Abstract
A male child with clinical features consistent with EEC/EECUT plus syndrome(ectrodactyly, ectodermal dysplasia, clefting, urinary tract abnormalities and thymic abnormalities) including mild ectodermal abnormalities, ectrodactyly of hands and feet, cleft palate, bilateral hydronephrosis and T cell lymphopenia is reported. He was noted to have T cell receptor excision circle (TREC) analysis below the cutoff for normal on newborn screening and T cell lymphopenia on further immunologic evaluation. A novel, presumably pathogenic de novo 3 base pair deletion in exon 7 of TP63 (c.970_972delATT) (NCBI Reference Sequence NM_003722.4) was identified. This observation provides supporting evidence for the association between TP63 mutations and EECUT plus syndrome. Clinicians caring for infants presenting with EEC spectrum disorders in the newborn period should also consider the possibility of T cell lymphopenia.
Keywords: ectrodactyly, ectodermal dysplasia, cleft palate, thymus, TP63, T cell, lymphopenia
Introduction
A prior clinical observation suggests an expanded spectrum for the EECUT syndrome spectrum of disorders (ectrodactyly, ectodermal dysplasia, clefting, urinary tract abnormalities) to include thymic involvement with a designated name EECUT plus syndrome [Frick, 1997]. We report a male child whose clinical features overlap with EECUT plus syndrome and who has a novel de novo mutation in TP63, providing molecular evidence for mutations in TP63 and EECUT plus syndrome. Implications for clinical management are discussed.
Clinical Report
The patient was born to a 29 year old gravida 2, para 1 female. Prenatal ultrasound was significant for bilateral ectrodactyly of hands and feet in addition to bilateral ureteropelvic junction obstruction. Amniocentesis was performed which revealed a normal, 46,XY chromosome constitution. Family history was negative for ectrodactyly, clefting disorders and ectodermal dysplasia. The family received genetic counseling, and a possible diagnosis of ectrodactyly-ectodermal dysplasia-clefting (EEC) syndrome was given to the family. Delivery was at term with a birth weight of 3.33 kg (~40%), length of 48 cm (~10%) and head circumference of 33 cm (3%). A soft palate cleft was noted after birth. At birth the baby was noted to have sparse hair, eyebrows and eyelashes. Both pinnae were small, measuring 3 × 2.5 cm each with overfolding of the top portion of the pinnae bilaterally. He was noted to have small ear canals. There was congenital absence of bilateral lacrimal puncta and canaliculi.
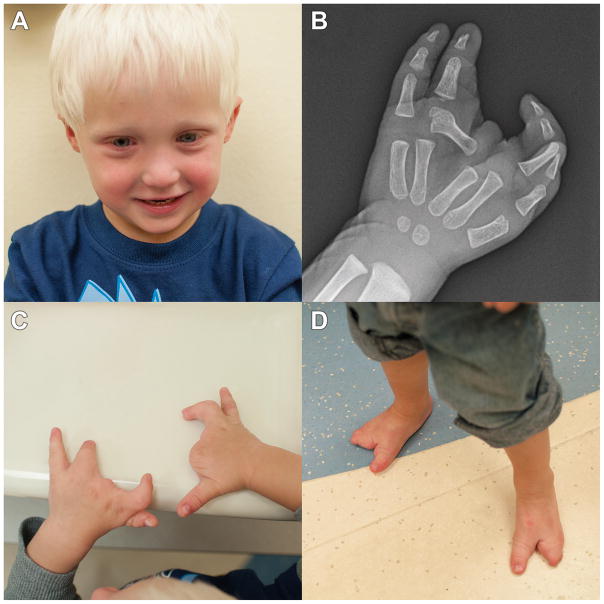
Ectrodactyly was clinically apparent at birth with a thumb present on the right hand, absence of the second and third digit, with digit 4 and 5 syndactyly. A thumb which was completely fused to the second digit was present on the left hand, followed by a rudimentary third digit and digits 4 and 5. On both feet, digit 1 was present, followed by a gap with digits 4 and 5 present. A nail bed was present on digit 5 on the left foot. A separate nail bed was present on digits 4 and 5 of the right foot. A right nasolacrimal sac abscess which required excision in addition to dacrocystectomy was performed on the right eye during the first several months of life. He was also noted to have left eye discharge during the first year of life. Insufficiency of nasolacrimal duct development was identified following probing. A photograph of the patient is shown in Fig 1a, in addition to right hand x-ray, right and left hands and feet. (1-b,c and d respectively).
Figure 1.
A: Photograph of facial features of patient at 27 months
B: Radiograph illustrating ectrodactyly of right hand.
C: Photograph illustrating ectrodactyly of right and left hands.
D: Photograph illustrating ectrodactyly of right and left feet.
The patient’s newborn screen was abnormal for severe combined immune deficiency (SCID)/ T cell lymphopenia testing determined by T cell receptor excision circle (TREC) analysis below the cutoff for normal. During the newborn period, he underwent a thorough immunologic evaluation demonstrating low for age T cell numbers (Table I). HIV PCR was negative. His T cell function was measured at 2 weeks of age and his phytohemagglutinin response was >100% of control (data not shown) and with serial monitoring remained normal. At two months of age he developed a periorbital cellulitis with Pseudomonas aeruginosa cultured from eye drainage with negative blood cultures. He was admitted for intravenous antibiotics, and he was treated with gamma globulin therapy secondary to the life threatening infection, T cell lymphopenia, and age-related limitations in thorough humoral function testing. He continued on monthly intravenous gamma globulin therapy and live vaccinations were held. Because he did not mount high fever with his infections along with ectodermal abnormalities, additional testing was obtained to interrogate intact NFkB function using Toll-like receptor (TLR) agonist response. This was found to be normal (data not shown). An MRI scan of the orbits, face and neck, which included a partial view of the thymus documented the presence of thymic tissue and did not demonstrate any obvious anatomic abnormalities. Serial immune studies demonstrated an improvement in T cell counts with sustained normal T cell function (Table I). Monthly gamma globulin therapy was discontinued after 14 months when T cell counts normalized, serum IgM and IgA levels were normal, and he was clinically well. After discontinuation of gamma globulin therapy his IgG levels were serially monitored and remained in the normal range. He was subsequently vaccinated with inactivated vaccines and found to have intact humoral function. He continues to have close immunologic monitoring.
Table 1.
Patient T cell values obtained at various age intervals
| Parameter Measured | 1 week | 1 month | 5 months | 8 months | 17 months | 21 months |
|---|---|---|---|---|---|---|
| Absolute CD3+ T cell count | 838 (2500–5500) | 1136 (2500–5500) | 835 (2500–5600) | 1932 (1900–5900) | 2244 (2100–6200) | 2185 (2100–6200) |
| Absolute CD4+ T cell count | 622 (1600–4000) | 829 (1600–4000) | 605 (1800–4000) | 1449 (1400–4300) | 1632 (1300–3400) | 1368 (1300–3400) |
| Absolute CD8+ T cell count | 231 (560–1700) | 368 (560–17000 | 543 (590–1600) | 527 (500–1700) | 561 (620–2000) | 721 (620–2000) |
His postnatal renal course was significant for bilateral hydronephrosis. Furosemide renal scan indicated that 85% of his kidney function was contributed by his right kidney and the remaining 15% from his left kidney. Dermatology and dental evaluations were performed and failed to demonstrate skin, hair or dental abnormalities. Motor milestones were normal and at 20 months he was speaking in phrases and had a 50 word vocabulary. OAE screening at 2000–6000 Hz failed in the right ear and passed in the left ear. He underwent bilateral myringotomies and tympanostomy tube insertion at 23 months of age because of chronic serous otitis media. Family history was negative for individual family members with ectrodactyly, cleft palate or ectodermal abnormalities.
FISH for 22q11.2 deletion was negative. Microarray CGH analysis was significant for a copy gain of chromosome 6q25.1 (150,499,134–150,822,353) x 3. This region includes four genes, two of which having OMIM entries. PPP1R4C, localized to this region, is an inhibitor of protein phosphatase-1 (PP1) which is a signal-transducing phosphatase that influences neuronal activity, protein synthesis, metabolism, muscle contraction and cell division.[Liu et al. 2002] Mutations in the second gene, IYD, result in congenital hypothyroidism secondary to iodotyrosine dehalogenase deficiency. [Affink et al. 2008] Parental studies indicated that the patient’s father had the identical copy number gain of chromosome 6q25.1, making the significance of this copy number gain less likely. Sequencing analysis of the TP63 gene was performed, and a 3 base pair deletion in exon 7 was identified beginning at nucleotide 970 (c.970_972delATT) (NCBI Reference Sequence NM_003722.4). This change results in the deletion of an isoleucine at amino acid position 324 (p.Ile324del) (p.Ile285del according to GenBank accession AF075430) and affects the DNA binding domain of the protein [Bokhoven and Brunner, 2002]. Parental analysis of TP63 failed to reveal the presence of the deletion in either parent indicating this was a de novo alteration. This deletion has not been reported in the NCBI dbSNP or HGMD databases. Comparison of the protein sequence for TP63 across species indicated conservation of the isoleucine at amino acid 324. The deletion was predicted to be deleterious with a p value of 1.0 using the Mutation Taster prediction algorithm (http://mutationtaster.org/MutationTaster/index.html). The p value is the probability of the prediction, and the closer the probability is to 1 (range: 0–1), the higher the “security” of the prediction. Based on the fact that this deletion is de novo in the patient, along with the highly conserved nature of this amino acid across species, its location within the DNA binding domain of the protein and the prediction by Mutation Taster that it is disease causing, this deletion is most likely a pathogenic mutation.
Discussion
Five syndromic disorders including EEC (ectrodactyly ectodermal dysplasia, clefting), LMS (limb mammary syndrome), ADULT (acro-dermato-ungual-lacrimal-tooth) syndrome, AEC (ankyloblepharon-ectodermal dysplasia-clefting) syndrome and RHS (Rapp-Hodgkin) syndrome and two non-syndromic disorders including SHFM4 (split hand-foot malformation 4) and NSCL (non-syndromic cleft lip) are associated with mutations in the TP63 gene, a transcription factor which regulates ectodermal, limb and orofacial development [Bokhaven and Brunner, 2002]. The components of EEC syndrome include limb abnormalities specifically characterized by ectrodactyly and syndactyly; ectodermal abnormalities characterized by alopecia, sparse to absent eyebrows and eyelashes; and orofacial clefting and maxillary hypoplasia may also occur. Dental abnormalities may include oligodontia and salivary gland abnormalities. Genitourinary abnormalities include structural kidney or ureteral malformations which have resulted in expansion of the EEC syndrome to EECUT (ectrodactyly, ectodermal dysplasia, clefting and urinary tract abnormalities) syndrome [Hecht, 1985]. A report was published in 1997 describing a newborn male child with EEC-related malformations including cystic enlargement of both kidneys secondary to a large ureterocele, short palpebral fissures, hypoplastic finger and toe nails, absent eyelashes, eyebrows, scalp hair and lanugo, bilateral syndactyly of fourth and fifth toes, genital abnormalities and hard palate cleft [Frick, 1997]. Hypoplastic lungs and cystic kidneys were detected on antenatal ultrasound. Because of preeclampsia, the pregnancy was terminated by caesarean section at 35 weeks gestation. The infant died at 90 minutes after birth due to respiratory insufficiency. Small rudimentary thymus tissue containing single epithelial cells was found on autopsy. There was complete absence of Hassall’s corpuscles. Splenic follicles were noted to be smaller than usual. Due to the presence of thymic abnormalities the term EEC/EECUT plus syndrome was suggested, consistent with an underlying abnormality in ectodermal development. At the time of this report, TP63 mutations had not been identified as a cause for EEC/EECUT plus syndrome.
Wisconsin became the first state to screen for SCID using the TREC assay in 2008 [Baker et al., 2009, Verbsky et al., 2012 TREC assay is a real-time quantitative polymerase chain reaction to measure TREC copy numbers using DNA isolated from dried NBS specimens. TRECs, generated during T cell development, are abundant in healthy infants’ dried blood specimens, and are very few or undetectable in the dried blood specimens of patients with SCID or T cell Lymphopenia (TCL).
The combined incidence of ectodermal dysplasias is approximately 1/10,000 [Rinne et al., 2007]. Of 207,396 infants who were screened on TREC assay in Wisconsin from 2008–2011, there were 5 infants with reversible T cell lymphopenia (TCL) as the described case here, with a calculated incidence of 0.0024%. The product of these two figures (0.001% × 0.0024%) is 0.000024% which represents a rough estimate of the number of infants who would be expected to have a TP63-mediated ectodermal dysplasia syndrome and reversible TCL identified through newborn screening for SCID/TCL.
The thymus has an epithelial component. Thymic epithelial cells are required for maturation of thymocytes into mature T lymphocytes.[Blackburn, 2004]. All isoforms of ΔTp63 are expressed in the mouse thymus. Severe thymic abnormalities observed in p63−/ − mice can be partially corrected with ΔNp63, providing evidence for direct involvement of ΔNp63 in thymic development.[Candi et al., 2007] Since the patient’s lymphopenia was reversible it is possible his TP63 mutation is mild enough to enable T cell function that is ultimately normal. The patient is currently being monitored by the immunology service.
The significance of the clinical and molecular findings in this patient provides supporting evidence for the expansion of EEC/EECUT plus syndrome to include a spectrum of thymic defects ranging from mild to severe. Practitioners caring for infants who display clinical features within the EEC/EECUT spectrum should follow up on newborn screening TREC assays if available. Those infants with a positive TREC screen should have careful immunology evaluation and follow-up. If TREC assay was not performed, newborns with EEC/EECUT should have screening immunologic studies to investigate for T cell lymphopenia.
Acknowledgments
We greatly appreciate the assistance and encouragement of the patient’s family. We appreciate the technical assistance of Robert J. Gordon, Michael Mulcahy and Gail Pearsall. We also thank Joanne Haun M.S. and Cynthia Essex for their assistance.
References
- Afink G, Kulik W, Overmars H, de Ranamie J, Veenboar T, van Cruchten A, Craen M, Ris-Stalpers C. Molecular characterization of iodotyrosine dehalogenase in patients with hypothryodism. JCEM. 2008;293:4894–4901. doi: 10.1210/jc.2008-0865. [DOI] [PubMed] [Google Scholar]
- Baker MW, Grossman WJ, Laessig RH, Hoffman GL, Brokopp CD, Kurtycz DF, Cogley MF, Litsheim TJ, Katcher ML, Routes JM. Development of a routine newborn screening protocol for severe combined immunodeficiency. J Allergy Clin Immunol. 2009;124:522–7. doi: 10.1016/j.jaci.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Blackburn CC, Manley N. Nat Rev Immunol. 2004;4:278–289. doi: 10.1038/nri1331. [DOI] [PubMed] [Google Scholar]
- Candi E, Rufini A, Terrinoni A, Giamboi-Miraglia A, Maria Lena A, Mantovani R, Knight R, Melino G. ΔNp63 regulates thymic development through enhanced expression of FgfR2 and Jag2. PNAS. 2007;124:11999–12004. doi: 10.1073/pnas.0703458104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bokhoven H, Brunner H. Splitting p63. Am J Hum Genet. 2002;71:1–13. doi: 10.1086/341450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick H, Münger DM, Fauchère JC, Stallmach T. Hypoplastic thymus and T-cell reduction in EECUT syndrome. Am J Med Genet. 1997;69:65–68. [PubMed] [Google Scholar]
- Hecht F. Updating a diagnosis: The EEC/EECUT syndrome. [Editorial] Am J Dis Child. 1985;139:1185. doi: 10.1001/archpedi.1985.02140140019014. [DOI] [PubMed] [Google Scholar]
- Liu QR, Zhang PW, Zhen Q, Walther D, Wang XB, Uh GR. KEP, a PKC-dependent protein phosphatase 1 inhibitor regulated by morphine. J Biol Chem. 2002;277:13312–13320. doi: 10.1074/jbc.M107558200. [DOI] [PubMed] [Google Scholar]
- Rinne T, Brunner HG, van Bokhoven H. Cell Cycle. 2007;6(2):262–268. doi: 10.4161/cc.6.3.3796. [DOI] [PubMed] [Google Scholar]
- Shearer WT. Lymphocyte subsets in healthy children from birth through 18 years of age: The Pediatric AIDS Clinical Trials Group. J Allergy Clin Immunol. 2003 doi: 10.1067/mai.1778. [DOI] [PubMed] [Google Scholar]
- Verbsky JW, Baker MW, Grossman WJ, Hintermeyer M, Dasu T, Bonacci B, Reddy S, Margolis D, Casper J, Gries M, Desantes K, Hoffman GL, Brokopp CD, Seroogy CM, Routes JM. Newborn Screening for Severe Combined Immunodeficiency: The Wisconsin Experience (2008–2011) J Clin Immunol. 2012;32:82–88. doi: 10.1007/s10875-011-9609-4. [DOI] [PubMed] [Google Scholar]