Abstract
The purpose of this study was to explore the effect of two months moderate exercise on levels of IFN-γ, IL-12, IL-6 and IL-4 in serum and supernatants of in vitro mitogen-activated (PHA for 48 h) whole blood (WB) and peripheral blood mononuclear cells (PBMCs). Sixteen healthy males participated in running program (30 min/day, 5 days/week). Blood samples were collected in three stages; 24 h before to start exercise, 48 h and two months after the last session of the exercise. The samples were analyzed for the cytokines by ELISA. The levels of IFN-γ and IL-12 were increased significantly in activated PBMCs culture after exercise and were back to normal level after two months rest. A significant elevation of IFN-γ/IL-4 ratio was observed in activated PBMCs culture by acting possibly on IFN-γ. The results suggest that short moderate intensity exercise enhances Th1 immune inflammatory and anti-allergic conditions in response to mitogen.
Keywords: Cytokine, IFN-γ, IL-12, Inflammation, Exercise
INTRODUCTION
The two major arms of the immune system are interferon (IFN)-γ producing T helper 1 (Th1) and interleukin (IL)-4 producing Th2 cells (1). IFN-γ provides help for humoral and cellular immune responses to clearing infections and are known as powerful cytokine that induce immunity, while IL-4 play a key role in promoting allergic inflammation (2,3,4).
Immune system is highly influenced by physical exercise. The clinical reported effects of moderate exercise on the immune system differ from that of excessive physical exercise. Short moderate exercise is correlated with reduced incidence of respiratory infections and asthma (5,6), while excessive amount of exercise is considered a major risk factor for allergic disorders (7,8). The molecular mechanism underling these differences in disease susceptibility based on exercise intensity are not clearly understood. The possible important mechanisms behind these phenomena are cytokines (9). During and after physical activity, levels of a variety of peripheral cytokines changes (10). For example, certain cytokines such as IL-1, IL-8, IL-6 and TNF-a are produced directly during exercise (11,12). On the other hand, Th1 immune response is differentially affected by intensity of exercise. Moderate exercise increased CD4+ Th1 cells and induced differentiation of naive T cells toward the Th1 phenotype (13,14,15), while excessive amount of exercise causes a decrease in the frequency of circulating CD4+ Th1 cells (16,17).
Based on this background, we hypothesized that short moderate exercise training in young men affects the balance between Th1-inflammatory and Th2-allergic cytokine response. As a result, we evaluated the effect of moderate exercise on behavior of typical Th1 and Th2 cytokines (IFN-γ and IL-4) as well as other cytokines (IL-12 and IL-6) in serum and supernatants of in vitro mitogen-activated WB and PBMCs, in three different pre-exercise, post-exercise and recovery periods.
MATERIALS AND METHODS
Participants
This study was a part of a larger study to explore the effect of exercise on immune systems. Sixteen healthy, untrained male university students (aged 19~23 years, mean 22.4±0.9 years, mean weight 71.5±2.5 kg, mean height 176±2 cm) with sedentary life style were selected and attended in this study. All volunteers completed a questionnaire assessing their physical activities, medical histories, demographic characteristics. The exclusion criteria were: presence of autoimmune disorders; cardiovascular diseases; uncontrolled or untreated hypo/hyperthyroidism; severe asthma; history of treatment with any medication (in the previous 6 mouths); known or suspected abuse of alcohol, drugs or narcotics; recent infections or presence of acute or chronic inflammatory disease. All the participants signed an informed written consent for the study which were approved by the Ethics Committee of Hamadan University of Medical Sciences.
Exercise protocol
The participants performed an exercise protocol on a treadmill. Before beginning the protocol, the primary exercise sessions were performed to detect the maximum heart rate (HR) of each participant. The target HR training zone were calculated for the intensity level of 60~65% using Karvonen formula as follows: [Target HR=((max HR–resting HR)×% intensity)+resting HR]. The participants took part in the exercise 5 days a week for two months at 17:30 to 18:00 (5 min warm-up, 20 min at target HR, and 5 min cool down). During training, maximal heart rate was measured, every 5 min throughout the trial to ensure that each participant was exercising at the correct relative intensity (60~65% max HR). During the study, participant did not perform any activities other than training.
Sample collection
Blood samples were collected in three different times for all participants including; 24 h before start the exercise protocol (pre-exercise), 48 h after the last session of the exercise (post-exercise) and two months rest after the exercise (recovery). The participants did not perform any physical activity during the recovery period and returned to normal sedentary life style. Blood samples were obtained from the cubital vein in the morning between 7~8 am, after a 12 h overnight fast. Samples were collected in two specimen containers, one containing ethylenediaminetetraacetic acid (EDTA) as anticoagulant (10 ml), and the other without anticoagulant (5 ml). Serums were collected and frozen at −80℃ for subsequent ELISA/hematological analysis, and anticoagulant blood used to culture experiments.
Isolation of PBMCs
Peripheral blood samples were collected in tubes with EDTA (Becton Dickinson). The PBMCs were isolated by Ficoll-Hypaque (Histopaque-1077, Biochrome) density-gradient centrifugation, described previously (18). PBMCs were collected into cell culture medium and washed twice with sterile phosphate buffer saline (PBS). Viability of cells was determined by trypan blue dye exclusion.
PBMCs and WB cells culture
For experiments using PBMCs, isolated cells (2×105 cells) were cultured in round bottom plates (Jet Biofil, Shanghai, PRC) and stimulated with 5 mg/ml of Phytohemagglutinin (PHA, Sigma) and incubated for 48 h at 37℃. The dose of mitogen was calculated based on previous studies (19,20). In parallel experiments, one ml of fresh blood containing anticoagulant was suspended in one ml complete culture media supplemented/stimulated with materials like for the PBMCs culture and incubated for 48 h. All culture experiments done in duplicate. To determine in vitro production of cytokines, the cell culture supernatant was collected and frozen at −80℃ until cytokine measurements. As a culture medium, RPMI 1640 (Gibco) supplemented with 2 mM L-glutamine (Gibco), 100 U/ml penicillin (Hayan, Iran), 100 µg/ml streptomycin (Hayan, Iran) and 10% heat inactivated fetal calf serum (Gibco) were used.
Measurement of cytokines
IFN-γ, IL-12, IL-6 and IL-4 were analyzed from serum and cell culture supernatants using a sandwich enzyme-linked immunosorbent assays (Invitrogen Corporation, Camarillo, CA, USA) according to the manufacturer's instructions. Before analysis all thawed samples were centrifuged to remove debris. The standard curves were generated by Smart Magellan™ data analysis software.
Hematological analyses
Total and differential circulating white blood cell (WBC) and platelet counts were determined by using Sysmex-KX21N (Diamond Diagnostics, USA) cell counter analyzer with standard laboratory procedures.
Statistical analysis
Results were expressed as mean±standard error of the mean. Data were checked for normality by the Shapiro-Wilk test, homogeneity of variance, and sphericity, before statistical analysis. ANOVA was used to assess differences among 3 time points. When appropriate, a post-hoc Bonferroni test was applied for multiple comparisons over time within the session. For all tests, p-value≤0.05 was considered statistically significant. Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS, Version 21) and graphs were drawn with Graph Pad Prism software (version 6.07).
RESULTS AND DISCUSSION
The effect of moderate exercise on cytokine production
The biological significance of alterations in the immune system by exercise are unknown but the possible important mechanisms behind that are cytokines. Most of the researches do only look at direct cytokine responses occurring immediately after different type of physical activity in serum or plasma. In this work, we attempted to evaluate the response of the IFN-γ, IL-12, IL-4 and IL-6 cytokines to short time moderate exercise in serum and supernatants of mitogen-stimulated WB and PBMCs. The boosting of cytokine responses in PBMCs by PHA in many ways mimics the initial adaptive immune response to infection. Three different samples of blood serum, WB and PBMCs were collected at three different stages; pre-exercise, post-exercise and after recovery period.
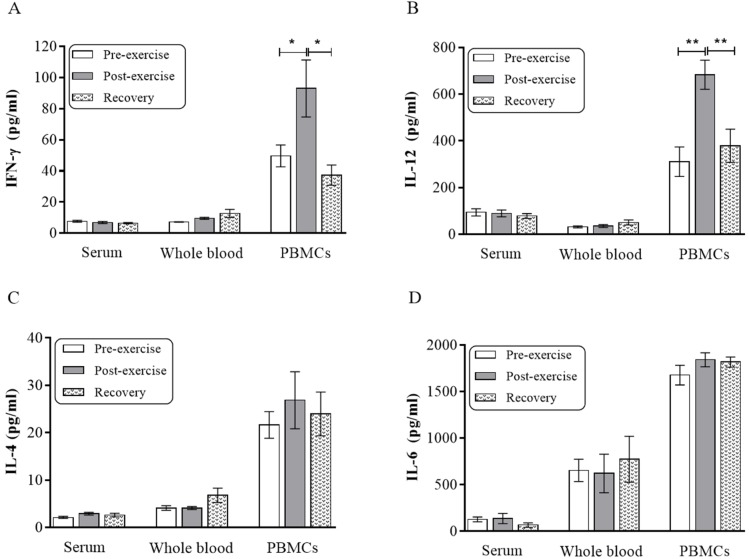
In the post-exercise PBMCs samples, a significant increase of IFN-γ was observed compared with pre-exercise samples (93±18.3 vs. 49.7±7.04, p=0.01). The level of IFN-γ was decreased significantly after two months resting in PBMCs samples (93±18.3 vs. 39.5±6.9, p=0.01) (Fig. 1A). Additional investigations including PHA stimulation of PBMCs showed increased production capacity for IL-12 in the post-exercise phase compared with preexercise phase (683±62 vs. 222±78.5, p<0.001) and returned to baseline values after recovery (311.1±63.3 vs. 683±62, p<0.001) (Fig. 1B). Data analysis of results for IL-6 and IL-4 showed that moderate exercise did not significantly affect the level of these cytokines in different samples (Fig. 1C, 1D).
Figure 1. The effect of moderate exercise on cytokine production. Three different samples of blood serum, WB and PBMCs were collected at three different phases. Serum and culture supernatants were assayed for IFN-γ (A), IL-12 (B), IL-4 (C) and IL-6 (D). Horizontal lines show mean±SEM. WB, whole blood; PBMCs, peripheral blood mononuclear cells; *p=0.01; **p<0.001.
Focusing on the effect of moderate exercise, our major findings are that there was significant difference between pre-exercise and post-exercise phase; that the level of IFN-γ was increased significantly in activated PBMCs culture after exercise; and that increased level of IL-12 was seen in post-exercise state compared with pre-exercise state in PBMCs culture. Our finding are in agreement with pervious study reported that moderate intensity exercise might promote the Th1-type cytokine in vitro (21).
Previous study on professional wrestlers with excessive amount of exercise revealed that production of IL-6 was elevated in mitogen-activated PBMCs culture supernatants, whereas IL-12 was decreased (19). Here, we observed that moderate intensity of exercise increase significantly production of IL-12 but has no effect on IL-6 in PBMCs culture after stimulated by mitogen. This finding provided another possible differences between high and moderate intensity of exercise related to cytokine response.
Moderate exercise alters IFN-γ/IL-4 ratio
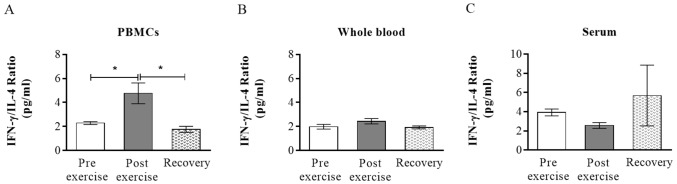
To be able to compare the effect of exercise on Th1/Th2 cells balance, the ratio of IFN-γ and IL-4 were assessed (13,17). When examining PHA-stimulated cytokine levels in PBMCs culture, the ratio of IFN-γ/IL-4 was increased after the exercise and returned to baseline level after recovery period, respectively (2.2±0.11 vs. 4.7±0.86, 4.7±0.86 vs. 1.8±0.24, p<0.001) (Fig. 2A). The ratio of IFN-γ/IL-4 did not fluctuate significantly in relation to moderate exercise in WB and serum samples (Fig. 2B, 2C).
Figure 2. Moderate exercise alters IFN-γ/IL-4 balance. To be able to compare the effect of exercise on Th1/Th2 balance, the ratio of IFN-γ and IL-4 were calculated in PBMCs (A), WB (B) and serum (C). Horizontal lines show mean±SEM. WB, whole blood; PBMCs, peripheral blood mononuclear cells; *p<0.001.
The balance between Th1 and Th2 responses is essential for maintaining normal immune system. IFN-γ and IL-4 are main cytokines of Th1 and Th2 cells, respectively (1). Due to the roles of IFN-γ and IL-4 in the initiation, expansion and regulation of the Th1/Th2 system, the ratio of IFN-γ/IL-4 has been deemed indicators between autoimmunity and allergy (22). IFN-γ is one of the most vital cytokines that induced immune shift towards Th1 and play a crucial role in clearing pathogens and preventing allergic inflammation (2). Moreover, increased level of IFN-γ and reduced IL-4 is an approach that physicians are trying to perform it for the treatment of allergies (23,24). It has been shown that exercise in moderate intensity can diminish the incidence of respiratory infections and allergies (5,6). However, molecular mechanisms that underlie this observation still need further explanation. Here we report that short time moderate exercise induces a temporary increase in the IFN-γ/IL-4 ratio on PBMCs cultures in response to mitogen, an effect that may contribute to our understanding of exercise-related immunity and anti-allergic hypothesis.
Hematological analyses
Total white blood cell counts did not change significantly after the exercise training course. We observed no changes in neutrophils, lymphocytes and platelets counts after the moderate exercise separately (Table I).
Table I. Comparison the effect of moderate exercise on WBC variables.
| Parameter | Pre-exercise | Post-exercise | Recovery | p-value |
|---|---|---|---|---|
| Total WBC count (×109/L) | 5.83±0.41 | 6.08±0.43 | 5.36±0.34 | NS |
| Neutrophils (%) | 62.23±2.58 | 63.2±2.97 | 61.18±3.3 | NS |
| Lymphocytes (%) | 34.69±2.77 | 34.5±2.95 | 36.2±3.17 | NS |
| Platelets (×109/L) | 264.2±16.1 | 271.9±13.5 | 270.4±13.5 | NS |
Results are presented as mean±SEM. NS, not statistically significant.
Overall, several studies were tested the effects of exercise on serum Th1 and Th2 cytokines. Some studies reported that cytokine enhances in response to exercises while others indicated a decrease (14,16,25,26). The inconsistent results from existing research may clarified by the use of a variety of exercise models, participants as well as intensity and duration of exercise. In this study, we focused to determine if short time moderate exercise was able to change the cytokine production levels in response to mitogen in vitro. The effect of exercise, reported here, was intense and observed in activated PBMCs but not detected significantly in other samples, suggesting that IFN-γ and IL-12 were intensely increased in mitogen-stimulated PBMCs after short time moderate exercise. A significant elevation of IFN-γ/IL-4 was observed in mitogen-activated PBMCs culture. This data suggest that moderate intensity exercise may divert the subtle balance in the immune system towards Th1, by acting possibly on IFN-γ. This may explain, at least partially, molecular mechanism behind the exercise-induced anti-allergic effect related to Th1/Th2 cytokines balance.
ACKNOWLEDGEMENTS
We are grateful to our participants. The study was funded by Vice-chancellor for Research and Technology, Hamadan University of Medical Sciences (No. 16355298).
Footnotes
CONFLICTS OF INTEREST: None of the authors declare competing financial interests.
References
- 1.Zhu J, Paul WE. Peripheral CD4+ T-cell differentiation regulated by networks of cytokines and transcription factors. Immunol Rev. 2010;238:247–262. doi: 10.1111/j.1600-065X.2010.00951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 3.Alahgholi-Hajibehzad M, Kasapoglu P, Jafari R, Rezaei N. The role of T regulatory cells in immunopathogenesis of myasthenia gravis: implications for therapeutics. Expert Rev Clin Immunol. 2015;11:859–870. doi: 10.1586/1744666X.2015.1047345. [DOI] [PubMed] [Google Scholar]
- 4.Jager A, Kuchroo VK. Effector and regulatory T-cell subsets in autoimmunity and tissue inflammation. Scand J Immunol. 2010;72:173–184. doi: 10.1111/j.1365-3083.2010.02432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia-Aymerich J, Varraso R, Danaei G, Camargo CA, Jr, Hernan MA. Incidence of adult-onset asthma after hypothetical interventions on body mass index and physical activity: an application of the parametric g-formula. Am J Epidemiol. 2014;179:20–26. doi: 10.1093/aje/kwt229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matthews CE, Ockene IS, Freedson PS, Rosal MC, Merriam PA, Hebert JR. Moderate to vigorous physical activity and risk of upper-respiratory tract infection. Med Sci Sports Exerc. 2002;34:1242–1248. doi: 10.1097/00005768-200208000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Robson-Ansley P, Howatson G, Tallent J, Mitcheson K, Walshe I, Toms C, DU Toit G, Smith M, Ansley L. Prevalence of allergy and upper respiratory tract symptoms in runners of the London marathon. Med Sci Sports Exerc. 2012;44:999–1004. doi: 10.1249/MSS.0b013e318243253d. [DOI] [PubMed] [Google Scholar]
- 8.Pedersen L, Elers J, Backer V. Asthma in elite athletes: pathogenesis, diagnosis, differential diagnoses, and treatment. Phys Sportsmed. 2011;39:163–171. doi: 10.3810/psm.2011.09.1932. [DOI] [PubMed] [Google Scholar]
- 9.Zhao G, Zhou S, Davie A, Su Q. Effects of moderate and high intensity exercise on T1/T2 balance. Exerc Immunol Rev. 2012;18:98–114. [PubMed] [Google Scholar]
- 10.Kruger K, Mooren FC, Pilat C. The Immunomodulatory effects of physical activity. Curr Pharm Des. 2016;22:3730–3748. doi: 10.2174/1381612822666160322145107. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki K, Nakaji S, Yamada M, Totsuka M, Sato K, Sugawara K. Systemic inflammatory response to exhaustive exercise. Cytokine kinetics. Exerc Immunol Rev. 2002;8:6–48. [PubMed] [Google Scholar]
- 12.Visser M, Pahor M, Taaffe DR, Goodpaster BH, Simonsick EM, Newman AB, Nevitt M, Harris TB. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2002;57:M326–M332. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- 13.Ibfelt T, Petersen EW, Bruunsgaard H, Sandmand M, Pedersen BK. Exercise-induced change in type 1 cytokine-producing CD8+ T cells is related to a decrease in memory T cells. J Appl Physiol (1985) 2002;93:645–648. doi: 10.1152/japplphysiol.01214.2001. [DOI] [PubMed] [Google Scholar]
- 14.Shimizu K, Kimura F, Akimoto T, Akama T, Tanabe K, Nishijima T, Kuno S, Kono I. Effect of moderate exercise training on T-helper cell subpopulations in elderly people. Exerc Immunol Rev. 2008;14:24–37. [PubMed] [Google Scholar]
- 15.Malm C. Exercise immunology: the current state of man and mouse. Sports Med. 2004;34:555–566. doi: 10.2165/00007256-200434090-00001. [DOI] [PubMed] [Google Scholar]
- 16.Lancaster GI, Khan Q, Drysdale PT, Wallace F, Jeukendrup AE, Drayson MT, Gleeson M. Effect of prolonged exercise and carbohydrate ingestion on type 1 and type 2 T lymphocyte distribution and intracellular cytokine production in humans. J Appl Physiol (1985) 2005;98:565–571. doi: 10.1152/japplphysiol.00754.2004. [DOI] [PubMed] [Google Scholar]
- 17.Rehm K, Sunesara I, Marshall GD. Increased Circulating Anti-inflammatory Cells in Marathon-trained Runners. Int J Sports Med. 2015;36:832–836. doi: 10.1055/s-0035-1547218. [DOI] [PubMed] [Google Scholar]
- 18.Alahgholi-Hajibehzad M, Oflazer P, Aysal F, Durmus H, Gulsen-Parman Y, Marx A, Deymeer F, Saruhan-Direskeneli G. Regulatory function of CD4+CD25++ T cells in patients with myasthenia gravis is associated with phenotypic changes and STAT5 signaling: 1,25-Dihydroxyvitamin D3 modulates the suppressor activity. J Neuroimmunol. 2015;281:51–60. doi: 10.1016/j.jneuroim.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Zamani A, Omidi M, Hemmatfar A, Salehi I, Bazmamoun H. Wrestlers' immune cells produce higher interleukin-6 and lower interleukin-12 and interleukin-13 in response to in vitro mitogen activation. Iran J Basic Med Sci. 2014;17:917–912. [PMC free article] [PubMed] [Google Scholar]
- 20.Deenadayalan A, Maddineni P, Raja A. Comparison of whole blood and PBMC assays for T-cell functional analysis. BMC Res Notes. 2013;6:120. doi: 10.1186/1756-0500-6-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baum M, Muller-Steinhardt M, Liesen H, Kirchner H. Moderate and exhaustive endurance exercise influences the interferon-gamma levels in whole-blood culture supernatants. Eur J Appl Physiol Occup Physiol. 1997;76:165–169. doi: 10.1007/s004210050229. [DOI] [PubMed] [Google Scholar]
- 22.Wagner B, Burton A, Ainsworth D. Interferongamma, interleukin-4 and interleukin-10 production by T helper cells reveals intact Th1 and regulatory TR1 cell activation and a delay of the Th2 cell response in equine neonates and foals. Vet Res. 2010;41:47. doi: 10.1051/vetres/2010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nguyen TH, Stokes JR, Casale TB. Future forms of immunotherapy and immunomodulators in allergic disease. Immunol Allergy Clin North Am. 2011;31:343–365. x–xi. doi: 10.1016/j.iac.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Jutel M, Van de Veen W, Agache I, Azkur KA, Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy and novel ways for vaccine development. Allergol Int. 2013;62:425–433. doi: 10.2332/allergolint.13-RAI-0608. [DOI] [PubMed] [Google Scholar]
- 25.Ogawa K, Oka J, Yamakawa J, Higuchi M. Habitual exercise did not affect the balance of type 1 and type 2 cytokines in elderly people. Mech Ageing Dev. 2003;124:951–956. doi: 10.1016/s0047-6374(03)00167-2. [DOI] [PubMed] [Google Scholar]
- 26.Steensberg A, Toft AD, Bruunsgaard H, Sandmand M, Halkjaer-Kristensen J, Pedersen BK. Strenuous exercise decreases the percentage of type 1 T cells in the circulation. J Appl Physiol. 2001;91:1708–1712. doi: 10.1152/jappl.2001.91.4.1708. [DOI] [PubMed] [Google Scholar]