Abstract
Objective
To assess the association between prophylactic indomethacin and bronchopulmonary dysplasia (BPD) in a recent, large cohort of extremely preterm infants.
Study design
Retrospective cohort study using prospectively collected data for infants with gestational ages < 29 weeks or birth weights of 401–1000g born between 2008 and 2012 at participating hospitals of the National Institute of Child Health and Human Development Neonatal Research Network. Infants treated with indomethacin in the first 24 hours of life were compared with those who were not. Study outcomes were BPD, defined as use of supplemental oxygen at 36 weeks postmenstrual age among survivors to that time point, death, and the composite of death or BPD. Pre-specified subgroup analyses were performed.
Results
Prophylactic indomethacin use varied by hospital. Treatment of a patent ductus arteriosus (PDA) after the first day of life was less common among 2,587 infants who received prophylactic indomethacin compared with 5,244 who did not (21.0% vs. 36.1%, p<0.001). After adjustment for potential confounders, use of prophylactic indomethacin was not associated with higher or lower odds of BPD (OR 0.89, 95% CI 0.72–1.10), death (OR 0.80, 95% CI 0.64–1.01), or death or BPD (OR 0.87, 95% CI 0.72–1.05). The only evidence of subgroup effects associated with prophylactic indomethacin were lower odds of death among infants with birth weights above the 10th percentile and those who were not treated for a PDA after the first day of life.
Conclusions
Prophylactic indomethacin was not associated with either reduced or increased risk for BPD or death.
Keywords: extreme prematurity, indomethacin, prophylaxis, bronchopulmonary dysplasia
The use of prophylactic indomethacin in preterm infants remains controversial.1 Prophylactic indomethacin reduces the incidence of severe intraventricular hemorrhage and subsequent symptomatic patent ductus arteriosus (PDA).2 However, prophylactic indomethacin has not been shown to prevent bronchopulmonary dysplasia (BPD), despite a strong association between PDA and the development of BPD.2–7 The available data from randomized trials are consistent with the hypothesis that prophylactic indomethacin may adversely affect respiratory outcomes. The most recent Cochrane Review on prophylactic indomethacin included 9 randomized controlled trials that assessed supplemental oxygen use at 28 days of life and only 1 trial, the Trial of Indomethacin Prophylaxis in Preterms (TIPP) that assessed supplemental oxygen use at 36 weeks postmenstrual age (PMA).2,8 Treatment with prophylactic indomethacin did not significantly reduce the rates of either BPD outcome, but the point estimates of the relative risks favored the control therapy for both definitions of BPD.8
A secondary analysis of TIPP data found that infants randomized to prophylactic indomethacin compared with placebo received a higher fraction of inspired oxygen (FiO2) during the first week of life.9 Van Overmeire et al demonstrated a similar phenomenon in a randomized trial of early (day 3) versus late (day 7) treatment of echocardiography confirmed PDA.10 These studies suggest that early treatment with indomethacin may adversely affect early respiratory function and could lead to a small but important increase in the risk for BPD.
It is unlikely that further large placebo controlled trials of prophylactic indomethacin will be conducted. Therefore, analyses of multi-center observational data may provide the only findings that can help resolve the remaining uncertainty about the risks and benefits of prophylactic indomethacin. To evaluate the potential association between prophylactic indomethacin and the risk for BPD at a postmenstrual age of 36 weeks, we conducted an analysis of data collected prospectively for the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Neonatal Research Network (NRN) Generic Database (GDB).
METHODS
The NRN GDB registry (ClinicalTrials.gov: NCT00063063) uses a predefined protocol to prospectively collect maternal and infant demographic and clinical data from birth through hospital discharge, death, or 120 days for all infants with gestational ages between 22 and 28 6/7 weeks or birth weights 401 to 1000g born at participating NRN centers. Live born infants who survived the first 12 hours of life and were delivered at the 35 hospitals included in the NRN were evaluated in this analysis. The institutional review board at each study center approved the collection of GDB data. Written or oral parental consent was obtained at 3 centers and a waiver of consent was granted at the remaining centers.
Outcome and Exposure Definitions
The primary study outcome was BPD, defined as the use of supplemental oxygen at 36 weeks postmenstrual age (PMA) among infants who survived to this time point. The secondary outcomes were death prior to 36 weeks PMA and the composite of death prior to 36 weeks PMA or BPD. We compared the risks for these outcomes between infants who received prophylactic indomethacin, defined as initiation of indomethacin within the first 24 hours of life, and infants who did not receive prophylactic indomethacin.
Statistical Analyses
Maternal complications of pregnancy and infant characteristics were compared between the infants who were treated with prophylactic indomethacin and those who were not using standard descriptive statistics. Rates of prophylactic indomethacin use at individual hospitals were calculated and reported graphically. The odds of the study outcomes were evaluated using logistic regression. The regression models were adjusted for several pre-specified potential confounding variables and those that differed between infants who were and were not treated with prophylactic indomethacin with a p-value <0.05 in the bivariate testing. These variables were included as fixed effects and fell into 1 of 3 groups: (1) maternal characteristics – gestational hypertension, multiple gestation pregnancy, rupture of maternal amniotic membranes for longer than 18 hours, treatment with antenatal antibiotics, treatment with antenatal corticosteroids, and cesarean delivery; (2) baseline neonatal characteristics - birth weight and gestational age (as continuous variables), sex, and birth weight < 10th percentile determined using the Alexander fetal growth curves11; (3) neonatal morbidities occurring in the first 24 hours of life - intubation, receipt of chest compressions, or epinephrine in the delivery room, and mechanical ventilation at 24 hours of life. Hospital was included in all models as a random effect. Data from small hospitals that participated in the NRN as part of a single center and had similar prophylactic indomethacin treatment rates were combined.
We evaluated 5 infant subgroups for potential differences in the association between the use of prophylactic indomethacin and the study outcomes: gestational age (< 26 wk versus ≥ 26 wk), birth weight percentile (< 10th percentile versus ≥ 10th percentile), sex, exposure to antenatal corticosteroids (any treatment versus none), and medical or surgical treatment of a PDA after the first 24 hours of life (any treatment versus none). The same logistic regression models described above with the addition of a treatment by subgroup interaction term were used in these analyses. An interaction p-value < 0.05 was considered to indicate a statistically important subgroup effect. No adjustments for multiple comparisons were made in this exploratory observational study.
Lastly, we performed a post-hoc analysis to assess the risk for the study outcomes associated with the rate of prophylactic indomethacin use at the birth hospital. A 3 level categorical variable with approximately equal numbers of patients per group was added to the multivariable regression models: no use: no infants treated with prophylactic indomethacin during the study period; moderate use: greater than 0% but less than 60% of infants treated with prophylactic indomethacin; and high use: greater than or equal to 60% of infants treated with prophylactic indomethacin. All statistical analyses were performed with SAS version 9.4 (SAS Institute, Cary, North Carolina).
RESULTS
Of the 7,831 infants included in this analysis recruited between January 1, 2008 and December 31, 2012, 6,749 (86.2%) were alive at 36 weeks PMA and were assessed for the primary outcome (Figure 1; available at www.jpeds.com). The characteristics of the study population are shown in Table I. Infants who received prophylactic indomethacin compared with those who did not were less mature at birth and were more likely to have a birth weight less than the 10th percentile, to be intubated or have received cardiopulmonary resuscitation in the delivery room, and to be intubated at 24 hours of life (Table I). Treatment with prophylactic indomethacin was associated with lower rates of subsequent medical or surgical therapy for a PDA (21.0% versus 36.1%, p<0.001).
Figure 1.
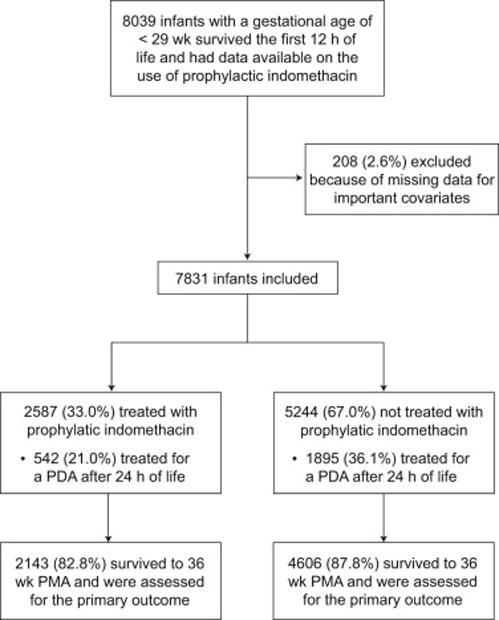
Flow diagram of the infants included in the analysis and those alive at 36 weeks postmenstrual age (PMA) and assessed for the primary study outcome.
Table 1.
Maternal and infant characteristics
| Characteristic | Prophylactic Indomethacin N = 2,587 |
No Prophylactic Indomethacin N = 5,244 |
P-value |
|---|---|---|---|
| Maternal | |||
| Gestational hypertension, n (%) | 741 (28.6) | 1346 (25.7) | 0.005 |
| Rupture of membranes > 18 hr, n (%) | 602 (23.3) | 1437 (27.4) | <0.001 |
| Antenatal antibiotics, n (%) | 1805 (69.8) | 3525 (67.2) | 0.02 |
| Antenatal corticosteroids, n (%) | 2377 (91.9) | 4645 (88.6) | <0.001 |
| Cesarean section, n (%) | 1740 (67.3) | 3485 (66.5) | 0.48 |
| Infant | |||
| Gestational age, wk - mean (SD) | 25.9 (1.5) | 26.7 (1.6) | <0.001 |
| Birth weight, g – mean (SD) | 777 (197) | 913 (246) | <0.001 |
| Male, n (%) | 1270 (49.1) | 2744 (52.3) | 0.007 |
| Birth weight < 10th percentile, n (%) | 276 (10.7) | 362 (6.9) | <0.001 |
| Delivery room intubation or CPR, n (%) | 1918 (74.1) | 3465 (66.1) | <0.001 |
| Intubated at 24 hr of life, n (%) | 1721 (66.5) | 2969 (56.6) | <0.001 |
CPR, cardiopulmonary resuscitation (includes chest compressions or treatment with epinephrine); SD, standard deviation
The proportion of infants treated with prophylactic indomethacin at each of the 35 participating hospitals ranged from 0% to 98% (Figure 2). The median treatment rate across all hospitals was 12%. Twelve hospitals (34.3%) did not prescribe prophylactic indomethacin to any infants included in this analysis.
Figure 2.

Proportion of infants treated with prophylactic indomethacin at each of the 35 study hospitals.
Primary Outcome
The rates of BPD among infants who survived to 36 weeks PMA were similar among those who received prophylactic indomethacin and those who did not (44.8% vs. 44.3%, p=0.72). After adjustment for hospital and 11 maternal and neonatal characteristics, the odds ratio for BPD associated with treatment with prophylactic indomethacin was 0.89 (95% CI 0.72–1.10; Figure 3). There was no evidence of differential treatment effects on the risk for BPD for any of the 5 infant subgroups (Figure 3).
Figure 3. BPD among survivors to 36 weeks postmenstrual age.

Unadjusted rates and risk-adjusted odds ratios for the full cohort and 5 infant subgroups are shown. n indicates the number of infants with the outcome and N indicates the total number of infants in each group. OR indicates odds ratio. CI indicates confidence interval. Odds ratios were determined with adjustment for hospital as a random affect and birth weight, gestational age, sex, birth weight < 10th percentile for sex and gestational age, maternal gestational hypertension, antenatal antibiotic exposure, antenatal corticosteroid exposure, rupture of amniotic membranes for greater than 18 hr, cesarean delivery, intubation or cardiopulmonary resuscitation in the delivery room, and invasive mechanical ventilation at 24hr of life as fixed effects.
Secondary Outcomes
In contrast to BPD, the rates of death prior to 36 weeks PMA (17.2% vs. 12.2%, p<0.001) and the composite of death prior to 36 weeks PMA or BPD (54.3% vs. 51.1%, p=0.008) were higher among the infants who received prophylactic indomethacin compared with untreated infants. However, after adjustment for potential confounding variables, treatment with prophylactic indomethacin was not associated with an increased risk of either study outcome (Table II).
Table 2.
Secondary outcomes for the full cohort and subgroups
| Prophylactic Indomethacin n/N (%) | No Prophylactic Indomethacin n/N (%) | Adjusted OR (95% CI)a For Prophylactic Indomethacin | Interaction p-value | |
|---|---|---|---|---|
| Death by 36 wk PMA | ||||
| Full cohort | 444/2587 (17.2) | 638/5244 (12.2) | 0.80 (0.64–1.01) | |
| Subgroups | ||||
| Gestational Age | 0.75 | |||
| < 26 weeks | 349/1334 (26.2) | 427/1592 (26.8) | 0.82 (0.64–1.04) | |
| ≥ 26 weeks | 95/1253 (7.6) | 211/3652 (5.8) | 0.86 (0.63–1.17) | |
| Birth weight | 0.007 | |||
| < 10th percentile | 91/276 (33.0) | 83/362 (22.9) | 1.32 (0.87–2.01) | |
| ≥ 10th percentile | 353/2311 (15.3) | 555/4882 (11.4) | 0.74 (0.59–0.93) | |
| Sex | 0.09 | |||
| Male | 257/1270 (20.2) | 357/2744 (13.0) | 0.90 (0.70–1.17) | |
| Female | 187/1317 (14.2) | 281/2500 (11.2) | 0.70 (0.53–0.92) | |
| Antenatal corticosteroids | 0.56 | |||
| Treated | 383/2377 (16.1) | 523/4645 (11.3) | 0.79 (0.62–1.00) | |
| Not treated | 61/210 (29.0) | 115/599 (19.2) | 0.90 (0.58–1.40) | |
| PDA treatment after 24 hr | 0.009 | |||
| Treated | 86/542 (15.9) | 196/1895 (10.3) | 1.03 (0.74–1.44) | |
| Not treated | 355/2040 (17.4) | 435/3342 (13.0) | 0.65 (0.51–0.83) | |
| Death by 36 wk PMA or BPD | ||||
| Full cohort | 1404/2587 (54.3) | 2680/5244 (51.1) | 0.87 (0.71–1.05) | |
| Subgroups | ||||
| Gestational Age | 0.42 | |||
| < 26 weeks | 933/1334 (69.9) | 1242/1592 (78.0) | 0.99 (0.78, 1.26) | |
| ≥ 26 weeks | 471/1253 (37.6) | 1438/3652 (39.4) | 0.88 (0.71, 1.10) | |
| Birth weight | 0.88 | |||
| < 10th percentile | 189/276 (68.5) | 288/362 (79.6) | 0.85 (0.55, 1.32) | |
| ≥ 10th percentile | 1512/2311 (65.4) | 2392/4882 (49.0) | 0.87 (0.71–1.06) | |
| Sex | 0.58 | |||
| Male | 742/1270 (58.4) | 1488/2744 (54.2) | 0.83 (0.66–1.05) | |
| Female | 662/1317 (50.3) | 1192/2500 (47.7) | 0.90 (0.72–1.13) | |
| Antenatal corticosteroids | 0.93 | |||
| Treated | 1269/2377 (53.4) | 2373/4645 (51.1) | 0.87 (0.71–1.06) | |
| Not treated | 135/210 (64.3) | 307/599 (51.3) | 0.86 (0.56–1.32) | |
| PDA treatment after 24 hr | 0.88 | |||
| Treated | 365/542 (67.3) | 1290/1895 (68.1) | 0.98 (0.74–1.31) | |
| Not treated | 1036/2040 (50.8) | 1383/3342 (41.4) | 1.00 (0.81–1.24) |
BPD, bronchopulmonary dysplasia; CI, confidence interval; OR, odds ratio; PDA, patent ductus arteriosus; PMA, postmenstrual age. n indicates the number of infants with the outcome and N indicates the total number of infants in each group
Model adjusted for hospital as a random affect and birth weight, gestational age, sex, birth weight < 10th percentile for sex and gestational age, maternal gestational hypertension, antenatal antibiotic exposure, antenatal corticosteroid exposure, rupture of amniotic membranes for greater than 18 hr, delivery by Cesarean section, intubation or cardiopulmonary resuscitation in the delivery room, and invasive mechanical ventilation at 24hr of life as fixed effects.
There was evidence of two subgroup effects for the outcome of death prior to 36 weeks PMA (Table II). Prophylactic indomethacin was associated with lower odds of death among infants with birth weights ≥ 10th percentile but not among those born < 10th percentile (interaction p=0.007). The odds of death were also lower for infants treated with prophylactic indomethacin among those who did not receive therapy for a PDA after the first 24 hours life (interaction p=0.009). Lastly, there was weak evidence of a potential subgroup effect for sex (interaction p=0.09), with lower odds of death among females but not males who were treated with prophylactic indomethacin. There was no evidence of heterogeneity of effects for the composite outcome of death prior to 36 weeks PMA or BPD (Table II).
Post-hoc Analysis by Hospital use of Prophylactic Indomethacin
BPD was less common among survivors to 36 weeks PMA cared for in a hospital with high prophylactic indomethacin treatment rates. After adjustment for potential confounders, delivery at a high use hospital compared with a no use hospital was associated with lower risk for BPD (odds ratio 0.31, 95% CI 0.14–0.69). The difference was not statistically significant for the comparison of moderate use with no use hospitals (odds ratio 0.60, 95% CI 0.29–1.22).
The frequency of death prior to 36 weeks PMA was similar when compared based on the hospital rate of prophylactic indomethacin use. However, rates of the composite outcome of death prior to 36 weeks PMA or BPD were lower at high use hospitals. In the adjusted analysis, only birth at a high use compared with no use hospital was associated with lower odds of death or BPD (high vs. none odds ratio 0.35, 95% CI 0.18–0.71; moderate vs. none odds ratio 0.58, 95% CI 0.31–1.09).
DISCUSSION
In this large, multicenter cohort of extremely preterm infants, the use of prophylactic indomethacin was not associated with either increased or decreased risks for BPD, death before 36 weeks postmenstrual age, or the combined outcome of death or BPD. As with all observational studies, these findings should be interpreted with appropriate caution. This study design cannot establish causality, only plausible associations. Moreover, in multicenter non-randomized studies such as this, center is often a strong predictor of both treatment and outcome.12,13 To account for potential confounding by center, we included birth hospital as a random effect in all models.12 However, residual confounding by hospital may persist. Finally, we are only able to adjust for measured clinical covariates and cannot account for potentially important but unknown factors that may differentially affect use of prophylactic indomethacin or outcome risk. Despite these limitations, this retrospective study of prospectively collected data provides contemporary results suggesting that prophylactic indomethacin use is not associated with either increased or decreased risk for BPD at 36 weeks postmenstrual age in extremely preterm infants.
Our finding of similar BPD rates among infants who received prophylactic indomethacin compared with those who did not is consistent with data from previous randomized controlled trials (RCTs).2,8 We observed this lack of an association between indomethacin prophylaxis and the risk for BPD despite a reduction in the frequency of later treatment for a PDA from 36% to 21%. Although the direction of effect in the present analysis favored prophylactic indomethacin for prevention of BPD while the previous studies did not,2,8 the interpretation of the trial and observational data is consistent. Prophylactic indomethacin is unlikely to have any beneficial or harmful effects on the risk for BPD among extremely preterm infants and if present, any effect is likely to be small.
The reasons for the apparent lack of a beneficial effect of prophylactic indomethacin on the risk for BPD are unclear. Although persistence of a PDA is associated with the development of BPD, pharmacological treatment of a PDA may not reduce this risk. It is also possible that the beneficial effects of ductal closure with prophylactic indomethacin may be offset by adverse drug effects such as water retention due to impaired renal function or direct toxicity to the lung.2, 14–16
In contrast to BPD, the unadjusted rates of death prior to 36 weeks PMA and the composite outcome of death or BPD were higher among the infants who received prophylactic indomethacin compared with those who did not. However, when these associations were evaluated in the multivariable models, the point estimates for the odds ratios favored treatment with prophylactic indomethacin but with confidence intervals that included the point of equivalence. A meta-analysis of RCTs that assessed death prior to hospital discharge also found a non-significant reduction in the risk of death among infants treated with prophylactic indomethacin compared with placebo.2 However, when all deaths occurring prior to the latest follow-up were considered, the potential benefit was no longer present.2
We found evidence of 2 subgroup effects among the 15 subgroup analyses we conducted. Prophylactic indomethacin compared with no therapy was associated with lower odds of death among infants with birth weights above the 10th percentile and among those who did not undergo medical or surgical therapy for a PDA after the first day of life. This latter finding suggests that prophylactic indomethacin may protect against mortality among infants who do not develop a symptomatic PDA. We also found weak evidence of heterogeneity of treatment effects based on sex, with lower risk of death among female but not male infants who received prophylactic indomethacin.
Two post-hoc analyses of TIPP data evaluated potential subgroup effects for mortality and neurodevelopmental outcomes; BPD was not assessed.17,18 Similar to our findings, TIPP investigators found no difference in the risk of death with prophylactic indomethacin compared with placebo based on a full course versus partial course or no treatment with antenatal corticosteroids.18 TIPP also found a possible subgroup effect for mortality by sex (interaction p-value = 0.054).17 However, in contrast to our results, the risk of death was increased for females who were treated with prophylactic indomethacin compared with placebo and was no different for males.17
Although the pitfalls of subgroup analyses are well described, when they are pre-specified and assessed with appropriate interaction testing, they can generate important hypotheses for future prospective studies.19,20 An additional consideration with subgroup analyses is the possibility of type-I error due to multiple testing. We assessed the risk associated with prophylactic indomethacin for 3 outcomes in 5 subgroups (15 total comparisons). Based on this number of tests, the probability of finding 1 or more nominally significant interaction terms (p=0.05) is approximately 54%.20 The probability of 2 or more subgroup effects being identified by chance is 17%.20
Finally, our post-hoc analysis suggested that delivery in a hospital in which at least 60% of extremely preterm infants received prophylactic indomethacin compared with one where no infants were treated was associated with reduced risk for BPD among survivors to 36 weeks PMA and the composite outcome of death prior to 36 weeks PMA or BPD. Although it is tempting to infer from this finding that high use of prophylactic indomethacin may reduce BPD risk, caution should be used when interpreting these results. Firstly, residual confounding by unmeasured differences in patient characteristics, clinical care, and other hospital factors cannot be excluded. Secondly, these findings are in contrast to and should not supersede the results of the primary analysis of this observational study or the available randomized trial data showing no benefit with prophylactic indomethacin for prevention of BPD.2,8
In conclusion, this study examined the possible association between prophylactic indomethacin and BPD. In this contemporary cohort of extremely preterm infants, we found no evidence that prophylactic indomethacin was associated with increased or decreased risks for BPD, death, or the combined outcome of death or BPD. The use of prophylactic indomethacin was associated with decreased mortality in two subgroups of infants. However, these findings require confirmation in prospective studies.
Acknowledgments
Funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network.
Appendix
The following investigators are additional members of the National Institute of Child Health and Human Development Neonatal Research Network, National Institutes of Health, Bethesda, MD:
NRN Steering Committee Chair: Michael S. Caplan, MD, University of Chicago, Pritzker School of Medicine; Richard A. Polin, MD, Division of Neonatology, College of Physicians and Surgeons, Columbia University, (2011-present).
Alpert Medical School of Brown University and Women & Infants Hospital of Rhode Island (U10 HD27904) – Abbot R. Laptook, MD; Martin Keszler, MD; Angelita M. Hensman, RN BSN; Kristin M. Basso, BSN MA; Elisa Vieira, RN BSN; Emily Little, BSN RN.
Case Western Reserve University, Rainbow Babies & Children’s Hospital (U10 HD21364, M01 RR80) – Avroy A. Fanaroff, MD; Anna Marie Hibbs, MD; Nancy S. Newman, BA RN.
Children’s Mercy Hospital, University of Missouri Kansas City School of Medicine (U10 HD68284) – William E. Truog, MD; Howard W. Kilbride, MD; Eugenia K. Pallotto, MD MSCE; Cheri Gauldin, RN BSN CCRC; Anne Holmes RN MSN MBA-HCM CCRC; Kathy Johnson RN, CCRC; Allison Knutson, BSN RNC-NIC.
Cincinnati Children’s Hospital Medical Center, University Hospital, and Good Samaritan Hospital (U10 HD27853, M01 RR8084) – Kurt Schibler, MD; Edward F. Donovan, MD; Barbara Alexander, RN; Cathy Grisby, BSN CCRC; Jody Hessling, RN; Estelle E. Fischer, MHSA MBA; Lenora D. Jackson, CRC; Kristin Kirker, CRC; Greg Muthig, BS.
Duke University School of Medicine, University Hospital, University of North Carolina, and Duke Regional Hospital (U10 HD40492, M01 RR30, UL1 TR83) – Ronald N. Goldberg, MD; C. Michael Cotten, MD MHS; Kimberley A. Fisher, PhD FNP-BC IBCLC; Kathy J. Auten, MSHS; Katherine A. Foy, RN; Sandra Grimes, RN BSN; Joanne Finkle, RN JD; Matthew M. Laughon, MD MPH; Carl L. Bose, MD; Janice Bernhardt, MS RN; Gennie Bose, RN.
Emory University, Children’s Healthcare of Atlanta, Grady Memorial Hospital, and Emory University Hospital Midtown (U10 HD27851, UL1 TR454) – David P. Carlton, MD; Ellen C. Hale, RN BS CCRC.
Eunice Kennedy Shriver National Institute of Child Health and Human Development – Stephanie Wilson Archer, MA.
Indiana University, University Hospital, Methodist Hospital, Riley Hospital for Children, and Wishard Health Services (U10 HD27856, M01 RR750, UL1 TR6) – Brenda B. Poindexter, MD MS; Gregory M. Sokol, MD; Leslie Dawn Wilson, BSN CCRC; Dianne E. Herron, RN CCRC.
Nationwide Children’s Hospital and the Ohio State University Medical Center (U10 HD68278) – Pablo J. Sánchez, MD; Leif D. Nelin, MD; Sudarshan R. Jadcherla, MD; Patricia Luzader, RN; Christine A. Fortney, PhD RN; Gail E. Besner; Nehal A. Parikh, MD.
RTI International (U10 HD36790) – Abhik Das, PhD; Dennis Wallace, PhD; Jeanette O’Donnell Auman, BS; Margaret M. Crawford, BS CCRP; Carolyn M. Petrie Huitema, MS CCRP; Kristin M. Zaterka-Baxter, RN BSN CCRP.
Stanford University, Dominican Hospital, El Camino Hospital, and Lucile Packard Children’s Hospital (U10 HD27880, M01 RR70, UL1 TR93) – Krisa P. Van Meurs, MD; Marian M. Adams, MD; David K. Stevenson, MD; M. Bethany Ball, BS CCRC; Andrew W. Palmquist, RN BSN; Melinda S. Proud, RCP.
Tufts Medical Center, Floating Hospital for Children (U10 HD53119, M01 RR54) – Ivan D. Frantz III, MD; John M. Fiascone, MD; Brenda L. MacKinnon, RNC; Ellen Nylen, RN BSN.
University of Alabama at Birmingham Health System and Children’s Hospital of Alabama (U10 HD34216, M01 RR32) – Waldemar A. Carlo, MD; Namasivayam Ambalavanan, MD; Monica V. Collins, RN BSN MaEd; Shirley S. Cosby, RN BSN. University of California - Los Angeles, Mattel Children’s Hospital, Santa Monica Hospital, Los Robles Hospital and Medical Center, and Olive View Medical Center (U10 HD68270) – Uday Devaskar, MD; Meena Garg, MD; Teresa Chanlaw, MPH; Rachel Geller, RN BSN.
University of Iowa and Mercy Medical Center (U10 HD53109, M01 RR59) – Edward F. Bell, MD; Dan L. Ellsbury, MD; John A. Widness, MD; Karen J. Johnson, RN BSN; Donia B. Campbell, RNC-NIC.
University of New Mexico Health Sciences Center (U10 HD53089, UL1 TR41) – Kristi L. Watterberg, MD; Robin K. Ohls, MD; Conra Backstrom Lacy, RN; Rebecca A. Montman, BSN RNC; Sandra Brown, BSN; Theresa Wussow, BSN; Carol Hartenberger, BSN MPH.
University of Pennsylvania, Hospital of the University of Pennsylvania, Pennsylvania Hospital, and Children’s Hospital of Philadelphia (U10 HD68244) – Barbara Schmidt, MD MSc; Haresh Kirpalani, MB MSc; Sara B. DeMauro, MD MSCE; Aasma S. Chaudhary, BS RRT; Soraya Abbasi, MD; Toni Mancini, RN BSN CCRC; Dara M. Cucinotta, RN.
University of Rochester Medical Center, Golisano Children’s Hospital, and the University of Buffalo Women’s and Children’s Hospital of Buffalo (U10 HD68263, M01 RR44, UL1 TR42) – Carl T. D’Angio, MD; Ronnie Guillet, MD PhD; Satyan Lakshminrusimha, MD; Ann Marie Reynolds, MD, MPH; Linda J. Reubens, RN CCRC; Rosemary Jensen; Deana Maffett, RN; Holly I.M. Wadkins; Michael G. Sacilowski, BS; Ashley Williams, MS Ed; Stephanie Guilford, BS; Cassandra A. Horihan, MS.
University of Texas Health Science Center at Houston Medical School, Children’s Memorial Hermann Hospital (U10 HD21373) – Kathleen A. Kennedy, MD MPH; Jon E. Tyson, MD MPH; Katrina Burson, RN BSN; Beverly Foley Harris, RN BSN; Georgia E. McDavid, RN; Patti L. Pierce Tate, RCP; Sharon L. Wright, MT.
University of Texas Southwestern Medical Center at Dallas, Parkland Health & Hospital System, and Children’s Medical Center Dallas (U10 HD40689, M01 RR633) – Pablo J. Sánchez, MD; Luc P. Brion, MD; Lijun Chen, PhD RN; Alicia Guzman; Melissa H. Leps, RN; Nancy A. Miller, RN; Janet S. Morgan, RN; Diana M. Vasil, MSN BSN RNC-NIC; Lizette E. Torres, RN.
University of Utah University Hospital, Intermountain Medical Center, LDS Hospital, and Primary Children’s Medical Center (U10 HD53124, M01 RR64, UL1 TR105) – Roger G. Faix, MD; Bradley A. Yoder, MD; Karen A. Osborne, RN BSN CCRC; Karie Bird, RN BSN; Jill Burnett, RNC BSN; Jennifer J. Jensen, RN BSN; Cynthia Spencer, RNC BSN; Kimberlee Weaver-Lewis, RN MS; Karen Zanetti, RN.
Wayne State University, University of Michigan, Hutzel Women’s Hospital, and Children’s Hospital of Michigan (U10 HD21385) – Seetha Shankaran, MD; John Barks, MD; Rebecca Bara, RN BSN; Mary Johnson, RN BSN; Mary Christensen, RT; Stephanie Wiggins, MS.
Yale University, Yale-New Haven Children’s Hospital, and Bridgeport Hospital (U10 HD27871, UL1 TR142) – Richard A. Ehrenkranz, MD; Harris Jacobs, MD; Patricia Cervone, RN; Monica Konstantino, RN BSN; JoAnn Poulsen, RN; Janet Taft, RN BSN.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Edited by Wright and WFB
The authors declare no conflicts of interest.
Trial registration ClinicalTrials.gov: NCT00063063
We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study.
References
- 1.Bose C, Laughon M. Patent ductus arteriosus: Lack of evidence for common treatments. Arch Dis Child Fetal Neonatal Ed. 2007;92:F498–502. doi: 10.1136/adc.2005.092734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fowlie PW, Davis PG, McGuire W. Prophylactic intravenous indomethacin for preventing mortality and morbidity in preterm infants. Cochrane Database Syst Rev. 2010:CD000174. doi: 10.1002/14651858.CD000174.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benitz WE. Treatment of persistent patent ductus arteriosus in preterm infants: Time to accept the null hypothesis? J Perinatol. 2010;30:241–52. doi: 10.1038/jp.2010.3. [DOI] [PubMed] [Google Scholar]
- 4.Benitz WE. Patent ductus arteriosus: To treat or not to treat? Arch Dis Child Fetal Neonatal Ed. 2012;97:F80–F2. doi: 10.1136/archdischild-2011-300381. [DOI] [PubMed] [Google Scholar]
- 5.Palta M, Gabbert D, Weinstein MR, Peters ME. Multivariate assessment of traditional risk factors for chronic lung disease in very low birth weight neonates. The Newborn Lung Project. J Pediatr. 1991;119:285–92. doi: 10.1016/s0022-3476(05)80746-2. [DOI] [PubMed] [Google Scholar]
- 6.Rojas MA, Gonzalez A, Bancalari E, Claure N, Poole C, Silva-Neto G. Changing trends in the epidemiology and pathogenesis of neonatal chronic lung disease. J Pediatr. 1995;126:605–10. doi: 10.1016/s0022-3476(95)70362-4. [DOI] [PubMed] [Google Scholar]
- 7.Trzaski J, Hagadorn J, Hussain N, Schwenn J, Wittenzellner C. Predictors of successful discontinuation of supplemental oxygen in very low-birth-weight infants with bronchopulmonary dysplasia approaching neonatal intensive care unit discharge. Am J Perinatol. 2011;29:79–86. doi: 10.1055/s-0031-1295646. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt B, Davis P, Moddemann D, Ohlsson A, Roberts RS, Saigal S, et al. Longterm effects of indomethacin prophylaxis in extremely-low-birth-weight infants. N Engl J Med. 2001;344:1966–72. doi: 10.1056/NEJM200106283442602. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt B, Roberts RS, Fanaroff A, Davis P, Kirpalani HM, Nwaesei C, et al. Indomethacin prophylaxis, patent ductus arteriosus, and the risk of bronchopulmonary dysplasia: Further analyses from the Trial of Indomethacin Prophylaxis in Preterms (TIPP) J Pediatr. 2006;148:730–4.e1. doi: 10.1016/j.jpeds.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 10.Van Overmeire B, Van de Broek H, Van Laer P, Weyler J, Vanhaesebrouck P. Early versu late indomethacin treatment for patent ductus arteriosus in premature infants with respiratory distress syndrome. J Pediatr. 2001;138:205–11. doi: 10.1067/mpd.2001.110528. [DOI] [PubMed] [Google Scholar]
- 11.Alexander G, Himes J, Kaufman R, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–8. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 12.Localio AR, Berlin JA, Ten Have TR, Kimmel SE. Adjustments for center in multicenter studies: An overview. Ann Intern Med. 2001;135:112–23. doi: 10.7326/0003-4819-135-2-200107170-00012. [DOI] [PubMed] [Google Scholar]
- 13.Ambalavanan N, Walsh M, Bobashev G, Das A, Levine B, Carlo WA, et al. Intercenter differences in bronchopulmonary dysplasia or death among very low birth weight infants. Pediatrics. 2011;127:e106–16. doi: 10.1542/peds.2010-0648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerdes J, Harris M, Polin R. Effects of dexamethasone and indomethacin on elastase, alpha 1-proteinase inhibitor, and fibronectin in bronchoalveolar lavage fluid from neonates. J Pediatr. 1988;113:727–31. doi: 10.1016/s0022-3476(88)80390-1. [DOI] [PubMed] [Google Scholar]
- 15.Lassus P, Viinikka L, Ylikorkala O, Pohjavuori M, Andersson S. Pulmonary prostacyclin is associated with less severe respiratory distress in preterm infants. Early Hum Dev. 2002;67:11–8. doi: 10.1016/s0378-3782(01)00244-4. [DOI] [PubMed] [Google Scholar]
- 16.Eronen M, Pesonen E, Kurki T, Teramo K, Ylikorkala O, Hallman M. Increased incidence of bronchopulmonary dysplasia after antenatal administration of indomethacin to prevent preterm labor. J Pediatr. 1994;124:782–8. doi: 10.1016/s0022-3476(05)81374-5. [DOI] [PubMed] [Google Scholar]
- 17.Ohlsson A, Roberts RS, Schmidt B, Davis P, Moddeman D, Saigal S, et al. Male/female differences in indomethacin effects in preterm infants. J Pediatr. 2005;147:860–2. doi: 10.1016/j.jpeds.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt B, Seshia M, Shankaran S, Mildenhall L, Tyson J, Lui K, et al. Effects of prophylactic indomethacin in extremely low-birth-weight infants with and without adequate exposure to antenatal corticosteroids. Arch Pediatr Adolesc Med. 2011;165:642–6. doi: 10.1001/archpediatrics.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin C, Guyatt G, Montori V. The sirens are singing: The perils of trusting trials stopped early and subgroup analyses. Crit Care Med. 2005;33:1870–1. doi: 10.1097/01.ccm.0000174484.77537.f2. [DOI] [PubMed] [Google Scholar]
- 20.Lagakos SW. The challenge of subgroup analyses - reporting without distorting. N Engl J Med. 2006;354:1667–9. doi: 10.1056/NEJMp068070. [DOI] [PubMed] [Google Scholar]


