minutes periods during the social trial we have uncovered a previously unknown feature of the Ube3amKO mouse: prolongation of sociability during a time when sociability is normally lost. Interestingly, humans with AS have a near total loss of language and yet still display increased smiling and laughing suggesting an increased social interest or motivation despite their impaired social skills. Pairs of Ube3amKO mice also emitted elevated ultrasonic vocalizations during a novel social encounter. The findings suggest that a simple measure of paired USV number and duration is not suitable for investigating the language disorder found in Angleman syndrome.
Our data show that neuronal loss of UBE3A leads to a prolonged interest in a social, but not to a novel non-social, stimulus. One potential explanation for this finding is the reciprocal changes in dopaminergic transmission in the striatum reported in Ube3amKO mice. Dopamine release is repressed in the dorsal striatum and enhanced in the ventral striatum (Berrios et al., 2016) paralleling the reduced preference for a novel object and the increased preference for a social stimulus, respectively. An alternate interpretation is that the decreased mobility observed in Ube3amKO mice somehow diminishes their ability or motivation to move away following an encounter with a novel stimulus. However, the decreased time spent interacting with a novel object argues that the ability to move away from a novel stimulus is intact and instead suggests UBE3A plays a role in regulating social motivation.
Both male and female Ube3amKO mice emitted more ultrasonic vocalizations when paired with a stranger female mouse. Interestingly, while female mice had a clear increase in social interaction in the second five minutes trial of the three chamber social approach test, male mice had only a trend for increased social approach duration. There were no sex differences in mobility that could explain this divergence. When male mice encounter a stranger male their interactions reflect both socialization and the establishment of dominance through aggression. Sex specific differences in the non-social aspects of the conspecific encounter in the three chamber social approach task may account for some of the difference observed between sexes and should be a focus of future studies.
The results of our manipulations of UBE3A and housing on sociability demonstrate that the amplitude of social preference is not static and can display a wide dynamic range under different genetic and environment conditions. Maternal Ube3a deletion increases sociability making them relatively resistant to the effects of group housing. Single housing wild type mice, by contrast, strongly increased their sociability, uncovering a strong intrinsic drive to socialize. These results also highlight the importance of carefully considering housing conditions when evaluating social behavior in rodent models of ASDs.
Conclusion
In this study we demonstrate that Ube3amKO mice have social behavioral features that may model some of the unusual social characteristics of individuals with Angelman syndrome. Both Ube3a-mKO mice display a prolonged social preference in the three chamber social approach task and increased vocalizations and physical interactions with a novel social stimulus. By contrast, these Angelman syndrome mice also showed reduced interest in exploring a novel object, dissociating the response to these two ethologically distinct stimuli. Wild-type mice exposed to brief periods of single housing display a prolonged social preference and increased ultrasonic vocalizations and physical interactions with a novel social stimulus resembling the effects of deleting maternal Ube3a. We suggest that UBE3A protein may be necessary for the repressive effects of social experience on subsequent social behavior and that the elevated sociability in mice and humans with maternal Ube3a deletion may reflect an enhanced social motivation.
Supplementary Material
Fig. 1s. Distance moved and chamber time during the acclimation trials in wild type and Angelman syndrome model mice. (A) Distance moved by wild type and pat-Ube3a-mKO mice during the first and second five minute three chamber trials. (B) Chamber time during the acclimation trial for female wild type and pat-Ube3a-mKO mice. (C) Distance moved by wild type and mat-Ube3a-mKO mice during the first and second five minutes three chamber trials. (D) Chamber time during the acclimation trial for female wild type and mat-Ube3a-mKO mice. All values are mean ± SEM.
Fig. 2s. Three chamber behavior and male-female ultrasonic vocalizations in male Angelman syndrome model mice. (A) Chamber time during the acclimation trial for male wild type and pat-Ube3a-mKO mice. (B) Time sniffing the social (novel mouse) or opposite (empty) chamber for male WT and pat-Ube3a-mKO mice. (C) Time spent in the social, middle, or opposite chamber for male WT and pat-Ube3a-mKO mice. (D) Distance moved by male wild type and pat-Ube3a-mKO mice during the first and second five minute three chamber trials. Number (E) and duration (F) of ultrasonic vocalizations emitted by male WT or pat-Ube3a-mKO mice paired with a novel female. Number (G) and duration (H) of ultrasonic vocalizations emitted by male WT or mat-Ube3a-mKO mice paired with a novel female. All values are mean ± SEM. *p < 0.05, **p < 0.01.
Highlights.
Opposing Ube3a gene dosage disorders have opposing effects on sociability
Angelman syndrome mice display increased social interactions and vocalizations
Angelman syndrome mice display reduced interactions with a novel object
Social experience represses sociability, an effect partially dependent on UBE3A
Acknowledgments
We thank Greg Salimando and Rebecca Broadhurst for assistance with genotyping and mouse husbandry. We also thank Kirsi Goldynia and Erica Choo for assistance with video analysis for physical contact during paired vocalizations. Supported by funding to M.P.A. from the NIH (1R01NS08916, 1R21MH100868, 1R21HD079249), Nancy Lurie Marks Family Foundation, Landreth Foundation, Simons Foundation, and Autism Speaks/National Alliance for Autism Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Financial Interests
The authors report no competing financial or other conflicts of interest.
Author Contributions
D.S. and M.P.A. designed the experiments and wrote the manuscript. D.S. performed all behavioral experiments.
References
- Adams D, Horsler K, Oliver C. Age related change in social behavior in children with Angelman syndrome. Am J Med Genet A. 2011;155A:1290–1297. doi: 10.1002/ajmg.a.33964. [DOI] [PubMed] [Google Scholar]
- Allensworth M, Saha A, Reiter LT, Heck DH. Normal social seeking behavior, hypoactivity and reduced exploratory range in a mouse model of Angelman syndrome. BMC Genet. 2011;12:7. doi: 10.1186/1471-2156-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrios J, Stamatakis AM, Kantak PA, McElligott ZA, Judson MC, Aita M, Rougie M, Stuber GD, Philpot BD. Loss of UBE3A from TH-expressing neurons suppresses GABA co-release and enhances VTA-NAc optical self-stimulation. Nat Commun. 2016;7:10702. doi: 10.1038/ncomms10702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonati MT, Russo S, Finelli P, Valsecchi MR, Cogliati F, Cavalleri F, Roberts W, Elia M, Larizza L. Evaluation of autism traits in Angelman syndrome: a resource to unfold autism genes. Neurogenetics. 2007;8:169–178. doi: 10.1007/s10048-007-0086-0. [DOI] [PubMed] [Google Scholar]
- Dölen G, Darvishzadeh A, Huang KW, Malenka RC. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature. 2013;501:179–184. doi: 10.1038/nature12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer PL, Hanayama R, Bloodgood BL, Mardinly AR, Lipton DM, Flavell SW, Kim TK, Griffith EC, Waldon Z, et al. The Angelman Syndrome protein Ube3A regulates synapse development by ubiquitinating arc. Cell. 2010;140:704–716. doi: 10.1016/j.cell.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heald M, Allen D, Villa D, Oliver C. Discrimination training reduces high rate social approach behaviors in Angelman syndrome: proof of principle. Res Dev Disabil. 2013;34:1794–1803. doi: 10.1016/j.ridd.2013.02.012. [DOI] [PubMed] [Google Scholar]
- Huang HS, Burns AJ, Nonneman RJ, Baker LK, Riddick NV, Nikolova VD, Riday TT, Yashiro K, Philpot BD, Moy SS. Behavioral deficits in an Angelman syndrome model: effects of genetic background and age. Behav Brain Res. 2013;243:79–90. doi: 10.1016/j.bbr.2012.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal I, Kumar V, Vatsa N, Singh BK, Shekhar S, Sharam A, Jana NR. Environmental enrichment improves behavioral abnormalities in a mouse model of Angelman syndrome. Mol Neurobiol. 2016 doi: 10.1007/s12035-016-0080-3. [DOI] [PubMed] [Google Scholar]
- Jiang YH, Armstrong D, Albrecht U, Atkins CM, Noebels JL, Eichele G, Sweatt JD, Beaudet AL. Mutation of the Angelman ubiquitin ligase in mice causes increased cytoplasmic p53 and deficits of contextual learning and long-term potentiation. Neuron. 1998;21:799–811. doi: 10.1016/s0896-6273(00)80596-6. [DOI] [PubMed] [Google Scholar]
- Maggio JC, Whitney G. Ultrasonic vocalizing by adult female mice (Mus musculus) J Comp Psychol. 1985;99:420–436. [PubMed] [Google Scholar]
- Matsuura T, Sutcliffe JS, Fang P, Galjaard RJ, Jiang YH, Benton CS, Rommens JM, Beaudet AL. De novo truncating mutations in E6-AP ubiquitin-protein ligase gene (UBE3A) in Angelman syndrome. Nat Genet. 1997;15:74–77. doi: 10.1038/ng0197-74. [DOI] [PubMed] [Google Scholar]
- Miura K, Kishino T, Li E, Webber H, Dikkes P, Holmes GL, Wagstaff J. Neurobehavioral and electroencephalographic abnormalities in Ube3a maternal-deficient mice. Neurobiol Dis. 2002;9:149–159. doi: 10.1006/nbdi.2001.0463. [DOI] [PubMed] [Google Scholar]
- Moss J, Howlin P, Hastings RP, Beaumont S, Griffith GM, Petty J, Tunnicliffe P, Yates R, Villa D, Oliver C. Social behavior and characteristics of autism spectrum disorder in Angelman, Cornelia de Lange, and Cri du Chat syndromes. Am J Intellect Dev Disabil. 2013;118:262–283. doi: 10.1352/1944-7558-118.4.262. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, Barbaro JR, Wilson LM, Threadgill DW, Lauder JM, Magnuson TR, Crawley JN. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler JJ, Moy SS, Dold G, Trang D, Simmons N, Perez A, Young NB, Barbaro RP, Piven J, Magnuson TR, Crawley JN. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 2004;3:303–14. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- Pelc K, Cheron G, Dan B. Behavior and neuropsychiatric manifestations in Angelman syndrome. Neuropsychiatr Dis Treat. 2008;4:577–584. doi: 10.2147/ndt.s2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters SU, Beaudet AL, Madduri N, Bacino CA. Autism in Angelman syndrome: implications for autism research. Clin Genet. 2004;66:530–536. doi: 10.1111/j.1399-0004.2004.00362.x. [DOI] [PubMed] [Google Scholar]
- Peters SU, Goddard-Finegold J, Beaudet AL, Madduri N, Turcich M, Bacino CA. Cognitive and adaptive behavior profiles of children with Angelman syndrome. Am J Hum Gen. 2004;128a:110–113. doi: 10.1002/ajmg.a.30065. [DOI] [PubMed] [Google Scholar]
- Ramocki MB, Peters SU, Tavyev YJ, Zhang F, Carvalho CM, Schaaf CP, Richman R, Fang P, Glaze DG, Lupski JR, Zoghbi HY. Autism and other neuropsychiatric symptoms are prevalent in individuals with MeCP2 duplication syndrome. Ann Neurol. 2009;66:771–782. doi: 10.1002/ana.21715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riby DM, Hancock PJ. Viewing it differently: Social scene perception in Williams syndrome and Autism. Neuropsychologia. 2008;46:2855–2860. doi: 10.1016/j.neuropsychologia.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Sanders SJ, Ercan-Sencicek AG, Hus V, Luo R, Murtha MT, Moreno-De-Luca D, Chu SH, Moreau MP, Gupta AR, Thomson SA, et al. Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron. 2011;70:863–885. doi: 10.1016/j.neuron.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SE, Zhou YD, Zhang G, Jin Z, Stoppel DC, Anderson M. Increased gene dosage of Ube3a results in autism traits and decreased glutamate synaptic transmission in mice. Sci Transl Med. 2011:3103ra97. doi: 10.1126/scitranslmed.3002627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valluy J, Bicker S, Aksoy-Aksel A, Lackinger M, Sumer S, Fiore R, Wüst T, Seffer D, Metge F, Dieterich C, Wöhr M, Schwarting R, Schratt G. A coding-independent function of an alternative Ube3a transcript during neuronal development. Nat Neurosci. 2015;18:666–673. doi: 10.1038/nn.3996. [DOI] [PubMed] [Google Scholar]
- Walz NC. Parent report of stereotyped behaviors social interaction, and developmental disturbances in individuals with Angelman syndrome. J Autism Dev Disord. 2007;37:940–947. doi: 10.1007/s10803-006-0233-8. [DOI] [PubMed] [Google Scholar]
- Wang H, Liang S, Burgdorf J, Wess J, Yeomans J. Ultrasonic vocalizations induced by sex and amphetamine in M2, M4, M5 muscarinic and D2 dopamine receptor knockout mice. PLoS One. 2008;3:e1893. doi: 10.1371/journal.pone.0001893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss LA, Shen Y, Korn JM, Arking DE, Miller DT, Fossdal R, Saemundsen E, Stefansson H, Ferreira MA, Green T, et al. Association between microdeletion and microduplication at 16p11.2 and autism. N Engl J Med. 2008;358:667–675. doi: 10.1056/NEJMoa075974. [DOI] [PubMed] [Google Scholar]
- Williams CA, Beaudet AL, Clayton-Smith J, Knoll JH, Kyllerman M, Laan LA, Magenis RE, Moncla A, Schinzel AA, Summers JA, Wagstaff J. Angelman syndrome 2005: updated consensus for diagnostic criteria. Am J Med Genet A. 2006;140:413–418. doi: 10.1002/ajmg.a.31074. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. 1s. Distance moved and chamber time during the acclimation trials in wild type and Angelman syndrome model mice. (A) Distance moved by wild type and pat-Ube3a-mKO mice during the first and second five minute three chamber trials. (B) Chamber time during the acclimation trial for female wild type and pat-Ube3a-mKO mice. (C) Distance moved by wild type and mat-Ube3a-mKO mice during the first and second five minutes three chamber trials. (D) Chamber time during the acclimation trial for female wild type and mat-Ube3a-mKO mice. All values are mean ± SEM.
Fig. 2s. Three chamber behavior and male-female ultrasonic vocalizations in male Angelman syndrome model mice. (A) Chamber time during the acclimation trial for male wild type and pat-Ube3a-mKO mice. (B) Time sniffing the social (novel mouse) or opposite (empty) chamber for male WT and pat-Ube3a-mKO mice. (C) Time spent in the social, middle, or opposite chamber for male WT and pat-Ube3a-mKO mice. (D) Distance moved by male wild type and pat-Ube3a-mKO mice during the first and second five minute three chamber trials. Number (E) and duration (F) of ultrasonic vocalizations emitted by male WT or pat-Ube3a-mKO mice paired with a novel female. Number (G) and duration (H) of ultrasonic vocalizations emitted by male WT or mat-Ube3a-mKO mice paired with a novel female. All values are mean ± SEM. *p < 0.05, **p < 0.01.


