Abstract
Herpesviruses infect cells by fusion of the viral envelope with cellular membranes, primarily the plasma membrane. During this process structural components of the mature virion are lost from the invading nucleocapsid, which then travels along microtubules to the nuclear pore. We examined the penetration process by immunoelectron microscopy and analyzed which of the major tegument proteins remained associated with the incoming capsid. We show that the UL36, UL37, and US3 proteins were present at intracytoplasmic capsids after penetration, whereas the UL11, UL47, UL48, and UL49 tegument proteins were lost. Thus, the three capsid-associated tegument proteins are prime candidates for viral proteins that interact with cellular motor proteins for transport of nucleocapsids to the nucleus.
Herpesviruses primarily infect their target cells by fusion of the viral envelope with the cellular plasma membrane at neutral pH (31). For penetration a set of envelope glycoproteins which are conserved throughout the Herpesviridae subfamilies is required that includes glycoproteins B (gB), H (gH), and L (gL). In addition, in the different viruses different receptor binding proteins are required, which for the prototypic alphaherpesvirus herpes simplex virus 1 (HSV-1) consist of gB, gC, and gD. gB and gC bind to cell surface proteoglycans, whereas gD interacts with cellular surface receptors from the tumor necrosis factor receptor or immunoglobulin superfamilies (31). Interaction of gD with its receptor then triggers the fusion machinery, which results in the formation of a continuous membrane consisting of the plasma membrane of the infected cell and the viral envelope. Whereas viral glycoproteins may reside for a short time at the fusion site (12), most of the tegument which surrounds the nucleocapsid in mature virions rapidly dissociates from the nucleocapsid (25). Among these proteins is the UL48 tegument component, which is translocated to the nucleus of the infected cells to activate immediate-early transcription from the viral genome (2), as well as the UL41 tegument protein, which mediates virus-induced host cell shutoff (21). However, it has not yet been determined exactly which tegument proteins may remain associated with the capsid during passage through the cytoplasm to the nuclear pore and which proteins readily dissociate from the nucleocapsid. This is particularly important since incoming capsids interact with the cellular dynein motor for transport along microtubules to the nuclear pore (5, 26, 30) where the viral genomic DNA is released into the nucleus. So far, no specific viral protein that mediates this capsid-motor interaction has been identified.
To directly assay for the presence or absence of specific tegument proteins at incoming capsids, we used immunoelectron microscopy. This is a powerful tool to examine the protein content of single virus particles at exact intracellular locations along the infectious pathway. In the past, we used this method primarily for analysis of the egress and virion maturation pathway of alphaherpesviruses, in particular pseudorabies virus (PrV) (10-12). Thus, we showed that after assembly of progeny nucleocapsids in the host cell nucleus they acquire a primary envelope by budding at the inner leaflet of the nuclear membrane, thereby incorporating three virally encoded proteins, the products of the conserved UL31 and UL34 genes and the nonconserved US3 gene (6, 10, 14). However, UL31 and UL34 proteins are lost during the subsequent fusion of the primary virion envelope with the outer leaflet of the nuclear membrane (reviewed in references 23 and 28). The US3 protein either may remain associated with the capsid during nuclear egress or is reacquired soon after translocation of nucleocapsids into the cytoplasm (10). Thereafter, virion formation presumably starts at two different sites. At the nucleocapsid, the capsid-associated inner ring of tegument most likely consists of the conserved UL36 gene product (15, 17, 34), the largest protein found in the herpesviruses, which interacts with the conserved UL37 protein (17). Thus, the hypothesis was put forward that the inner, capsid-proximal part of the tegument consists of the UL36, UL37, and US3 proteins. In contrast, the products of the UL46, UL47, UL48, and UL49 genes, which are present only in the alphaherpesviruses but constitute the bulk of the tegument in HSV-1 (32) and PrV (23), associate with membranes of the trans-Golgi network, presumably by interacting with the cytoplasmic tails of virion glycoproteins that assemble within these membranes. Indeed, the UL49 protein of PrV has been shown to interact with the cytoplasmic tails of the gE and gM envelope proteins (8). These data support the picture of a nucleation of tegumentation at two sites, the capsid and the future budding site. The conserved HSV-1 UL11 tegument protein has intrinsic Golgi-targeting properties (3), and deletion of HSV-1 or PrV UL11 or its homolog in human cytomegalovirus impairs virion formation (1, 20, 29). Immunoelectron microscopic analyses indicated that the PrV UL11 protein is associated with the inner side of the virion envelope (20), which is in line with the assumption that UL11 binds to the future viral envelope at the budding site. In summary, the virion architecture that has hypothetically been derived from these studies separated the capsid-proximal tegument components UL36, UL37, and US3 from the distal UL11, UL46, UL47, UL48, and UL49 proteins (23).
If this vision of the tegument composition of the herpesvirus particle is correct, one would assume that after infectious entry into target cells it may be possible that the different proteins disassemble in the same order as they assemble and that the capsid-proximal UL36, UL37, and US3 proteins may remain associated with incoming capsids for longer periods of time than the capsid-distal tegument proteins do. To analyze this in detail, we capitalized on several highly potent monospecific antisera that we generated to PrV tegument proteins in the past few years (7, 8, 15, 16, 19, 20). To this end, rabbit kidney (RK13) cells were infected with PrV strain Kaplan (PrV-Ka [13]) at a multiplicity of infection of 100 at 4°C to obtain a synchronized infection. Cells were then incubated at 37°C for 1 to 5 min and fixed immediately thereafter. Fixation for epoxy embedding in Glycid ether 100 (Serva, Heidelberg, Germany) was performed with 2.5% buffered glutardialdehyde and 1% osmium tetroxide, whereas that for acrylic embedding was performed in Lowicryl K4 M (Lowi, Waldkraiburg, Germany) with only 0.5% buffered glutardialdehyde. Intermediate embedding in low-melting-point agarose (Biozym, Oldendorf, Germany), dehydration, and resin embedding were performed as described earlier (10-12, 14). For intracellular postembedding labeling Lowicryl ultrathin sections were blocked at the surface with 1% cold-water fish gelatin-0.02% M glycine-1% bovine serum albumin (Sigma, Deisenhofen, Germany) in phosphate-buffered saline for 2 h and then incubated with monospecific antisera for 2 h at room temperature. Labeling with 10-nm goat anti-rabbit colloidal gold or protein A-gold (GAR 10 or PAG 10, respectively; British Biocell International, Cambridge, United Kingdom) was performed for 1 h at room temperature. Labeling was scored as positive when extracellular virions or intracellular nucleocapsids were decorated with at least three gold dots per particle.
Ultrathin sections of epoxy-embedded cells and labeled Lowicryl sections, counterstained with uranyl acetate and lead salts, were examined with a transmission electron microscope (EM 400 T, Tecnai 12; Philips, Eindhoven, The Netherlands).
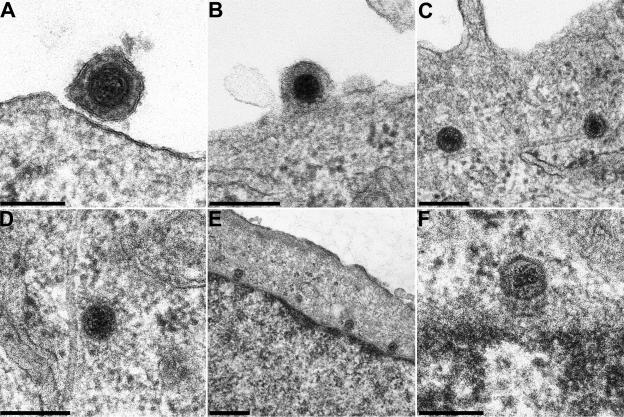
In Fig. 1 the important entry stages are demonstrated in ultrathin sections of epoxy-embedded cells. PrV virions attach to the cell surface (Fig. 1A) by interaction of gC with cellular heparan sulfate proteoglycans (22) and by interaction of gD with cellular nectin (9). The viral envelope then fuses with the plasma membrane (Fig. 1B), which requires gB, gD, and the gH/gL complex (31). Capsids then associate with microtubules (Fig. 1C and D) (30) and finally reach nuclear pores (Fig. 1E and F). Interestingly, capsids are invariably oriented with one vertex towards the nuclear pore.
FIG. 1.
Ultrastructure of the entry process of PrV. PrV virions were documented during attachment (A) and penetration (B), associated with microtubules in the cytoplasm (C and D), and at the nuclear pore (E and F). Bars, 150 (A and F), 200 (B to D), and 500 (E) nm.
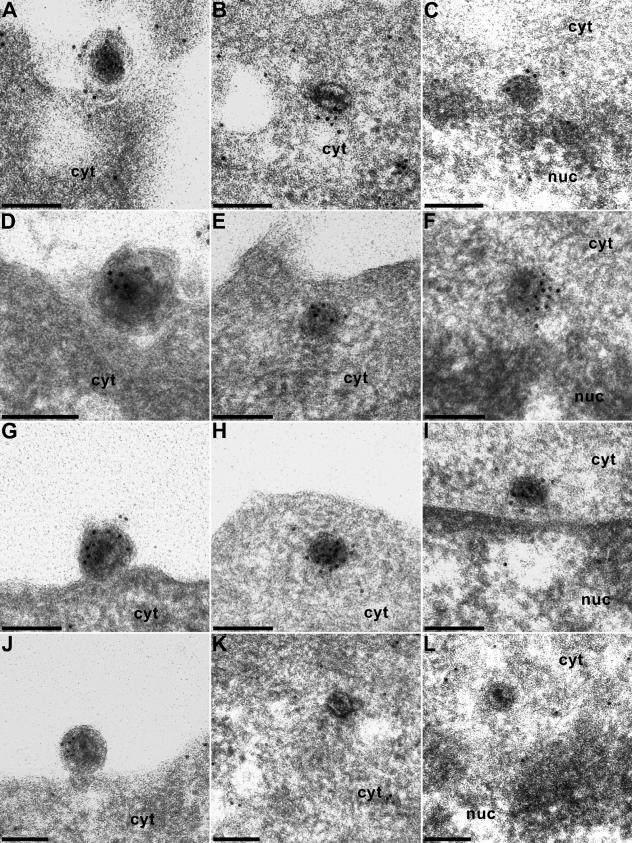
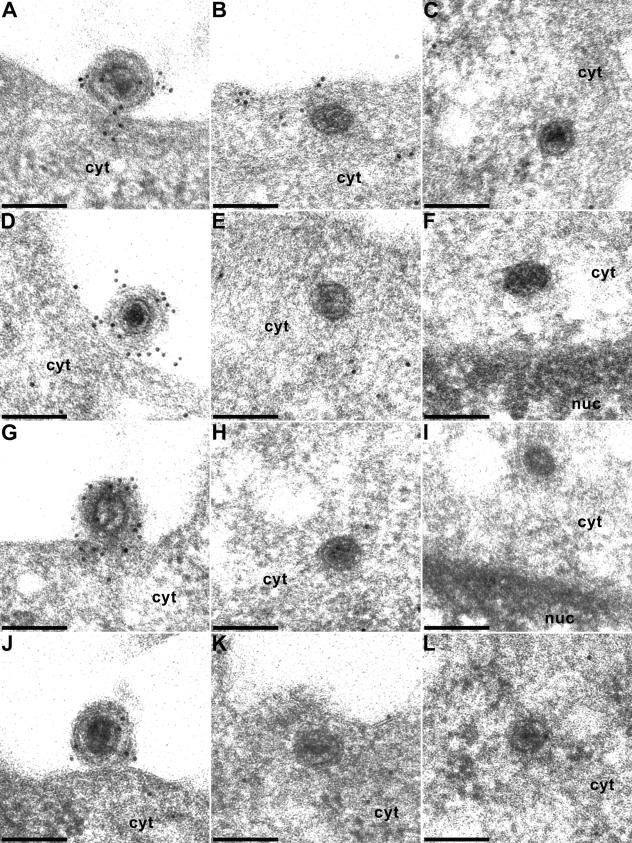
Due to the different embedding technique, which tries to preserve as much antigenicity as possible, images of immunogold-stained capsids in Lowicryl are ultrastructurally not as clear as in double-fixed epoxy embedding (Fig. 1). Because of the rather small amount of antigen at the surface of the section, labeling density was frequently low. Moreover, it required multiple experiments and assays to find the capsids at the intended locations. If possible, per labeling we investigated ca. 10 capsids at the nuclear pore and ca. 30 to 50 capsids in the cytoplasm or in the extracellular space. For each labeling, 10 to 20 ultrathin sections were analyzed. Nevertheless, we were able to record the presence of most of the different tegument antigens on nucleocapsids in three different entry stages: at extracellular virions undergoing fusion of the virion envelope and the plasma membrane (Fig. 2 and 3, panels A, D, G, and J), within the cytoplasm near the plasma membrane (Fig. 2B, E, H, and K; Fig. 3B, C, E, H, K, and L), and at the nuclear pore (Fig. 2C, F, I, and L; Fig. 3F and I).
FIG. 2.
Immunogold labeling of virions during entry. Extracellular PrV virions during attachment or fusion (A, D, G, and J), in the cytoplasm after penetration (B, E, H, and K), and at the nuclear pore (C, F, I, and L) were immunogold labeled with antisera to the US3 (A to C), UL36 (D to F), UL37 (G to I), and UL11 (J to L) proteins. cyt, cytoplasm; nuc, nucleus. Bars, 150 nm.
FIG. 3.
Immunogold labeling of virions during entry. Extracellular PrV virions during attachment or fusion (A, D, G, and J), in the cytoplasm after penetration (B, C, E, H, K, and L), and at the nuclear pore (F and I) were immunogold labeled with antisera to the UL46 (A to C), UL47 (D to F), UL48 (G to I), and UL49 (J to L) proteins. cyt, cytoplasm; nuc, nucleus. Bars, 150 nm.
Extracellular virions in the process of fusion were labeled with antisera to all the virion components tested: US3 (Fig. 2A), UL36 (Fig. 2D), UL37 (Fig. 2G), UL11 (Fig. 2J), UL46 (Fig. 3A), UL47 (Fig. 3D), UL48 (Fig. 3G), and UL49 (Fig. 3J). In contrast, intracytoplasmic capsids either immediately after fusion or at the nuclear pore reacted only with the anti-US3 (Fig. 2B and C), anti-UL36 (Fig. 2E and F), or anti-UL37 (Fig. 2H and I) antisera. None of the other antisera showed any specific labeling of capsids after entry, whereas label may rarely be detected at the plasma membrane (see, e.g., with the anti-UL46 antiserum in Fig. 3B), indicating that tegument proteins remain briefly associated with the stripped envelope.
In summary, our data on the composition of the incoming virus particle are congruent with the hypothesis of a two-part tegument containing a capsid-proximal portion consisting of the UL36, UL37, and US3 proteins and a capsid-distal portion containing the UL11, UL46, UL47, UL48, and UL49 proteins. Moreover, since the UL36, UL37, and US3 proteins remain associated with the capsid during its passage through the cytoplasm to the nuclear pore, they are prime candidates for viral proteins interacting with the cellular dynein motor (5, 30) that propels the capsids through the cytoplasm to the nuclear pore and for interaction with nuclear pore proteins.
Our findings may also have relevance for transport of capsids to the periphery after virion formation in the cytoplasm. In particular, in the axons of infected neurons it has been demonstrated that partially tegumented capsids are transported separately from glycoprotein-containing vesicles (24, 27, 33) by interaction with the kinesin motor. Although the HSV-1 US11 protein has been shown to interact with conventional kinesin heavy chain (4), this protein is not conserved in all neurotropic alphaherpesviruses and, e.g., PrV does not specify this protein (18). Thus, it remains to be analyzed which other protein(s) is important for axonal transport of capsids. In the light of our data, the UL36, UL37, and US3 proteins may well play a role in this process.
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft (Me 854/4-3).
We thank Mandy Jörn, Petra Meyer, Diana Werner, and Elke Zorn for expert technical assistance.
REFERENCES
- 1.Baines, J. D., and B. Roizman. 1992. The UL11 gene of herpes simplex virus encodes a function that facilitates nucleocapsid envelopment and egress from cells. J. Virol. 66:5168-5174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Batterson, W., and B. Roizman. 1983. Characterization of the herpes simplex virion-associated factor responsible for the induction of α-genes. J. Virol. 46:371-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowzard, J. B., R. J. Visalli, C. B. Wilson, J. S. Loomis, E. M. Callahan, R. J. Courtney, and J. W. Wills. 2000. Membrane targeting properties of a herpesvirus tegument protein-retrovirus Gag chimera. J. Virol. 74:8692-8699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diefenbach, R. J., M. Miranda-Saksena, E. Diefenbach, D. J. Holland, R. A. Boadle, P. J. Armati, and A. L. Cunningham. 2002. Herpes simplex virus tegument protein US11 interacts with conventional kinesin heavy chain. J. Virol. 76:3282-3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Döhner, K. A. Wolfstein, U. Prank, C. Echeverri, D. Dujardin, R. Vallee, and B. Sodeik. 2002. Function of dynein and dynactin in herpes simplex virus capsid transport. Mol. Biol. Cell 13:2795-2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuchs, W., B. G. Klupp, H. Granzow, N. Osterrieder, and T. C. Mettenleiter. 2002. The interacting UL31 and UL34 gene products of pseudorabies virus are involved in egress from the host-cell nucleus and represent components of primary enveloped but not mature virions. J. Virol. 76:364-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuchs, W., H. Granzow, B. G. Klupp, M. Kopp, and T. C. Mettenleiter. 2002. The UL48 tegument protein of pseudorabies virus is critical for intracytoplasmic assembly of infectious virions. J. Virol. 76:6729-6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuchs, W., B. G. Klupp, H. Granzow, C. Hengartner, A. Brack, A. Mundt, L. W. Enquist, and T. C. Mettenleiter. 2002. Physical interaction between envelope glycoproteins E and M of pseudorabies virus and the major tegument protein UL49. J. Virol. 76:8208-8217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geraghty, R. J., C. Krummenacher, G. H. Cohen, R. J. Eisenberg, and P. G. Spear. 1998. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science 280:1618-1620. [DOI] [PubMed] [Google Scholar]
- 10.Granzow, H., B. G. Klupp, and T. C. Mettenleiter. 2004. The pseudorabies virus US3 protein is a component of primary and of mature virions. J. Virol. 78:1314-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Granzow, H., B. G. Klupp, W. Fuchs, J. Veits, N. Osterrieder, and T. C. Mettenleiter. 2001. Egress of alphaherpesviruses: comparative ultrastructural study. J. Virol. 75:3675-3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Granzow, H., F. Weiland, A. J.öns, B. G. Klupp, A. Karger, and T. C. Mettenleiter. 1997. Ultrastructural analysis of the replication cycle of pseudorabies virus in cell culture: a reassessment. J. Virol. 71:2072-2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaplan, A. S., and A. E. Vatter. 1959. A comparison of herpes simplex and pseudorabies virus. Virology 7:394-407. [DOI] [PubMed] [Google Scholar]
- 14.Klupp, B. G., H. Granzow, and T. C. Mettenleiter. 2000. Primary envelopment of pseudorabies virus at the nuclear membrane requires the UL34 gene product. J. Virol. 74:10063-10073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klupp, B. G., H. Granzow, E. Mundt, and T. C. Mettenleiter. 2001. Pseudorabies virus UL37 gene product is involved in secondary envelopment. J. Virol. 75:8927-8936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klupp, B. G., H. Granzow, and T. C. Mettenleiter. 2001. Effect of the pseudorabies virus US3 protein on nuclear membrane localization of the UL34 protein and virus egress from the nucleus. J. Gen. Virol. 82:2363-2371. [DOI] [PubMed] [Google Scholar]
- 17.Klupp, B. G., W. Fuchs, H. Granzow, R. Nixdorf, and T. C. Mettenleiter. 2002. The pseudorabies virus UL36 tegument protein physically interacts with the UL37 protein. J. Virol. 76:3065-3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klupp, B. G., C. Hengartner, T. C. Mettenleiter, and L. W. Enquist. 2004. The complete, annotated sequence of the pseudorabies virus genome. J. Virol. 78:424-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kopp, M., B. G. Klupp, H. Granzow, W. Fuchs, and T. C. Mettenleiter. 2002. Identification and characterization of the pseudorabies virus tegument proteins UL46 and UL47: role for UL47 in virion morphogenesis in the cytoplasm. J. Virol. 76:8820-8833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kopp, M., H. Granzow, W. Fuchs, B. G. Klupp, E. Mundt, A. Karger, and T. C. Mettenleiter. 2003. The pseudorabies virus UL11 protein is a virion component involved in secondary envelopment in the cytoplasm. J. Virol. 77:5339-5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwong, A., and N. Frenkel. 1989. The herpes simplex virus virion host shutoff function. J. Virol. 63:4834-4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mettenleiter, T. C., L. Zsak, F. Zuckermann, N. Sugg, H. Kern, and T. Ben-Porat. 1990. Interaction of glycoprotein gIII with a cellular heparin-like substance mediates adsorption of pseudorabies virus. J. Virol. 64:278-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mettenleiter, T. C. 2002. Herpesvirus assembly and egress. J. Virol. 76:1537-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miranda-Saksena, J., R. A. Boadle, P. Armati, and A. L. Cunningham. 2002. In rat dorsal root ganglia neuron, herpes simplex virus type 1 tegument forms in the cytoplasm of the cell body. J. Virol. 76:9934-9951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morrison, E., Y.-F. Wang, and D. M. Meredith. 1998. Phosphorylation of structural components promotes dissociation of the herpes simplex virus 1 tegument. J. Virol. 72:7108-7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogawa-Goto, K., K. Tanaka, W. Gibson, E. Moriishi, Y. Miura, T. Kurata, S. Irie, and T. Sata. 2003. Microtubule network facilitates nuclear targeting of human cytomegalovirus capsid. J. Virol. 77:8541-8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Penfold, M. E., P. Armati, and A. L. Cunningham. 1994. Axonal transport of herpes simplex virus to epidermal cells: evidence for a specialized mode of virus transport and assembly. Proc. Natl. Acad. Sci. USA 91:6529-6533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanchez, V., and D. H. Spector. 2002. CMV makes a timely exit. Science 297:778-779. [DOI] [PubMed] [Google Scholar]
- 29.Silva, M.-C., Q.-C. Yu, L. Enquist, and T. Shenk. 2003. Human cytomegalovirus UL99-encoded pp28 is required for the cytoplasmic envelopment of tegument-associated capsids. J. Virol. 77:10594-10605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sodeik, B., M. Ebersold, and A. Helenius. 1997. Microtubule-mediated transport of incoming herpes simplex virus 1 capsids to the nucleus. J. Cell Biol. 136:1007-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spear, P. G., and R. Longnecker. 2003. Herpesvirus entry: an update. J. Virol. 77:10179-10185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stevens, A. C., and P. G. Spear. 1997. Herpesvirus capsid assembly and envelopment, p. 312-351. In W. Chiu, R. M. Burnett, and R. Garcea (ed.), Structural biology of viruses. Oxford University Press, New York, N.Y.
- 33.Tomishima, M., G. A. Smith, and L. W. Enquist. 2001. Sorting and transport of alpha herpesviruses in axons. Traffic 2:429-436. [DOI] [PubMed] [Google Scholar]
- 34.Zhou, H., D. Chen, J. Jakana, F. J. Rixon, and W. Chiu. 1999. Visualization of tegument-capsid interactions and DNA in intact herpes simplex virus type 1 virions. J. Virol. 73:3210-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]