Abstract
End-stage renal disease (ESRD) with immune disorder involves complex interactions between the innate and adaptive immune responses. ESRD is associated with various alterations in immune function such as a reduction in polymorphonuclear leukocyte bactericidal activity, a suppression of lymphocyte proliferative response to stimuli, and a malfunction of cell-mediated immunity at the molecular level. ESRD also increases patients' propensity for infections and malignancies as well as causing a diminished response to vaccination. Several factors influence the immunodeficiency in patients with ESRD, including uremic toxins, malnutrition, chronic inflammation, and the therapeutic dialysis modality. The alteration of T-cell function in ESRD has been considered to be a major factor underlying the impaired adaptive cellular immunity in these patients. However, cumulative evidence has suggested that the immune defect in ESRD can be caused by an Ag-presenting dendritic cell (DC) dysfunction in addition to a T-cell defect. It has been reported that ESRD has a deleterious effect on DCs both in terms of their number and function, although the precise mechanism by which DC function becomes altered in these patients is unclear. In this review, we discuss the effects of ESRD on the number and function of DCs and propose a possible molecular mechanism for DC dysfunction. We also address therapeutic approaches to improve immune function by optimally activating DCs in patients with ESRD.
Keywords: Antigen presenting cells, Costimulatory molecule, Dendritic cells, End-stage renal disease, Immunodeficiency
INTRODUCTION
Chronic kidney disease (CKD) is defined by a reduced glomerular filtration rate (GFR) and/or the presence of pathological damage or markers of kidney damage such as proteinuria or hematuria for more than 3 months (1). End-stage renal disease (ESRD) is the final stage of CKD in which the kidneys no longer function well enough to meet the needs of daily life. Therefore, patients with ESRD need renal replacement therapies (RRTs) such as hemodialysis (HD), peritoneal dialysis (PD), or kidney transplantation. (1). A progressive loss of kidney function causes complications in all bodily organs and affects the cardiovascular, neuromuscular, hematologic, endocrinologic, and immunologic systems through the retention of uremic toxins (1).
From the immunologic perspective, ESRD is characterized by disorders of both the innate and adaptive immune systems. That is, the functions of polymorphonuclear leukocytes, monocytes, macrophages, APCs, and lymphocytes in maintaining an efficient immune response are affected by multiple causes including increased oxidative stress and inflammation, priming of leukocytes, accumulation of uremic toxins, increased apoptosis of immune cells, disturbed renal metabolic effects, and dialysis-related factors (e.g., interactions between the blood and dialysis equipment, the presence of endotoxins in the water used for dialysis, access-related infections, and PD solutions with a high glucose concentration, low pH, or the presence of glucose degradation products represent chronic stimuli for the inflammatory response (2,3,4,5,6,7,8,9,10). These abnormalities of immune cells lead to an increased propensity to infection, a diminished response to vaccination, a decreased production of antibodies in response to specific stimuli by B cells, and an impaired cell-mediated immunity (8,9,10). Furthermore, it has been reported that dysfunctions of the immune system are associated with a high prevalence of cardiovascular disease and mortality in patients with ESRD (1). Among these various immunological disorders in patients with ESRD, the inappropriate production of protective antibodies in response to vaccination implies that an alteration in the function of APCs and/or lymphocytes might contribute to the immune dysfunction in patients with ESRD. Although T-cell functions have been considered as essential factors underlying the impaired cellular immunity in patients with ESRD (7,11), several recent studies have shown that the immune defect in patients with ESRD may also be caused by the dysfunction of APCs, including dendritic cells (DCs). For example, an impaired T-cell activity may be restored in the presence of DCs from healthy donors (12). In addition, stimulation with phytohaemagglutinin together with phorbol myristate acetate, which is a potent mitogen for human T cells, results in a similarly high level of T-cell proliferation for both patients with CKD and healthy controls (13). Moreover, the serological response to strong antigenic stimuli such as cytomegalovirus is not affected in these patients (14). All these data suggest that a functional defect of DCs is one of the key contributors to the immunodeficiency in patients with ESRD and adequate responses can be achieved in these patients when their DCs are optimally stimulated.
DCs are professional APCs that coordinate both the innate and adaptive immune systems (15,16), and alterations in the number and function of DCs have been established in patients with ESRD (3,17,18,19,20). Several studies over the past decade have demonstrated abnormal DC function in these patients. However, the detailed mechanisms have not been fully elucidated. In this review, we describe the effects of ESRD on the number and function of DCs and suggest how to improve the immune disturbance in patients with ESRD by optimally activating DCs.
SOURCES AND FUNCTIONS OF DCs
DCs are the major APCs that bridge the innate and adaptive immune responses by inducing naïve T-cell responses to captured foreign Ags, and several sources of DCs have been identified: Langerhans cells within the skin, monocytes that can differentiate into DCs (monocyte-derived DCs), and immature DCs within the circulation (15,16). DCs can be activated directly via pathogen recognition receptors such as TLRs or indirectly through exposure to specific cytokines. After sensing a foreign Ag via pathogen recognition receptors and then capturing and processing it, DCs undergo maturation, resulting in the secretion of inflammatory cytokines such as IL-12 and IFN, and the upregulation of costimulatory molecules such as CD80 and CD86. After undergoing maturation, DCs migrate to secondary lymphoid tissues where they present processed Ag/peptide coupled to MHCs, which allows for the selection and expansion of Ag-specific CD4+ Th cells (15,16).
The subsets of circulating DC precursors include precursor myeloid DCs and precursor plasmacytoid DCs (21,22,23). These cells express complementary sets of TLRs and respond to different pathogen-associated molecular patterns by upregulating CCR7 expression and producing distinct combinations of cytokines. Myeloid DCs express most TLRs, except for TLR7 and TLR9, and drive a potent Th1-polarized immune response to LPS, which is a TLR4 agonist, through the increased production of IL-12. By contrast, plasmacytoid DCs constitutively express intracellular TLR7 and TLR9, and play an important role in antiviral immunity by producing large amounts of type I-IFNs such as IFN-α (24).
ABNORMALITIES OF DCs IN PA TIENTS WITH ESRD
Number of DCs
Several earlier studies have shown significant reductions in the number of circulating DCs in patients with ESRD compared with that in healthy controls (3,17,18,25). Verkade et al. reported that the number of circulating DCs was reduced by 50%, on average, in patients with ESRD on HD compared with that in healthy controls (3). In a study by Hesselink et al., the authors detected a decrease in the number of circulating DCs in patients with ESRD on either HD or PD, compared with that in healthy volunteers (17). They also revealed that patients on HD had lower myeloid DC counts than patients on PD, patients not receiving RRT, or controls. Similar results were obtained that showed decreased relative numbers of DC precursors in patients with CKD, including those on either HD or PD (18). Moreover, Agrawal et al. found that the plasmacytoid and total DC counts of patients with ESRD on HD were significantly lower than those of controls. The reduction in the number of plasmacytoid DCs was more striking than that of myeloid DCs, and both DC counts declined further after HD (25).
A reduction in the number of circulating DCs has also been reported in patients with CKD not receiving RRT. The two studies mentioned above both reported that the circulating DC counts in patients with CKD who were not undergoing RRT were lower compared with those of healthy controls (17,18). However, there were some differences between the two studies. The study by Hesselink et al. found a lower number of plasmacytoid DCs in patients with CKD than in normal controls, but there was no difference in the myeloid DC count between patients with CKD and controls. In addition, the plasmacytoid DC count, but not the myeloid DC count, was correlated with the GFR in the CKD group who were not receiving RRT. However, Lim et al. showed that the numbers of both plasmacytoid and myeloid DC precursors from patients with CKD were significantly lower than those of healthy controls. The association between GFR and the counts of myeloid DC precursors, but not those of plasmacytoid DC precursors, was statistically significant. A recent study by Paul et al. reported that, compared with healthy controls, patients with CKD 3 showed significantly decreased numbers of circulating myeloid DC precursors (−29%), plasmacytoid DC precursors (−43%), and total DC precursors (−38%) (all p<0.001). Furthermore, the counts of myeloid (r=0.33), plasmacytoid (r=0.38), and total DC precursors (r=0.41) were positively associated with the estimated GFR (all p<0.001) (26). Taken together, all these data indicate that renal dysfunction has a negative effect on either some or all populations of DCs in patients with CKD, including those on HD or PD and those in predialysis.
Although the precise mechanism of the decreased number of DCs in patients with ESRD is unclear, some possible mechanisms have been proposed. First, the retention of uremic toxins may shorten the lifespan of DCs. Indeed, Lim et al. found that uremic serum induces increases in the apoptosis and necrosis of monocyte-derived DCs (20). A second hypothesis states that the reduction of DCs in patients with ESRD may be caused by their diminished production despite elevated levels of GM-CSF, which is a DC-mobilizing cytokine (18,27). Third, an increased recruitment and elevated turnover of circulating DCs in chronically inflamed tissues throughout the body, including the vessel wall, may reduce the number of DCs. For instance, some studies have reported that the recruitment of myeloid DCs from the blood into the vascular intima is induced by several pro-atherogenic factors that also suppress the recirculation of myeloid DCs from the vessel wall into the blood in coronary heart diseases (28,29,30,31). Because advanced CKD is characterized by a chronic inflammatory state and accompanied by an increased prevalence of atherosclerosis, this may lead to a reduction in the number of circulating DCs owing to their sequestration in the atherosclerotic vessel wall. Alternatively, the number of DCs in patients with ESRD on HD could be caused by losses of DCs incurred by the HD procedure. Indeed, some data have indicated that the migration of DCs into tissues or their accumulation in the dialysis equipment and tubing may cause reduced DC counts (3,25).
Functional abnormalities of DCs
It has been reported that while the primary immunostimulatory signal determines the Ag specificity of a cellular immune reaction, the secondary signal induced by costimulatory molecules on DCs is a critical regulator of T-cell differentiation and the resulting immune response (32). In the absence of costimulatory molecules such as CD80 and CD86, T cells become anergic to the presented Ag (33). Immature monocyte-derived DCs can subsequently undergo maturation after treatment with agents such as LPS, CD40 ligand, or a cytokine cocktail (13,19,34). Thus, the decreased Ag-specific T-cell proliferation using immature monocyte-derived DCs from patients may be interpreted as a functional consequence of the suboptimal expression of costimulatory molecules.
Several studies have shown that cultured monocyte-derived DCs from patients with ESRD on HD are less able to stimulate T cells than those from healthy controls, owing to a decreased expression of the pivotal costimulatory molecules (13,19,20). Verkade et al. revealed that the expression of MHC class I and II, CD83, CD86, and CCR7 was significantly lower on mature monocyte-derived DCs from patients on HD in comparison with that in controls (13). Additionally, Lim et al. showed that uremic LPS-stimulated monocyte-derived DCs cultured in control medium or autologous uremic medium demonstrated reduced endocytosis and a decreased expression of costimulatory molecules (CD40, CD80, and CD86) and maturation markers (CD83) compared with those of normal monocyte-derived DCs cultured in control medium. These findings indicated that the terminal differentiation of monocyte-derived DCs (i.e., the transition from an immature to a mature stage) in patients with ESRD on HD was impaired (20). Concordantly with the above data, the terminal differentiation of monocyte-derived DCs was impaired in patients with CKD stage IV to V who were not undergoing RRT (13). Similar results have been obtained for circulating myeloid and plasmacytoid DC precursors directly isolated from patients with ESRD on HD. Lim et al. reported that the expression of surface costimulatory molecules (CD40, CD80, and CD86), IL-12p70 production following LPS stimulation, and IFN-α production following HSV stimulation were impaired in those cells as compared with those in DCs isolated from healthy controls (18). Moreover, some clinical findings have also supported the presence of a DC dysfunction in patients with ESRD, showing that the expression of DC-related cell surface molecules (CD83, CD86, and CCR) was significantly lower on mature monocyte-derived DCs from both hepatitis B virus (HBV) vaccination responders and non-responders compared with that on DCs from healthy controls (13). Additionally, mature monocyte-derived DCs from non-responders to HBV vaccination had a less mature phenotype than DCs from responders and healthy volunteers (13). All these data suggest that the terminal differentiation and maturation capacities of DCs are negatively affected in patients with ESRD, which may cause an immune dysfunction in these patients. However, a few studies found no significant difference in the expression levels of costimulatory molecules on DCs (25,34). Agrawal et al. observed that the magnitude of the LPS-induced upregulation of CD86 on circulating DCs isolated from patients on HD did not differ from that of controls, although their expression of MHC class II and an activation marker were significantly reduced (25). In addition, another recent study showed that the cell surface expression of MHC class II, CD83, CD86, and CCR7 on monocyte-derived DCs did not differ between patients on HD and healthy controls. Furthermore, mature monocyte-derived DCs from patients on HD showed significantly enhanced allogeneic T-cell proliferation compared with those from healthy controls (34). The exact reasons for the discrepancies among studies regarding the expression of costimulatory and maturation markers by DCs upon stimulation are unclear. As previously suggested, multiple factors including differences in the kinds of stimulation, the concentrations of the cytokines used for stimulation, the degrees of mismatch in MHC typing between DC and T cell donors, and the ethnic backgrounds or age groups of enrolled subjects might be responsible for those heterogeneous results (34). In addition, most of the studies that have examined the number and function of DCs have used circulating DCs or monocyte-derived DCs from peripheral blood samples. Because DCs are present in nearly all bodily tissues and form a dense network (18,25,35), the results for DCs from the peripheral tissues of patients with ESRD may differ from those for circulating or monocyte-derived DCs.
Etiology of DC dysfunction
Although most of the results from experimental studies and clinical findings have suggested that the effects of ESRD on DCs are deleterious in terms of both their number and function, the precise reasons for the alteration of DC function in ESRD are unclear. However, some studies have suggested plausible explanations.
First, uremia is a likely candidate for causing altered DC function. In support of this notion, Lim et al. demonstrated that when monocyte-derived DCs from healthy controls were cultured in the presence of either normal or uremic sera, they exhibited decreased endocytosis and a reduced cell-surface expression of costimulatory markers (CD40, CD80, and CD86) and a maturation marker (CD83) only when cultured with uremic sera (20,36). However, the same study found no significant improvement in the functions of monocyte-derived DCs from patients on HD when they were cultured in control media, as compared to DCs from the same source that were cultured in autologous uremic media. These observations indicate that although a uremic milieu has a negative impact on DC functions, there is a likelihood of an underlying intrinsic functional defect of monocyte-derived DCs in patients on HD.
Second, alterations of TLRs may be a cause of DC dysfunction in patients with ESRD. TLRs play a pivotal role in pathogen recognition and subsequent cytokine synthesis, and they are key regulators of DC function and maturation (37). That is, DCs can be activated directly via TLRs, which regulate the expression of costimulatory molecules on DCs. Therefore, it is possible that an impaired function of TLRs is associated with the impaired function of DCs. Indeed, TLR4 expression on monocytes was shown to be constitutively diminished in patients with CKD stage III to IV (38), or stage V not on RRT, especially in subjects who are predisposed to infections, and diminished TLR4 expression has been associated with a reduced synthesis of tumor necrosis factor-α, IL-1β, IL-6, and IL-8 in response to LPS challenge compared with those from control subjects (39). Similar results have been observed in patients on HD, suggesting that, apart from uremia, endotoxins contained in the dialysate might eventually lead to a decrease in TLR4 expression by continuously stimulating DCs, causing a diminished ability to respond to LPS (38,40). Conversely, an increased expression of TLR2 and TLR4 on monocytes from patients on HD has also been reported (41). The underlying mechanisms of this altered TLR4 expression in patients with ESRD are unknown. However, it is possible that differences among the models of dialysis equipment used for HD might result in these heterogeneous results. Moreover, the above studies evaluated the effect of renal dysfunction on the monocytes, rather than DCs, isolated from patients with CKD. Thus, the results obtained for monocytes may differ from those for DCs.
Third, hyperparathyroidism may bring about a DC dysfunction in patients with ESRD. Several previous studies have found that parathyroid hormone receptors exist on most immunologic cells, including neutrophils, B cells, and T cells, and a chronic excessive level of parathyroid hormone, as in uremia, affects the functions of neutrophil, B cells, and T cells via the sustained elevation of their intracellular calcium concentration (42). However, no study has reported on the expression of parathyroid hormone receptor on APCs including DCs; therefore, this needs to be elucidated.
MODULATION OF DC FUNCTION IN PATIENTS WITH ESRD
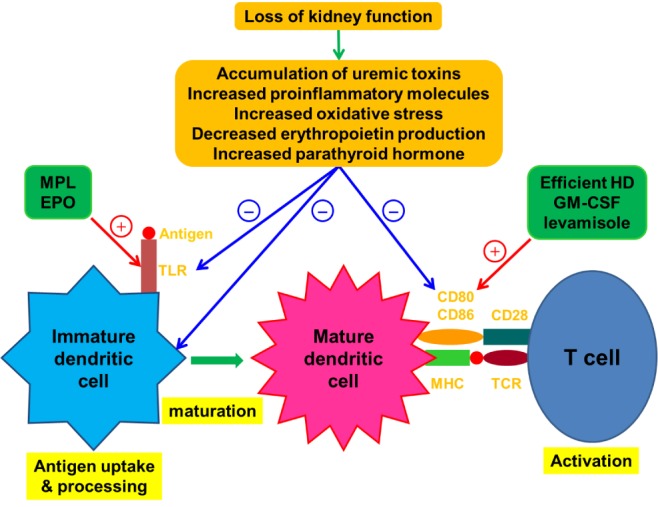
As indicated above, dysfunctional Ag presentation by DCs can be a major cause of immune dysfunction in patients with ESRD, and recent studies on basic immunology have determined that early innate immune signals can shape subsequent adaptive responses (43). Thus, various attempts to overcome the immune dysfunction in patients with ESRD by stimulating DCs have been carried out by several researchers (Fig. 1).
Figure 1. Effect of kidney failure on dendritic cells and therapeutic approaches involving the modulation of dendritic cell maturation. The loss of kidney function causes an accumulation of uremic toxins and proinflammatory molecules, leading to chronic low grade inflammation and increased oxidative stress. Kidney failure also leads to disturbed renal metabolic and endocrinologic activities, resulting in abnormalities such as increased parathyroid hormone production and a decreased circulating concentration of erythropoietin. The results of these defects associated with renal failure have detrimental effects on dendritic cells. After sensing a foreign Ag via TLRs and then capturing and processing it, dendritic cells undergo maturation and begin to express Ag–MHCs and appropriate costimulatory molecules like CD80 and CD86 at the cell surface. This process is associated with T-cell activation. Several therapeutic modalities can induce the terminal differentiation of immature dendritic cells into their fully matured immunogenic form through activating TLRs or inducing the upregulation of costimulatory molecules. The red line and plus sign indicate activation. The blue line and minus sign indicate inhibition. EPO, erythropoietin; GM-CSF, granulocyte macrophage colony-stimulating factor; HD, hemodialysis; MHC, major histocompatibility complex; MPL, monophosphoryl lipid; TCR, T-cell receptor; TLR, Toll-like receptor.
Efficient dialysis
Since it has been clear that the effects of uremia on DCs are detrimental (20,36), several therapeutic interventions have been proposed to prevent or at least limit uremiarelated cellular and/or biological alterations of immunologic cells, such as the use of biocompatible HD membranes and the introduction of hemofiltration (44,45,46,47). In addition, it has been suggested that improving HD clearance using a highly efficient dialyzer has the potential to improve the immunologic dysfunction in patients with ESRD. A previous study showed that uremic toxins of varying m.w. (low, medium, and high m.w.) contained in the sera of patients on HD inhibited the functions of normal myeloid and plasmacytoid DCs. Improving the clearance of low-m.w. uremic toxins via the use of a more efficient dialysis membrane improved myeloid DC function, as indicated by the DCs displaying an appropriate expression of costimulatory molecules, CD83, and allogeneic T-cell proliferation, although this did not alter plasmacytoid DC function (36). In addition, a preliminary retrospective analysis found that a prolonged treatment duration is associated with a normalization of the defective expression of CD86 via increased HD adequacy (11).
The effects of the removal of medium- or high-m.w. molecules on DC function in these patients have not been determined, especially at the molecular level. Medium- and high-m.w. uremic toxins also inhibit the function of DCs (36). Emerging evidence from other studies suggests that the failure of conventional HD to remove medium-sized molecules may contribute to an exacerbated inflammatory state and consequently cause impaired immune responses (10). Thus, it has been suggested that using high-flux membranes or introducing hemodiafiltration may lead to further improvements in the function of DCs in patients on HD, because both of those methods can efficiently remove medium-m.w. uremic toxins through convection (48,49). In accordance with this suggestion, a study of 1480 patients on HD showed that the rate of seroconversion to HBV vaccine was significantly higher in patients receiving HD with high-flux membranes than in patients receiving HD with low-flux membranes, and HBV surface Ab titers were higher in the group on HD with high-flux membranes (50). Hence, the authors suggested that HD with high-flux membranes can improve patients' responses to HBV vaccine, although they did not evaluate whether those beneficial effects are caused by an improvement of DC function through the removal of medium-m.w. uremic toxins. In addition, a recent study by Rama et al. showed that switching from conventional HD to the on-line hemodiafiltration technique led to a reduction in the circulating concentration of pro-inflammatory cytokines, suggesting that this therapeutic modality could inhibit the immuno-inflammatory state in patients on HD (47). Taken together, although DC function can be enhanced by efficient HD, its clinical impact has not been fully examined, and there are only a few data about the roles of high-flux membranes or hemodiafiltration on DC functions. Therefore, further studies are needed to evaluate the effects of HD with high-flux membranes and hemodiafiltration on DC functions.
Erythropoietin (EPO)
Although EPO, which is produced by the kidney in response to hypoxia, mainly regulates the survival, proliferation, and differentiation of erythroid progenitor cells, accumulating data suggest that EPO has pleiotropic activities including the immunopotentiation of both the cell-mediated and humoral immune responses (51). These findings have been supported by the demonstration of EPO receptors on various non-erythropoietic (non-hematopoietic) cells and tissues including DCs (52,53,54). In addition, most patients with ESRD have anemia that is mainly caused by EPO deficiency (55). Thus, several studies have suggested that EPO deficiency is a causative factor of immune dysfunction, and EPO replacement may improve the impaired immune responses of patients with ESRD. Prutchi-Sagiv et al. reported that EPO treatment increased the numbers of peripheral blood DCs and monocyte-derived DCs isolated from healthy volunteers (56). The EPO treatment of monocyte-derived DCs was also found to be associated with an increased cell surface expression of CD80, CD86, and MHC class II, increased Ag uptake, and elevated IL-12 secretion compared with those of untreated DCs (56). Similar results were also observed in an animal experiment using transgenic mice overexpressing human EPO, which had more numerous DC populations with a higher cell surface expression of CD80 and CD86 (57). In another animal study using mice, which was carried out by Rocchetta et al., the authors showed that DCs treated with EPO followed by LPS acquired a stronger allostimulatory activity than EPO-treated or LPS-treated DCs alone through the upregulation of TLR4 in differentiating DCs, which subsequently rendered them more sensitive to stimulation by LPS (54). Conversely, the opposite results were reported by Cravedi et al., who did not detect EPO receptor expression on in vitro-generated monocyte-derived DCs from healthy donors after maturation with LPS, and the expression levels of MHC class II, CD40, CD80, and CD86 on the DCs were similar regardless of EPO treatment (58).
From the clinical perspective, it has been reported that EPO improves the response to vaccinations such as HBV in patients with ESRD (59,60,61). Similar results were found in an experimental study using mice, which showed that EPO-treated mice had an enhanced immune response to the clinically-relevant HBV surface Ag (62). Oster et al. also reported that EPO treatment is associated with an improved immune response to influenza vaccine in hematologic patients, with titers similar to those of healthy subjects, although they did not examine the renal function of the patients (63). However, a meta-analysis of 11 studies failed to detect any benefit of EPO treatment regarding the response to HBV vaccine (64). Therefore, the role of EPO treatment in improving the function of DCs and their responsiveness to HBV vaccine remains to be elucidated. However, most of the studies on this topic have used monocyte-derived DCs isolated from healthy volunteers, rather than patients with ESRD, and bone marrow-derived DCs from mice. Thus, further studies are needed to define the effect of EPO on the function of DCs isolated from patients with ESRD.
Vaccine adjuvants
Whereas vaccination against HBV is recommended for all patients with CKD (65), several previous studies have shown that a considerable proportion of these patients develop inefficient immune protection following standard vaccination procedures (66,67). Consequently, the addition of some immune modulators as vaccine adjuvants have been adopted to enhance the efficacy of HBV vaccination in patients on HD, because most of these adjuvants exert their effects, at least in part, by activating DCs (43).
GM-CSF
It was shown that the in vitro exposure of human monocytes to GM-CSF for 24 to 48 h upregulated the expression of the costimulatory molecules CD86 and CD40 as well as MHC class II (68). Verkade et al. also revealed that monocytes treated with GM-CSF exhibited an increased expression of MHC class II, CD54, and CD40, but their expression of CD86 was unchanged. Moreover, they showed that after GM-CSF treatment, DCs virtually disappeared from the circulation, suggesting that DCs leave the peripheral blood and most likely enter the lymphoid tissues (3). In addition, a few studies using mice have shown that GM-CSF administration increased the number of splenic DCs (69,70).
Based on the above findings, some clinical reports have revealed that GM-CSF can be useful as an adjuvant to HBV vaccination in patients with ESRD. Kapoor et al. showed that GM-CSF is a safe vaccine adjuvant that can stimulate an earlier and stronger Ab response to HBV vaccination in patients on HD (71). Similar results were reported showing that most patients developed a protective Ab response to HBV after two booster vaccinations with GM-CSF (3). Moreover, two meta-analyses of 7 studies (187 patients) and 13 studies (734 patients) showed a significantly increased vaccination response rate for patients with CKD who were treated with GM-CSF plus HBV vaccine vs. those who were treated with HBV vaccine alone (72,73), suggesting that GM-CSF can be administered with HBV vaccine to improve the immunologic response in patients with CKD via the activation of DCs.
Levamisole
Levamisole, a synthetic phenylimidazolthiazole with an antihelminthic effect, has been reported to stimulate depressed T-cell activity and enhance the production of antibodies by B cells (74,75). In addition, Chen et al. demonstrated that the treatment of monocyte-derived DCs from healthy donors with levamisole increased the presentation of CD80, CD86, CD83 and MHC class II molecules on the cell membrane and the production of IL-12 p40. Furthermore, neutralization with antibodies against TLR2 inhibited the levamisole-induced production of IL-12 p40, suggesting that TLR2 has a vital role in mediating the stimulation of DCs by levamisole (76). Similar results were found in an experimental study using mice, which showed that levamisole treatment promoted the expression of the DC activation markers CD86 and MHC class II (77).
Based on these molecular mechanisms, several clinical studies have shown that when administered together with certain vaccines such as HBV and tetanus-diphtheria, levamisole can improve seroprotection in patients with ESRD on HD (78,79,80,81), whereas a few other studies did not show a beneficial effect (82,83). Moreover, a meta-analysis of four studies (328 patients) showed that the oral administration of levamisole at a dose of 80~120 mg for 4~6 months significantly increased seroconversion after HBV vaccination (75). These observations suggest that levamisole could significantly improve the response rates of patients on HD to several vaccines through, at least in part, activating DCs.
HB-AS04 and HB-AS02
The vaccines HB-AS04 and HB-AS04 contain recombinant HBV surface Ag formulated with monophosphoryl lipid (MPL), which is a purified and detoxified derivative of the LPS from the bacterial wall of Salmonella minnesota (84). MPL-stimulated APCs express increased levels of costimulatory molecules and secrete cytokines, inducing strong humoral or cellular responses depending on the Ag considered (85,86). Thus, these vaccines can trigger the TLR4 signaling of APCs including DCs, shaping a more effective subsequent adaptive immune response (87).
Clinical observations have shown that in patients with ESRD who are either pre-HD or undergoing HD, compared to four double-doses of a standard recombinant HBV surface Ag vaccine, the HBV-AS04 vaccine provided a faster response and a higher initial response rate (78% vs. 51%). Additionally, the decline in seroprotection over time was significantly delayed in the HBV-AS04 group, and significantly fewer primed patients required a booster dose during follow-up to 42 months (88,89). Surquin et al. reported that compared to four doses of HB-AS04 vaccine, three doses of HB-AS02 vaccine at 0, 1, and 6 months induced a more rapid seroprotection and a higher HBV Ab concentration in patients who were pre-dialysis or undergoing HD or PD (90). All these results indicate that TLR4 activation by vaccine adjuvants such as MPL may improve the function of DCs in patients with ESRD.
CONCLUSIONS
ESRD-related immune dysfunction is complex and diverse. In this review, we summarized multiple studies demonstrating that patients with ESRD show profound alterations in the number and function of DCs. Based on the accumulated evidence, we suggest that DC dysfunction is one of the major causes of the immunologic disturbance in patients with ESRD. Over the past decades, various research groups have explored ways to stimulate DCs, such as high efficiency dialysis, the use of EPO, and administering vaccine adjuvants such as GM-CSF and levamisole. However, the results to date are incomplete and inconsistent. Thus, the further investigation of DC immunobiology in patients with ESRD may help clinicians to understand the complications related to impaired immunity in these patients, such as a frequent incidence of infection or malignancy and a high rate of mortality.
ACKNOWLEDGEMENTS
This work was supported by the National Research Foundation of Korea (NRF-2015R1A2A2A01003472, -2014M 3A6A4075058, -2015R1A2A1A15051472), BK21 plus project fund, and this paper was written as part of Konkuk University's research support program for its faculty on sabbatical leave in 2014. This study was supported by Veterinary Science Research Institute of The Konkuk University.
Abbreviations
- APC
antigen presenting cell
- CCR
chemokine receptor
- CKD
chronic kidney disease
- DC
Dendritic cell
- EPO
erythropoietin
- ESRD
end-stage renal disease
- HBV
hepatitis B virus
- HD
hemodialysis
- GFR
glomerular filtration rate
- MPL
monophosphoryl lipid
- PD
peritoneal dialysis
- RRT
renal replacement therapy
Footnotes
CONFLICTS OF INTEREST: The authors have declared that they have no conflict of interest.
References
- 1.Webster AC, Nagler EV, Morton RL, Masson P. Chronic Kidney Disease. Lancet. 2017;389:1238–1252. doi: 10.1016/S0140-6736(16)32064-5. [DOI] [PubMed] [Google Scholar]
- 2.Anding K, Gross P, Rost JM, Allgaier D, Jacobs E. The influence of uraemia and haemodialysis on neutrophil phagocytosis and antimicrobial killing. Nephrol Dial Transplant. 2003;18:2067–2073. doi: 10.1093/ndt/gfg330. [DOI] [PubMed] [Google Scholar]
- 3.Verkade MA, van de Wetering J, Klepper M, Vaessen LM, Weimar W, Betjes MG. Peripheral blood dendritic cells and GM-CSF as an adjuvant for hepatitis B vaccination in hemodialysis patients. Kidney Int. 2004;66:614–621. doi: 10.1111/j.1523-1755.2004.00781.x. [DOI] [PubMed] [Google Scholar]
- 4.Sester M, Sester U, Clauer P, Heine G, Mack U, Moll T, Sybrecht GW, Lalvani A, Köhler H. Tuberculin skin testing underestimates a high prevalence of latent tuberculosis infection in hemodialysis patients. Kidney Int. 2004;65:1826–1834. doi: 10.1111/j.1523-1755.2004.00586.x. [DOI] [PubMed] [Google Scholar]
- 5.Yoon JW, Gollapudi S, Pahl MV, Vaziri ND. Naïve and central memory T-cell lymphopenia in end-stage renal disease. Kidney Int. 2006;70:371–376. doi: 10.1038/sj.ki.5001550. [DOI] [PubMed] [Google Scholar]
- 6.Lim WH, Kireta S, Leedham E, Russ GR, Coates PT. Uremia impairs monocyte and monocyte-derived dendritic cell function in hemodialysis patients. Kidney Int. 2007;72:1138–1148. doi: 10.1038/sj.ki.5002425. [DOI] [PubMed] [Google Scholar]
- 7.Litjens NH, Huisman M, van den Dorpel M, Betjes MG. Impaired immune responses and antigen-specific memory CD4+ T cells in hemodialysis patients. J Am Soc Nephrol. 2008;19:1483–1490. doi: 10.1681/ASN.2007090971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hauser AB, Stinghen AE, Kato S, Bucharles S, Aita C, Yuzawa Y, Pecoits-Filho R. Characteristics and causes of immune dysfunction related to uremia and dialysis. Perit Dial Int. 2008;(Suppl 3):S183–S187. [PubMed] [Google Scholar]
- 9.Kato S, Chmielewski M, Honda H, Pecoits-Filho R, Matsuo S, Yuzawa Y, Tranaeus A, Stenvinkel P, Lindholm B. Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol. 2008;3:1526–1533. doi: 10.2215/CJN.00950208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen G, Hörl WH. Immune dysfunction in uremia–an update. Toxins (Basel) 2012;4:962–990. doi: 10.3390/toxins4110962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Girndt M, Sester M, Sester U, Kaul H, Köhler H. Molecular aspects of T- and B-cell function in uremia. Kidney Int Suppl. 2001;78:S206–S211. doi: 10.1046/j.1523-1755.2001.59780206.x. [DOI] [PubMed] [Google Scholar]
- 12.Girndt M, Köhler H, Schiedhelm-Weick E, Meyer zum Büschenfelde KH, Fleischer B. T cell activation defect in hemodialysis patients: evidence for a role of the B7/CD28 pathway. Kidney Int. 1993;44:359–365. doi: 10.1038/ki.1993.252. [DOI] [PubMed] [Google Scholar]
- 13.Verkade MA, van Druningen CJ, Vaessen LM, Hesselink DA, Weimar W, Betjes MG. Functional impairment of monocyte-derived dendritic cells in patients with severe chronic kidney disease. Nephrol Dial Transplant. 2007;22:128–138. doi: 10.1093/ndt/gfl519. [DOI] [PubMed] [Google Scholar]
- 14.Betjes MG, Litjens NH, Zietse R. Seropositivity for cytomegalovirus in patients with end-stage renal disease is strongly associated with atherosclerotic disease. Nephrol Dial Transplant. 2007;22:3298–3303. doi: 10.1093/ndt/gfm348. [DOI] [PubMed] [Google Scholar]
- 15.Hubo M, Trinschek B, Kryczanowsky F, Tuettenberg A, Steinbrink K, Jonuleit H. Costimulatory molecules on immunogenic versus tolerogenic human dendritic cells. Front Immunol. 2013;4:82. doi: 10.3389/fimmu.2013.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ganguly D, Haak S, Sisirak V, Reizis B. The role of dendritic cells in autoimmunity. Nat Rev Immunol. 2013;13:566–577. doi: 10.1038/nri3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hesselink DA, Betjes MG, Verkade MA, Athanassopoulos P, Baan CC, Weimar W. The effects of chronic kidney disease and renal replacement therapy on circulating dendritic cells. Nephrol Dial Transplant. 2005;20:1868–1873. doi: 10.1093/ndt/gfh897. [DOI] [PubMed] [Google Scholar]
- 18.Lim WH, Kireta S, Thomson AW, Russ GR, Coates PT. Renal transplantation reverses functional deficiencies in circulating dendritic cell subsets in chronic renal failure patients. Transplantation. 2006;81:160–168. doi: 10.1097/01.tp.0000188620.72969.56. [DOI] [PubMed] [Google Scholar]
- 19.Verkade MA, van Druningen CJ, de Hoek OP, Weimar W, Betjes MG. Decreased antigen-specific T-cell proliferation by moDC among hepatitis B vaccine non-responders on haemodialysis. Clin Exp Med. 2007;7:65–71. doi: 10.1007/s10238-007-0127-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim WH, Kireta S, Leedham E, Russ GR, Coates PT. Uremia impairs monocyte and monocyte-derived dendritic cell function in hemodialysis patients. Kidney Int. 2007;72:1138–1148. doi: 10.1038/sj.ki.5002425. [DOI] [PubMed] [Google Scholar]
- 21.Robinson SP, Patterson S, English N, Davies D, Knight SC, Reid CD. Human peripheral blood contains two distinct lineages of dendritic cells. Eur J Immunol. 1999;29:2769–2778. doi: 10.1002/(SICI)1521-4141(199909)29:09<2769::AID-IMMU2769>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 22.Martínez del Hoyo G, Martín P, Anjuère F, Arias CF, Marín AR, Ruiz S, Parrillas V, Hernández H. Origin and differentiation of dendritic cells. Trends Immunol. 2001;22:691–700. doi: 10.1016/s1471-4906(01)02059-2. [DOI] [PubMed] [Google Scholar]
- 23.Chowdhury F, Johnson P, Williams AP. Enumeration and phenotypic assessment of human plasmacytoid and myeloid dendritic cells in whole blood. Cytometry A. 2010;77:328–337. doi: 10.1002/cyto.a.20872. [DOI] [PubMed] [Google Scholar]
- 24.Jarrossay D, Napolitani G, Colonna M, Sallusto F, Lanzavecchia A. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur J Immunol. 2001;31:3388–3393. doi: 10.1002/1521-4141(200111)31:11<3388::aid-immu3388>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 25.Agrawal S, Gollapudi P, Elahimehr R, Pahl MV, Vaziri ND. Effects of end-stage renal disease and haemodialysis on dendritic cell subsets and basal and LPS-stimulated cytokine production. Nephrol Dial Transplant. 2010;25:737–746. doi: 10.1093/ndt/gfp580. [DOI] [PubMed] [Google Scholar]
- 26.Paul K, Kretzschmar D, Yilmaz A, Bärthlein B, Titze S, Wolf G, Busch M GCKD-Study Investigators. Circulating dendritic cell precursors in chronic kidney disease: a cross-sectional study. BMC Nephrol. 2013;14:274. doi: 10.1186/1471-2369-14-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merad M, Manz MG. Dendritic cell homeostasis. Blood. 2009;113:3418–3427. doi: 10.1182/blood-2008-12-180646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yilmaz A, Weber J, Cicha I, Stumpf C, Klein M, Raithel D, Daniel WG, Garlichs CD. Decrease in circulating myeloid dendritic cell precursors in coronary artery disease. J Am Coll Cardiol. 2006;48:70–80. doi: 10.1016/j.jacc.2006.01.078. [DOI] [PubMed] [Google Scholar]
- 29.Yilmaz A, Schaller T, Cicha I, Altendorf R, Stumpf C, Klinghammer L, Ludwig J, Daniel WG, Garlichs CD. Predictive value of the decrease in circulating dendritic cell precursors in stable coronary artery disease. Clin Sci (Lond) 2009;116:353–363. doi: 10.1042/CS20080392. [DOI] [PubMed] [Google Scholar]
- 30.Kretzschmar D, Betge S, Windisch A, Pistulli R, Rohm I, Fritzenwanger M, Jung C, Schubert K, Theis B, Petersen I, Drobnik S, Mall G, Figulla HR, Yilmaz A. Recruitment of circulating dendritic cell precursors into the infarcted myocardium and pro-inflammatory response in acute myocardial infarction. Clin Sci (Lond) 2012;123:387–398. doi: 10.1042/CS20110561. [DOI] [PubMed] [Google Scholar]
- 31.Wen J, Wen Y, Zhiliang L, Lingling C, Longxing C, Ming W, Qiang F. A decrease in the percentage of circulating mDC precursors in patients with coronary heart disease: a relation to the severity and extent of coronary artery lesions? Heart Vessels. 2013;28:135–142. doi: 10.1007/s00380-011-0218-1. [DOI] [PubMed] [Google Scholar]
- 32.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harding FA, McArthur JG, Gross JA, Raulet DH, Allison JP. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature. 1992;356:607–609. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- 34.Choi HM, Woo YS, Kim MG, Jo SK, Cho WY, Kim HK. Altered monocyte-derived dendritic cell function in patients on hemodialysis: a culprit for underlying impaired immune responses. Clin Exp Nephrol. 2011;15:546–553. doi: 10.1007/s10157-011-0424-2. [DOI] [PubMed] [Google Scholar]
- 35.Cao Q, Zheng D, Wang YP, Harris DC. Macrophages and dendritic cells for treating kidney disease. Nephron Exp Nephrol. 2011;117:e47–e52. doi: 10.1159/000320595. [DOI] [PubMed] [Google Scholar]
- 36.Lim WH, Kireta S, Russ GR, Coates PT. Uremia impairs blood dendritic cell function in hemodialysis patients. Kidney Int. 2007;71:1122–1131. doi: 10.1038/sj.ki.5002196. [DOI] [PubMed] [Google Scholar]
- 37.Gluba A, Banach M, Hannam S, Mikhailidis DP, Sakowicz A, Rysz J. The role of Toll-like receptors in renal diseases. Nat Rev Nephrol. 2010;6:224–235. doi: 10.1038/nrneph.2010.16. [DOI] [PubMed] [Google Scholar]
- 38.Koc M, Toprak A, Arikan H, Odabasi Z, Elbir Y, Tulunay A, Asicioglu E, Eksioglu-Demiralp E, Glorieux G, Vanholder R, Akoglu E. Toll-like receptor expression in monocytes in patients with chronic kidney disease and haemodialysis: relation with inflammation. Nephrol Dial Transplant. 2011;26:955–963. doi: 10.1093/ndt/gfq500. [DOI] [PubMed] [Google Scholar]
- 39.Ando M, Shibuya A, Tsuchiya K, Akiba T, Nitta K. Reduced expression of Toll-like receptor 4 contributes to impaired cytokine response of monocytes in uremic patients. Kidney Int. 2006;70:358–362. doi: 10.1038/sj.ki.5001548. [DOI] [PubMed] [Google Scholar]
- 40.Kuroki Y, Tsuchida K, Go I, Aoyama M, Naganuma T, Takemoto Y, Nakatani T. A study of innate immunity in patients with end-stage renal disease: special reference to toll-like receptor-2 and -4 expression in peripheral blood monocytes of hemodialysis patients. Int J Mol Med. 2007;19:783–790. [PubMed] [Google Scholar]
- 41.Gollapudi P, Yoon JW, Gollapudi S, Pahl MV, Vaziri ND. Leukocyte toll-like receptor expression in endstage kidney disease. Am J Nephrol. 2010;31:247–254. doi: 10.1159/000276764. [DOI] [PubMed] [Google Scholar]
- 42.Geara AS, Castellanos MR, Bassil C, Schuller-Levis G, Park E, Smith M, Goldman M, Elsayegh S. Effects of parathyroid hormone on immune function. Clin Dev Immunol. 2010;2010:pii: 418695. doi: 10.1155/2010/418695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guy B. The perfect mix: recent progress in adjuvant research. Nat Rev Microbiol. 2007;5:505–517. doi: 10.1038/nrmicro1681. [DOI] [PubMed] [Google Scholar]
- 44.Girndt M, Lengler S, Kaul H, Sester U, Sester M, Köhler H. Prospective crossover trial of the influence of vitamin E-coated dialyzer membranes on T-cell activation and cytokine induction. Am J Kidney Dis. 2000;35:95–104. doi: 10.1016/S0272-6386(00)70307-6. [DOI] [PubMed] [Google Scholar]
- 45.Contin-Bordes C, Lacraz A, de Précigout V. Potential role of the soluble form of CD40 in deficient immunological function of dialysis patients: new findings of its amelioration using polymethylmethacrylate (PMMA) membrane. NDT Plus. 2010;3(Suppl 1):i20–i27. doi: 10.1093/ndtplus/sfq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martínez-Miguel P, de Sequera P, Albalate M, Medrano D, Sánchez-Villanueva R, Molina A, Sousa F, Benito J, Nuñez J, Vozmediano C, Aragoncillo I, Barril G, Rodríguez-Puyol D, Pérez-García R, López-Ongil S. Evaluation of a polynephron dialysis membrane considering new aspects of biocompatibility. Int J Artif Organs. 2015;38:45–53. doi: 10.5301/ijao.5000380. [DOI] [PubMed] [Google Scholar]
- 47.Rama I, Llaudó I, Fontova P, Cerezo G, Soto C, Javierre C, Hueso M, Montero N, Martínez-Castelao A, Torras J, Grinyó JM, Cruzado JM, Lloberas N. Online haemodiafiltration improves inflammatory state in dialysis patients: A longitudinal study. PLoS One. 2016;11:e0164969. doi: 10.1371/journal.pone.0164969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ward RA, Schmidt B, Hullin J, Hillebrand GF, Samtleben W. A comparison of on-line hemodiafiltration and high-flux hemodialysis: a prospective clinical study. J Am Soc Nephrol. 2000;11:2344–2350. doi: 10.1681/ASN.V11122344. [DOI] [PubMed] [Google Scholar]
- 49.Karkar A, Abdelrahman M, Locatelli F. A randomized trial on health-related patient satisfaction level with high-efficiency online hemodiafiltration versus high-flux dialysis. Blood Purif. 2015;40:84–91. doi: 10.1159/000381255. [DOI] [PubMed] [Google Scholar]
- 50.Dede F, Yıldız A, Aylı D, Colak N, Odabaş AR, Akoğlu H, Eskioğlu E, Covic A. Modulation of the immune response to HBV vaccination by hemodialysis membranes. Int Urol Nephrol. 2010;42:1069–1075. doi: 10.1007/s11255-009-9616-z. [DOI] [PubMed] [Google Scholar]
- 51.Sasaki R. Pleiotropic functions of erythropoietin. Intern Med. 2003;42:142–149. doi: 10.2169/internalmedicine.42.142. [DOI] [PubMed] [Google Scholar]
- 52.Arcasoy MO. The non-haematopoietic biological effects of erythropoietin. Br J Haematol. 2008;141:14–31. doi: 10.1111/j.1365-2141.2008.07014.x. [DOI] [PubMed] [Google Scholar]
- 53.Nairz M, Sonnweber T, Schroll A, Theurl I, Weiss G. The pleiotropic effects of erythropoietin in infection and inflammation. Microbes Infect. 2012;14:238–246. doi: 10.1016/j.micinf.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rocchetta F, Solini S, Mister M, Mele C, Cassis P, Noris M, Remuzzi G, Aiello S. Erythropoietin enhances immunostimulatory properties of immature dendritic cells. Clin Exp Immunol. 2011;165:202–210. doi: 10.1111/j.1365-2249.2011.04417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kazmi WH, Kausz AT, Khan S, Abichandani R, Ruthazer R, Obrador GT, Pereira BJ. Anemia: an early complication of chronic renal insufficiency. Am J Kidney Dis. 2001;38:803–812. doi: 10.1053/ajkd.2001.27699. [DOI] [PubMed] [Google Scholar]
- 56.Prutchi Sagiv S, Lifshitz L, Orkin R, Mittelman M, Neumann D. Erythropoietin effects on dendritic cells: potential mediators in its function as an immunomodulator? Exp Hematol. 2008;36:1682–1690. doi: 10.1016/j.exphem.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 57.Lifshitz L, Prutchi-Sagiv S, Avneon M, Gassmann M, Mittelman M, Neumann D. Non-erythroid activities of erythropoietin: Functional effects on murine dendritic cells. Mol Immunol. 2009;46:713–721. doi: 10.1016/j.molimm.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 58.Cravedi P, Manrique J, Hanlon KE, Reid-Adam J, Brody J, Prathuangsuk P, Mehrotra A, Heeger PS. Immunosuppressive effects of erythropoietin on human alloreactive T cells. J Am Soc Nephrol. 2014;25:2003–2015. doi: 10.1681/ASN.2013090945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sennesael J, van Der Niepen P, Verbeelen DL. Treatment with recombinant human erythropoietin increases antibody titers after hepatitis B vaccination in dialysis patients. Kidney Int. 1991;40:121–128. doi: 10.1038/ki.1991.189. [DOI] [PubMed] [Google Scholar]
- 60.Anandh U, Thomas PP, Shastry JC, Jacob CK. A randomised controlled trial of intradermal hepatitis B vaccination and augmentation of response with erythropoietin. J Assoc Physicians India. 2000;48:1061–1063. [PubMed] [Google Scholar]
- 61.Hassan K, Shternberg L, Alhaj M, Giron R, Reshef R, Barak M, Kristal B. The effect of erythropoietin therapy and hemoglobin levels on the immune response to Engerix-B vaccination in chronic kidney disease. Ren Fail. 2003;25:471–478. doi: 10.1081/jdi-120021160. [DOI] [PubMed] [Google Scholar]
- 62.Katz O, Gil L, Lifshitz L, Prutchi-Sagiv S, Gassmann M, Mittelman M, Neumann D. Erythropoietin enhances immune responses in mice. Eur J Immunol. 2007;37:1584–1593. doi: 10.1002/eji.200637025. [DOI] [PubMed] [Google Scholar]
- 63.Oster HS, Prutchi-Sagiv S, Halutz O, Shabtai E, Hoffman M, Neumann D, Mittelman M. Erythropoietin treatment is associated with an augmented immune response to the influenza vaccine in hematologic patients. Exp Hematol. 2013;41:167–171. doi: 10.1016/j.exphem.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 64.Fabrizi F, Dixit V, Martin P, Messa P. Erythropoietin use and immunogenicity of hepatitis B virus vaccine in chronic kidney disease patients: A meta-analysis. Kidney Blood Press Res. 2012;35:504–510. doi: 10.1159/000335956. [DOI] [PubMed] [Google Scholar]
- 65.Kausz A, Pahari D. The value of vaccination in chronic kidney disease. Semin Dial. 2004;17:9–11. doi: 10.1111/j.1525-139x.2004.17104.x. [DOI] [PubMed] [Google Scholar]
- 66.Stevens CE, Alter HJ, Taylor PE, Zang EA, Harley EJ, Szmuness W. Hepatitis B vaccine in patients receiving hemodialysis. Immunogenicity and efficacy. N Engl J Med. 1984;311:496–501. doi: 10.1056/NEJM198408233110803. [DOI] [PubMed] [Google Scholar]
- 67.Peces R, Laurés AS. Persistence of immunologic memory in long-term hemodialysis patients and healthcare workers given hepatitis B vaccine: role of a booster dose on antibody response. Nephron. 2001;89:172–176. doi: 10.1159/000046064. [DOI] [PubMed] [Google Scholar]
- 68.Hornell TM, Beresford GW, Bushey A, Boss JM, Mellins ED. Regulation of the class II MHC pathway in primary human monocytes by granulocyte-macrophage colony-stimulating factor. J Immunol. 2003;171:2374–2383. doi: 10.4049/jimmunol.171.5.2374. [DOI] [PubMed] [Google Scholar]
- 69.Storozynsky E, Woodward JG, Frelinger JG, Lord EM. Interleukin-3 and granulocyte-macrophage colony-stimulating factor enhance the generation and function of dendritic cells. Immunology. 1999;97:138–149. doi: 10.1046/j.1365-2567.1999.00741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hanada K, Tsunoda R, Hamada H. GM-CSF-induced in vivo expansion of splenic dendritic cells and their strong costimulation activity. J Leukoc Biol. 1996;60:181–190. doi: 10.1002/jlb.60.2.181. [DOI] [PubMed] [Google Scholar]
- 71.Kapoor D, Aggarwal SR, Singh NP, Thakur V, Sarin SK. Granulocyte-macrophage colony-stimulating factor enhances the efficacy of hepatitis B virus vaccine in previously unvaccinated haemodialysis patients. J Viral Hepat. 1999;6:405–409. doi: 10.1046/j.1365-2893.1999.00180.x. [DOI] [PubMed] [Google Scholar]
- 72.Fabrizi F, Ganeshan SV, Dixit V, Martin P. Meta-analysis: the adjuvant role of granulocyte macrophage-colony stimulating factor on immunological response to hepatitis B virus vaccine in end-stage renal disease. Aliment Pharmacol Ther. 2006;24:789–796. doi: 10.1111/j.1365-2036.2006.03035.x. [DOI] [PubMed] [Google Scholar]
- 73.Cruciani M, Mengoli C, Serpelloni G, Mazzi R, Bosco O, Malena M. Granulocyte macrophage colony-stimulating factor as an adjuvant for hepatitis B vaccination: a metaanalysis. Vaccine. 2007;25:709–718. doi: 10.1016/j.vaccine.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 74.Alavian SM, Tabatabaei SV. Effects of oral levamisole as an adjuvant to hepatitis B vaccine in adults with end-stage renal disease: a meta-analysis of controlled clinical trials. Clin Ther. 2010;32:1–10. doi: 10.1016/j.clinthera.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 75.Fabrizi F, Dixit V, Messa P, Martin P. Meta-analysis: levamisole improves the immune response to hepatitis B vaccine in dialysis patients. Aliment Pharmacol Ther. 2010;32:756–762. doi: 10.1111/j.1365-2036.2010.04410.x. [DOI] [PubMed] [Google Scholar]
- 76.Chen LY, Lin YL, Chiang BL. Levamisole enhances immune response by affecting the activation and maturation of human monocyte-derived dendritic cells. Clin Exp Immunol. 2008;151:174–1781. doi: 10.1111/j.1365-2249.2007.03541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Niu X, Yang Y, Wang J. Synergistic and additive effects of cimetidine and levamisole on cellular immune responses to hepatitis B virus DNA vaccine in mice. Scand J Immunol. 2013;77:84–91. doi: 10.1111/sji.12018. [DOI] [PubMed] [Google Scholar]
- 78.Deniz Ayli M, Ensari C, Ayli M, Mandiroglu F, Mut S. Effect of oral levamisole supplementation to hepatitis B vaccination on the rate of immune response in chronic hemodialysis patients. Nephron. 2000;84:291–292. doi: 10.1159/000045598. [DOI] [PubMed] [Google Scholar]
- 79.Kayataş M. Levamisole treatment enhances protective antibody response to hepatitis B vaccination in hemodialysis patients. Artif Organs. 2002;26:492–496. doi: 10.1046/j.1525-1594.2002.06928.x. [DOI] [PubMed] [Google Scholar]
- 80.Argani H, Akhtarishojaie E. Levamizole enhances immune responsiveness to intra-dermal and intra-muscular hepatitis B vaccination in chronic hemodialysis patients. J Immune Based Ther Vaccines. 2006;4:3. doi: 10.1186/1476-8518-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fallahzadeh MK, Sajjadi S, Singh N, Khajeh M, Sagheb MM. Effect of levamisole supplementation on tetanus vaccination response rates in haemodialysis patients: a randomized double-blind placebo-controlled trial. Nephrology (Carlton) 2014;19:27–31. doi: 10.1111/nep.12158. [DOI] [PubMed] [Google Scholar]
- 82.Sali S, Alavian SM, Hajarizadeh B. Effect of levamisole supplementation on hepatitis B virus vaccination response in hemodialysis patients. Nephrology (Carlton) 2008;13:376–379. doi: 10.1111/j.1440-1797.2008.00952.x. [DOI] [PubMed] [Google Scholar]
- 83.Sanadgol H, Khoshnoodi M, Mashhadi MA, Forghani MS. Effect of adding levamisole on seroconversion response to hepatitis B virus vaccination in hemodialysis patients: a single-center experience. Iran J Kidney Dis. 2011;5:338–341. [PubMed] [Google Scholar]
- 84.Garçon N, Chomez P, Van Mechelen M. Glaxo-SmithKline adjuvant systems in vaccines: concepts, achievements and perspectives. Expert Rev Vaccines. 2007;6:723–739. doi: 10.1586/14760584.6.5.723. [DOI] [PubMed] [Google Scholar]
- 85.Ulrich JT, Myers KR. Monophosphoryl lipid A as an adjuvant. Past experiences and new directions. Pharm Biotechnol. 1995;6:495–524. [PubMed] [Google Scholar]
- 86.Evans JT, Cluff CW, Johnson DA, Lacy MJ, Persing DH, Baldridge JR. Enhancement of antigen-specific immunity via the TLR4 ligands MPL adjuvant and Ribi.529. Expert Rev Vaccines. 2003;2:219–229. doi: 10.1586/14760584.2.2.219. [DOI] [PubMed] [Google Scholar]
- 87.Eleftheriadis T, Pissas G, Liakopoulos V, Stefanidis I, Lawson BR. Toll-like receptors and their role in renal pathologies. Inflamm Allergy Drug Targets. 2012;11:464–477. doi: 10.2174/187152812803589994. [DOI] [PubMed] [Google Scholar]
- 88.Tong NK, Beran J, Kee SA, Miguel JL, Sánchez C, Bayas JM, Vilella A, de Juanes JR, Arrazola P, Calbo-Torrecillas F, de Novales EL, Hamtiaux V, Lievens M, Stoffel M. Immunogenicity and safety of an adjuvanted hepatitis B vaccine in pre-hemodialysis and hemodialysis patients. Kidney Int. 2005;68:2298–2303. doi: 10.1111/j.1523-1755.2005.00689.x. [DOI] [PubMed] [Google Scholar]
- 89.Kong NC, Beran J, Kee SA, Miguel JL, Sánchez C, Bayas JM, Vilella A, Calbo-Torrecillas F, López de Novales E, Srinivasa K, Stoffel M, Hoet B. A new adjuvant improves the immune response to hepatitis B vaccine in hemodialysis patients. Kidney Int. 2008;73:856–862. doi: 10.1038/sj.ki.5002725. [DOI] [PubMed] [Google Scholar]
- 90.Surquin M, Tielemans CL, Kulcsár I, Ryba M, Vörös P, Mat O, Treille S, Dhaene M, Stolear JC, Kuriyakose SO, Leyssen MX, Houard SA. Rapid, enhanced, and persistent protection of patients with renal insufficiency by AS02(V)-adjuvanted hepatitis B vaccine. Kidney Int. 2010;77:247–255. doi: 10.1038/ki.2009.454. [DOI] [PubMed] [Google Scholar]


