Abstract
Clinical and preclinical data demonstrate that altered pulmonary physiology (including increased inflammation, increased blood flow, airway resistance and hyperreactivity) is an intrinsic component of SCD and may contribute to excess SCD morbidity and mortality. Inhaled corticosteroids (ICS), a safe and effective therapy for pulmonary inflammation in asthma, may ameliorate the altered pulmonary physiologic milieu in SCD. With this single-center, longitudinal, randomized, triple-blind, placebo controlled trial we studied the efficacy and feasibility of ICS in 54 non-asthmatic individuals with SCD. Participants received once daily mometasone furoate 220 mcg dry powder inhalation or placebo for 16 weeks. The primary outcome was feasibility (the number who complete the trial divided by the total number enrolled) with pre-specified efficacy outcomes including daily pain score over time (patient reported) and change in soluble vascular cell adhesion molecule (sVCAM) levels between entry and 8-weeks. For the primary outcome of feasibility, the result was 96% (52 of 54, 95% CI 87% – 99%) for the intent-to-treat analysis and 83% (45 of 54, 95% CI 71% – 91%) for the per-protocol analysis. The adjusted treatment effect of mometasone was a reduction in daily pain score of 1.42 points (95%CI 0.61 – 2.21, p = 0.001). Mometasone was associated with a reduction in sVCAM levels of 526.94 ng/mL more than placebo (95% CI 50.66 – 1,003.23, p = 0.03). These results support further study of ICS in SCD including multi-center trials and longer durations of treatment. www.clinicaltrials.gov (NCT02061202)
Keywords: Sickle Cell Disease
Introduction
Sickle Cell Disease (SCD) is an autosomal recessive disorder of hemoglobin characterized by chronic hemolysis and altered blood rheology with clinical manifestations that include pain, progressive organ damage and shortened lifespan.1 The lung plays an essential role in SCD pathophysiology as it is the only organ that reverses red cell sickling, which occurs during hemoglobin deoxygenation. A growing body of clinical and preclinical data demonstrates that altered pulmonary physiology (including increased inflammation, increased blood flow, airway resistance and hyperreactivity) is an intrinsic component of SCD and may contribute to excess SCD morbidity and mortality.2–6 Therapies to ameliorate the altered pulmonary physiologic milieu in SCD are a promising avenue for disease modification.
Comorbid asthma is a well-established risk factor for increased SCD morbidity and mortality, and more recent observational research identified high rates of airway hyperreactivity, obstruction and wheezing in individuals with SCD who do not have asthma.7–16 Several studies demonstrated a link between the presence of respiratory symptoms and SCD complications including pain, acute chest syndrome and death.9,17–19 In one prospective cohort of non-asthmatic individuals with SCD, the rate of pain episodes doubled during 8-week periods after self-reported cough or wheeze (mostly in the setting of upper respiratory infection as patients with asthma were excluded) and there was a trend towards increased rates of acute chest syndrome.13 These data and other observational studies suggested a temporal link between respiratory symptoms and SCD pain, raising the question of whether therapies aimed at reversing airway obstruction and inflammation could benefit non-asthmatic individuals with SCD, particularly after periods of self-reported wheeze or cough.
The etiology of non-asthmatic lung function abnormalities in SCD is an area of ongoing investigation. Sickle mice demonstrate elevated airway inflammation, higher levels of pulmonary CD4+ and CD8+ T cells and myeloid activation chemokines.6,20,21 This inflammation does not appear to be driven exclusively by eosinophilic processes in humans as levels of exhaled nitric oxide (eNO) are only slightly higher in SCD compared with healthy controls but not as high as individuals with poorly controlled asthma.22,23 Given these data, anti-inflammatory asthma therapies (oral leukotriene inhibitors24 and inhaled corticosteroids) are promising as adjuvant SCD therapy. Emerging data suggest that elevated pulmonary capillary blood volume and flow (resulting from chronic anemia) are important contributors to the observed increase in airway resistance in SCD25 and therefore therapies (such as hydroxyurea and transfusion) aimed at ameliorating the high flow state may have greater benefit than bronchodilators. While hydroxyurea is associated with improvements in lung function, transfusion resulted in further increases in pulmonary capillary blood volume and airway resistance.26 With this study, we aimed to evaluate the efficacy and feasibility of inhaled corticosteroids (ICS) for individuals with SCD who do not have asthma.
Methods
Study design and conduct
With this single-site, randomized, placebo-controlled, triple-blind phase II trial, we tested the hypothesis that in non-asthmatic individuals with SCD who report recent cough or wheeze, ICS administered for a 16-week period will reduce SCD-related complications, improve patient-reported outcomes and reduce biological markers of pulmonary inflammation and vascular injury in comparison to placebo. We also tested protocol fidelity and feasibility in preparation for a future multi-site trial. The single primary endpoint for the trial was feasibility (defined as the number of participants who meet criteria for per-protocol analysis of all clinical and biologic outcomes divided by the total number enrolled) however we did specify a priori patient-reported (daily pain diary scores over time) and biologic outcomes (change in sVCAM and change in eNO between zero and eight weeks). To meet criteria for per-protocol analyses, participants were required to maintain at least 70% adherence to study medication, complete the 8-week (+/− 2 weeks) assessment of biological outcomes (spirometry, blood draw and questionnaires), and fill out at least 30 pain diaries. The intent-to-treat analysis included all participants except those who were lost to follow up prior to the first data collection point (two individuals were lost, and had no data for analysis). The target sample size was 45 participants with a 2:1 allocation to drug vs. placebo. A sample size calculation was performed for the a priori patient-reported outcome of daily pain diary scores. To detect a difference in mean pain score of 1 point for ICS vs. placebo we estimated a power of 0.80 with a sample size of 45 (30 drug, 15 placebo) at a two-sided alpha level of 0.05 and autoregressive correlation of intra-individual scores of 0.20. This study was approved by the Mount Sinai Program for Protection of Human Subjects. The trial was conducted under an FDA investigational new drug application (IND 117997) and registered with clinicaltrials.gov (NCT02061202). Each participant provided written informed consent. A complete schedule of protocol changes is included in the supplement. Protocol changes were minor and had no effect on the intervention, study outcomes or the intent to treat analyses.
Patients
Eligibility criteria for the study were HbSS or HbSβ0 thalassemia (confirmed by electrophoresis), age 15 or older and a patient self-report of cough or wheeze over the preceding two months. Exclusion criteria included a diagnosis of asthma, incarceration, pregnancy, more than 15 ED visits for SCD pain over the prior 12 months and discharge from the hospital within the previous 7 days. Specifically, patients with asthma were excluded because it was not considered ethical to randomize patients with asthma and wheezing to placebo. A multistep algorithm with high sensitivity in adults and children13,27 was used to identify and exclude all individuals with asthma or possible asthma and is included as a supplement. Briefly, any individual who carried a diagnosis of asthma or a history of using asthma medications was excluded. For individuals with no asthma diagnosis, they were excluded if they had any three of the following criteria 1) Wheeze causing shortness of breath, 2) wheeze without colds, 3) wheeze with exercise, 4) family history of asthma in a parent. All data were collected at the Mount Sinai Medical Center (inpatient, ED, ambulatory clinics) or via telephone.
Study therapy and adaptive randomization
Participants were randomized in a 2:1 fashion to receive once-daily inhaled mometasone furoate 220 mcg twisthaler or placebo for 16 weeks in addition to standard SCD care. The 2:1 allocation ratio was chosen to 1) demonstrate the feasibility of unequal allocation schema if necessary in future trials and 2) to generate safety data for mometasone (by exposing more patients to active drug). Participants were observed for an additional 4-week washout period after study completion (up to 20 weeks) however if patients felt marked symptom improvement they were allowed to continue the study medication (instead of washing out) at the discretion of their healthcare provider. To ensure equal allocation of patients on hydroxyurea and equal allocation of patients with low, medium and high rates of ED utilization, a covariate-balancing, adaptive, biased-coin randomization algorithm was employed.28 Briefly, the adaptive, biased-coin changed the probability of treatment assignment based on the degree of existing imbalance between groups (with respect to hydroxyurea use and prior rate of ED utilization) for participants already in the trial. Unlike a stratified block randomization, the biased coin algorithm altered probability (between 10% and 90%) but never made deterministic assignments to maintain balance. This approach was chosen because it made masking of treatment assignment more feasible but maintained a similar degree of covariate balance to traditional stratified block randomization. Randomization assignments were performed by a separate, unblinded research coordinator who was not responsible for data collection or assessment. Participants, physicians, investigators, and data collectors were all blinded to treatment assignment.
Measures to improve adherence and reduce attrition
Adherence was assessed with dose counters on mometasone twisthalers (placebo inhalers did not have dose counters and had a different appearance than mometasone inhalers – detailed description of procedures to maintain complete blinding of patients, providers, data collectors and analyzers are available in the supplement) and self-reported adherence via follow up phone calls for placebo. In addition, the medication adherence report scale for asthma (a 10 question tool scored between 0 and 5 with higher scores indicating greater adherence)29,30 was administered to all patients to evaluate its utility in measuring inhaler adherence in people with SCD. To improve adherence, a training module was adapted from the National Asthma Education and Prevention Program and the NIH Breathe Easier module.31 The module included training and education on proper use of ICS, medication side effects and mechanism of action (in lay-person terms), inhaler technique and a ten minute structured coaching session to improve medication adherence (see supplement).
Efficacy and safety assessments
Participants were assessed in-person at trial entry, at 8-weeks and at 16-weeks (study completion). At study entry and 8-weeks, assessments included ASCQ-Me (NHLBI developed SCD quality of life measurement tool) pain domain, spirometry with bronchodilator, eNO, peripheral blood testing (standard-of-care tests and peripheral-blood markers of inflammatory activation) and side-effect questionnaires. Procedures for pulmonary function testing were identical to those used for the Sleep and Asthma Cohort22 and are included as a supplement. Adverse events were assessed for every hospital visit, via questionnaire at each in-person assessment, and at follow-up phone calls, which occurred at 2-weeks, 4-weeks and 12-weeks. Participants also filled out daily pain diaries (derived from the Pain in Sickle Cell Epidemiology Study - PiSCES)32 for the 16-week duration of the study and the 4-week washout period. Clinical events including hospital admissions, ED visits and ICU stays were assessed via chart review and via in-person assessments at 2, 8, 10, 16 and 20 weeks. Trial safety was assessed via an independent data safety and monitoring board (DSMB). The DSMB met every six months with an interim analysis performed after 20 participants to assess trial safety (only adverse events were reviewed including deaths, pneumonia and ICU visits without frequentist hypothesis testing). There were no a priori safety or futility parameters for the study. There were no serious adverse events at the interim analysis and the study was allowed to continue to completion.
Serum Quantification of Cytokines
Blood for serum was collected in Vacutainer serum separator tubes (BD, Franklin Lakes, NJ, USA). Tubes were stored cold for less than 6 hrs until centrifugation. Serum was separated and stored at −80°C until analysis. All cytokines were analyzed by a bead-based ELISA method (R&D Systems, Minneapolis, MN) using a Luminex 200 (Luminex Corporation, Austin, TX, USA). All samples were run in duplicate, and both visits of each subject were analyzed simultaneously on the same plate, eliminating any variability between assays.
Statistical analyses
All statistical analyses were performed using SAS version 9.3. All data were checked for missingness and statistical outliers and confirmed by rechecking paper case-report forms for data-entry errors. Missing data were missing at random and missingness was not related to treatment assignment. Data were reviewed for distributional characteristics to ensure that statistical assumptions were met prior to hypothesis testing. Data were checked for statistically significant differences in important variables (age, gender, steady-state fetal hemoglobin, steady-state hemoglobin, steady-state WBC, prior rate of ED utilization) across treatment groups. Bivariate analyses were performed to determine if covariates were significantly associated with outcome data. The a priori plan was to statistically adjust only for variables found to be associated with the outcome variable (at a level of p value below 0.2) or if significant between-group differences were found. For the primary patient-reported outcome of pain scores over time, a random-intercepts, mixed effect model was used with patient as a random effect. Treatment assignment was considered as a fixed effect. An unstructured variance-covariance structure was chosen for the model because it yielded the lowest Akaike information criterion. Because a significant difference in age between groups was found, all results were adjusted for age. For continuous outcomes, linear regression was used; for count data, negative binomial regression; and for binary data, logistic regression. To determine the effect of mometasone on the odds of admission to the hospital during any visit, a generalized estimating equation was used (proc GENMOD with a repeated statement for subject ID, predictors included treatment assignment and age, dependent variable was admission to the hospital) with robust estimation of variances and a binary logit link. No adjustments were made for multiple comparisons of secondary outcome data.
Results
Patient and treatment characteristics
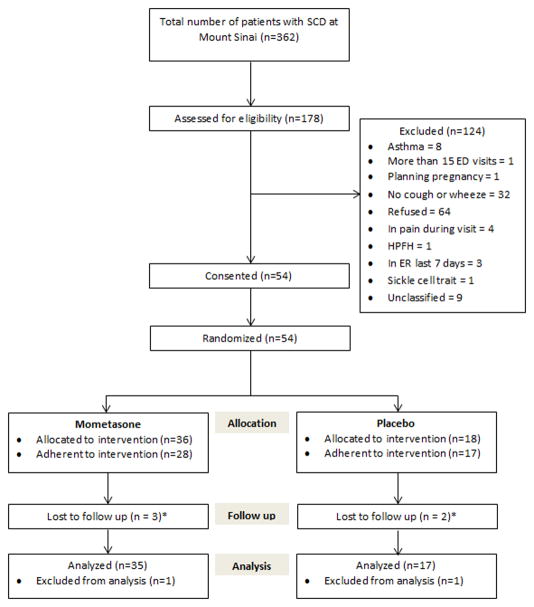
Between February 2014 and October 2016, a total of 54 patients were consented and randomized into the study, with two patients lost to follow up prior to data collection leaving 52 patients available for the intent-to-treat analysis and 45 patients eligible for the per-protocol analysis (figure 1). For the primary outcome of feasibility (calculated as the number who complete the study divided by the number consented), the result was 96% (52 of 54, 95% CI 87% – 99%) for the intent-to-treat analysis and 83% (45 of 54, 95% CI 71% – 91%) for the per-protocol analysis. Patient characteristics are provided in table 1. At study entry, participants in the placebo group were older than the mometasone group (mean 36 vs. 30 years, p= 0.02). There were no other statistically significant differences in baseline characteristics between treatment groups nor were there statistically significant associations between covariates (age, gender, baseline laboratory values) and outcome variables. Rates of obstructive lung disease were low in both groups and there were no cases of response to bronchodilator during spirometry. ENO levels were normal in both groups at baseline.
Figure 1. Patient Flow CONSORT Diagram.
* 1 participant in each group was lost to follow up prior to data collection and excluded from analyses. Others (n=3) were still included in the intent to treat analysis because they completed follow up questionnaires, spirometry, eNO, and blood draws prior to being lost
Table I.
Participant Characteristics
| Characteristic | Placebo (N = 17) | Mometasone (N = 35) | P |
|---|---|---|---|
| Male sex, N(%) | 11 (64%) | 17 (49%) | 0.4 |
| Age (y) | |||
| Mean (SD) | 36 (9.81) | 30 (8.56) | 0.02 |
| Hemoglobin diagnosis | 1.0 | ||
| HbSS | 16 | 34 | |
| Hb Sβ0 thalassemia | 1 | 1 | |
| Medical History | |||
| HU therapy N (%) | 11 (64.7%) | 23 (65.7%) | 1.0 |
| History of eczema | 1 (6%) | 4 (11%) | 1.0 |
| History of allergies | 6 (35%) | 14 (40%) | 0.3 |
| Smoking | 0.8 | ||
| Never, N(%) | 9 (52%) | 22 (63%) | |
| Smoked in past but not now, N(%) | 6 (35%) | 10 (29%) | |
| Current smoker | 2 (12%) | 3 (9%) | |
| Steady state Hemoglobin | |||
| Mean(SD) | 9.01 (1.13) | 8.71 (1.49) | 0.5 |
| Steady State Hemoglobin F % | |||
| Mean (SD) | 10.56 (7.32) | 9.03 (6.56) | 0.5 |
| Spirometry at study entry | |||
| FEV1 % Predicted mean (SD) | 86.67(21.88) | 87.83 (12.90) | 0.9 |
| FVC % Predicted mean (SD) | 87.47 (12.36) | 92.00 (16.08) | 0.3 |
| FEV1/FVC mean (SD) | 80.14 (14.95) | 83.24 (3.56) | 0.4 |
| Positive bronchodilator response* | 0 | 0 | 0.6 |
| Exhaled NO ppb mean (SD) | 15.35 (10.11) | 14.00 (6.27) | |
| Prior ED Utilization (past 12 months) | 0.4 | ||
| 0–5 visits – n (%) | 12 (71%) | 30 (86%) | |
| 6–10 visits – n (%) | 4 (24%) | 4 (11%) | |
| 11–15 visits – n (%) | 1 (6%) | 1 (3%) | |
| Laboratory values at study entry | |||
| Hemoglobin g/dL | |||
| Mean (SD) | 8.92 (1.59) | 8.55 (1.54) | 0.4 |
| WBC count, × 103/μL | |||
| Mean (SD) | 11.84 (4.48) | 10.55 (3.81) | 0.3 |
| Hemoglobin F % | |||
| Mean (SD) | 9.19 (7.27) | 8.52 (6.09) | 0.7 |
| Reticulocyte count % | |||
| Mean (SD) | 7.71 (2.86) | 7.74 (4.03) | 0.97 |
| Lactate dehydrogenase IU/I | |||
| Mean (SD) | 367.60 (207.08) | 440.21 (182.47) | 0.5 |
| sVCAM mg/mL | |||
| Mean (SD) | 2378.9 (987.4) | 2712.7 (1478.8) | 0.4 |
Positive bronchodilator response defined as an improvement of greater than 12% in FEV1
Inhaled Corticosteroids Reduce Patient-Reported Pain
For the outcome of pain score over time, the adjusted treatment effect of mometasone was a reduction in daily pain score of 1.42 points (95%CI 0.61 – 2.21, p = 0.001). Figure 2 shows pain scores by week stratified by treatment group. The following secondary clinical outcomes numerically favored mometasone over placebo but were not statistically significant. Changes in ASCQ-Me pain impact were 5.2 points (on a 100-point scale) better (reductions indicate improved quality of life) for the mometasone group than placebo (95% CI −18.90 – 8.49, p = 0.46). For patients who had at least one ED visit, the absolute difference in rate of admission (or 24-hour observation) to the hospital was 12% lower for the mometasone group (Risk Ratio= 0.79, 95% CI 0.45 – 1.38, p = 0.40). Patients in the mometasone group spent, on average after statistical adjustment, 1.88 fewer days in the hospital (95% CI −5.95 – 2.19, p = 0.36).
Figure 2. Weekly mean pain diary scores of Mometasone vs. Placebo groups.
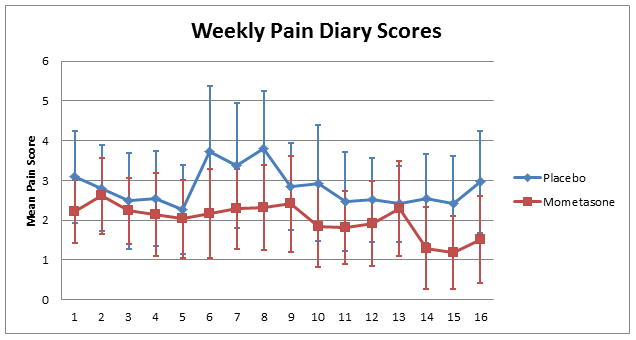
Mometasone was associated with a mean pain score reduction of 1.42 points over time (95%CI 0.61-2.21, P>0.001). Error bars indicate 95% confidence intervals
Secondary biomarker outcomes
For the a priori biomarker outcome of change in sVCAM (table 2) as a surrogate for vascular injury, the placebo group experienced a mean increase of 170.25 ng/ml and the mometasone group experienced a decrease of 182.47 ng/ml (adjusted treatment effect 526.94 ng/mL, 95% CI 1,003.23 – 50.66, p = 0.03). This corresponded to a percent change in sVCAM for the placebo vs. mometasone group of 8.82% vs −4.86%. There were no significant between-group differences in TNF-α, IFN-γ, IL1β, IL-6, E-selectin, P-selectin, IL-4, IL-2 or IL-13 (see supplement). Change in reticulocyte percentage significantly favored the mometasone group (absolute difference −1.86%, 95% CI −3.66 – −0.06, p = 0.04) however change in absolute reticulocyte count was not significant. Other clinical markers of hemolysis were not statistically significant including change in hemoglobin levels (absolute difference = 0.5 g/dL, 95% CI −0.21 – 1.20, p = 0.16) and change in LDH levels (−111.99, 95% CI −272.49 – 48.51, p = 0.16). The primary marker of pulmonary inflammation, change in eNO, was not significantly different between groups. There were no significant differences between groups with respect to changes in spirometry over time.
Table II.
Outcomes
| Adjusted Intent to treat analyses | |||||
|---|---|---|---|---|---|
| Placebo (17) | Drug (35) | Treatment Effect | 95%CI | P value | |
| Patient-reported outcomes | |||||
| Pain score over time † | |||||
| Mean (SD) | 2.82 (2.21) | 2.09 (2.55) | −1.42 | −0.61 – −2.21 | 0.001 |
| LS mean (SE)*** | 3.09 (0.23) | 1.67 (0.34) | |||
| Δ ASCQ-Me Pain domain †† | |||||
| Mean (SD) | 6.9 (24.0) | 2.8 (22.7) | −5.2 | −19.7 – 9.29 | 0.47 |
| LS mean (SE)*** | 6.9 (6.80) | 2.8 (4.86) | |||
| Asthma control test (0–25) †† | |||||
| • Total score | 17.1 (1.78) | 17.7 (2.25) | 0.20 | −1.13 – 1.53 | 0.3 |
| • Respiratory symptoms interfering with work? | 7 (41.2%) | 9 (26.5%) | |||
| • Shortness of breath? | 6 (35.3%) | 5 (14.7%) | |||
| • Nighttime symptoms? | 6 (35.3%) | 7 (20.6%) | |||
| • Wheezing controlled? | 12 (75%) | 22 (66.7%) | |||
| Clinical outcomes | |||||
| ED Visits ††† | |||||
| Mean (SD) | 1.12 (1.87) | 0.97 (1.52) | 0.80* | 0.35 – 1.84 | 0.60 |
| Predicted count (SE)** | 1.05 (0.44) | 1.00 (0.31) | |||
| Observation admits ††† | |||||
| Mean (SD) | 0.59 (1.18) | 0.37 (0.77) | 0.58* | 0.21 – 1.62 | 0.30 |
| Predicted count (SE)** | 0.57 (0.52) | 0.38 (0.41) | |||
| Admissions ††† | |||||
| Mean (SD) | 0.47 (1.23) | 0.37 (0.77) | 0.73* | 0.25 – 2.13 | 0.56 |
| Predicted count (SE)** | 0.45 (0.54) | 0.38 (0.41) | |||
| Overnight stays (admit or obs) ††† | |||||
| Mean (SD) | 0.82 (1.67) | 0.60 (1.09) | 0.69* | 0.28 – 1.71 | 0.43 |
| Predicted count (SE)** | 0.79 (0.45) | 0.61 (0.34) | |||
| Admit rate †††† | |||||
| Mean (SD) | 0.42 (0.39) | 0.38 (0.36) | 0.85* | 0.33 – 2.15 | 0.74 |
| Predicted rate (SE) | 0.42 (0.46) | 0.36 (0.31) | |||
| Admit/obs rate †††† | |||||
| Mean (SD) | 0.74 (0.36) | 0.62 (0.38) | 0.79* | 0.45 – 1.38 | 0.40 |
| Predicted rate (SE) | 0.73 (0.25) | 0.58 (0.21) | |||
| Inpatient days ††† | |||||
| Mean (SD) | 4.09 (9.25) | 2.67 (5.11) | −1.88 | −5.95 – 2.19 | 0.36 |
| LS Mean (SE)*** | 3.95 (1.91) | 2.68 (1.38) | |||
| Biological outcomes | |||||
| Δ Hemoglobin g/dL †† | |||||
| Mean (SD) | −0.01 (1.31) | 0.30 (0.96) | 0.50 | −0.21 – 1.20 | 0.16 |
| LS mean (SE)*** | 0.00 (0.32) | 0.29 (0.23) | |||
| Δ Reticulocytes % †† | |||||
| Mean (SD) | 0.39 (2.85) | −1.16 (2.63) | −1.86 | −3.66 – −0.06 | 0.04 |
| LS mean (SE)*** | 0.37 (0.82) | −1.12 (0.62) | |||
| Δ Reticulocytes × 103/μL †† | |||||
| Mean (SD) | 0.07 (0.52) | −0.15 (0.39) | −0.21 | −0.52 – 0.10 | 0.18 |
| LS mean (SE)*** | 0.07 (0.14) | −0.15 (0.10) | |||
| Δ WBC × 103/μL †† | |||||
| Mean (SD) | −0.61 (3.70) | −0.55 (2.80) | 0.15 | −1.94 – 2.23 | 0.89 |
| LS mean (SE)*** | −0.54 (0.94) | −0.57 (0.67) | |||
| Δ Platelets †† | |||||
| Mean (SD) | 4.33 (127.75) | −41.36 (88.14) | −27.44 | −93.79 – 38.92 | 0.41 |
| LS mean (SE)*** | 2.50 (29.98) | −41.09 (21.28) | |||
| Δ Neutrophils × 103/μL †† | |||||
| Mean (SD) | −0.82 (3.08) | −0.31 (2.92) | 0.51 | −1.54 – 2.56 | 0.50 |
| LS mean (SE)*** | −0.77 (0.93) | −0.33 (0.64) | |||
| Δ LDH †† | |||||
| Mean (SD) | 47.5 (117.15) | −64.81 (132.37) | −111.99 | −272.49 – 48.51 | 0.16 |
| LS mean (SE)*** | 47.39 (76.06) | −65.19 (43.30) | |||
| Δ sVCAM ng/mL †† | |||||
| Mean (SD) | 170.25 (814.74) | −182.47 (785.21) | −526.94 | −1,003.23 – −50.66 | 0.03 |
| LS mean (SE)*** | 169.88 (215.59) | −182.39 (158.85) | |||
| Percent change (SD) | 8.82% (34.60) | −4.86% (29.17) | 13.67 | ||
| Δ eNO (ppb) †† | |||||
| Mean (SD) | 2.71 (6.92) | 0.63 (9.63) | −2.47 | −8.06 – 3.12 | 0.38 |
| LS mean (SE)*** | 2.73 (2.52) | 0.58 (1.84) | |||
| Δ FEV1 %predicted †† | |||||
| Mean (SD) | −2.94 (5.15) | 0.58 (7.69) | 2.67 | −1.70 – 70.4 | 0.23 |
| LS mean (SE)*** | −2.99 (1.96) | 0.66 (1.50) | |||
| Δ FVC % predicted †† | |||||
| Mean (SD) | −1.29 (4.37) | 0.48 (6.53) | 1.39 | −2.37 – 5.15 | 0.46 |
| LS mean (SE)*** | −1.32 (1.69) | 0.60 (1.29) | |||
| Δ FEV1/FVC †† | |||||
| Mean (SD) | −1.41 (3.39) | −0.71 (3.42) | 0.39 | −1.78 – 2.56 | 0.72 |
| LS mean (SE)*** | −1.44 (0.97) | −0.73 (0.74) | |||
All values adjusted for participant age
RR – rate ratio
Predicted counts obtained via negative binomial regression
Least squares mean obtained via linear regression
Random-intercepts, mixed effect model with patient as a random effect
Linear Regression
Negative Binomial Regression
Generalized estimating equation with “REPEATED” statement for subject ID and binary logit link
Safety, adherence and attrition
There were no deaths, cases of pneumonia or acute chest syndrome during the study period. Rates of non-serious adverse events including hoarseness of voice, thrush and sore throat were numerically higher for the mometasone group but were not statistically significant (table 3). The rate of overall medication adherence was 92.6% for the placebo group and 84.7% in the mometasone group (mean difference 7.9%, 95% CI −1.35 – 17.14, p = 0.09). Individuals in the mometasone group had a significantly lower average score on the medication adherence report scale (4.51 vs. 4.18, mean difference = 0.33, 95% CI 0.06 – 0.60, p = 0.02). Post hoc analyses stratified by patient-reported side effects (including hoarseness, thrush and sore throat) did not show a relationship between lower medication adherence and presence of side effects. The medication adherence report scale had a statistically significant but moderate correlation with actual medication adherence (r = 0.40, p = 0.01). With true medication adherence defined as having taken more than 70% of prescribed doses, the medication adherence report scale had an area under the receiver operating characteristic curve of 0.74 (95% CI 0.56 – 0.92) and the cutoff with maximal discrimination was a score of 4.5 out of 5 which had 47% sensitivity and 100% specificity for identification of medication adherence.
Table III.
Adverse events
| Intent to treat analysis | |||||
|---|---|---|---|---|---|
| Placebo (17) | Drug (35) | Odds Ratio | 95%CI | P value | |
| Hoarseness of voice n(%) | 4 (23.5%) | 15 (42.9%) | 1.34 | 0.48 – 3.74 | 0.57 |
| Thrush n(%) | 0 | 2 (5.7%) | 1.16 | 0.08 – 17.42 | 0.91 |
| Sore throat n(%) | 5 (29.4%) | 15 (42.9%) | 1.14 | 0.42 – 3.12 | 0.80 |
| Pneumonia | 0 | 0 | NA | ||
| Acute chest syndrome | 0 | 0 | NA | ||
With respect to trial attrition, there were 2 patients lost to follow up prior to collection of any outcome data at 1 (placebo) and 4 (mometasone) weeks (one dropped out of the trial after one week, the other moved away) who did not contribute data and were not included in the analyses. Of the remaining 52 participants, 3 were lost to follow up between 8 and 10-weeks but were included in all the biological and clinical intent-to-treat analyses (figure 1). A complete description of all 5 cases of attrition is provided in the supplement.
Discussion
Here we report results of a single center, randomized, placebo-controlled, triple-blinded feasibility study of ICS for non-asthmatic individuals with SCD. While the primary goals of the study were to demonstrate protocol fidelity and feasibility, there was also a statistically significant benefit with respect to the a priori clinical outcome: daily pain diary score. Prior studies have found a minimum clinically significant difference between two pain scores to be 1.3 points on a 0–10 scale for acute pain33 and participants in the current study experienced a mean daily improvement in pain score of 1.42 points over four months. With multiple measurements (as were done in this study), the minimum clinically important difference has been shown to decrease further.34 The findings reported herein provide an initial indication that ICS may be a useful adjuvant therapy for non-asthmatic individuals with SCD.
In addition to the main clinical outcome of pain diary scores, the trial also showed a statistically significant benefit for ICS with respect to change in sVCAM. This cytokine was chosen as the primary marker of vascular injury for because higher sVCAM levels are correlated with increased SCD morbidity and mortality.35–39 It was also chosen because sVCAM was measured in peripheral blood away from the site of action of the drug (the lung) and the dose of inhaled mometasone used in this trial does not achieve detectable levels in the blood. Reticulocyte percent, a clinical marker of hemolytic burden, also showed a statistically significant benefit for ICS and a similar, non-significant result was seen with serum LDH and absolute reticulocyte count. These biological assays worsened for the placebo group during the trial. This was not unexpected as we enrolled only individuals who had recent episodes of cough and wheeze; a group we have previously demonstrated to have high rates of vaso-occlusive complications.13 Other biomarkers including IFN-γ and several interleukins did not show substantial changes with mometasone. We speculate that this is because mometasone works by improving red cell oxygenation in the lung thereby reducing red cell sickling, membrane damage and hemolysis. Many of the cytokines which did not show a difference between groups were markers of the innate and adaptive immune response which might not be as directly affected by red cell damage as are sVCAM and the other markers of hemolysis (LDH and reticulocyte count) which did show a difference. With respect to ED visits, admissions and inpatient days, the results numerically favored ICS but were not statistically significant. There were no clinically meaningful changes in either group with eNO. This is consistent with the findings of Cohen et al. in the Sleep and Asthma Cohort22 and suggests that eNO levels in patients with SCD may not be a marker of steroid response, eosinophilic inflammation. The lack of difference between groups with spirometry was likely because individuals with asthma were screened out and most had normal spirometry at entry. Another important strength of the study is that there was no observed increase in the rate of pulmonary infections or serious adverse events which provides important preliminary safety data for ICS in SCD.
To our knowledge, there are no other published clinical trials of asthma controller medications for treatment of SCD. With respect to protocol fidelity, we demonstrated high rates of adherence to study medication with low rates of loss to follow up. The once-daily dosing of inhaled mometasone furoate may have contributed to the high rates of adherence observed in this trial. We also demonstrated the feasibility of an adaptive, covariate-balancing, biased coin randomization to minimize potential bias without making deterministic treatment assignments. Maintaining adherence to asthma control medicine is a tremendous clinical challenge, and our trial evaluated, in individuals with SCD, techniques validated in the general population for improving adherence and measuring adherence to ICS. In our cohort, the medication adherence report scale had similar correlation to actual medication adherence (r = 0.40 in the current trial vs. 0.42 previously) similar area under the ROC curve (0.74 in the current trial vs. 0.75 previously) but we found higher specificity (100% vs 69.4% previously) and lower sensitivity (47% vs. 82.4% previously).29,30 As with previous studies of the medication adherence report scale, a cutoff of 4.5 had the greatest discriminatory value. The National Asthma Education and Prevention Program has a number of carefully constructed tools to improve adherence to asthma controller medication and this trial used an adapted version of these materials. These adapted tools were easy to administer and may be useful for future studies of ICS in SCD and for clinical use of ICS in SCD.
The most important limitations of the study are the small sample size and the fact that it was conducted at a single center. Single center trials historically provide larger estimates of treatment effect than multi-center trials40 so the results reported here must be interpreted with caution. Additionally, rates of adherence to medication were lower in the mometasone group. This would be expected to bias the intent to treat analyses towards the null hypothesis. Stratified analyses did not suggest that measured side effects played a role in the lower adherence, however anecdotally some participants reported an unpleasant taste of the mometasone (something we did not measure in the trial) which may have contributed to lower adherence. Increased pulmonary capillary blood volume has emerged as an important potential etiology of the alterations in lung function in SCD.25,26 Unfortunately this trial was designed prior to the publication of this literature, however the effects of ICS on pulmonary capillary blood flow should be assessed in future studies. Further investigation into the potential mechanisms of benefit for ICS in SCD are also warranted.
In conclusion, this single center, triple blind, placebo controlled trial provides preliminary evidence of clinical and biological benefit of ICS in patients with SCD who do not have asthma but report recent cough or wheeze. Future studies to improve generalizability of these findings should be conducted at multiple centers and should evaluate longer durations of treatment.
Supplementary Material
Acknowledgments
This research was funded by the National Heart Lung and Blood Institute (K23 HL119351 Inhaled Mometasone to Promote Reduction in Vaso-Occlusive Events). Lead research coordinator, Alexa Punzalan, served as the blinded-research coordinator and was in charge of collecting data and managing all data bases. Aria Mattias, the blinded research coordinator was responsible for randomizing all enrolled participants and training them on proper inhaler technique.
Authorship and Conflict of Interest Disclosures
Jeffrey Glassberg MD MA
No conflicts of interest to report
Conception and design of the study
Participant recruitment
Trial oversight and execution
Data analyses
Drafting of the first version of the manuscript
Revision of the manuscript for important intellectual content
Caterina Minnitti MD
No conflicts of interest to report
Conception and design of the study
Participant recruitment
Data analyses
Drafting of the manuscript
Revision of the manuscript for important intellectual content
Caroline Cromwell MD
No conflicts of interest to report
Conception and design of the study
Data analyses
Drafting of the manuscript
Revision of the manuscript for important intellectual content
Lawrence Cytryn MD
No conflicts of interest to report
Data analyses
Drafting of the manuscript
Revision of the manuscript for important intellectual content
Thomas A. Kraus PhD
No conflicts of interest to report
Conception and design of the study
Luminex assays
Data analyses
Drafting of the manuscript
Revision of the manuscript for important intellectual content
Gwen Skloot MD
No conflicts of interest to report
Conception and design of the study
Interpretation of pulmonary function testing
Data analyses
Drafting of the manuscript
Revision of the manuscript for important intellectual content
Jason T. Connor PhD
No conflicts of interest to report
Conception and design of the study
Management of biased coin randomization
Data analyses
Drafting of the manuscript
Revision of the manuscript for important intellectual content
Adeeb Habibur Rahman PhD
No conflicts of interest to report
Conception and design of the study
Data analyses
Drafting of the manuscript
Revision of the manuscript for important intellectual content
William J. Meurer MD MS
No conflicts of interest to report
Conception and design of the study
Management of biased coin randomization
Data analyses
Drafting of the manuscript
Revision of the manuscript for important intellectual content
References
- 1.Leikin SL, Gallagher D, Kinney TR, Sloane D, Klug P, Rida W. Mortality in children and adolescents with sickle cell disease. Cooperative Study of Sickle Cell Disease Pediatrics. 1989;84:500–8. [PubMed] [Google Scholar]
- 2.Glassberg JA, Strunk R, DeBaun MR. Wheezing in children with sickle cell disease. Curr Opin Pediatr. 2014;26:9–18. doi: 10.1097/MOP.0000000000000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petrov VP. Complications of sickle-cell anemia. Klin Med (Mosk) 1966;44:94–8. [PubMed] [Google Scholar]
- 4.Pianosi P, D’Souza SJ, Charge TD, Esseltine DE, Coates AL. Pulmonary function abnormalities in childhood sickle cell disease. J Pediatr. 1993;122:366–71. doi: 10.1016/s0022-3476(05)83418-3. [DOI] [PubMed] [Google Scholar]
- 5.Siddiqui AK, Ahmed S. Pulmonary manifestations of sickle cell disease. Postgrad Med J. 2003;79:384–90. doi: 10.1136/pmj.79.933.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nandedkar SD, Feroah TR, Hutchins W, et al. Histopathology of experimentally induced asthma in a murine model of sickle cell disease. Blood. 2008;112:2529–38. doi: 10.1182/blood-2008-01-132506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galadanci NA, Liang WH, Galadanci AA, et al. Wheezing is common in children with sickle cell disease when compared with controls. J Pediatr Hematol Oncol. 2015;37:16–9. doi: 10.1097/MPH.0000000000000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shilo NR, Alawadi A, Allard-Coutu A, et al. Airway hyperreactivity is frequent in non-asthmatic children with sickle cell disease. Pediatr Pulmonol. 2016;51:950–7. doi: 10.1002/ppul.23374. [DOI] [PubMed] [Google Scholar]
- 9.Cohen RT, Madadi A, Blinder MA, DeBaun MR, Strunk RC, Field JJ. Recurrent, severe wheezing is associated with morbidity and mortality in adults with sickle cell disease. Am J Hematol. 2011;86:756–61. doi: 10.1002/ajh.22098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glassberg JA, Spivey JF, Strunk R, Boslaugh S, DeBaun MR. Painful episodes in children with sickle cell disease and asthma are temporally associated with respiratory symptoms. Blood. 2005;106:28b-b. doi: 10.1097/01.mph.0000212968.98501.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glassberg JA, Chow A, Wisnivesky J, Hoffman R, Debaun MR, Richardson LD. Wheezing and asthma are independent risk factors for increased sickle cell disease morbidity. Br J Haematol. 2012 doi: 10.1111/bjh.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glassberg JA, Wang J, Cohen R, Richardson LD, DeBaun MR. Risk factors for increased ED utilization in a multinational cohort of children with sickle cell disease. Acad Emerg Med. 2012;19:664–72. doi: 10.1111/j.1553-2712.2012.01364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diep RT, Busani S, Simon J, Punzalan A, Skloot GS, Glassberg JA. Cough and wheeze events are temporally associated with increased pain in individuals with sickle cell disease without asthma. Br J Haematol. 2015 doi: 10.1111/bjh.13325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knight-Madden JM, Forrester TS, Lewis NA, Greenough A. Asthma in children with sickle cell disease and its association with acute chest syndrome. Thorax. 2005;60:206–10. doi: 10.1136/thx.2004.029165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knight-Madden JM, Forrester TS, Lewis NA, Greenough A. The impact of recurrent acute chest syndrome on the lung function of young adults with sickle cell disease. Lung. 2010;188:499–504. doi: 10.1007/s00408-010-9255-2. [DOI] [PubMed] [Google Scholar]
- 16.Knight-Madden JM, Barton-Gooden A, Weaver SR, Reid M, Greenough A. Mortality, asthma, smoking and acute chest syndrome in young adults with sickle cell disease. Lung. 2013;191:95–100. doi: 10.1007/s00408-012-9435-3. [DOI] [PubMed] [Google Scholar]
- 17.Boyd JH, Moinuddin A, Strunk RC, DeBaun MR. Asthma and acute chest in sickle-cell disease. Pediatr Pulmonol. 2004;38:229–32. doi: 10.1002/ppul.20066. [DOI] [PubMed] [Google Scholar]
- 18.Boyd JH, Macklin EA, Strunk RC, DeBaun MR. Asthma is associated with acute chest syndrome and pain in children with sickle cell anemia. Blood. 2006;108:2923–7. doi: 10.1182/blood-2006-01-011072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glassberg J, Spivey JF, Strunk R, Boslaugh S, DeBaun MR. Painful episodes in children with sickle cell disease and asthma are temporally associated with respiratory symptoms. J Pediatr Hematol Oncol. 2006;28:481–5. doi: 10.1097/01.mph.0000212968.98501.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andemariam B, Adami AJ, Singh A, et al. The sickle cell mouse lung: proinflammatory and primed for allergic inflammation. Transl Res. 2015;166:254–68. doi: 10.1016/j.trsl.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pritchard KA, Jr, Feroah TR, Nandedkar SD, et al. Effects of experimental asthma on inflammation and lung mechanics in sickle cell mice. Am J Respir Cell Mol Biol. 2012;46:389–96. doi: 10.1165/rcmb.2011-0097OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen RT, Rodeghier M, Kirkham FJ, et al. Exhaled nitric oxide: Not associated with asthma, symptoms, or spirometry in children with sickle cell anemia. J Allergy Clin Immunol. 2016;138:1338–43. e4. doi: 10.1016/j.jaci.2016.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chaudry RA, Rosenthal M, Bush A, Crowley S. Reduced forced expiratory flow but not increased exhaled nitric oxide or airway responsiveness to methacholine characterises paediatric sickle cell airway disease. Thorax. 2014;69:580–5. doi: 10.1136/thoraxjnl-2013-204464. [DOI] [PubMed] [Google Scholar]
- 24.Haynes J, Jr, Obiako B, King JA, Hester RB, Ofori-Acquah S. Activated neutrophil-mediated sickle red blood cell adhesion to lung vascular endothelium: role of phosphatidylserine-exposed sickle red blood cells. Am J Physiol Heart Circ Physiol. 2006;291:H1679–85. doi: 10.1152/ajpheart.00256.2006. [DOI] [PubMed] [Google Scholar]
- 25.Wedderburn CJ, Rees D, Height S, et al. Airways obstruction and pulmonary capillary blood volume in children with sickle cell disease. Pediatr Pulmonol. 2014;49:724. doi: 10.1002/ppul.22952. [DOI] [PubMed] [Google Scholar]
- 26.Lunt A, McGhee E, Robinson P, Rees D, Height S, Greenough A. Lung function, transfusion, pulmonary capillary blood volume and sickle cell disease. Respiratory physiology & neurobiology. 2016;222:6–10. doi: 10.1016/j.resp.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 27.Strunk RC, Cohen RT, Cooper BP, et al. Wheezing symptoms and parental asthma are associated with a physician diagnosis of asthma in children with sickle cell anemia. J Pediatr. 2014;164:821–6. e1. doi: 10.1016/j.jpeds.2013.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meurer WJ, Connor JT, Glassberg J. Simulation of various randomization strategies for a clinical trial in sickle cell disease. Hematology. 2016 doi: 10.1080/10245332.2015.1101966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen JL, Mann DM, Wisnivesky JP, et al. Assessing the validity of self-reported medication adherence among inner-city asthmatic adults: the Medication Adherence Report Scale for Asthma. Ann Allergy Asthma Immunol. 2009;103:325–31. doi: 10.1016/s1081-1206(10)60532-7. [DOI] [PubMed] [Google Scholar]
- 30.Mora PA, Berkowitz A, Contrada RJ, et al. Factor structure and longitudinal invariance of the Medical Adherence Report Scale-Asthma. Psychol Health. 2011;26:713–27. doi: 10.1080/08870446.2010.490585. [DOI] [PubMed] [Google Scholar]
- 31.Asthma Guidelines. National Institutes of Health, U.S. Department of Health and Human Services; Feb, 2011. www.nhlbi.nih.gov/health-pro/resources/lung/naci/asthma-info/asthma-guidelines.htm. [Google Scholar]
- 32.Smith WR, Bovbjerg VE, Penberthy LT, et al. Understanding pain and improving management of sickle cell disease: the PiSCES study. J Natl Med Assoc. 2005;97:183–93. [PMC free article] [PubMed] [Google Scholar]
- 33.Bernstein SL, Bijur PE, Gallagher EJ. Relationship between intensity and relief in patients with acute severe pain. Am J Emerg Med. 2006;24:162–6. doi: 10.1016/j.ajem.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 34.Olsen MF, Bjerre E, Hansen MD, et al. Pain relief that matters to patients: systematic review of empirical studies assessing the minimum clinically important difference in acute pain. BMC medicine. 2017;15:35. doi: 10.1186/s12916-016-0775-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duits AJ, Pieters RC, Saleh AW, et al. Enhanced levels of soluble VCAM-1 in sickle cell patients and their specific increment during vasoocclusive crisis. Clin Immunol Immunopathol. 1996;81:96–8. doi: 10.1006/clin.1996.0163. [DOI] [PubMed] [Google Scholar]
- 36.Duits AJ, Rojer RA, van Endt T, et al. Erythropoiesis and serum sVCAM-1 levels in adults with sickle cell disease. Ann Hematol. 2003;82:171–4. doi: 10.1007/s00277-003-0610-8. [DOI] [PubMed] [Google Scholar]
- 37.Schnog JB, Rojer RA, Mac Gillavry MR, Ten Cate H, Brandjes DP, Duits AJ. Steady-state sVCAM-1 serum levels in adults with sickle cell disease. Ann Hematol. 2003;82:109–13. doi: 10.1007/s00277-003-0609-1. [DOI] [PubMed] [Google Scholar]
- 38.Sakhalkar VS, Rao SP, Weedon J, Miller ST. Elevated plasma sVCAM-1 levels in children with sickle cell disease: impact of chronic transfusion therapy. Am J Hematol. 2004;76:57–60. doi: 10.1002/ajh.20016. [DOI] [PubMed] [Google Scholar]
- 39.Liem RI, O’Gorman MR, Brown DL. Effect of red cell exchange transfusion on plasma levels of inflammatory mediators in sickle cell patients with acute chest syndrome. Am J Hematol. 2004;76:19–25. doi: 10.1002/ajh.20054. [DOI] [PubMed] [Google Scholar]
- 40.Unverzagt S, Prondzinsky R, Peinemann F. Single-center trials tend to provide larger treatment effects than multicenter trials: a systematic review. J Clin Epidemiol. 2013;66:1271–80. doi: 10.1016/j.jclinepi.2013.05.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.