Abstract
In patients with Crohn’s disease (CD), perianal fistulas frequently recur, causing substantial morbidity. We performed a 12 patient, 6 month phase I trial to determine whether autologous mesenchymal stem cells (MSCs), applied in a bioabsorbable matrix, can heal the fistula. Fistula repair was not associated with any serious adverse events related to MSCs or plug placement. At 6 months, 10/12 patients (83%) had complete clinical healing and radiographic markers of response. We found placement of MSC-coated matrix fistula plugs in 12 patients with chronic perianal fistulas to be safe and lead to clinical healing and radiographic response in 10 patients.
Keywords: STOMP trial, IBD, cell therapy, clinical trial
Perianal fistulizing Crohn’s Disease (CD), a particularly refractory disease complication, occurs in up to 20% of CD patients and has a cumulative risk of 26% over a 20 year period1. Novel therapies include the use of biologic and artificial matrices as well as other biological approaches such as MSC therapy. A recent Phase III trial demonstrated that injection of allogeneic MSCs into a fistula tract appears safe and efficacious (50% remission rate at week 24)2. We developed an approach to deliver concentrated MSC to the fistula via attachment of autologous MSCs to a bioabsorbable matrix for definitive surgical placement. Subsequently, we designed a Phase I clinical trial (STem cells On Matrix Plugs; STOMP) to test feasibility and safety of this therapy.
Details of product manufacturing and patient enrollment are available in supplemental methods. Briefly, approval for a Phase I study of autologous MSC-coated fistula plugs in patients with fistulizing CD was obtained through Mayo Clinic Institutional Review Board and the FDA (IND #15356). Patients with CD ages 18–65, with a single draining fistula for at least 3 months without proctitis, and who had failed anti-TNF therapy were eligible. Autologous adipose tissue was obtained and cells were processed and cryo-preserved in the Human Cell Therapy Lab. Upon scheduling of plug placement, MSCs were thawed and returned to culture in the presence of a Gore® Bio-A® Fistula Plug (MATRIX) in a polypropylene bioreactor for 3–6 days. The average dose was approximately 20×106 cells per plug. Patients underwent intraoperative placement of the stem cell loaded plug (MSC-MATRIX) 6 weeks following the MSC harvest by the same surgeon (EJD).
The primary endpoint of this study was to determine the safety and feasibility of using autologous MSC-MATRIX for treatment of refractory CD fistulas. The secondary endpoint of efficacy was defined in 2 ways: 1) clinically and 2) radiographically. Clinically, a partial response was defined as decreased drainage and symptoms as reported by the patient and complete clinical healing was defined as complete cessation of drainage both spontaneously and upon gentle compression upon physical exam at the Week 24 (6 month) visit. Radiographic response was defined by decrease in the diameter and length of the T2-weighted hyperintense fistula tract on T2-weighted fast spin-echo images (percent change from baseline), without development of abscess or additional ramifications off the treated fistula, and without increase in the Van Aasche MRI perianal fistula severity score3.
Twelve of 18 screened patients were treated. Enrolled patients had diverse demographics (Table 1) and had persistent refractory disease (median of 5 years of perianal disease). All patients remained on biologic therapy through the 6 month study duration. There was 1 serious adverse event, which was related to underlying CD, and not related to study treatment. This serious event was debridement of granulation tissue in the fistula tract unrelated to the placement of MSC-MATRIX and did not result in study withdrawal. There were 2 non-serious adverse events related to seromas at the site of fat collection. In addition, there were 11 non-serious adverse events of which 4 were related to underlying CD and 5 were unrelated to underlying CD or the study interventions.
Table 1.
| Subject | Age | Sex | Disease Duration | Previous Management | Previous Surgical Management | No. Prior EUA | Clinic findings at EUA | Clinical response | Drainage | Incontinence |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 21 | F | 13 | IFX, ADA, 6-MP, steroids | seton; fistulotomy | 9 since 2011 | Transsphincteric with puborectalis/levator plate extensions | yes | no | no |
| 2 | 58 | M | 4 | IFX, ADA; AZA | seton; fistulotomy | 8 since 2012 | Suprasphincteric | no | Yes | no |
| 3 | 40 | F | 2 | IFX; ADA | seton | 7 since 2012 | Intersphincteric fistula | yes | no | no |
| 5 | 18 | M | 6 | IFX; ADA | seton | 12 since 2011 | Intersphincteric fistula | yes | no | no |
| 7 | 24 | M | 4 | 6-MP; ADA | seton; diversion | 7 since 2012 | Transsphincteric with puborectalis/levator extension | yes | no | no |
| 8 | 25 | F | 7 | IFX; Cimzia; steroids | seton | 4 since 2008 | Transsphincteric | yes | no | no |
| 9 | 33 | F | 2 | IFX; ADA; AZA; steroids | seton | 5 since 2013 | Transsphincteric | yes | no | no |
| 12 | 51 | F | 6 | IFX+6MP | seton | 1 since 2009 | Transsphincteric | no | Yes | no |
| 13 | 31 | M | 17 | ADA; AZA | I and D | 5 since 1998 | Transsphincteric | yes | no | no |
| 14 | 56 | M | 10 | IFX; AZA; steroids | none | none | Intersphincteric | yes | no | no |
| 17 | 21 | F | 3 | IFX; MTX; ADA; | seton | 5 since 2014 | Transsphincteric | yes | no | no |
| 18 | 42 | M | 4 | ADA | seton; I and D; fistulotomy | 3 since 2013 | Transsphincteric | yes | no | no |
| Mean Age | 35 |
| Median Age | 32 |
| Mean Disease Duration | 6.5 |
| Median Disease Duration | 5 |
IFX: Infliximab
ADA: adalimumab
AZA: Azathioprine
6-MP: 6-Mercaptopurine
MXT: Methotrexate
EUA: Examination under Anesthesia
I and D: Incision and Drainage
Nine of 12 patients had complete clinical healing by 3 months, and 10 of 12 patients (83.3%) had complete clinical healing at 6 months. Of the 2 patients without clinical healing, one developed an abscess at three months requiring seton placement, and the other experienced persistent drainage. Other than 1 patient switching from infliximab to adalimumab therapy (patient preference), no patients underwent a change in primary anti-Crohn’s therapy throughout the 6 months; however 4 patients received antibiotics (<30-day course) at the discretion of the clinical management team.
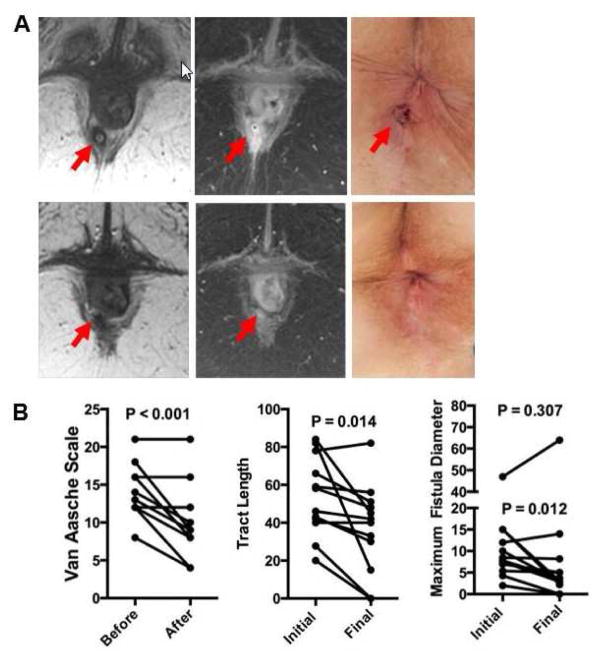
MRI was used to define the characteristics of the treated fistula tracts at baseline and six months (Figure 1A, B). Changes in Van Assche Score, and the length and diameter of T2-weighted hyperintensity within the fistula are shown in Figure 1. Radiographic criteria for response were demonstrated in 10 of 12 patients (83%). Mean absolute changes for length and diameter of fistula tract decreased by a mean of 23.5 and 5.0 mm, respectively, in responding patients, and increased by a mean of 0.2 and 10 mm in treatment failures, respectively. There was a significant decrease in the length of T2-weighted hyperintensity within the fistula (median decrease 22%, range −5 to 100%, p=0.01), and a non-significant decrease in diameter (median decrease 57%, range −36 to 100%, p=0.307), with negative values representing an increase in fistula size in the treatment failures. Similarly, Van Assche perianal severity scores also decreased (median 13 to median 9, p=0.0008), without worsening in any patients. These data collectively demonstrate the therapeutic potential for MSC-MATRIX in this refractory disease. We now plan a larger study as the feasibility, safety and preliminary evidence of efficacy, suggest a promising new approach to the treatment of patients with fistulizing perianal CD.
Figure 1. Fistula response upon treatment with MSC-MATRIX.
(A) Pre and post treatment imaging in a representative patient. Red arrow indicates intersphincteric fistula in 39 year-old female Crohn’s patient prior to treatment and six months after therapy, along with images from perianal examination at time of plug placement (top row) and follow-up MRI. (B) Cumulative results of the changes in Van Aasche score, tract length and fistula diameter. P values represent paired T test before and six months after plug placement. For the fistula diameter, the P value on the upper is representative of all samples while the P value below is for the 11 samples with a starting diameter less than 20 mm.
Supplementary Material
Acknowledgments
GRANT SUPPORT: We would like to thank the visionary support of this work by the Ehrlich Family Foundation. This work was also supported by National Institutes of Health grants R01 (R01AI089714 to WAF), as well as intramural grants from the Center of Regenerative Medicine at the Mayo Clinic and direct support from the Department of Lab Medicine and Pathology. We also appreciate the generous philanthropic support of William H. and Karen J. Eby, as well as the Richard M. Schulze Family Foundation. We thank the members of our research groups, including Peggy Bulur, Adam Armstrong and Scott Riester for technical assistance and/or stimulating discussions.
ABBREVIATIONS
- ABD
Allan B. Dietz
- anti-TNF
Anti-tumor necrosis factor
- CBC
complete blood count with differential
- CD
Crohn’s Disease
- CRP
C reactive protein
- D-PBS
Dulbecco’s phosphate-buffered saline
- EJD
Eric J. Dozois, colorectal surgeon
- ESR
erythrocyte sedimentation rate
- EUA
exam under anesthesia
- GMP
Good Manufacturing Practices
- GWB
Greg W. Butler
- HIV
human immunodeficiency virus
- IND
Investigational New Drug
- IRB
Institutional Review Board
- MATRIX
Gore® Bio-A® Fistula Plug
- MRI
magnetic resonance imaging
- MSC-MATRIX
stem cell loaded plug
- MSCs
MSC, mesenchymal stem cell(s)
- SOP
Standard Operating Procedure
- STOMP
Stem cells On Matrix Plug
Footnotes
AUTHOR CONTRIBUTIONS:
Allan B. Dietz -Study concept and design, supervision, data analysis, writing and editing
Eric J. Dozois - Study concept and design, supervision, writing and editing
Joel G. Fletcher - Imaging collection and analysis, writing and editing
Greg W. Butler – Technical support
Darcie Radel – Technical support
Amy L. Lightner– Drafting and editing manuscript
Maneesh Dave - Manuscript writing and editing
Jessica Friton– Patient management,
Asha Nair– Data collection
Emily T. Camilleri– Data collection
Amel Dudakovic - Data collection and analysis
Andre J van Wijnen– Data collection and analysis, manuscript writing and editing
William A. Faubion - Study concept and design, supervision, data analysis, writing and editing
DISCLOSURES: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. Allan Dietz and Greg Butler are inventors of technology used as a tool in this research; the technology has been licensed to a commercial entity (PLTMax; Mill Creek LifeScienes). ABD and Mayo Clinic have equity in the company and ABD and GWB have contractual rights to receive royalties from the licensing of this technology. ABD has governance responsibilities within this company. These conflicts have been disclosed to and are managed by the Mayo Clinic Conflict of Interest Board and are included here as directed by them. No other authors have a conflict to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ClinicalTrials.gov Identifier: NCT01915927
References
- 1.Schwartz DA, et al. Gastroenterology. 2002;122:875–80. doi: 10.1053/gast.2002.32362. [DOI] [PubMed] [Google Scholar]
- 2.Panes J, et al. Lancet. 2016;388:1281–90. doi: 10.1016/S0140-6736(16)31203-X. [DOI] [PubMed] [Google Scholar]
- 3.Van Assche G, et al. American Journal of Gastroenterology. 2003;98:332–339. doi: 10.1111/j.1572-0241.2003.07241.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.