Abstract
One of the cell types first encountered by human immunodeficiency virus type 1 (HIV-1) following sexual transmission are dendritic cells (DC). DC capture HIV-1 through C-type lectin receptors, of which the best studied example is DC-SIGN, which mediates HIV-1 internalization. DC can keep the virus infectious for several days and are able to transmit HIV-1 to CD4+ T cells. We tested proteins from milk and serum for their ability to block DC-mediated HIV-1 transmission, of which bovine lactoferrin (bLF) is the most potent inhibitor. bLF binds strongly to DC-SIGN, thus preventing virus capture and subsequent transmission. Interestingly, bLF is a much more efficient inhibitor of transmission than human lactoferrin. Since bLF is nontoxic and easy to purify in large quantities, it is an interesting candidate microbicide against HIV-1. Another advantage of bLF is its ability to block HIV-1 replication in T cells. DC-mediated capture of a bLF-resistant HIV-1 variant that was selected during long-term culturing in T cells could still be blocked by bLF. This underscores the usefulness of bLF as a microbicide drug to prevent HIV-1 transmission.
Human immunodeficiency virus type 1 (HIV-1) infects CD4+ T cells through interaction of its envelope protein gp120 with CD4 and a chemokine coreceptor on the target cell (6). However, sexual transmission of HIV-1 first requires the crossing of mucosal tissue, and the precise mechanism of HIV-1 transmission across this barrier is poorly understood. One possibility is that HIV-1 enters via lesions caused by other diseases (12). However, studies with rhesus macaques show that lesions are not absolutely required for transmission and that simian immunodeficiency virus (SIV) can cross the intact epithelium (29, 30, 38). One of the cell types first encountered by HIV-1 or SIV are intraepithelial and submucosal dendritic cells (DC) (10, 20, 23, 34, 38). If not occurring via lesions, virus uptake could occur via the processes that extend into the luminal surface. DC are professional antigen-presenting cells that sample the environment at sites of pathogen entry. Sentinel immature DC develop into mature effector DC upon activation by microorganisms and migrate to the draining lymph nodes where they stimulate naive Th cells (2, 3). HIV-1 has been proposed to make use of this migratory process, being captured by the DC and delivered to the lymph node, where the virus is transmitted to CD4+ T cells. The lymph node then becomes the principal site of virus production (18, 33). DC capture HIV-1 through C-type lectin receptors, of which the best studied example is DC-SIGN (CD209), which mediates HIV-1 internalization such that the virus remains infectious for several days (14, 25). Subsequent transmission to T cells takes place via an “infectious synapse,” but virus that has not been internalized can also be transmitted to T cells (14, 28).
Milk proteins are known to cover a wide range of biological functions. For example, milk proteins and derivates thereof show antifungal, antibacterial, and antiviral properties (7, 8, 13, 19, 39, 44). Positively charged macromolecules can inhibit the binding of HIV-1 to the CD4 receptor (27, 32), and negatively charged macromolecules can inhibit HIV-1 by binding to the positively charged V3 loop of gp120 (40). Most of these proteins need to be chemically modified in order to become inhibitory, but native lactoferrin (LF) inhibits HIV-1 replication in T cells (7, 13). LF binds strongly to the V3 loop of gp120, but it may also bind to the (co)receptors on the target cell (8, 40). LF is a protein of approximately 80 kDa, consisting of two symmetric lobes (the N and C lobes), and is thought to be an important component of the innate immune system (26, 44). Prior HIV-1 inhibition studies were performed by use of HIV-1 replication assays. In the present study, we tested proteins from milk and serum for their ability to block DC-mediated HIV-1 transmission. We found that bovine LF (bLF) is the most potent inhibitor. bLF binds to DC-SIGN, thus preventing virus capture and subsequent transmission. Interestingly, bLF is a much more efficient inhibitor of transmission than human LF (hLF). Since LF is nontoxic, available in large quantities, and inexpensive, it is an interesting candidate microbicide against HIV-1. The usefulness of bLF is underscored by the fact that DC-mediated transmission of a bLF-resistant HIV-1 variant, which was selected during replication studies, can still be blocked by bLF.
MATERIALS AND METHODS
Generation of monocyte-derived dendritic cells.
Peripheral blood mononuclear cells were isolated by density centrifugation on Lymphoprep (Nycomed, Torshov, Norway). Subsequently, peripheral blood mononuclear cells were layered on a Percoll gradient (Pharmacia, Uppsala, Sweden) with three density layers (1.076, 1.059, and 1.045 g/ml). The light fraction with predominantly monocytes was collected, washed, and seeded in 24- or 6-well culture plates (Costar, Cambridge, Mass.) at a density of 5 × 105 or 2.5 × 106 cells per well, respectively. After 60 min at 37°C, nonadherent cells were removed, and adherent cells were cultured to obtain immature DC in Iscove's modified Dulbecco's medium (Life Technologies Ltd., Paisley, United Kingdom) with gentamicin (86 μg/ml) (Duchefa, Haarlem, The Netherlands) and 10% fetal clone serum (HyClone, Logan, Utah) and supplemented with granulocyte-macrophage colony-stimulating factor (500 U/ml) (Schering-Plough, Uden, The Netherlands) and interleukin-4 (IL-4) (250 U/ml) (Strathmann Biotec AG, Hannover, Germany). At day 3, the culture medium with supplements was refreshed. At day 6, maturation was induced by culturing the cells with the following factors alone or in combination as indicated: IL-1β (10 ng/ml) (Strathmann Biotec AG), tumor necrosis factor alpha (TNF-α) (50 ng/ml) (Strathmann Biotec AG), poly(I·C) (20 μg/ml) (Sigma-Aldrich, St. Louis, Mo.), gamma interferon (1,000 U/ml) (Strathmann Biotec AG), or prostaglandin E2 (PGE2) (10−6 M) (Sigma-Aldrich). After 2 days, mature CD14− CD1b+ CD83+ DC were obtained. All subsequent tests were performed after harvesting and extensive washing of the cells to remove all factors.
Flow cytometry.
Mature DC were analyzed for the expression of cell surface molecules by fluorescence-activated cell sorting. Mouse anti-human monoclonal antibodies were used against the following molecules: CD14 (IgG2b; BD Biosciences, San Jose, CA), CD1b (B-B5, immunoglobulin G1 [IgG1]; Diaclone, Besançon, France), CD83 (HB15a, IgG2b; Immunotech, Marseille, France), CD86 (1G10, IgG2a; Innogenetics, Ghent, Belgium), and ICAM-1 (CD54) (R&D Systems, Abingdon, United Kingdom). All monoclonal antibody incubations were followed by incubation with fluorescein isothiocyanate-conjugated goat F(ab′)2 anti-mouse IgG and IgM (Jackson ImmunoResearch Laboratories, West Grove, Pa.). Samples were analyzed on a FACScan (BD Biosciences).
Virus stocks and cells.
The SupT1 T-cell line was transfected by electroporation with 5 μg of the molecular clone of T-tropic HIV-1 LAI. The virus-containing supernatant was harvested at 3 to 5 days posttransfection, filtered, and stored at −80°C. The concentration of virus was determined by CA-p24 enzyme-linked immunosorbent assay (ELISA). SupT1 cells were maintained in RPMI 1640 (Life Technologies), supplemented with 10% fetal calf serum, 2 mM sodium pyruvate, 10 mM HEPES, 2 mM l-glutamine, penicillin (100 U/ml), and streptomycin (100 μg/ml).
The LuSIV cell with an integrated long terminal repeat-luciferase reporter construct has been described previously (36). Cells were maintained in the same medium as the SupT1 cells, but with 300 μg of hygromycin B per ml to maintain the luciferase genetic construct. One day before the transmission experiments, the cells were split 1:3 in fresh medium without hygromycin B.
Milk proteins and derivatives.
bLF, C-lobe bLF, human serum albumin (HSA), HSA modified by 13-kDa heparin (Hep1-HSA), succinylated HSA (Suc-HSA), β-lactoglobulin (βLG), βLG modified by 3-hydroxyphthalic anhydride (3HP-βLG), and nisin Z were prepared as described below. bLF was obtained from Broculo Domo Ingredients (Beilen, The Netherlands). Hydrolysis of 5% bLF by trypsin (TPCK [tosylsulfonyl phenylalanyl chloromethyl ketone] treated; obtained from Sigma Chemical Co., St. Louis, Mo.) was performed at a bLF/trypsin ratio of 1:20 for 20 h at 37°C in 0.05 M citrate-bicarbonate buffer with 5 mM CaCl2 and 5 mM FeCl3 at pH 8.0. The peptide mixture was further purified by reversed-phase high-pressure liquid chromatography (35). The purified C-lobe (obtained as a peak eluting between 23 and 27 min) was analyzed by means of N-terminal sequencing and mass spectrometry (Quattro II; Micromass, Chershire, United Kingdom). This C-lobe was identified as the fragment of bLF consisting of residues 342 to 689, to which iron was still bound. HSA, consisting of at least 95% monomeric protein, was obtained from the Central Laboratory of the Blood Transfusion Services (Sanquin). HSA was covalently modified with heparin and succinic acid anhydride to yield Hep1-HSA and Suc-HSA, respectively (5). βLG was purified as described previously (9). 3HP-βLG was produced through modification of βLG with 3-hydroxyphthalic anhydride as described previously (7). Nisin Z was produced and purified as described previously (24). hLF was expressed in transgenic rice as described previously (22, 31); in brief, a synthetic hLF gene with a signal sequence was linked to the rice glutelin 1 promoter and transformed into rice cells to produce recombinant hLF.
Single-cycle transmission assay.
Fully matured DC were incubated in a 96-well-plate (35 × 103 to 50 × 103 DC/50 μl/well) with virus (5 ng of CA-p24/well) for 2 h at 37°C. The DC were washed with phosphate-buffered saline after centrifugation at 400 × g to remove unbound virus. Washing was repeated, followed by addition of 50 × 103 LuSIV cells. All candidate transmission inhibitors were preincubated with either DC, virus, or LuSIV cells for 30 min at 37°C. After 24 h, LuSIV cells were harvested for luciferase measurement. The assay of transmission from DC to SupT1 cells has been described elsewhere (37). After washing of DC, SupT1 T cells were added and cocultured. Viral replication was monitored by measuring CA-p24 in the supernatant by ELISA.
LuSIV cells were collected in Eppendorf tubes, spun down at 500 × g, and resuspended in 50 μl of lysis buffer (25 mM Tris-HCl [pH 7.8], 2 mM dithiothreitol, 2 mM CDTA, 10% glycerol, 1% Triton X-100). The cells were incubated for 45 min at room temperature while shaking, followed by 10 min of centrifugation at 16,000 × g. The supernatant was transferred to a white solid 96-well plate (Corning Costar), and 150 μl of luci-buffer (100 μg of bovine serum albumin per ml, 6.6 mM ATP, 15 mM MgSO4, 25 mM glycylglycine) was added. The Lumistar control was used to determine the amount of luciferase. One hundred microliters of DE(-)Luciferin (Roche Diagnostics GmbH) was injected per well (0.28 mg/ml of luci-buffer excluding ATP), and 50 × 103 LuSIV cells grown without DC or HIV-1 were used to obtain the background luciferase value, which was subtracted from all data.
HIV-1 capture by DC.
Fully matured DC were incubated in a 96-well plate (35 × 103 DC/50 μl/well) with bLF or medium for 30 min, followed by incubation with virus for 2 h at 37°C. After centrifugation at 400 × g, the DC were washed with phosphate-buffered saline to remove unbound virus. This step was performed twice and was followed by CA-p24 ELISA to determine the amount of HIV-1 captured by the DC.
DC-SIGN adhesion assay.
The DC-SIGN adhesion assay has been described elsewhere (15, 16). To test bLF, HSA, βLG, 3HP-βLG, and nisin Z for the ability to inhibit the gp120-DC-SIGN interaction, gp120 (1 μg/ml) was applied to an ELISA plate and left for 1 h at 4°C, followed by incubation with the candidate inhibitors and soluble DC-SIGN-Fc (1.5 μg/ml) for 30 min at room temperature. After washing, binding of DC-SIGN to gp120 was measured by anti-IgG1 ELISA.
To determine the DC-SIGN affinity for different forms of LF, we applied different amounts of the bLF and hLF variants (and 1 μg of gp120 per ml as a positive control) to an ELISA plate and measured the binding of soluble DC-SIGN-Fc after 30 min of incubation at room temperature.
RESULTS
Native bLF inhibits DC-mediated HIV-1 transmission.
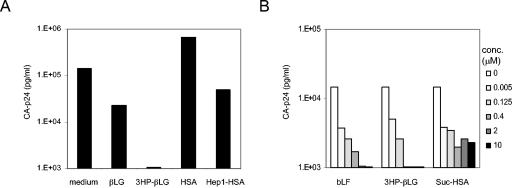
We tested several modified and native proteins from human serum and bovine milk for their ability to inhibit in vitro HIV-1 transmission from DC to T cells. We have previously shown that DC subsets differ significantly in their ability to transmit HIV-1 to SupT1 T cells (37). Based on their ability to induce a Th1 or Th2 response, mature DC are designated DC1 and DC2, respectively (11). For our experiments, we used the DC1 subset, which is most efficient at HIV-1 transmission. A total of 50,000 DC were incubated with a candidate inhibitor for 30 min at 37°C, followed by 2 h of incubation with HIV-1. The DC were washed twice to remove inhibitors and unbound virus and cocultured with SupT1 T cells. Virus transmission and subsequent spread in the T-cell culture were monitored by measuring CA-p24 in the supernatant by ELISA. First, we tested 10 μM native βLG, 3HP-βLG, native HSA, and Hep1-HSA (Fig. 1A). Only the modified 3HP-βLG protein was able to inhibit DC-mediated HIV-1 transmission to SupT1 T cells, most likely because of its negative charge that binds to the positively charged HIV-1 envelope gp120. We next tested native bLF, which was compared to the negatively charged compounds 3HP-βLG and Suc-HSA at different concentrations (Fig. 1B). Suc-HSA was not as efficient at blocking transmission as 3HP-βLG. Most importantly, we observed a complete block of HIV-1 transmission and subsequent replication with 2 μM native bLF protein. We therefore decided to focus on the mechanism of bLF inhibition.
FIG. 1.
Inhibitory properties of modified and native proteins from human serum and bovine milk for DC-mediated HIV-1 transmission. (A) Mature DC were incubated with candidate inhibitors for 30 min at 37°C, followed by 2 h of incubation with HIV-1. The DC were washed twice to remove unbound virus and subsequently cocultured with SupT1 T cells. Virus transmission and subsequent replication were measured by CA-p24 ELISA at day 6. We tested βLG and HSA as well as chemically modified forms thereof (3HP-βLG and Hep1-HSA) at 10 μM. (B) Inhibition by native bLF and the modified proteins 3HP-βLG and Hep1-HSA. Mature DC were preincubated with a concentration range of these proteins (0 to 10 μM), followed by 2 h of incubation with HIV-1, washing, and coculturing with SupT1 T cells. Virus transmission and subsequent replication were measured by CA-p24 ELISA at day 4.
Single-cycle transmission assay to quantify HIV-1 transmission efficiency.
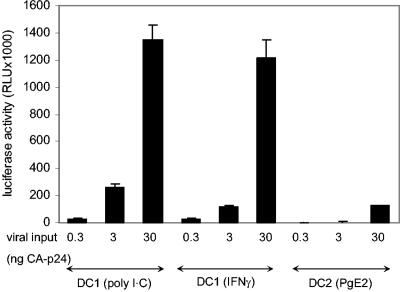
The transmission experiments shown in Fig. 1 involve DC-mediated transmission and multiple subsequent rounds of virus replication in SupT1 T cells. Thus, it cannot be formally excluded that the inhibitors target the latter process and not the DC-mediated transmission to T cells. We therefore set up a single-cycle transmission assay with reporter LuSIV cells, CEMx174-derived cells that contain the firefly luciferase reporter gene downstream of the SIVmac239 long terminal repeat. This cell line is susceptible to infection by HIV or SIV, which results in Tat-mediated expression of luciferase (36). When the LuSIV cells are harvested within 24 h for luciferase measurement, there is no significant T-cell spread of newly produced HIV-1 virions, such that luciferase activity is a quantitative measure of the amount of virus that is transmitted by DC. We first tested this new transmission assay with three different subsets of mature DC: two types of DC1 [matured by poly(I·C) or gamma interferon plus lipopolysaccharide (LPS) and maturation factors IL-1β and TNF-α] and one DC2 type (matured by PgE2 and LPS plus maturation factors). Consistent with previous results (37), both DC1 subsets were much more efficient in transmitting HIV-1 than DC2 cells (Fig. 2). Further inhibition experiments were performed with the most efficient DC1 subset stimulated with poly(I·C).
FIG. 2.
Single-cycle HIV-1 transmission assay. Immature DC were stimulated for 48 h with poly(I·C); gamma interferon (IFNγ) plus LPS, IL-1β, and TNF-α; and PgE2 plus LPS, IL-1β, and TNF-α to produce two types of DC1 and one type of DC2, respectively. The DC were incubated for 2 h with different amounts of HIV-1 (0.3, 3, and 30 ng of CA-p24), followed by washing steps to remove unbound virus. DC were cocultured with reporter LuSIV cells, and luciferase activity was measured after 24 h. RLU, relative light units. Error bars indicate standard deviations.
bLF inhibits transmission more efficiently when preincubated with DC.
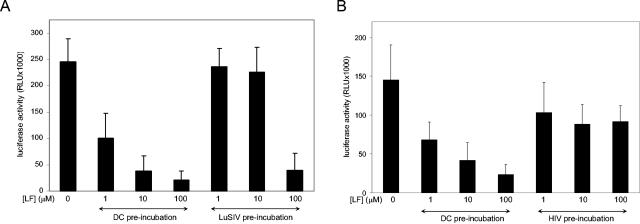
To study the mechanism of bLF-mediated inhibition of transmission by DC, we varied the time of bLF addition in the single-cycle transmission assay. We compared bLF preincubation of DC with preincubation of reporter LuSIV cells (Fig. 3A). DC were incubated for 30 min at 37°C at different bLF concentrations. This preincubation was followed by a 2-h incubation with HIV-1, two washes to remove unbound virus and bLF, and coculturing with LuSIV cells. This resulted in severe inhibition of HIV-1 transmission. In contrast, when the LuSIV cells were preincubated with bLF before the coculture with DC-bound HIV-1, we observed inhibition only at the highest bLF concentration. The results indicate that bLF is a more effective inhibitor of transmission when incubated with DC rather than with LuSIV cells. This suggests that bLF interferes with the DC-HIV interaction. Next, we examined whether bLF exerts its inhibitory effect through interaction with the DC or the virus (Fig. 3B). Either DC or HIV was preincubated for 30 min with bLF, followed by mixing and incubation for 2 h. After washing twice, the DC were cocultured with LuSIV cells. The results clearly show that bLF inhibits more potently when it is preincubated with DC, suggesting a cellular target.
FIG. 3.
bLF blocks transmission when incubated with DC. (A) DC were incubated with HIV-1 for 2 h, followed by washing and coculturing with LuSIV cells. We preincubated either DC or the target LuSIV cells with bLF at 1, 10, or 100 μM. (B) DC or HIV-1 were preincubated with 1, 10, or 100 μM bLF, followed by mixing and incubation for 2 h. After washing, DC were cocultured with LuSIV cells. RLU, relative light units. Error bars indicate standard deviations.
bLF prevents DC-mediated HIV-1 capture.
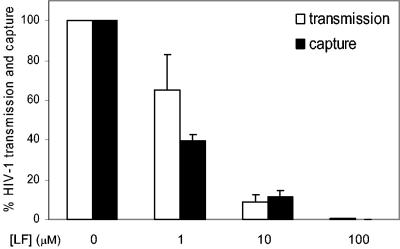
bLF could have an indirect impact on DC function. We tested this by culturing immature DC at 10 and 100 μM bLF for 2 days [with or without LPS plus maturation factors (IL-1β and TNF-α) or poly(I·C)]. We observed no induction of apoptosis (by annexin and propidium iodide staining), and bLF did not influence cytokine (IL-6, IL-12 p70, and TNF-α) production, DC maturation (CD83 expression), or the expression level of the costimulatory molecule CD86. bLF also did not affect ICAM-1 expression on the DC, which is an important factor for DC-mediated HIV-1 transmission (37). In addition, bLF did not bias naive T-cell outgrowth induced by the differentially cultured DC (results not shown). Thus, it seemed more likely that bLF interferes directly with DC-HIV interaction. To test this possibility, we measured the ability of DC to capture HIV-1 in the presence of bLF (Fig. 4). DC were preincubated for 30 min with 0, 1, 10, or 100 μM bLF, followed by HIV-1 incubation and washing steps. Half of the DC population was subsequently cocultured with LuSIV reporter cells to determine the transmission efficiency. The other half was used to measure the amount of captured HIV-1 by CA-p24 ELISA. The amount of transmission and virus capture was set at 100% for the control incubation without bLF. The drop in transmission efficiency coincided with inhibition of HIV-1 capture, demonstrating that bLF blocks virus-cell contact.
FIG. 4.
bLF blocks DC-mediated HIV-1 capture. DC were preincubated with 0, 1, 10, or 100 μM bLF for 30 min, followed by incubation with HIV-1 for 2 h. After washing, half of the DC population was cocultured with LuSIV cells for 24 h. The other half was used to quantify the amount of captured HIV-1 by CA-p24 ELISA. The amount of transmission and virus capture is set at 100% for the mock incubation without bLF. Error bars indicate standard deviations.
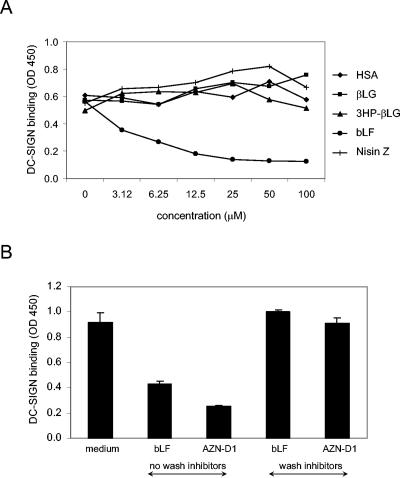
bLF blocks the DC-SIGN-gp120 interaction.
Since DC-SIGN plays an important role in the capture of HIV-1 by monocyte-derived DC, it is possible that bLF interacts with this surface molecule and thus interferes with HIV-1 binding. This was tested directly in a DC-SIGN-gp120 interaction assay (Fig. 5A). gp120 was applied to an ELISA plate, and bLF or control proteins (HSA, βLG, 3HP-βLG, and nisin Z) were added together with soluble DC-SIGN-Fc. After washing, the binding of DC-SIGN to gp120 was determined by anti-IgG1 ELISA. This experiment clearly shows that bLF blocks the interaction between gp120 and DC-SIGN. Next, we tested whether bLF acts by binding either DC-SIGN or gp120 (Fig. 5B). Using the same type of binding assay, we performed time-of-addition experiments with bLF and a blocking antibody against DC-SIGN (AZN-D1) (15). The adhesion of soluble DC-SIGN-Fc to coated gp120 could be blocked by bLF and AZN-D1. However, we measured no inhibition when bLF or AZN-D1 was preincubated with the coated gp120, followed by washing and subsequent addition of DC-SIGN-Fc. These results suggest that bLF binds the DC-SIGN molecule.
FIG. 5.
bLF blocks the DC-SIGN-gp120 interaction. (A) gp120 was applied to an ELISA plate. bLF and control proteins (HSA, βLG, 3HP-βLG, or nisin Z) were added, as well as soluble DC-SIGN-Fc. After washing, the binding of DC-SIGN to gp120 was determined by anti-IgG1 ELISA. (B) Adhesion of DC-SIGN-Fc to gp120 was determined in the presence of bLF or DC-SIGN-blocking antibody AZN-D1 or after bLF and AZN-D1 had been preincubated with gp120, followed by washing. OD 450, optical density at 450 nm. Error bars indicate standard deviations.
bLF binds DC-SIGN and is the most potent HIV-1 transmission inhibitor.
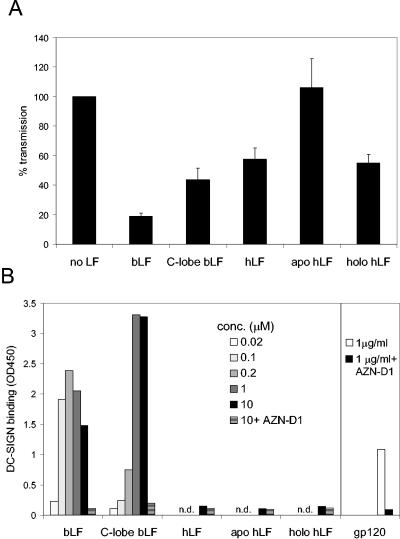
We next compared several LF variants for their inhibitory potential in the single-cycle transmission assay. We tested bLF, the C-lobe fragment of bLF, hLF, iron-depleted hLF (apo-hLF), and iron-saturated hLF (holo-hLF) (Fig. 6A). bLF is the most potent transmission inhibitor in comparison to the other variants. Partial inhibition was observed for the C-lobe fragment of bLF, which consists of the C-terminal residues 342 to 689 to which iron is still bound. The iron-depleted hLF showed no inhibitory capabilities, whereas the iron-saturated variant did inhibit transmission to a small extent. This suggests that iron binding and protein conformation are important for inhibition. The transmission results correlate with the ability of these proteins to inhibit HIV-1 capture by DC (results not shown). In addition, we tested these LF variants for their ability to bind DC-SIGN in a direct binding assay (Fig. 6B). We applied several amounts of the LF variants to an ELISA plate and measured the adhesion of soluble DC-SIGN-Fc to the plate. gp120 was included as a positive control. Both bLF and the C-lobe appear to bind DC-SIGN. None of the hLF variants bind DC-SIGN. The blocking antibody AZN-D1 could prevent binding of DC-SIGN-Fc, demonstrating the specificity of the assay.
FIG. 6.
LF variants and inhibition of transmission. (A) DC were preincubated with bLF, the C-lobe fragment of bLF, hLF, apo-hLF, or holo-hLF. After HIV-1 incubation and washing, DC were cocultured with LuSIV cells. The results of eight independent experiments are combined; transmission without inhibitor was set at 100%. Error bars indicate standard deviations. (B) Binding of LF variants to DC-SIGN. Several amounts of the LF variants were applied to an ELISA plate, and the adhesion of soluble DC-SIGN-Fc was measured by anti-IgG1 ELISA. At the highest concentration of LF, DC-SIGN-blocking antibody AZN-D1 was also added. As a positive control, we applied gp120. OD 450, optical density at 450 nm; n.d., not done.
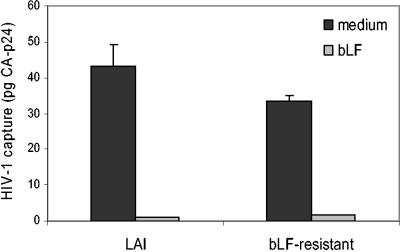
bLF blocks DC-mediated capture of bLF-resistant HIV-1.
During previous prolonged culturing of HIV-1 in the presence of bLF, a resistant HIV-1 variant was selected (8). The envelope protein of this variant contains two mutations (T188I and G431R) that have been suggested to play a role in the virus-cell interaction. Since HIV-1 replication involves virus-cell interactions other than DC-mediated transmission, we tested whether DC-mediated capture of the bLF-resistant HIV-1 could be blocked by bLF (Fig. 7). DC were preincubated with bLF or mock treated, and binding of wild-type or bLF-resistant HIV-1 was determined by CA-p24 ELISA. Both viruses can be captured by DC, indicating that the resistance mutations in the envelope protein do not interfere with DC-SIGN binding. Most importantly, bLF can block DC-mediated capture of the resistant HIV-1. This indicates that different bLF-mediated inhibitory mechanisms affect the processes of HIV-1 transmission and replication.
FIG. 7.
DC-mediated capture of bLF-resistant HIV-1. DC that were preincubated with bLF or medium were incubated with wild-type HIV-1 or bLF-resistant HIV-1, which was selected during prolonged culturing in the presence of bLF. After washing twice, virus capture by DC was measured by CA-p24 ELISA. Error bars indicate standard deviations.
DISCUSSION
To slow the HIV-1 pandemic, preventing HIV-1 from establishing a persistent infection after sexual intercourse may be more effective than treating HIV-1-seropositive patients with antiretroviral medicines. In countries where women are indecisive about condom usage, alternatives such as microbicides that can be applied before intercourse should be considered. Sexual transmission of HIV-1 requires the help of DC in the mucosal tissues (10, 20, 23, 34, 38): DC capture HIV-1 through a range of receptors, of which DC-SIGN is the best studied; it mediates HIV-1 internalization and transmission to T cells (14, 25). Upon activation by microorganisms, DC migrate to the draining lymph nodes, where they can transmit HIV-1 to CD4+ T cells. The lymph node then becomes the principal site of virus production (18, 33).
In this study, we investigated the capacities of several proteins from bovine milk and human serum to block HIV-1 transmission from DC to T cells. Proteins become potent inhibitors of virus replication upon chemical modification that introduces negative charges. We now demonstrate that these proteins also inhibit DC-mediated HIV-1 transmission. Interestingly, we also found that bLF blocks DC-mediated HIV-1 transmission in its native form. Time-of-addition experiments with bLF in a single-cycle transmission assay showed that the inhibitory effect of bLF is mediated through the DC and not through the virus or the target T cells. We therefore tested whether bLF had any effects on DC physiology that could explain the loss of transmission capacity. bLF does not induce apoptosis in DC, and we found no influence of bLF on the maturation status of DC or the expression of ICAM-1 and the costimulatory molecule CD86. We also did not find any effect of bLF on naive T-cell stimulation or cytokine (IL-6, IL-12 p70, and TNF-α) production. These results are consistent with our alternative explanation that bLF inhibits attachment of HIV-1 to the DC. Indeed, DC preincubation with bLF could inhibit HIV-1 capture, and bLF blocked the adhesion of soluble DC-SIGN to gp120. The effect of bLF was mediated through DC-SIGN binding, as preincubation of coated gp120 with bLF followed by washing and subsequent addition of soluble DC-SIGN did not result in blocking. Direct binding of DC-SIGN to bLF is demonstrated in Fig. 6B, for which we applied bLF and measured DC-SIGN binding.
We tested LF variants for their ability to block HIV-1 transmission by DC. We compared bLF with the C-lobe fragment of bLF, hLF, apo-hLF, and iron-saturated hLF (holo-hLF). All LF variants except apo-hLF could block DC-mediated HIV-1 transmission to a certain extent, but bLF was the most potent inhibitor. This remarkable result is in accordance with HIV-1 replication studies, in which bLF also has a higher anti-HIV-1 activity than hLF (13, 19). The inhibition of transmission by the different LF variants correlates with the ability of these proteins to inhibit HIV-1 capture by DC.
Both bLF and the C-lobe fragment bind equally well to DC-SIGN, but the C-lobe fragment is less efficient in blocking transmission of HIV-1. The complete bLF may be more efficient than C-lobe in shielding DC-SIGN due to steric hindrance. Besides this, the conformation of the LF protein might also be crucial, since iron-depleted hLF has no inhibitory capability. Iron binding by LF is accompanied by substantial conformational changes between the open apo form and the closed holo form (1, 17). hLF and holo-hLF are partially able to block DC-mediated transmission of HIV-1, but the adhesion experiment shows no binding to DC-SIGN. Native LF isolated from milk is known to interact with a range of viruses (8, 13, 19, 44), and it is possible that hLF and holo-hLF prevent HIV-1 binding to DC through alternative routes. Moreover, HIV-1 binding to monocyte-derived DC takes place predominantly via DC-SIGN, but other C-type lectins such as the mannose receptor and an unidentified trypsin resistant C-type lectin may also play a role (4, 41-43).
bLF is abundantly available and easy to purify, and toxicity and immunogenicity problems with this native protein may be limited. It is therefore a candidate microbicide to prevent sexual HIV-1 transmission, possibly in combination with other compounds such as entry inhibitors or neutralizing antibodies (21, 45). Future research in this direction should determine the half-life of mucosally applied bLF, as well as the minimal required concentration to prevent SIV transmission in vivo in the macaque model. Besides blocking transmission by DC, bLF has the advantage of blocking HIV-1 via interactions with the virus itself and with the target T cells (7, 8, 13, 40). In case of lesions in the mucosal tissue, bLF could thus also prevent initial rounds of HIV-1 replication in submucosal CD4+ T cells. Although prolonged in vitro culturing of HIV-1 in the presence of bLF led to the selection of escape variants (8), we could still block DC-mediated capture of this mutant with bLF. Using a microbicide that interferes with both HIV-1 transmission and replication might be a promising protective strategy.
Acknowledgments
We thank Ventria Bioscience for the kind gift of the recombinant human lactoferrin variants, J. E. Clements for the LuSIV cells, and L. Beljaars for the donation of modified HSA proteins.
The initial phase of this bLF project was supported by a grant from the Dutch Ministry of Economic Affairs (program Industrial Proteins IIE98-011). This research has been funded by grant 7008 from Aids Fonds Netherlands.
REFERENCES
- 1.Anderson, B. F., H. M. Baker, G. E. Norris, S. V. Rumball, and E. N. Baker. 1990. Apolactoferrin structure demonstrates ligand-induced conformational change in transferrins. Nature 344:784-787. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767-811. [DOI] [PubMed] [Google Scholar]
- 3.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 4.Baribaud, F., S. Pohlmann, G. Leslie, F. Mortari, and R. W. Doms. 2002. Quantitative expression and virus transmission analysis of DC-SIGN on monocyte-derived dendritic cells. J. Virol. 76:9135-9142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beljaars, L., R. Floris, B. Berkhout, C. Smit, D. K. Meijer, and G. Molema. 2002. The influence of charge clustering on the anti-HIV-1 activity and in vivo distribution of negatively charged albumins. Biochem. Pharmacol. 63:1663-1673. [DOI] [PubMed] [Google Scholar]
- 6.Berger, E. A., P. M. Murphy, and J. M. Farber. 1999. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 17:657-700. [DOI] [PubMed] [Google Scholar]
- 7.Berkhout, B., G. C. H. Derksen, N. K. T. Back, B. Klaver, C. G. de Kruif, and S. Visser. 1997. Structural and functional analysis of negatively charged milk proteins with anti-HIV activity. AIDS Res. Hum. Retroviruses 13:1101-1107. [DOI] [PubMed] [Google Scholar]
- 8.Berkhout, B., J. L. van Wamel, L. Beljaars, D. K. Meijer, S. Visser, and R. Floris. 2002. Characterization of the anti-HIV effects of native lactoferrin and other milk proteins and protein-derived peptides. Antiviral Res. 55:341-355. [DOI] [PubMed] [Google Scholar]
- 9.Caessens, P. W. J. R., S. Visser, and H. Gruppen. 1997. Method for the isolation of bovine b-lactoglobulin from a cheese whey protein fraction and physicochemical characterisation of the purified product. Int. Dairy J. 7:229-235. [Google Scholar]
- 10.Choi, Y. K., K. M. Whelton, B. Mlechick, M. A. Murphey-Corb, and T. A. Reinhart. 2003. Productive infection of dendritic cells by simian immunodeficiency virus in macaque intestinal tissues. J. Pathol. 201:616-628. [DOI] [PubMed] [Google Scholar]
- 11.de Jong, E. C., P. L. Vieira, P. Kalinski, J. H. Schuitemaker, Y. Tanaka, E. A. Wierenga, M. Yazdanbakhsh, and M. L. Kapsenberg. 2002. Microbial compounds selectively induce Th1 cell-promoting or Th2-cell promoting dendritic cells in vitro with diverse Th cell-polarizing signals. J. Immunol. 168:1704-1709. [DOI] [PubMed] [Google Scholar]
- 12.Dickerson, M. C., J. Johnston, T. E. Delea, A. White, and E. Andrews. 1996. The causal role for genital ulcer disease as a risk factor for transmission of human immunodeficiency virus. An application of the Bradford Hill criteria. Sex. Transm. Dis. 23:429-440. [DOI] [PubMed] [Google Scholar]
- 13.Floris, R., I. Recio, B. Berkhout, and S. Visser. 2003. Antibacterial and antiviral effects of milk proteins and derivatives thereof. Curr. Pharm. Des. 9:1257-1275. [DOI] [PubMed] [Google Scholar]
- 14.Geijtenbeek, T. B., D. S. Kwon, R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, J. Middel, I. L. Cornelissen, H. S. Nottet, V. N. KewalRamani, D. R. Littman, C. G. Figdor, and Y. van Kooyk. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100:587-597. [DOI] [PubMed] [Google Scholar]
- 15.Geijtenbeek, T. B., R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, G. J. Adema, Y. van Kooyk, and C. G. Figdor. 2000. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell 100:575-585. [DOI] [PubMed] [Google Scholar]
- 16.Geijtenbeek, T. B., G. C. van Duijnhoven, S. J. van Vliet, E. Krieger, G. Vriend, C. G. Figdor, and Y. van Kooyk. 2002. Identification of different binding sites in the dendritic cell-specific receptor DC-SIGN for ICAM-3 and HIV-1. J. Biol. Chem. 277:11314-11320. [DOI] [PubMed] [Google Scholar]
- 17.Grossmann, J. G., M. Neu, E. Pantos, F. J. Schwab, R. W. Evans, E. Townes-Andrews, P. F. Lindley, H. Appel, W. G. Thies, and S. S. Hasnain. 1992. X-ray solution scattering reveals conformational changes upon iron uptake in lactoferrin, serum and ovo-transferrins. J. Mol. Biol. 225:811-819. [DOI] [PubMed] [Google Scholar]
- 18.Haase, A. T. 1999. Population biology of HIV-1 infection: viral and CD4+ T cell demographics and dynamics in lymphatic tissues. Annu. Rev. Immunol. 17:625-656. [DOI] [PubMed] [Google Scholar]
- 19.Harmsen, M. C., P. J. Swart, M.-P. De Bethune, R. Pauwels, E. De Clerq, T. Hauw The, and D. K. F. Meijer. 1995. Antiviral effects of plasma and milk proteins: lactoferrin shows potent activity against both human immunodeficiency virus and human cytomegalvirus replication in vitro. J. Infect. Dis. 172:380-388. [DOI] [PubMed] [Google Scholar]
- 20.Hu, J., M. B. Gardner, and C. J. Miller. 2000. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J. Virol. 74:6087-6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu, Q., I. Frank, V. Williams, J. J. Santos, P. Watts, G. E. Griffin, J. P. Moore, M. Pope, and R. J. Shattock. 2004. Blockade of attachment and fusion receptors inhibits HIV-1 infection of human cervical tissue. J. Exp. Med. 199:1065-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Humphrey, B. D., N. Huang, and K. C. Klasing. 2002. Rice expressing lactoferrin and lysozyme has antibiotic-like properties when fed to chicks. J. Nutr. 132:1214-1218. [DOI] [PubMed] [Google Scholar]
- 23.Jameson, B., F. Baribaud, S. Pohlmann, D. Ghavimi, F. Mortari, R. W. Doms, and A. Iwasaki. 2002. Expression of DC-SIGN by dendritic cells of intestinal and genital mucosae in humans and rhesus macaques. J. Virol. 76:1866-1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuipers, O. P., H. S. Rollema, W. M. Yap, H. J. Boot, R. J. Siezen, and W. M. de Vos. 1992. Engineering dehydrated amino acid residues in the antimicrobial peptide nisin. J. Biol. Chem. 267:24340-24346. [PubMed] [Google Scholar]
- 25.Kwon, D. S., G. Gregorio, N. Bitton, W. A. Hendrickson, and D. R. Littman. 2002. DC-SIGN-mediated internalization of HIV is required for trans-enhancement of T cell infection. Immunity 16:135-144. [DOI] [PubMed] [Google Scholar]
- 26.Lonnerdal, B., and S. Iyer. 1995. Lactoferrin: molecular structure and biological function. Annu. Rev. Nutr. 15:93-110. [DOI] [PubMed] [Google Scholar]
- 27.Matthews, T. H. J., C. D. G. Nair, M. K. Lawrence, and D. A. J. Tyrrell. 1976. Antiviral activity in milk of possible clinical importance. Lancet ii:1387-1389. [DOI] [PubMed] [Google Scholar]
- 28.McDonald, D., L. Wu, S. M. Bohks, V. N. KewalRamani, D. Unutmaz, and T. J. Hope. 2003. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science 300:1295-1297. [DOI] [PubMed] [Google Scholar]
- 29.Miller, C. J. 1994. Animal models of viral sexually transmitted diseases. Am. J. Reprod. Immunol. 31:52-63. [DOI] [PubMed] [Google Scholar]
- 30.Miller, C. J., N. J. Alexander, S. Sutjipto, S. M. Joye, A. G. Hendrickx, M. Jennings, and P. A. Marx. 1990. Effect of virus dose and nonoxynol-9 on the genital transmission of SIV in rhesus macaques. J. Med. Primatol. 19:401-409. [PubMed] [Google Scholar]
- 31.Nandi, S., Y. A. Suzuki, J. Huang, D. Yalda, P. Pham, L. Wu, G. Bartley, N. Huang, and B. Lonnerdal. 2002. Expression of human lactoferrin in transgenic rice grains for the application in infant formula. Plant Sci. 163:713-722. [Google Scholar]
- 32.Newburg, D. S., R. P. Viscidi, A. Ruff, and R. H. Yolken. 1992. A human milk factor inhibits binding of human immunodeficiency virus to the CD4 receptor. Pediatr. Res. 31:22-28. [DOI] [PubMed] [Google Scholar]
- 33.Pantaleo, G., C. Graziosi, L. Butini, P. A. Pizzo, S. M. Schnittman, D. P. Kotler, and A. S. Fauci. 1991. Lymphoid organs function as major reservoirs for human immunodeficiency virus. Proc. Natl. Acad. Sci. USA 88:9838-9842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pope, M., and A. T. Haase. 2003. Transmission, acute HIV-1 infection and the quest for strategies to prevent infection. Nat. Med. 9:847-852. [DOI] [PubMed] [Google Scholar]
- 35.Recio, I., and S. Visser. 1999. Two ion-exchange chromatographic methods for the isolation of antibacterial peptides from lactoferrin. In situ enzymatic hydrolysis on an ion-exchange membrane. J. Chromatogr. 831:191-201. [DOI] [PubMed] [Google Scholar]
- 36.Roos, J. W., M. F. Maughan, Z. Liao, J. E. Hildreth, and J. E. Clements. 2000. LuSIV cells: a reporter cell line for the detection and quantitation of a single cycle of HIV and SIV replication. Virology 273:307-315. [DOI] [PubMed] [Google Scholar]
- 37.Sanders, R. W., E. C. de Jong, C. E. Baldwin, J. H. Schuitemaker, M. L. Kapsenberg, and B. Berkhout. 2002. Differential transmission of human immunodeficiency virus type 1 by distinct subsets of effector dendritic cells. J. Virol. 76:7812-7821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spira, A. I., P. A. Marx, B. K. Patterson, J. Mahoney, R. A. Koup, S. M. Wolinsky, and D. D. Ho. 1996. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J. Exp. Med. 183:215-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swart, P. J., M. C. Harmsen, M. E. Kuipers, A. A. van Dijk, B. W. A. van der Strate, P. H. C. van Berkel, J. H. Nuijens, C. Smit, and D. K. F. Meijer. 1999. Charge modification of plasma and milk proteins results in antiviral active compounds. J. Peptide Sci. 5:563-576. [DOI] [PubMed] [Google Scholar]
- 40.Swart, P. J., M. E. Kuipers, C. Smit, R. Pauwels, M. P. De Bethune, E. De Clerq, D. K. F. Meijer, and J. G. Huisman. 1996. Antiviral effects of milk proteins: acylation results in polyanionic compounds with potent activity against human immunodeficiency virus types 1 and 2 in vivo. AIDS Res. Hum. Retroviruses 12:769-775. [DOI] [PubMed] [Google Scholar]
- 41.Turville, S. G., J. Arthos, K. M. Donald, G. Lynch, H. Naif, G. Clark, D. Hart, and A. L. Cunningham. 2001. HIV gp120 receptors on human dendritic cells. Blood 98:2482-2488. [DOI] [PubMed] [Google Scholar]
- 42.Turville, S. G., P. U. Cameron, J. Arthos, K. MacDonald, G. Clark, D. Hart, and A. L. Cunningham. 2001. Bitter-sweet symphony: defining the role of dendritic cell gp120 receptors in HIV infection. J. Clin. Virol. 22:229-239. [DOI] [PubMed] [Google Scholar]
- 43.Turville, S. G., P. U. Cameron, A. Handley, G. Lin, S. Pohlmann, R. W. Doms, and A. L. Cunningham. 2002. Diversity of receptors binding HIV on dendritic cell subsets. Nat. Immunol. 3:975-983. [DOI] [PubMed] [Google Scholar]
- 44.van der Strate, B. W., L. Beljaars, G. Molema, M. C. Harmsen, and D. K. Meijer. 2001. Antiviral activities of lactoferrin. Antiviral Res. 52:225-239. [DOI] [PubMed] [Google Scholar]
- 45.Veazey, R. S., R. J. Shattock, M. Pope, J. C. Kirijan, J. Jones, Q. Hu, T. Ketas, P. A. Marx, P. J. Klasse, D. R. Burton, and J. P. Moore. 2003. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat. Med. 9:343-346. [DOI] [PubMed] [Google Scholar]