Abstract
Objectives
1) To describe the use of occupational therapy (OT), physical therapy (PT) and speech-language pathology (SLP) services in a level IV neonatal intensive care unit (NICU), 2) to describe predictors of early therapy usage, and 3) to test the hypothesis that more NICU-based therapy will relate to better neurobehavioral outcomes.
Methods
Seventy-nine infants born ≤ 32 weeks gestation had therapy interventions, as standard of care, tracked across NICU hospitalization. Infants received neurobehavioral testing prior to NICU discharge.
Results
All (100%) received OT and PT, and 41 (51%) received SLP. The average age at initiation of OT, PT, and SLP was 30.4 ± 1.4, 30.3 ± 1.4, and 35.9 ± 2.3 weeks postmenstrual age, respectively. Infants received therapy an average of 1.8 ± .44, 1.8 ± .4 and 1.1 ± .53 times per week for OT, PT and SLP, respectively. There were 56 different therapeutic interventions performed. There was overlap in the interventions provided by different NICU therapists; however, interventions unique to each discipline were identified. More therapy was not related to better neurobehavioral outcomes, but rather more frequent therapy could be attributed to more complex medical conditions (p<0.05).
Conclusion
Early therapy services in the NICU can start early in gestation and continue routinely until NICU discharge in order to optimize outcomes. These findings can aid our understanding of how neonatal therapy services are implemented in a level IV NICU.
Introduction
Prematurity is a serious public health concern affecting 1 in 9 infants, amounting to approximately a half million infants per year in the United States alone 1. Complications of premature birth include long-term developmental problems, such as attention deficit hyperactivity disorder, learning disabilities, motor delays, visual perception and visual-motor problems, executive functioning deficits, cerebral palsy, and vision and hearing impairments 2–7. Up to 74% of extremely low birth weight infants experience alterations in functional outcome at five years of age 8. While the use of therapy is well-understood following neonatal intensive care unit (NICU) discharge, developmental alterations are already present by term equivalent age, which warrants intervention during NICU hospitalization 9–11.
Developmental challenges are present prior to NICU discharge12–16. Compared to their full term counterparts, preterm infants at term equivalent age are more likely to demonstrate alterations in neurobehavior with abnormal reflexes, more hypotonia and hypertonia, poorer quality of movement, poorer orientation, more abnormal signs, lower tolerance of handling, poorer self-regulation, more excitability, and more stress 15–17. Therefore, a multidisciplinary team of occupational therapists (OT), physical therapists (PT), and speech-language pathologists (SLP) can potentially impact alterations in early experiences that can influence development as well as can promote foundational skills for optimizing outcomes in high risk infants in the NICU.
The American Academy of Pediatrics (AAP) has defined guidelines that require a neonatal therapist (OT or PT) to be on staff in NICUs with level III or IV designation 18. A level III or IV designation signifies the highest level of neonatal care, including care of infants at any gestational age at birth and with complex medical and surgical needs. The roles of OT, PT, and SLP in the NICU have been defined, and the use of different therapeutic interventions carried out by neonatal therapists with high-risk infants in the NICU have been described 19–37, 38,39. Due to the vulnerability of premature infants, NICU-based therapists necessitate advanced skills to optimize outcomes of the infant, while understanding and adapting to medical interventions that occur simultaneous to therapy interventions 29,35,38–40. Despite the growing number of therapists involved in treating infants in the NICU 41 and growing evidence on the benefits of specific interventions, no studies to our knowledge have identified the usage of therapy services in the NICU.
The aims of this study were to describe the type, timing, and frequency of therapy services in a level IV NICU and to determine if there are relationships between NICU-based therapy services and demographic and medical factors as well as preterm infant neurobehavior. It was hypothesized that infants who received more therapy would demonstrate better neurobehavioral outcomes.
Methods
This study consisted of a cohort of 79 premature infants who were prospectively enrolled at birth as part of an overarching study investigating the effects of neonatal positioning 42. Infants received routine medical care and therapy services, which were documented in the electronic medical record. Infants underwent neurobehavioral testing at >/= 35 weeks postmenstrual age (PMA). This study was approved by the Washington University Human Research Protection Office, and parents signed informed consent.
Participants
Consecutive admissions of preterm infants born ≤ 32 weeks estimated gestational age (EGA) in 2011 were recruited. The study site was a 75-bed, level IV NICU, and infants were excluded if they had a congenital anomaly. The parent study enrolled an additional 12 infants admitted to a secondary level III NICU, but those infants were excluded from this investigation.
Infant and Medical Factors
Infant factors collected included EGA at birth, multiple birth (twin or triplet), race (Caucasian or non-Caucasian), mother’s marital status, PMA at discharge, length of stay (LOS), and gender. Medical factors included days on ventilation, days on continuous positive airway pressure (CPAP), days on oxygen (including days on a ventilator, CPAP and oxygen delivered via a nasal cannula), presence of necrotizing enterocolitis (NEC; all stages), confirmed sepsis, and presence of brain injury (having either a grade III-IV intraventricular hemorrhage, cystic periventricular leukomalacia, or cerebellar hemorrhage from routine cranial ultrasound or magnetic resonance imaging).
NICU-Based Therapy
At the study site, automatic orders for OT and PT were generated from the medical team at birth for infants born ≤ 32 weeks EGA. Routine therapy services were not initiated until 30 weeks PMA, but for those born <30 weeks EGA OTs and PTs provided positioning and parent education consults prior to routine, continuous therapy services that started at 30 weeks PMA. SLPs received referrals from the medical team on a case-by-case basis, most often related to the infant demonstrating feeding or swallowing dysfunction.
Positioning consultations were often the first time the infant was seen by therapy and occurred much earlier than the initiation of continuous therapy. Therefore, positioning consults were documented separately from the initial evaluation that led to subsequent initiation of continuous therapy. Positioning consults included education on positioning equipment needed to promote optimal development, as well as education on developmental care, sensory development, appropriate touch, reading behavioral cues, and therapy in the NICU.
Each discipline used an electronic form to document services, in which there were prewritten options of common types of therapy. Therapists also had an option to type in specific intervention(s) that were not listed (see Table 1 for a complete list of interventions). At the study site NICU, there were 6 OTs, 6 PTs, 2 SLPs, and 1 physical therapy assistant (PTA) dedicated to the NICU in 2011, for a total of 2 full time equivalent (FTE) positions for OT, 3 FTEs for PT and 1 FTE for SLP. Therapy services that were provided as part of routine care and documented in each infant’s medical record were tracked. Because each therapy evaluation and treatment session was a different length of time, the number of sessions along with the time for each were collected.
Table 1.
Categorization of NICU therapy interventions documented in the medical record.
| Behavioral Organization | Developmental Interventions |
|---|---|
|
| |
| Behavioral Organization | Balance and Proprioception Skills Training |
| Calming | Developmental Skills |
| Regulation/State | Developmental Abilities |
| Response to the Environment | Developmental Progression |
| Fine Motor Skills | |
| Functional Mobility | |
| Functional Motor Skills | |
| Gross Motor Skills | |
| Head Control | |
| Midline Orientation | |
| Midline Movement | |
| Upper Extremity Functioning | |
| Visual | |
| Visual Motor/Perceptual | |
|
| |
| Feeding | Handling and Activity Tolerance |
|
| |
| Feeding | Activity Tolerance/Endurance |
| Swallowing | Diaper Change |
| P.O. Feeding Skills | Graded Handling |
| Therapeutic Tasting | Handling Tolerance |
| Holding | |
|
| |
| Neurodevelopment | Oral Motor |
|
| |
| Joint Approximation | Non Nutritive Sucking |
| Handling Skills | Oral Motor |
| Weight Bearing | Oral Sensory-Motor |
|
| |
| Parent and Team Involvement | Positioning |
|
| |
| Educated Parents on P.O. Readiness and Cue- | Boundaries |
| Based Feeding | Containment |
| Parent/Caregiver Education | Positioning |
| Team Conference | Tolerance of Prone |
| Tolerated Semi-Prone Position | |
|
| |
| Range of Motion | Sensory Motor Activities |
|
| |
| Isolated Extension in Prone | Deep Pressure |
| Range of Motion | Graded Touch |
| Splinting Tolerance | INFANIB Assessment |
| Stretching
|
Manual Lymphatic Drainage |
|
Strengthening
|
Scar Massage |
| Strength Training/Therapeutic Exercise | Sensory-Motor |
| Strengthening | Therapeutic Massage |
| Vestibular Stimulation | |
56 interventions found in the electronic medical record were categorized into the eleven central groupings based on their similarities
Interventions in italics are those that were written in as an intervention by the therapists. All others were part of the electronic drop down menu
Cognition, ADL’s, general, gait training, transfer training, splinting (splint fabrication and training), pain management, receptive language, expressive language, speech/articulation, voice/resonance/fluency, and cognition/memory were additional interventions prewritten into the electronic treatment form but were not documented by therapists in this study
The following neonatal therapy variables were collected for each infant during NICU hospitalization:
Type of therapy: Whether the infant received OT, PT, and/or SLP
Timing of therapy initiation: PMA at initial evaluation completed
Duration of initial evaluation: Minutes documented for the initial evaluation
Administration of positioning consult: Whether the infant received a positioning consult; PMA at first positioning consult visit, number of sessions, average minutes, and discipline(s) completing the positioning consult
Frequency of therapy: Average number of minutes (total therapy time/number of sessions) and total number of sessions an infant received OT, PT, and/or SLP at each PMA across hospitalization; total minutes and number of therapy sessions
Specific interventions administered: The types of interventions documented in the medical record were identified. Whether each infant received each identified intervention; PMA and the total number of times that an infant received a specific intervention were documented. These variables were collected for all therapies combined and separately.
Infant Neurobehavior
The NICU Network Neurobehavioral Scale (NNNS) was administered at the infant’s bedside prior to NICU discharge (starting at 35 weeks PMA or when the infant was able to tolerate the assessment, whichever came first), by a certified evaluator (author, RP). During the neurobehavioral exam, the infant is placed in different positions, reflexes are tested, and behavior is observed. The NNNS was chosen, because it is a comprehensive assessment of infant neurobehavior with 13 subscales: Habituation, Orientation, Tolerance of Handling, Arousal, Self-Regulation, Asymmetry, Excitability, Lethargy, Hypotonia, Hypertonia, Quality of Movement, Stress Signs, and Non-Optimal Reflexes 43. Habituation items were not administered. Neurobehavioral impairment, defined as having ≥ 3 NNNS summary scores more than 2 standard deviations from the mean, based on established norms, was also documented for each infant 44,45.
Statistical Analyses
IBM Statistical Package for the Social Sciences (SPSS 21, IBM, Chicago IL) was used for statistical analyses. Relationships between medical factors and the timing of therapy initiation and usage were investigated using regression models and independent samples t-tests. Relationships between therapy initiation and frequency of therapy (that was provided until 35 weeks PMA) and neurobehavioral outcomes were investigated using univariate and multivariate linear regression (controlling for EGA, brain injury, and PMA at time of neurobehavioral testing). Relationships between early therapy and neurobehavioral impairment were also investigated using logistic regression models. All factors were explored using p= 0.05.
Results
Eighty-eight infants from the study site’s level IV NICU were enrolled. Of those, four infants expired prior to NICU discharge, three withdrew, and two transferred to a different facility. Sample characteristics are summarized in Table 2. Seventy-nine (100%) infants received OT, and 79 (100%) infants received PT prior to NICU discharge. Forty-one (51%) infants received a SLP evaluation with 4 (10%) of those receiving no further intervention following the initial SLP evaluation.
Table 2.
Characteristics of the cohort and therapy factors.
| Infant factors | Mean ± S.D.; n (%) | |
| Estimated gestational age | 28.3 ± 2.7 | |
| Multiple | 20 (25%) | |
| Non Caucasian race | 47 (60%) | |
| Single mother | 65 (82%) | |
| PMA at discharge | 38.7 ± 3.6 | |
| Length of stay (in weeks) | 11.4 ± 5.6 | |
| Female gender | 46 (58%) | |
| Medical factors | Median (IQ range); n (%) | |
| Days on ventilation | 1 (1–8) | |
| Days on CPAP | 1 (0–4) | |
| Days on oxygen | 18 (7–84) | |
| Necrotizing enterocolitis | 7 (9%) | |
| Sepsis | 26 (33%) | |
| Cerebral injury | 17 (22%) | |
| Developmental factors | Mean ± S.D.; n (%) | |
| Neurobehavioral impairment | 38 (48%) | |
| Orientation | 3.5 ± 1.0 | |
| Tolerance to Handling | 0.7 ± 0.1 | |
| Quality of Movement | 3.1 ± 0.7 | |
| Self–regulation | 4.0 ± 0.8 | |
| Non-Optimal Reflexes | 7.4 ± 2.3 | |
| Stress Signs | 0.4 ± 0.1 | |
| Arousal | 3.7 ±1.0 | |
| Hypertonia | 1.7 ± 1.3 | |
| Hypotonia | 1.2 ± 1.2 | |
| Asymmetry | 2.9 ± 2.0 | |
| Excitability | 6.0 ± 2.8 | |
| Lethargy | 7.6 ± 3.0 | |
| Occupational Therapy | Min-Max | Mean ± SD |
| PMA at initiation | 26–34 | 30.4 ± 1.4 |
| Time spent on evaluation | 15–45 | 29.3 ± 5.8 |
| Time of treatment sessions (minutes) | 24–38 | 28.8 ± 2.7 |
| Total treatment sessions | 2–44 | 17.0 ± 9.2 |
| Total treatment hours | 1–23 | 8.2 ± 4.7 |
| Average number of sessions per week | 0.7–3 | 1.8 ± 0.4 |
| Physical Therapy | Min–Max | Mean ± SD |
| PMA at initiation | 26–34 | 30.3 ± 1.4 |
| Time spent on evaluation | 15–45 | 30.3 ± 1.4 |
| Time of treatment sessions (minutes) | 23–31 | 27.2 ± 1.8 |
| Total treatment sessions | 2–50 | 16.8 ± 8.8 |
| Total treatment hours | 0.9–23 | 7.7 ± 4.3 |
| Average number of sessions per week | 1–2.9 | 1.8 ± 0.4 |
| Speech Language Pathology | Min–Max | Mean ± SD |
| PMA at initiation | 31–43 | 35.9 ± 2.3 |
| Time spent on evaluation | 15–45 | 31.0 ± 8.2 |
| Time of treatment sessions (minutes) | 18–45 | 28.5 ± 3.9 |
| Total treatment sessions | 1–22 | 6.5 ± 4.9 |
| Total treatment hours | 0.5–11 | 3 ± 2.2 |
| Average number of sessions per week | 0.1–2 | 1.1 ± 0.5 |
Oxygen defined as days on a ventilator in addition to days on CPAP, and oxygen delivered via a nasal cannula
Multiple; if the infant was a twin or a triplet
Single mother; unmarried mother
CPAP indicates continuous positive airway pressure
‘Moderate to severe cerebral injury’ is defined as having a grade III-IV intraventricular hemorrhage, cystic periventricular leukomalacia, or cerebellar hemorrhage from routine cranial ultrasound or magnetic resonance imaging.
Neurobehavioral impairment defined as having ≥ 3 summary scores from the NNNS more than 2 SD from the mean based on established norms 53; NNNS subscale scores can be referenced in the NNNS manual 54
OT, PT, and SLP Provided During NICU Hospitalization
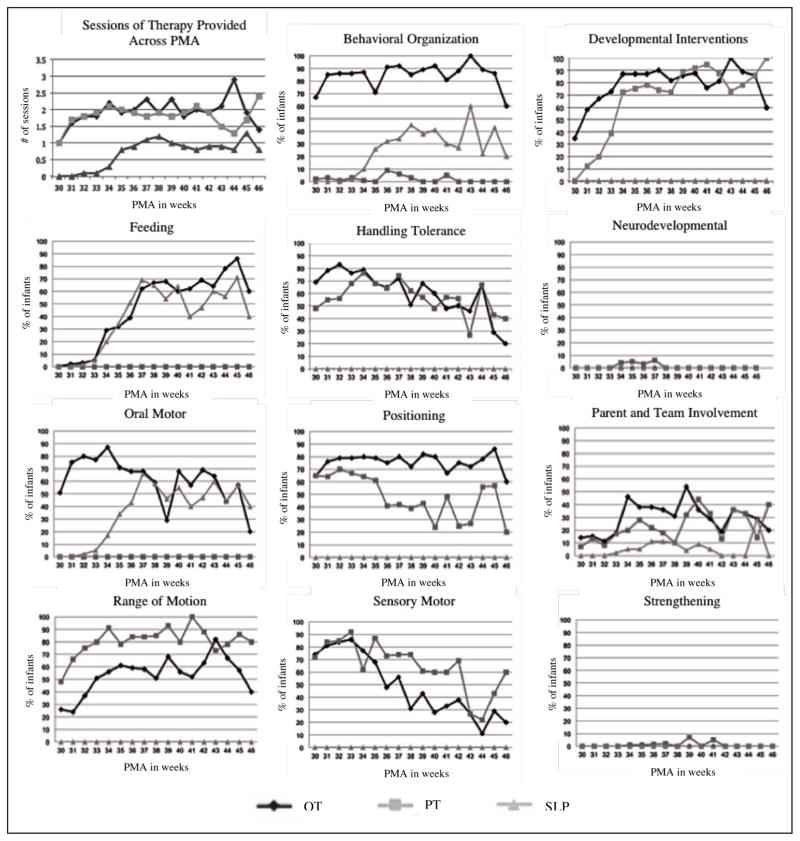
See Table 3 for descriptives outlining the average timing of therapy initiation, the amount of time spent on the evaluation and treatment sessions, the total number of sessions and hours of treatment over NICU hospitalization, and the frequency of therapy visits. See Figure 1 for the patterns of average frequency of therapy visits per week across PMA.
Table 3.
Medical and demographic factors associated with therapy initiation, therapy sessions, and therapy time.
| OT Initiation | PT Initiation | SLP Initiation | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 95% CI | Beta | P Value | 95% CI | Beta | P Value | 95% CI | Beta | P Value | |
| EGA | .22–.41 | .62 | < .001* | .22–.41 | .60 | < .001* | −.44−.07 | −.22 | .157 |
| Multiple birth | −.5–.91 | … | .560 | −.54–.90 | … | .623 | −1.04–2.8 | … | .358 |
| Non Caucasian Race | −1.17–.05 | … | .072 | −1.10–.173 | … | .151 | −1.17– 1.67 | … | .725 |
| Marital status | −.98–.62 | … | .662 | −.94–.71 | … | .780 | −2.57– 1.07 | …. | .412 |
| PMA at discharge | −.14–.03 | −.14 | .237 | −.12-.06 | −.07 | .544 | .26–.53 | .68 | < .001* |
| Length of stay (in weeks) | −.15- −.04 | −.38 | < .001* | −.14- −.03 | −.33 | .003* | .11–.31 | .54 | < .001* |
| Female gender | −.86–.37 | … | .434 | −.85–.43 | … | .514 | −2.27–.52 | … | .212 |
| Ventilation days | −.03–.01 | −.16 | .162 | −.02–.01 | −.08 | .463 | .00–.06 | .31 | .038* |
| CPAP days | −.09–.00 | −.21 | .068 | −.09–.01 | −.191 | .094 | −.07–.11 | .063 | .683 |
| Oxygen days | −.02- −.00 | −.35 | .002* | −.01- −.00 | −.29 | .010* | .01–.03 | .49 | .001* |
| NEC | −1.93–.195 | … | .108 | −2.13–.03 | … | .057 | −4.09–.22 | … | .077 |
| Sepsis | −.32–.81 | … | .396 | −.49–.68 | … | .743 | −2.4–.36 | … | .143 |
| Brain injury | −.05–1.4 | … | .069 | −.07–1.22 | … | .077 | −2.79–.15 | … | .077 |
| Minutes of Therapy | Sessions of Therapy | |||||
|---|---|---|---|---|---|---|
| 95% CI | Beta | P Value | 95% CI | Beta | P Value | |
| EGA | −195.00- −118.02 | −.69 | < .001* | −6.76- −4.17 | −.69 | < .001* |
| Multiple birth | −132.17–499.91 | … | .250 | −5.6–16.08 | … | .339 |
| Non Caucasian race | −210.03–353.76 | … | .613 | −6.70–12.57 | … | .545 |
| Marital status | −244.40–479.66 | … | .520 | −8.22–16.55 | … | .51 |
| PMA at discharge | 138.18–171.02 | .91 | < .001* | 4.70–5.84 | .90 | < .001* |
| Length of stay (in weeks) | 90.66–111.23 | .91 | < .001* | 3.13–3.81 | .92 | < .001* |
| Female gender | −63.96– 489.79 | … | .130 | −1.87–17.05 | … | .114 |
| Ventilation days | 18.08–29.27 | .69 | < .001* | 0.61–1.0 | .69 | < .001* |
| CPAP days | 13.14–54.79 | .35 | .002* | 0.54–2.0 | .37 | < .001* |
| Oxygen days | −0.01–0.00 | .85 | < .001* | 0.31–0.41 | .86 | < .001* |
| NEC | −607.40–366.63 | … | .624 | −19.88–13.46 | … | .702 |
| Sepsis | −802.16- −264.07 | … | < .001* | −27.6- −9.24 | … | < .001* |
| Brain Injury | −852.94- −224.17 | … | .001* | −29.01- −7.47 | … | .001* |
| Average OT therapy sessions per week | 347.89–919.94 | .449 | < .001* | 12.35–31.83 | .458 | < .001* |
| Average PT therapy sessions per week | 381.96–1016.03 | .447 | < .001* | 13.79–35.33 | .459 | < .001* |
| Average SLP therapy sessions per week | −70.41 | .254 | .109 | −2.91–22.60 | .243 | .126 |
Therapy initiation defined as PMA at initial evaluation
Minutes and sessions include total minutes and total sessions of therapy provided by OT, PT, and SLP combined for LOS
Significant associations at p= <0.05
P values and Beta values are from investigations of medical and demographic factors related to timing of OT, PT, and SLP initiation, minutes, and number of treatment sessions using linear regression models.
Figure 1.
Differences in the provision of therapeutic services across PMA including the average number of therapy sessions provided each week of hospitalization from 30 to 46 weeks PMA and differences in the provision of therapeutic interventions by OT, PT, and SLP for each week of hospitalization from 30 to 46 weeks PMA.
Positioning Consults
In this cohort of preterm infants, 51(65%) received a positioning consult with the PMA at time of positioning consult ranging between 23–32 weeks PMA with a mean (standard deviation) of 26.8 ± 2.1 weeks PMA. The time spent on positioning consults ranged from 10 to 45 minutes with a mean of 17.0 ± 7.7 minutes.
Provision of Therapeutic Interventions
A total of 56 different interventions were documented in the medical record among the cohort (see Table 1). To understand which interventions were exclusively provided by each discipline and which were provided by more than one discipline, interventions that were conducted by a discipline (>1% of the time) were identified. PTs were the only discipline to document functional motor skills (3.5%), gross motor skills (1.3%), and stretching (2%). OTs were the only discipline to document upper extremity functioning (7.9%), visual development (6.4%), head control (6.2%), and non-nutritive sucking (9.9%). SLP’s were the only discipline who documented swallowing (26.6%). After consolidation into 11 groupings of interventions (see Table 1), OTs completed all categories of interventions except for neurodevelopmental and strengthening, and PTs completed all intervention categories except for feeding and oral motor. SLP’s delivered interventions including behavioral organization, feeding, oral motor, and parent and team involvement. All three disciplines documented behavioral organization and parent and team involvement. The provision of therapeutic interventions for OT, PT, and SLP across PMA are shown in Figure 1.
Medical and Infant Factors and Associations with NICU Therapy Initiation and Therapy Sessions and Time
See Table 4 for the relationships of medical and demographic factors with timing of therapy initiation and therapy sessions and time. Those that reached statistical significance (p< .05) are listed in the table, and there were no other significant relationships.
Associations between Timing of Therapy Initiation, Frequency of Therapy, and Neurobehavior
See Table 2 for descriptives of neurobehavioral outcome of the sample. Earlier initiation of SLP was related to better self-regulation (p= .02), and higher PMA at SLP initiation was related to more stress (p= .02). Better self-regulation remained significant in the multivariate regression (p= .04). There were no significant relationships between PMA at OT or PT initiation and neurobehavior in either the univariate or multivariate analyses. More minutes of total therapy were associated with greater tolerance of handling (p= .04), poorer self-regulation (p= .04), and more lethargy (p= .03). There were no longer significant relationships after controlling for EGA, brain injury, PMA at time of neurobehavioral testing, and frequency of therapy. There were no relationships between the total therapy sessions and neurobehavior. Infants who had neurobehavioral impairment had more sessions (p= .04) and minutes (p= .04) of SLP and more minutes of OT across the LOS (p= .05). There were no further relationships between early therapy and neurobehavioral outcomes. Controlling for treatment arm (based on grouping of alternative neonatal positioning versus standard infant positioning, from the overarching study) did not alter the findings.
Discussion
The key findings of this study are: 1) OTs, PTs, and SLPs have a role in providing therapeutic interventions to high risk infants hospitalized in the NICU, 2) therapy in the NICU was conducted early in gestation and done in concert with concurrent medical interventions, 3) neonatal therapists provided a diverse repertoire of developmentally-appropriate interventions for infants born premature in the NICU, and 4) there was some overlap in the interventions provided by each discipline, but each discipline also provided interventions unique to their profession.
We were unable to support our hypothesis that infants who received more therapy would have better neurobehavior. Few associations between neonatal therapy usage and neurobehavioral outcomes were observed, but this was a challenging relationship to untangle. Provision of therapy services could be confounded by multiple factors including earlier discharge, poor tolerance due to medical stability, delayed discharge, and/or medically complex infants with significant impairment. In addition, 100% of infants in the cohort received therapy, making this relationship difficult to untangle in a unit where therapy services are standard of care.
While the role of OT, PT, and SLP has been well described in the literature 41, no studies to date have reported neonatal therapy usage in a US-based, level IV NICU. In the current study, 100% of infants received PT, 100% received OT, and 51% received SLP in the NICU. The patterns of therapy involvement are consistent with AAP recommendations; however, more research is needed to identify the patterns of therapy in NICUs of different sizes, demographic make ups, levels, and in different locations. Additionally, further work is needed to determine how many neonatal therapists should be used to adequately address the needs of high risk infants in different sizes and levels of NICUs. In the current study, there was 1 FTE of an OT for every 37.5 beds, 1 FTE of a PT for every 25 beds, and 1 FTE of a SLP for the entire 75-bed unit. Understanding gaps in the use of neonatal therapy, as well as ways to improve continuity of services following NICU discharge, can aid in consistent provision of therapy services for infants at risk of or already demonstrating signs of impaired development.
While there may be variations in practice across settings, our findings demonstrated that neonatal therapy can be initiated early in gestation, with OT and PT being initiated at 30 weeks PMA. SLP was initiated later in PMA at 36 weeks, which coincides with the timing of oral feeding, a common focus of SLP intervention. It also remains unclear if the later time at SLP initiation may be related to differences in referral patterns (automatic orders versus case-by-case referral). This warrants more research to determine if automatic referrals may generate more timely therapeutic interventions in the NICU in order to promote optimal outcomes. Although our findings may be confounded by the fact that therapy was initiated at different times, it is interesting to note that sicker infants (those on respiratory supports, sepsis, and brain injury) received more therapy services before discharge and had an earlier initiation of OT and PT services. Some NICUs may be wary of using neonatal therapy because of the infant’s vulnerable state, particularly if they have neurologic involvement or are dependent on respiratory supports; however, this study identified that OT and PT can be initiated at an early PMA, despite the presence of medical challenges. Recently, a process of neonatal therapy certification was implemented that can ensure a standard of appropriate knowledge and expertise for neonatal therapists working with high risk infants (ntncb.com). Advanced training ensures that therapists have the skill-set to address complex infant behaviors at a vulnerable time in development. The high incidence of neurobehavioral impairment (48%) in the current cohort further warrants the multidisciplinary team of neonatal therapists.
It is important to note that a wide repertoire of therapeutic interventions were provided by neonatal therapists in the NICU. Fifty-six different interventions were identified. The overlap and duplication of interventions provided by neonatal therapists in the present study falls in line with previous reports aimed at defining and delineating the roles of neonatal therapists 41,46. In 2012, the NANT Professional Collaborative, a group of neonatal therapists who work to help define guidelines for practice, found that practice guidelines outlining the role of each neonatal therapist exhibit overlap of skills, but also illustrate skills unique to their scope of practice; an important distinction when many institutions view some of the therapy disciplines as interchangeable 41,46. Just as therapists working with other populations have a unique role to play in the rehabilitation process, each therapy discipline has a unique role in optimizing outcomes of vulnerable preterm infants in the NICU. The current study offers a glimpse at role delineation among neonatal therapists by identifying specific interventions provided exclusively by each therapy discipline. OTs were the only discipline to address components of development aimed at optimizing occupational participation for infants born premature including upper extremity functioning, visual development, head control, and non-nutritive sucking. PTs aimed to optimize movement, addressing functional motor skills, gross motor skills, and stretching 47. SLPs were the only discipline to address swallowing performance in preterm infants. How the NICU team functions influences infant outcomes, as use of a multidisciplinary care team can improve patient and caregiver satisfaction; promote collaboration, coordination, quality improvement, communication, continuity, and competence among healthcare providers; and facilitate a positive outlook towards the provision of developmental care 48–50.
This study provides preliminary data describing the neonatal therapist’s role within an urban Level IV NICU environment, identifies when neonatal therapy was initiated, and gives insight into which therapeutic interventions were being conducted across PMA. Gaining an understanding of how neonatal therapy is structured within the NICU, as well as how the developmental team can operate successfully with the inclusion of an OT, PT, and SLP is a positive step toward better integration of neonatal therapists in NICUs and progress toward addressing the unique developmental needs of preterm infants in an effort to reverse the high rates of morbidity.
Limitations of the present study include that it was a descriptive study. Data on therapy usage was derived from therapy documentation consisting of prewritten treatment modalities and those added in by the therapist, however, additional interventions may have been described in expanded text form and could have been missed. In addition, a large number of the interventions discovered were those that had been written in by the therapist, and it remains unclear if a comprehensive list of interventions to choose from would have changed the findings. Additionally, data was gathered from a single study site with a diverse and large number of neonatal therapists delivering care, and this may not be representative of all NICUs, although the AAP has formulated a recent guideline requiring an OT or PT to be on staff in all Level III and IV NICUs 18. The findings should be interpreted carefully, as they may not be generalizable. Findings may not be applicable in settings that have different demographic populations where there are lower rates of single mothers, lower rates of diverse infant populations or lower rates of medically complex preterm infants. To gain a better understanding of the provision of neonatal therapy, as well as increase generalizability, further directions should expand on the current findings by looking at NICUs across the country, the timing of specific interventions, and how specific interventions relate to outcome. Finally, studies that aim to understand the effects of NICU-based therapy on outcomes are warranted. Due to the significant influence of co-morbid conditions on neurobehavior during the neonatal period, exploring long term outcomes is ideal.
What’s Known on this Subject
Current AAP guidelines stipulate that an OT or PT should be on staff in level III and IV NICUs. Despite the growing numbers of neonatal therapists, no studies to date have defined the use of early therapy in the NICU.
What This Study Adds
This is the first study to identify when therapy is initiated in the NICU, to define the amount of therapy and types of therapeutic interventions provided, and to explore how different medical factors relate to provision of NICU-based therapy services.
Acknowledgments
Thank you to Cindy Albrecht, Anna Annecca, Rebecca Armitage, Dr. Carolyn Baum, Dr. Christine Berg, Gabrielle Blenden, Katie Bogan, Hayley Chrzastowski, Kelsey Dewey, Polly Durant, Felicia Foci, Bailey Hall, Rachel Harris, Laura Madlinger-Lewis, Laura Mazelis, Odo Nwabara, Sarah Oberle, Jessica Roussin, Justin Ryckman, Lauren Reynolds, Zachary Ross, Lisa Shabosky, Dr. Joan Smith, Patricia Spener, Elaine Ward, Sarah Wolf for their contributions to this manuscript. Also, thank you to the neonatal therapy team at St. Louis Children’s Hospital for their valued involvement.
Funding Source: This project was supported by the National Institute of Health Comprehensive Opportunities for Rehabilitation Research Training (CORRT) Grant (K12 HD055931) and the Washington University Intellectual and Developmental Disabilities Research Center (NIH/NICHD P30 HD062171).
Abbreviations
- NICU
neonatal intensive care unit
- OT
occupational therapy
- PT
physical therapy
- SLP
speech-language pathology
- AAP
American Academy of Pediatrics
- EGA
estimated gestational age
- CPAP
continuous positive airway pressure
- PMA
postmenstrual age
- LOS
length of stay
- NEC
necrotizing enterocolitis
- PTA
physical therapy assistant
- NNNS
NICU Network Neurobehavioral Scale
- NANT
National Association of Neonatal Therapy
Footnotes
Financial Disclosure: Authors have indicated they have no financial relationships to disclose.
Conflict of Interest: Authors have indicated there are no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.March of Dimes. Prematurity Campaign. 2013 http://www.marchofdimes.com/mission/prematurity_indepth.html. [PMC free article] [PubMed]
- 2.Johnson S, Fawke J, Hennessy E, et al. Neurodevelopmental disability through 11 years of age in children born before 26 weeks of gestation. Pediatrics. 2009;124(2):e249–257. doi: 10.1542/peds.2008-3743. [DOI] [PubMed] [Google Scholar]
- 3.Delobel-Ayoub M, Arnaud C, White-Koning M, et al. Behavioral problems and cognitive performance at 5 years of age after very preterm birth: the EPIPAGE Study. Pediatrics. 2009;123(6):1485–1492. doi: 10.1542/peds.2008-1216. [DOI] [PubMed] [Google Scholar]
- 4.Marlow N, Wolke D, Bracewell MA, Samara M. Neurologic and developmental disability at six years of age after extremely preterm birth. The New England journal of medicine. 2005;352(1):9–19. doi: 10.1056/NEJMoa041367. [DOI] [PubMed] [Google Scholar]
- 5.McCormick MC, Litt JS, Smith VC, Zupancic JA. Prematurity: an overview and public health implications. Annual review of public health. 2011;32:367–379. doi: 10.1146/annurev-publhealth-090810-182459. [DOI] [PubMed] [Google Scholar]
- 6.Aylward GP. Neurodevelopmental outcomes of infants born prematurely. Journal of developmental and behavioral pediatrics : JDBP. 2014;35(6):394–407. doi: 10.1097/01.DBP.0000452240.39511.d4. [DOI] [PubMed] [Google Scholar]
- 7.Prevention CfDCa. Preterm Birth. 2013 http://www.cdc.gov/reproductivehealth/MaternalInfantHealth/PretermBirth.htm.
- 8.Mikkola K, Ritari N, Tommiska V, et al. Neurodevelopmental Outcome at 5 Years of Age of a National Cohort of Extremely Low Birth Weight Infants Who Were Born in 1996–1997. Pediatrics. 2005;116(6):1391–1400. doi: 10.1542/peds.2005-0171. [DOI] [PubMed] [Google Scholar]
- 9.Pineda RG, Neil J, Dierker D, et al. Alterations in brain structure and neurodevelopmental outcome in preterm infants hospitalized in different neonatal intensive care unit environments. The Journal of pediatrics. 2014;164(1):52–60. e52. doi: 10.1016/j.jpeds.2013.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pineda RG, Tjoeng TH, Vavasseur C, Kidokoro H, Neil JJ, Inder T. Patterns of altered neurobehavior in preterm infants within the neonatal intensive care unit. J Pediatr. 2013;162(3):470–476. e471. doi: 10.1016/j.jpeds.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pitcher JB, Schneider LA, Drysdale JL, Ridding MC, Owens JA. Motor system development of the preterm and low birthweight infant. Clinics in perinatology. 2011;38(4):605–625. doi: 10.1016/j.clp.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Limperopoulos C, Soul JS, Gauvreau K, et al. Late gestation cerebellar growth is rapid and impeded by premature birth. Pediatrics. 2005;115(3):688–695. doi: 10.1542/peds.2004-1169. [DOI] [PubMed] [Google Scholar]
- 13.Bodensteiner JB, Johnsen SD. Cerebellar injury in the extremely premature infant: newly recognized but relatively common outcome. Journal of child neurology. 2005;20(2):139–142. doi: 10.1177/08830738050200021101. [DOI] [PubMed] [Google Scholar]
- 14.Messerschmidt A, Brugger PC, Boltshauser E, et al. Disruption of cerebellar development: potential complication of extreme prematurity. AJNR. American journal of neuroradiology. 2005;26(7):1659–1667. [PMC free article] [PubMed] [Google Scholar]
- 15.Brown NC, Doyle LW, Bear MJ, Inder TE. Alterations in neurobehavior at term reflect differing perinatal exposures in very preterm infants. Pediatrics. 2006;118(6):2461–2471. doi: 10.1542/peds.2006-0880. [DOI] [PubMed] [Google Scholar]
- 16.Smith GC, Gutovich J, Smyser C, et al. Neonatal intensive care unit stress is associated with brain development in preterm infants. Annals of neurology. 2011;70(4):541–549. doi: 10.1002/ana.22545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pineda RG, Tjoeng TH, Vavasseur C, Kidokoro H, Neil JJ, Inder T. Patterns of altered neurobehavior in preterm infants within the neonatal intensive care unit. The Journal of pediatrics. 2013;162(3):470–476. e471. doi: 10.1016/j.jpeds.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riley LE, Stark AR, Kilpatrick SJ, Papile LA. Guidelines for Perinatal Care. 7. Elk Grove Village, IL: American Academy of Pediatrics; 2012. [Google Scholar]
- 19.Oberg GK, Blanchard Y, Obstfelder A. Therapeutic encounters with preterm infants: interaction, posture and movement. Physiotherapy theory and practice. 2014;30(1):1–5. doi: 10.3109/09593985.2013.806621. [DOI] [PubMed] [Google Scholar]
- 20.Case–Smith J. An efficacy study of occupational therapy with high-risk neonates. American Journal of Occupational Therapy. 1988;42(8):499–506. doi: 10.5014/ajot.42.8.499. [DOI] [PubMed] [Google Scholar]
- 21.Caretto V, Topolski KF, Linkous CM, Lowman DK, Murphy SM. Current parent education on infant feeding in the neonatal intensive care unit: The role of the occupational therapist. American Journal of Occupational Therapy. 2000;54(1):59–64. doi: 10.5014/ajot.54.1.59. [DOI] [PubMed] [Google Scholar]
- 22.Anderson J. Sensory intervention with the preterm infant in the neonatal intensive care unit. American Journal of Occupational Therapy. 1986;40(1):19–26. doi: 10.5014/ajot.40.1.19. [DOI] [PubMed] [Google Scholar]
- 23.Matthews CL. Supporting suck-swallow-breath coordination during nipple feeding. The American journal of occupational therapy : official publication of the American Occupational Therapy Association. 1994;48(6):561–562. doi: 10.5014/ajot.48.6.561. [DOI] [PubMed] [Google Scholar]
- 24.Glass RP, Wolf LS. A global perspective on feeding assessment in the neonatal intensive care unit. The American journal of occupational therapy : official publication of the American Occupational Therapy Association. 1994;48(6):514–526. doi: 10.5014/ajot.48.6.514. [DOI] [PubMed] [Google Scholar]
- 25.Garber J. Oral–Motor Function and Feeding Intervention. Physical & Occupational Therapy in Pediatrics. 2013;33(1):111–138. doi: 10.3109/01942638.2012.750864. [DOI] [PubMed] [Google Scholar]
- 26.Sheppard JJ, Fletcher KR. Evidence-based interventions for breast and bottle feeding in the neonatal intensive care unit. Seminars in speech and language. 2007;28(3):204–212. doi: 10.1055/s-2007-984726. [DOI] [PubMed] [Google Scholar]
- 27.Limperopoulos C, Majnemer A. The role of rehabilitation specialists in Canadian NICUs: A national survey. Physical & occupational therapy in pediatrics. 2002;22(1):57–72. [PubMed] [Google Scholar]
- 28.Sweeney JK, Gutierrez T. Musculoskeletal implications of preterm infant positioning in the NICU. The Journal of perinatal & neonatal nursing. 2002;16(1):58–70. doi: 10.1097/00005237-200206000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Byrne E, Garber J. Physical Therapy Intervention in the Neonatal Intensive Care Unit. Physical & Occupational Therapy in Pediatrics. 2013;33(1):75–110. doi: 10.3109/01942638.2012.750870. [DOI] [PubMed] [Google Scholar]
- 30.Olson JA, Baltman K. Infant mental health in occupational therapy practice in the neonatal intensive care unit. American Journal of Occupational Therapy. 1994;48(6):499–505. doi: 10.5014/ajot.48.6.499. [DOI] [PubMed] [Google Scholar]
- 31.Frank A, Maurer P, Shepherd J. Light and sound environment: A survey of neonatal intensive care units. Physical & Occupational Therapy in Pediatrics. 1991;11(2):27–45. [Google Scholar]
- 32.Als H. A synactive model of neonatal behavioral organization: framework for the assessment of neurobehavioral development in the premature infant and for support of infants and parents in the neonatal intensive care environment. Physical & Occupational Therapy in Pediatrics. 1986;6(3–4):3–53. [Google Scholar]
- 33.Oberg GK, Blanchard Y, Obstfelder A. Therapeutic encounters with preterm infants: interaction, posture and movement. Physiotherapy theory and practice. 2013 doi: 10.3109/09593985.2013.806621. [DOI] [PubMed] [Google Scholar]
- 34.Zarem C, Crapnell T, Tiltges L, et al. Neonatal nurses' and therapists' perceptions of positioning for preterm infants in the neonatal intensive care unit. Neonatal network : NN. 2013;32(2):110–116. doi: 10.1891/0730-0832.32.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grenier IR, Bigsby R, Vergara ER, Lester BM. Comparison of motor self-regulatory and stress behaviors of preterm infants across body positions. The American journal of occupational therapy : official publication of the American Occupational Therapy Association. 2003;57(3):289–297. doi: 10.5014/ajot.57.3.289. [DOI] [PubMed] [Google Scholar]
- 36.Monfort K, Case-Smith J. The effects of a neonatal positioner on scapular rotation. The American journal of occupational therapy : official publication of the American Occupational Therapy Association. 1997;51(5):378–384. doi: 10.5014/ajot.51.5.378. [DOI] [PubMed] [Google Scholar]
- 37.Mahoney MC, Cohen MI. Effectiveness of Developmental Intervention in the Neonatal Intensive Care Unit: Implications for Neonatal Physical Therapy. Pediatric Physical Therapy. 2005;17(3):194–208. doi: 10.1097/01.pep.0000176574.70254.60. [DOI] [PubMed] [Google Scholar]
- 38.Vergara E, Anzalone M, Bigsby R, et al. Specialized knowledge and skills for occupational therapy practice in the neonatal intensive care unit. American Journal of Occupational Therapy. 2006;60(6):659–668. doi: 10.5014/ajot.60.6.659. [DOI] [PubMed] [Google Scholar]
- 39.Dewier JL. The Speech-Language Pathologist's Role in the Neonatal Intensive Care Unit. Research Papers. 2012 [Google Scholar]
- 40.Baumgartner CA, Bewyer E, Bruner D. Management of communication and swallowing in intensive care: the role of the speech pathologist. AACN advanced critical care. 2008;19(4):433–443. doi: 10.1097/01.AACN.0000340724.80280.31. [DOI] [PubMed] [Google Scholar]
- 41.Sturdivant C. A collaborative approach to defining neonatal therapy. Newborn and Infant Nursing Reviews. 2013;13(1):23–26. [Google Scholar]
- 42.Madlinger-Lewis L, Reynolds L, Zarem C, Crapnell T, Inder T, Pineda R. The effects of alternative positioning on preterm infants in the neonatal intensive care unit: A randomized clinical trial. Research in developmental disabilities. 2014;35(2):490–497. doi: 10.1016/j.ridd.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lester BM, Tronick EZ. NICU network neurobehavioral scale manual. Baltimore, MD: Paul H Brooks Publishing; 2004. [Google Scholar]
- 44.Tronick EZ, Olson K, Rosenberg R, Bohne L, Lu J, Lester BM. Normative neurobehavioral performance of healthy infants on the neonatal intensive care unit network neurobehavioral scale. Pediatrics. 2004;113(Supplement 2):676–678. [PubMed] [Google Scholar]
- 45.Lester BM, Tronick E. NICU Network Neurobehavioral Scale (NNNS) manual. Paul H Brookes Pub Co; 2004. [Google Scholar]
- 46.Barbosa VM. Teamwork in the neonatal intensive care unit. Physical & occupational therapy in pediatrics. 2013;33(1):5–26. doi: 10.3109/01942638.2012.729556. [DOI] [PubMed] [Google Scholar]
- 47.Sweeney JK, Heriza CB, Reilly MA, Smith C, VanSant AF. Practice Guidelines for the Physical Therapist in the Neonatal Intensive Care Unit (NICU) Pediatric Physical Therapy. 1999;11(3):119–132. [Google Scholar]
- 48.Hearn J, Higginson IJ. Do specialist palliative care teams improve outcomes for cancer patients? A systematic literature review. Palliative medicine. 1998;12(5):317–332. doi: 10.1191/026921698676226729. [DOI] [PubMed] [Google Scholar]
- 49.Hendricks-Munoz KD, Prendergast CC. Barriers to provision of developmental care in the neonatal intensive care unit: neonatal nursing perceptions. American journal of perinatology. 2007;24(2):71–77. doi: 10.1055/s-2006-958156. [DOI] [PubMed] [Google Scholar]
- 50.Ohlinger J, Brown MS, Laudert S, Swanson S, Fofah O. Development of potentially better practices for the neonatal intensive care unit as a culture of collaboration: communication, accountability, respect, and empowerment. Pediatrics. 2003;111(Supplement E1):e471–e481. [PubMed] [Google Scholar]