Abstract
Extracellular vesicles (EVs), lipid bilayer-enclosed structures that contain a variety of biological molecules shed by cells, are increasingly becoming appreciated as a major form of cell-to-cell communication. Indeed, EVs have been shown to play important roles in several physiological processes, as well as diseases such as cancer. EVs dock on to the surfaces of recipient cells where they transmit signals from the cell surface and/or transfer their contents into cells to elicit functional responses. EV docking and uptake by cells represent critical, but poorly understood processes. Here, we focus on the mechanisms by which EVs dock and transfer their contents to cells. Moreover, we highlight how these findings may provide new avenues for therapeutic intervention.
Keywords: extracellular vesicles, microvesicles, exosomes, intercellular communication, cancer, metastasis, docking, uptake, targeting
1. Introduction
Cells release extracellular vesicles (EVs), which are lipid-enclosed vesicles ranging from ∼ 30–1000 nm in diameter. EVs contain a variety of cargo, including mRNA [1-3], microRNA [1, 4, 5], long non-coding RNA [6, 7], DNA [7], and proteins [2, 4, 8-13] (Figure 1A). In order to elicit functional effects, EVs dock onto recipient (target) cells, at which point the EVs can initiate signaling events at the cell surface or are internalized by cells. In either case, EVs are capable of promoting phenotypic changes in recipient cells, which are dependent on their cargo [1, 3-5, 10, 14, 15].
Figure 1.
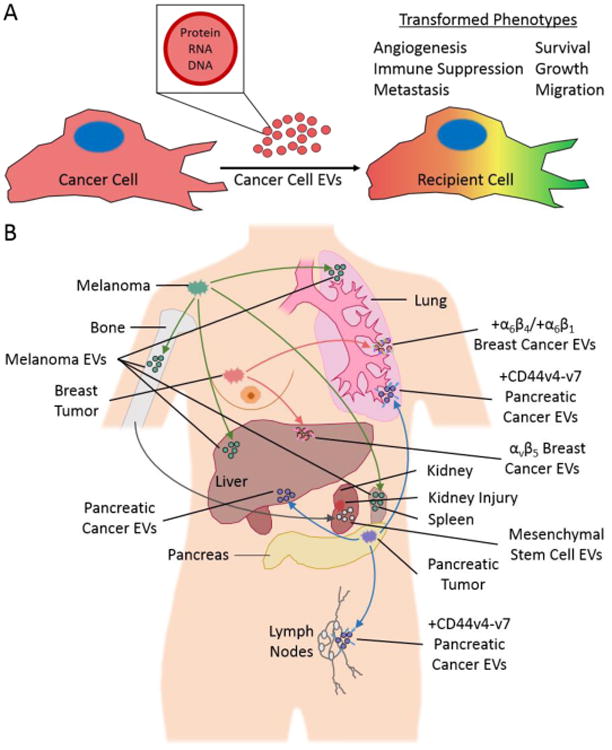
(A) EVs generated by cancer cells contain a variety of cargo (i.e., protein, RNA, and DNA) that can be transferred to other cells. This causes the phenotypes of recipient cells to change (denoted by the color change) in ways that promote cancer progression. (B) EVs derived from bone marrow or different types of cancer cells accumulate in specific organs in animal models. For example, EVs generated by bone marrow cells accumulate in the kidney where they promote injury recovery (grey arrow and EVs). However, cancer-derived EVs appear to promote metastasis in a variety of organs. Specifically, EVs from melanoma cells preferentially accumulate in the bone, liver, spleen, and lung (green arrows and EVs), EVs from breast cancer cells accumulate in the liver and lungs (pink arrows and EVs), and EVs from pancreatic cancer cells accumulate in the liver, lung, and lymph nodes (blue arrows and EVs).
At least two different types of EVs have been identified: microvesicles (MVs) and exosomes. Size is one distinguishing feature between these two classes of EVs. MVs typically range in size from 200–1000 nm in diameter, while exosomes are smaller, averaging between 30 and 120 nm. MVs and exosomes also differ in how they are formed. Exosome biogenesis involves the redirection of multivesicular bodies (MVBs) within the traditional endosomal sorting pathway, from the lysosome where they would typically be degraded, to the cell surface. These redirected MVBs fuse with the plasma membrane and release their contents (i.e., exosomes) into the extracellular environment [16, 17]. Consistent with the idea that exosomes originate from MVBs, it has been shown that interfering with the machinery in the endosomal sorting pathway, such as endosomal sorting complexes required for transport (ESCRT) proteins, blocks exosome formation and release [16]. In contrast, MVs are thought to bud from the plasma membrane through Arf6-[18] and RhoA-dependent [19] rearrangements of the actin cytoskeleton. Although exosomes and MVs appear to be shed via different mechanisms, it is not known whether these two major types of EVs are capable of mediating distinct biological outcomes. However, one study found that MVs deliver functional plasmid DNA and proteins to recipient cells more efficiently than exosomes [20], suggesting that different classes of EVs may be functionally distinct.
It is also worth emphasizing that the field is still debating what properties define exosomes versus MVs and how to best isolate each class of EVs. Moreover, it is becoming increasingly clear that several sub-types of exosomes and MVs likely exist, adding an additional layer of complexity to this issue. As a result, many studies claiming to specifically study either exosomes or MVs are, instead, isolating a mixture of EVs. This has prompted the EV community to adopt new guidelines that include using the term EVs, rather than MVs or exosomes, in cases where it is not absolutely clear that a particular class of EVs is being isolated and studied. However, for the purposes of this review, we decided to use the terminologies (i.e., EVs, MVs, or exosomes) chosen by the authors when describing their work.
EVs participate in a variety of physiological processes, including pregnancy [21], stem cell differentiation [22], inflammation [23, 24], and blood coagulation [23, 24]. For instance, MVs play a role in implantation, one of the earliest and most important stages of pregnancy where a blastocyst-stage embryo attaches to the maternal uterine lining and migrates into the tissue. Although generally thought to be directed by maternal signals, it now appears that the embryo also contributes to implantation. Specifically, it was shown that embryonic stem cells (ESCs) located within the inner cell mass of the blastocyst generate MVs that interact with the surrounding layer of trophoblasts. The ESC-derived MVs docked onto trophoblasts and stimulated signaling events that promoted trophoblast migration, an essential step for implantation. These effects were mediated by interactions between laminin and fibronectin extracellular matrix (ECM) proteins located on the surfaces of ESC-derived MVs, and integrins and laminin receptors expressed along the plasma membranes of trophoblasts [21], suggesting that the docking of MVs onto trophoblasts is critical for MV-promoted implantation. Consistent with this idea, when blastocyst-stage embryos were incubated with MVs isolated from ESC cultures, and then surgically introduced into the uterus of pseudo-pregnant mouse, the rates of implantation significantly increased, underscoring the importance of MVs in this process [21].
In addition to their roles in normal biology, EVs are also involved in diseases, such as viral infection [25, 26], prion and amyloid diseases [27, 28], and cancer [29, 30]. In the context of cancer, EVs have been extensively studied and shown to promote a wide range of processes that underlie cancer progression, including inflammatory responses [5], angiogenesis [2, 31-33], metastasis [34, 35], cell migration [36], proliferation [10, 37, 38], and immune suppression [39, 40] (Figure 1A). One study demonstrating the potential effects of EVs on cancer progression showed that EVs from cancer cells can affect normal cells that are adjacent to tumors. MVs derived from the highly aggressive MDAMB231 breast cancer cell line promoted the proliferation, survival, and anchorage-independent growth of immortalized fibroblasts and normal mammary epithelial cells [10], major cell types found in the breast tumor microenvironment. These results suggest that cancer-derived MVs can cause normal (i.e., non-cancerous) cell types to acquire some properties of cancer/transformed cells. These effects were shown to be dependent on a cross-linked form of fibronectin present on the surface of MDAMB231-derived MVs. The cross-linked fibronectin engaged integrins expressed on the recipient fibroblasts and mammary epithelial cells and stimulated signaling events that promoted their growth under anchorage-free conditions [10].
Although the EV field is still young, a great deal has been learned regarding MV and exosome biogenesis, the cargo that they contain, and the biological effects that they promote. However, the mechanisms that mediate the docking of EVs to recipient (target) cells and EV cargo internalization by cells are still not well understood [41]. Here, we highlight the current knowledge on these important processes.
2. EV Targeting and Docking on Cells
EVs derived from different sources have been reported to interact preferentially with specific cell types. For example, exosomes from oligodendroglial precursor cells or mature oligodendrocytes were internalized by microglia but not by astrocytes, neurons, or other oligodendrocytes [42]. Similarly, bone marrow dendritic cell (DC) exosomes were preferentially internalized by splenic conventional DCs, rather than by plasmacytoid DCs, B lymphocytes, macrophages, or splenic T cells [4]. Interestingly, the interaction and uptake of EVs by recipient cells may be dependent on the specific properties of the recipient cells. For example, epithelial cells and astrocytes were unable to internalize EVs from transformed cells [43]. However, when the same cell types were transformed through ectopic expression of oncogenic forms of Ras or c-Src, they efficiently internalized the EVs [43]. Thus, the induction of cellular transformation may cause some cell types to change in ways that make them more prone to EV uptake.
In cases where EVs are capable of influencing the functions of multiple types of cells, the mechanisms by which the EVs bind to and are internalized by recipient cells varies between different cell types [44, 45]. One such case involves MVs isolated from microglia cells. When these MVs attached to other microglia, they moved along the surface of the cell toward its nucleus prior to being internalized. However, when microglia-derived MVs attached to astrocytes, they remained stationary and were not internalized [45]. Similarly, leukemia-derived exosomes were internalized by phagocytic cells, but primarily attached to and remained on the surfaces of non-phagocytic cells [44]. Thus, the mechanisms that regulate the targeting of EVs to specific cell lineages, and/or the fates of EVs once they attach to cells, can determine the biological effects that EVs have on recipient cells.
The preferential interactions between EVs and certain cell types have also been observed in vivo (Figure 1B), through the accumulation of specific EVs in distinct organs. When EVs isolated from human embryonic kidney (HEK) 293T cells and DCs were injected into the blood stream of mice, they primarily localized to the liver and the spleen [3, 46, 47], whereas EVs from human mesenchymal stem cells (known to aid in tissue recovery following injury) accumulated in the liver, spleen, and sites of acute kidney injury (Figure 1B), where they facilitated injury recovery [48, 49]. Similarly, melanoma-derived exosomes accumulated in the lungs, bone, liver, and spleen and increased the frequency of metastasis at these sites [38]. The accumulation of EVs at sites of injury or metastasis suggests that the specific targeting of these vesicles likely contributes heavily to their functional effects.
Overall, the preferential interactions of EVs with recipient cells, and their selective accumulation in specific organs seems to indicate that EVs are targeted to certain cell lineages. Much of this specificity can be explained by protein surface receptors and adhesion molecules (i.e., tetraspanins, integrins, proteoglycans, and lectins) that are enriched in EVs (Figure 2A). Integrins, ECM proteins, lectins, proteoglycans, or glycolipids on EVs allow them to dock with cells expressing appropriate receptors on their surfaces [41]. Here, we describe the surface receptors, adhesion molecules, and ECM proteins that mediate EV-cell binding.
Figure 2.
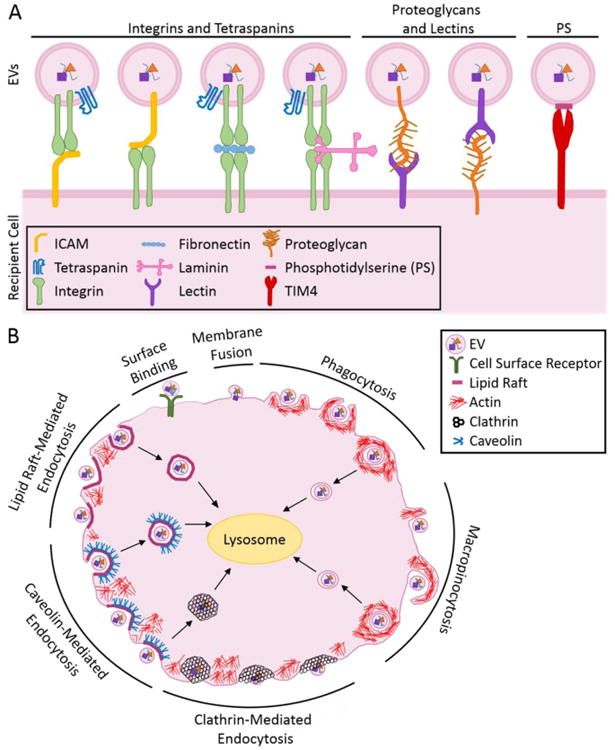
(A) EVs bind to the surfaces of recipient cells using various lipids and adhesion proteins, including tetraspanins, integrins, ECM proteins, and proteoglycans. (B) EVs interact with, and are internalized by, recipient cells via cell surface binding, membrane fusion, phagocytosis, macropinocytosis, as well as through clathrin-, caveolin-, and lipid raft-mediated endocytosis.
2.1 Tetraspanins, ECM Proteins, and Integrins
Tetraspanins are small transmembrane proteins that mediate cell adhesion, migration, and signaling [50]. Certain tetraspanins, e.g., CD63 and CD81, are routinely found in exosomes [51, 52] and, thus, are frequently used as exosomal markers. The expression of other members of the tetraspanin family in exosomes may help target the exosomes to certain cell types [53, 54] by recruiting additional adhesion proteins into the exosomes [55]. For instance, vascular cell adhesion molecule 1 (VCAM-1) and integrin α4 were recruited into pancreatic adenocarcinoma-derived exosomes via associations with tetraspanin 8. The enrichment of VCAM-1 and integrin α4 in the exosomes enhanced the docking and uptake of the exosomes by endothelial cells [55].
Integrins are transmembrane proteins that are receptors for ECM proteins, including laminin and fibronectin. They often interact with tetraspanins and appear to mediate many cellular outcomes [50, 56]. Moreover, ECM-integrin interactions also play major roles in EV binding and uptake by cells [10, 21, 36, 49, 57, 58] (Figure 2A). Thus, inhibiting fibronectin on the surfaces of MDAMB231-derived MVs from binding or activating α5β1 integrins on recipient fibroblasts, by treating the cells with the RGD peptide (a peptide that blocks fibronectin-integrin interactions), inhibited the MVs from inducing the anchorage-independent growth of fibroblasts [10]. Similarly, the increase in trophoblast cell migration caused by ESC-derived MVs was reduced by treating trophoblasts with the RGD and YIGSR peptides, which blocked cellular integrins from binding to fibronectin and laminin associated with the MV surface [21]. In addition, the docking and uptake of exosomes by recipient cells are also dependent on exosomal ECM proteins and cellular ECM protein receptors (e.g., β1, αv, β3, and αL integrins and intercellular adhesion molecule 1 [ICAM-1]) [47].
Integrins on the surfaces of recipient cells also play a role in targeting exosomes to specific cell types in vivo. Exosomes with ICAM-1 on their surfaces bind to T cells and lymph node DCs expressing integrin β2 [12, 58, 59]. Similarly, pancreatic ductal adenocarcinoma exosomes preferentially bind F4/80+ (adhesion G protein-coupled receptor E1), CD11b+ (integrin αM) Kupffer cells found in the liver (Figure 1B), possibly via exosomal ICAM, which is a CD11b ligand [60], and promote liver metastases [61].
The findings described above collectively suggest that adhesion proteins, such as tetraspanins and ECM proteins (e.g., fibronectin and laminin), found along the surfaces of EVs can interact with their corresponding receptors (e.g., integrins) on recipient cells and promote EV-cell docking. However, there have also been suggestions that some EVs contain integrins that mediate the docking of EVs onto certain cell types [57, 62, 63]. This has been demonstrated in the context of cancer metastasis. It is well-established that certain types of cancer cells preferentially colonize secondary sites, a process called the “seed and the soil” hypothesis [64]. For example, the 4175-LuT breast cancer and BxPC-3 pancreatic cancer cell lines metastasize to the lung and liver, respectively, when introduced as xenografts into nude mice. Interestingly, when exosomes derived from these cancer cells were injected into the blood stream of mice, they too accumulated in the lung or liver [57]. This organ-tropic effect was dependent on the expression of specific integrins on the exosome surface [57]. Exosomes from the lung-tropic 4175-LuT breast cancer cells contained α6β4 and α6β1 integrins, accumulated in regions of the lung that were rich in laminin (a ligand for these integrins), and promoted lung metastases (Figure 1B) [57]. The authors showed that knocking down integrin β4 in the exosomes or pre-incubating the exosomes with the HYD-1 peptide, which blocks interactions between laminin and its receptors (i.e., integrins), reduced the accumulation of the exosomes in the lung [57]. Importantly, blocking the accumulation of 4175-LuT breast cancer-derived exosomes in the lung (via an integrin β4 knockdown) led to a reduction in metastasis [57].
Exosomes from the liver-tropic BxPC-3 pancreatic cancer cell line contained integrin αvβ5 and preferentially accumulated in regions of the liver that were rich in fibronectin (an integrin αvβ5 ligand) (Figure 1B). Moreover, exosomes from the liver-tropic Pan02 pancreatic cancer cells also contained integrins αvβ5, accumulated in the liver, and promoted liver metastases. Accumulation of exosomes in the liver was reduced by knocking down integrin β5 in the BxPC-3-derived exosomes, or by treating the BxPC-3- or Pan02-derived exosomes with the RGD peptide, which blocks fibronectin-integrin interactions. Furthermore, the ability of Pan02-derived exosomes to promote liver metastases was ameliorated by treating the exosomes with the RGD peptide [57]. Overall, these data suggest that EV localization in vivo is determined by adhesion molecules, such as integrins, and metastasis can be reduced by blocking integrins responsible for EV localization.
2.2 Proteoglycans and Lectins
Emerging evidence suggests that proteoglycans, cell surface proteins with carbohydrate modifications, and lectins are enriched in EVs and likely contribute to their ability to attach to recipient cells [65-68]. Cell surface proteoglycans may play a role in exosome docking, given that proteoglycan-deficient recipient cells internalize exosomes less efficiently than cells expressing proteoglycans [69]. Accordingly, lectins, such as galectins 1, 3, and 5, and E-selectin that recognize and bind to proteoglycans or glycolipids [65], are found in EVs [58, 70-72]. Furthermore, it appears that exosomal galectin-5 may mediate exosome uptake by binding to cell surface proteoglycans, since treating exosomes with excess asialofetuin (a proteoglycan that is a galectin-5 ligand) reduced reticulocyte exosome uptake by macrophages [72].
It has also been demonstrated that proteoglycan receptors (e.g., lectins) along the plasma membranes of cells and proteoglycans on exosome surfaces [49, 73] help mediate exosome docking. Blocking cellular heparan sulfate proteoglycan (HSPG) receptors decreased exosome uptake [74, 75]. Similarly, treating exosomes with excess galectin-5 reduced reticulocyte exosome uptake by macrophages, suggesting that galectin-5 in recipient cells may bind with proteoglycans on exosomes [72]. Furthermore, blocking the proteoglycan CD44 in EVs reduced their uptake by recipient cells [49]. However, there are also examples showing that HSPGs (such as syndecan and glypican) in exosomes are not required for uptake [69], suggesting that proteoglycan-mediated EV uptake may be cell-type dependent.
In addition to facilitating EV-cell interactions in vitro, proteoglycans such as CD44v4-v7 (a CD44 isoform [76]) in exosomes and cell surface lectins (e.g., CD169) influence EV distribution in vivo [15, 77]. The localization of pancreatic carcinoma exosomes to lymph node stroma cells and lung fibroblasts (Figure 1B) was impaired when CD44v4-v7 was knocked down in the exosomes [15]. Similarly, exosomes derived from primary B cells localize to CD169+ cells, and CD169 null mice have an altered exosome distribution [77], suggesting that exosomal proteoglycans and cell surface lectins may target exosomes to specific cells.
3. Mechanisms of EV-Cell Interactions and Uptake
Once an EV attaches to the cell surface it has two possible fates. In some cases, proteins on the EV bind to and activate receptors expressed on the recipient cell without being internalized [78-81] (Figure 2B). In other instances, the EV contents are transferred to the recipient cell via direct fusion with the plasma membrane or endocytosis (Figure 2B). Both EV-mediated signaling from the cell surface, and the transfer of EV contents to cells, can elicit functional effects. Herein, we describe the mechanisms that cells use to internalize EVs.
3.1 EV Internalization via Membrane Fusion and Endocytosis
There are two mechanisms by which EVs can be internalized by cells: direct membrane fusion [4, 13, 82] or endocytosis [4, 47, 83, 84] (Figure 2B). The most commonly studied mechanism of EV uptake is endocytosis, an active process where cells engulf particles or molecules [85]. Several pieces of evidence suggest that exosomes are internalized via an active endocytic process, as opposed to passive membrane fusion. These include the reduced uptake of exosomes by cells incubated at 4°C [4, 47, 83, 84], the detection of exosomes enclosed in double membrane structures in cells following their internalization [83, 84], and the colocalization of exosomes with various endosomal and lysosomal markers [5, 42, 44, 82-84, 86-88]. It should be noted that MVs are similarly internalized by cells [20].
3.1.1 Clathrin-Dependent Endocytosis, Phagocytosis, and Macropinocytosis
EV uptake can be reduced by disrupting actin dynamics [4, 44, 47, 72, 86, 88], suggesting that endocytic mechanisms requiring cytoskeletal remodeling, including clathrin-dependent endocytosis, phagocytosis, and macropinocytosis [89], are responsible for EV uptake (Figure 2B). Clathrin-dependent endocytosis begins when accessory proteins recruited to the plasma membrane induce membrane curvature. The membrane invagination is then coated with clathrin to create a clathrin-coated pit, which is released from the plasma membrane via membrane scission by dynamin [90]. EV endocytosis is at least partially dependent on clathrin-mediated endocytosis because expression of dominant-negative forms of clathrin in cells, or treating recipient cells with clathrin inhibitors (e.g., chlorpromazine or pitstop-2), reduced exosome uptake [44, 67, 86].
Phagocytosis, the actin-dependent, receptor-mediated ingestion of large particles by cells, is also used to internalize EVs. This process begins when a cell recognizes an EV and rearranges its cytoskeleton to create a cup-shaped extension that surrounds the vesicle [91, 92]. The tips of the cup fuse with each other and membrane scission occurs to create an endosome (Figure 2B). Several lines of evidence support the idea that exosomes are internalized via phagocytosis. Exosomes colocalized with phagocytic markers, such as lysosomal-associated membrane protein 1 (LAMP-1), but not with transferrin, a clathrin-coated pit marker [44]. In addition, several proteins known to be involved in phagocytosis, such as T cell immunoglobulin and mucin domain containing (TIM4), are necessary for exosome uptake [44, 47, 57, 91]. More specifically, TIM4 is a phagocytic receptor that recognizes phosphatidylserine (PS), a phospholipid that is abundant in EVs. Blocking TIM4 in macrophages reduced the uptake of leukemia cell-derived exosomes, which suggests a possible role for PS in exosome internalization [8, 9, 13, 45, 47, 62, 93].
Macropinocytosis also contributes to EV endocytosis [83, 94-96] and may be used in conjunction with other endocytic mechanisms [42, 44]. Macropinocytosis occurs when cytoskeletal rearrangements cause membrane ruffles, which extend into lamellipodia that fold back on themselves and fuse with the plasma membrane [95] (Figure 2B). Inhibiting macropinocytosis by disrupting the functions of dynamin, Na+/H+ exchange, or Rac1 function reduced exosome uptake [42], whereas activating signaling pathways known to enhance macropinocytosis (e.g., via epidermal growth factor or stromal cell-derived factor 1α) promoted exosome uptake [96], suggesting that exosomes are internalized via macropinocytosis.
3.1.2 Lipid Rafts and Caveolins
EVs may also be endocytosed at distinct regions along the plasma membrane known as lipid rafts [86], which are required for the formation of caveolae (small cave-like invaginations in the plasma membrane) [85, 89] (Figure 2B). Lipid rafts appear to be involved in exosome internalization because exosomes colocalized with lipid raft markers on recipient cells [83, 97], and disrupting lipid rafts by depleting cells of cholesterol reduced EV uptake by cells [67, 83, 97]. Lipid raft-mediated endocytosis of EVs has been observed, both in the presence and absence of caveolins, which are proteins that aid in caveolae formation [67, 83, 94]. Caveolin-1 knockdowns in recipient cells significantly reduced exosome uptake, suggesting that caveolins mediate exosome internalization [94]. However, in other systems, exosomes did not colocalize with caveolin-1 in recipient cells, even though lipid raft disruption reduced exosome internalization [67]. These results suggest that exosome endocytosis may occur via lipid raft-dependent pathways that sometimes require caveolin proteins.
Collectively, these studies demonstrate that the mechanisms of EV uptake are extremely varied and, depending on the cell type, rely on a variety of internalization mechanisms [44, 45, 67, 83, 94]. Regardless of their route of uptake, EVs are capable of stimulating signaling pathways that cause changes in the recipient cells, either by activating surface receptors or through the delivery of cargo. Once inside the cell, cargo, such as transcripts, signaling proteins, and transcription factors, can elicit a variety of functional effects, which in the context of cancer, promote cancer progression.
4. Conclusions and Future Directions
In this review, we highlighted some of the mechanisms that regulate EV targeting and uptake. In many cases, the specific proteins, proteoglycans, and lipids that are responsible for mediating these events have not yet been identified. It has been demonstrated that integrins help target EVs to certain organs in vivo and that tetraspanins, proteoglycans (e.g., HSPGs), and lectins may also be involved in cell recognition and targeting. Furthermore, little is known about the differences in the mechanisms responsible for exosome versus MV targeting and uptake. To date, most of the work regarding EV uptake has been performed using exosomes, and further study is needed to determine if targeting and internalization mechanisms differ between the two EV classes, especially due to emerging evidence that diverse classes of EVs exist and may differ in function [20].
A better understanding of how EVs are targeted to recipient cells and the mechanisms of uptake is critical to understand EV function in multiple areas of biology and for the development of therapeutics that target EVs, or treatments that take advantage of EVs as vehicles for delivering therapeutic reagents [98, 99]. It has been shown that the intrinsic distribution patterns of EVs in vivo can be altered so that EVs target certain cells, which raises exciting therapeutic possibilities. For instance, exosomes engineered to express the GE11 peptide (an epidermal growth factor receptor [EGFR] ligand) on their surfaces preferentially bound to EGFR-expressing breast cancer cells and successfully delivered microRNA to recipient cells [98]. This selective exosome targeting demonstrates that EV-cell interactions are specific and EVs can be targeted to certain recipient cells for use in therapeutic applications.
Although this review primarily focused on EV targeting, docking, and uptake in cancer, these processes have broad implications in other fields of biology. For example, EV targeting, docking, and uptake during development must be tightly controlled for EVs to properly function. MVs released by ESCs promote the migration of trophoblasts and enhance blastocyst implantation in the uterus during development [21]. In this case, the selective uptake of EVs may be critical for the EVs to function properly and implantation to occur without deleterious side effects. If targeting and uptake are not specific, the EVs released by ESCs may lead to aberrant growth, improper development, and miscarriage. It is not known if ESC-derived EVs are selectively internalized by certain cell types. However, ESC-derived MVs localized to the trophectoderm (the outer layer of trophoblasts) in the blastocyst and laminin- and fibronectin-integrin interactions appear to be important for the docking of ESC-derived MVs to trophoblasts [21]. In other systems, it has been demonstrated that integrins are important in determining the selective docking and uptake of EVs by recipient cells [12, 57, 59, 61], suggesting that integrins may play a role in the specificity of ESC MV-recipient cell interactions during development. Thus, the recruitment, docking, and uptake of EVs are fundamental to their functions and will require a better understanding in order to take advantage of their significant potential as therapeutic targets and diagnostic markers.
Acknowledgments
We would like to thank Cindy Westmiller for helping us prepare this manuscript.
Funding: Some of the research reported in this publication was supported by the National Institutes of Health under grants GM040654, CA201402, and F32CA196321. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 2.Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10(12):1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lai CP, Mardini O, Ericsson M, Prabhakar S, Maguire CA, Chen JW, Tannous BA, Breakefield XO. Dynamic Biodistribution of Extracellular Vesicles in Vivo Using a Multimodal Imaging Reporter. ACS Nano. 2014;8(1):483–494. doi: 10.1021/nn404945r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montecalvo A, Larregina AT, Shufesky WJ, Stolz DB, Sullivan MLG, Karlsson JM, Baty CJ, Gibson GA, Erdos G, Wang Z, Milosevic J, Tkacheva OA, Divito SJ, Jordan R, Lyons-Weiler J, Watkins SC, Morelli AE. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood. 2012;119(3):756–766. doi: 10.1182/blood-2011-02-338004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fabbri M, Paone A, Calore F, Galli R, Gaudio E, Santhanam R, Lovat F, Fadda P, Mao C, Nuovo GJ, Zanesi N, Crawford M, Ozer GH, Wernicke D, Alder H, Caligiuri MA, Nana-Sinkam P, Perrotti D, Croce CM. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci USA. 2012;109(31):E2110–E2116. doi: 10.1073/pnas.1209414109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qu L, Ding J, Chen C, Wu ZJ, Liu B, Gao Y, Chen W, Liu F, Sun W, Li XF, Wang X, Wang Y, Xu ZY, Gao L, Yang Q, Xu B, Li YM, Fang ZY, Xu ZP, Bao Y, Wu DS, Miao X, Sun HY, Sun YH, Wang HY, Wang LH. Exosome-Transmitted lncARSR Promotes Sunitinib Resistance in Renal Cancer by Acting as a Competing Endogenous RNA. Cancer Cell. 2016;29(5):653–668. doi: 10.1016/j.ccell.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Balaj L, Lessard R, Dai L, Cho YJ, Pomeroy SL, Breakefield XO, Skog J. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat Commun. 2011;2:180. doi: 10.1038/ncomms1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, Rak J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10(5):619–624. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- 9.Al-Nedawi K, Meehan B, Kerbel RS, Allison AC, Rak J. Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proc Natl Acad Sci USA. 2009;106(10):3794–3799. doi: 10.1073/pnas.0804543106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antonyak MA, Li B, Boroughs LK, Johnson JL, Druso JE, Bryant KL, Holowka DA, Cerione RA. Cancer cell-derived microvesicles induce transformation by transferring tissue transglutaminase and fibronectin to recipient cells. Proc Natl Acad Sci USA. 2011;108(12):4852–4857. doi: 10.1073/pnas.1017667108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hawari FI, Rouhani FN, Cui X, Yu ZX, Buckley C, Kaler M, Levine SJ. Release of full-length 55-kDa TNF receptor 1 in exosome-like vesicles: A mechanism for generation of soluble cytokine receptors. Proc Natl Acad Sci USA. 2004;101(5):1297–1302. doi: 10.1073/pnas.0307981100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nolte-'t Hoen ENM, Buschow SI, Anderton SM, Stoorvogel W, Wauben MHM. Activated T cells recruit exosomes secreted by dendritic cells via LFA-1. Blood. 2009;113(9):1977–1981. doi: 10.1182/blood-2008-08-174094. [DOI] [PubMed] [Google Scholar]
- 13.del Conde I, Shrimpton CN, Thiagarajan P, López JA. Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood. 2005;106(5):1604–1611. doi: 10.1182/blood-2004-03-1095. [DOI] [PubMed] [Google Scholar]
- 14.Stoeck A, Keller S, Riedle S, Sanderson Michael P, Runz S, Le Naour F, Gutwein P, Ludwig A, Rubinstein E, Altevogt P. A role for exosomes in the constitutive and stimulus-induced ectodomain cleavage of L1 and CD44. Biochem J. 2006;393(3):609–618. doi: 10.1042/BJ20051013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rana S, Malinowska K, Zöller M. Exosomal Tumor MicroRNA Modulates Premetastatic Organ Cells. Neoplasia. 2013;15(3):281–295. doi: 10.1593/neo.122010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colombo M, Moita C, van Niel G, Kowal J, Vigneron J, Benaroch P, Manel N, Moita LF, Théry C, Raposo G. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci. 2013;126(24):5553–5565. doi: 10.1242/jcs.128868. [DOI] [PubMed] [Google Scholar]
- 17.Hurley JH. ESCRTs are everywhere. EMBO J. 2015;34(19):2398–2407. doi: 10.15252/embj.201592484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muralidharan-Chari V, Clancy J, Plou C, Romao M, Chavrier P, Raposo G, D'Souza-Schorey C. ARF6-Regulated Shedding of Tumor Cell-Derived Plasma Membrane Microvesicles. Curr Biol. 2009;19(22):1875–1885. doi: 10.1016/j.cub.2009.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li B, Antonyak MA, Zhang J, Cerione RA. RhoA triggers a specific signaling pathway that generates transforming microvesicles in cancer cells. Oncogene. 2012;31(45):4740–4749. doi: 10.1038/onc.2011.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanada M, Bachmann MH, Hardy JW, Frimannson DO, Bronsart L, Wang A, Sylvester MD, Schmidt TL, Kaspar RL, Butte MJ, Matin AC, Contag CH. Differential fates of biomolecules delivered to target cells via extracellular vesicles. Proc Natl Acad Sci USA. 2015;112(12):E1433–E1442. doi: 10.1073/pnas.1418401112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desrochers LM, Bordeleau F, Reinhart-King CA, Cerione RA, Antonyak MA. Microvesicles provide a mechanism for intercellular communication by embryonic stem cells during embryo implantation. Nat Commun. 2016;7:11958. doi: 10.1038/ncomms11958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quesenberry P, Aliotta J, Deregibus M, Camussi G. Role of extracellular RNA-carrying vesicles in cell differentiation and reprogramming. Stem Cell Res Ther. 2015;6:153. doi: 10.1186/s13287-015-0150-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dignat-George F, Boulanger CM. The Many Faces of Endothelial Microparticles. Arterioscler Thromb Vasc Biol. 2011;31:27–33. doi: 10.1161/ATVBAHA.110.218123. [DOI] [PubMed] [Google Scholar]
- 24.Puddu P, Puddu GM, Cravero E, Muscari S, Muscari A. The involvement of circulating microparticles in inflammation, coagulation and cardiovascular diseases. The Canadian Journal of Cardiology. 2008;26(4):e140–e145. doi: 10.1016/s0828-282x(10)70371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alenquer M, Amorim M. Exosome Biogenesis, Regulation, and Function in Viral Infection. Viruses. 2015;7(9):2862. doi: 10.3390/v7092862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meckes DG, Raab-Traub N. Microvesicles and Viral Infection. J Virol. 2011;85(24):12844–12854. doi: 10.1128/JVI.05853-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Toro J, Herschlik L, Waldner C, Mongini C. Emerging Roles of Exosomes in Normal and Pathological Conditions: New Insights for Diagnosis and Therapeutic Applications. Front Immunol. 2015;6:203. doi: 10.3389/fimmu.2015.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aguzzi A, Lakkaraju AKK. Cell Biology of Prions and Prionoids: A Status Report. Trends Cell Biol. 2015;26(1):40–51. doi: 10.1016/j.tcb.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 29.Minciacchi VR, Freeman MR, Di Vizio D. Extracellular Vesicles in Cancer: Exosomes, Microvesicles and the Emerging Role of Large Oncosomes. Sem Cell Dev Biol. 2015;40:41–51. doi: 10.1016/j.semcdb.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ciardiello C, Cavallini L, Spinelli C, Yang J, Reis-Sobreiro M, de Candia P, Minciacchi V, Di Vizio D. Focus on Extracellular Vesicles: New Frontiers of Cell-to-Cell Communication in Cancer. Int J Mol Sci. 2016;17(2):175. doi: 10.3390/ijms17020175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park JE, Tan HS, Datta A, Lai RC, Zhang H, Meng W, Lim SK, Sze SK. Hypoxic Tumor Cell Modulates Its Microenvironment to Enhance Angiogenic and Metastatic Potential by Secretion of Proteins and Exosomes. Mol Cell Proteomics. 2009;9(6):1085–1099. doi: 10.1074/mcp.M900381-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hood JL, San RS, Wickline SA. Exosomes Released by Melanoma Cells Prepare Sentinel Lymph Nodes for Tumor Metastasis. Cancer Res. 2011;71(11):3792–3801. doi: 10.1158/0008-5472.CAN-10-4455. [DOI] [PubMed] [Google Scholar]
- 33.Benameur T, Tual-Chalot S, Andriantsitohaina R, Martínez MC. PPARα Is Essential for Microparticle-Induced Differentiation of Mouse Bone Marrow-Derived Endothelial Progenitor Cells and Angiogenesis. PLoS ONE. 2010;5(8):e12392. doi: 10.1371/journal.pone.0012392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCready J, Sims JD, Chan D, Jay DG. Secretion of extracellular hsp90α via exosomes increases cancer cell motility: a role for plasminogen activation. BMC Cancer. 2010;10:294. doi: 10.1186/1471-2407-10-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luga V, Zhang L, Viloria-Petit Alicia M, Ogunjimi Abiodun A, Inanlou Mohammad R, Chiu E, Buchanan M, Hosein Abdel N, Basik M, Wrana Jeffrey L. Exosomes Mediate Stromal Mobilization of Autocrine Wnt-PCP Signaling in Breast Cancer Cell Migration. Cell. 2012;151(7):1542–1556. doi: 10.1016/j.cell.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 36.Sung BH, Ketova T, Hoshino D, Zijlstra A, Weaver AM. Directional cell movement through tissues is controlled by exosome secretion. Nat Commun. 2015;6:7168. doi: 10.1038/ncomms8164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bobrie A, Krumeich S, Reyal F, Recchi C, Moita LF, Seabra MC, Ostrowski M, Théry C. Rab27a Supports Exosome-Dependent and -Independent Mechanisms That Modify the Tumor Microenvironment and Can Promote Tumor Progression. Cancer Res. 2012;72(19):4920–4930. doi: 10.1158/0008-5472.CAN-12-0925. [DOI] [PubMed] [Google Scholar]
- 38.Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, Garcia-Santos G, Ghajar CM, Nitadori-Hoshino A, Hoffman C, Badal K, Garcia BA, Callahan MK, Yuan J, Martins VR, Skog J, Kaplan RN, Brady MS, Wolchok JD, Chapman PB, Kang Y, Bromberg J, Lyden D. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18(6):883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee HM, Choi EJ, Kim JH, Kim TD, Kim YK, Kang C, Gho YS. A membranous form of ICAM-1 on exosomes efficiently blocks leukocyte adhesion to activated endothelial cells. Biochem Biophys Res Commun. 2010;397(2):251–256. doi: 10.1016/j.bbrc.2010.05.094. [DOI] [PubMed] [Google Scholar]
- 40.Aung T, Chapuy B, Vogel D, Wenzel D, Oppermann M, Lahmann M, Weinhage T, Menck K, Hupfeld T, Koch R, Trümper L, Wulf GG. Exosomal evasion of humoral immunotherapy in aggressive B-cell lymphoma modulated by ATP-binding cassette transporter A3. Proc Natl Acad Sci USA. 2011;108(37):15336–15341. doi: 10.1073/pnas.1102855108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mulcahy LA, Pink RC, Carter DRF. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014;3:24641. doi: 10.3402/jev.v3.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fitzner D, Schnaars M, van Rossum D, Krishnamoorthy G, Dibaj P, Bakhti M, Regen T, Hanisch UK, Simons M. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J Cell Sci. 2011;124(3):447–458. doi: 10.1242/jcs.074088. [DOI] [PubMed] [Google Scholar]
- 43.Lee TH, Chennakrishnaiah S, Meehan B, Montermini L, Garnier D, Asti E, Hou W, Magnus N, Gayden T, Jabado N, Eppert K, Majewska L, Rak J. Barriers to horizontal cell transformation by extracellular vesicles containing oncogenic H- ras. Oncotarget. 2016;7(32):51991–52002. doi: 10.18632/oncotarget.10627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feng D, Zhao WL, Ye YY, Bai XC, Liu RQ, Chang LF, Zhou Q, Sui SF. Cellular Internalization of Exosomes Occurs Through Phagocytosis. Traffic. 2010;11(5):675–687. doi: 10.1111/j.1600-0854.2010.01041.x. [DOI] [PubMed] [Google Scholar]
- 45.Prada I, Amin L, Furlan R, Legname G, Verderio C, Cojoc D. A New Approach to Follow a Single Extracellular Vesicle-Cell Interaction using Optical Tweezers. Biotechniques. 2016;60(1):35–41. doi: 10.2144/000114371. [DOI] [PubMed] [Google Scholar]
- 46.Wiklander OPB, Nordin JZ, O'Loughlin A, Gustafsson Y, Corso G, Mäger I, Vader P, Lee Y, Sork H, Seow Y, Heldring N, Alvarez-Erviti L, Smith CIE, Le Blanc K, Macchiarini P, Jungebluth P, Wood MJA, Andaloussi SEL. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles. 2015;4:26316. doi: 10.3402/jev.v4.26316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morelli AE, Larregina AT, Shufesky WJ, Sullivan MLG, Stolz DB, Papworth GD, Zahorchak AF, Logar AJ, Wang ZL, Watkins SC, Falo LD, Thomson AW. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood. 2004;104(10):3257–3266. doi: 10.1182/blood-2004-03-0824. [DOI] [PubMed] [Google Scholar]
- 48.Grange C, Tapparo M, Bruno S, Chatterjee D, Quesenberry PJ, Tetta C, Camussi G. Biodistribution of mesenchymal stem cell-derived extracellular vesicles in a model of acute kidney injury monitored by optical imaging. Int J Mol Med. 2014;33(5):1055–1063. doi: 10.3892/ijmm.2014.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, Morando L, Busca A, Falda M, Bussolati B, Tetta C, Camussi G. Mesenchymal Stem Cell-Derived Microvesicles Protect Against Acute Tubular Injury. J Am Soc Nephrol. 2009;20(5):1053–1067. doi: 10.1681/ASN.2008070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hemler ME. Tetraspanin functions and associated microdomains. Nat Rev Mol Cell Biol. 2005;6(10):801–811. doi: 10.1038/nrm1736. [DOI] [PubMed] [Google Scholar]
- 51.Heijnen HFG, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated Platelets Release Two Types of Membrane Vesicles: Microvesicles by Surface Shedding and Exosomes Derived From Exocytosis of Multivesicular Bodies and α-Granules. Blood. 1999;94(11):3791–3799. [PubMed] [Google Scholar]
- 52.Escola JM, Kleijmeer MJ, Stoorvogel W, Griffith JM, Yoshie O, Geuze HJ. Selective Enrichment of Tetraspan Proteins on the Internal Vesicles of Multivesicular Endosomes and on Exosomes Secreted by Human B-lymphocytes. J Biol Chem. 1998;273(32):20121–20127. doi: 10.1074/jbc.273.32.20121. [DOI] [PubMed] [Google Scholar]
- 53.Rana S, Yue S, Stadel D, Zöller M. Toward tailored exosomes: The exosomal tetraspanin web contributes to target cell selection. Int J Biochem Cell Biol. 2012;44(9):1574–1584. doi: 10.1016/j.biocel.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 54.Rana S, Claas C, Kretz CC, Nazarenko I, Zoeller M. Activation-induced internalization differs for the tetraspanins CD9 and Tspan8: Impact on tumor cell motility. Int J Biochem Cell Biol. 2010;43(1):106–119. doi: 10.1016/j.biocel.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 55.Nazarenko I, Rana S, Baumann A, McAlear J, Hellwig A, Trendelenburg M, Lochnit G, Preissner KT, Zöller M. Cell Surface Tetraspanin Tspan8 Contributes to Molecular Pathways of Exosome-Induced Endothelial Cell Activation. Cancer Res. 2010;70(4):1668–1678. doi: 10.1158/0008-5472.CAN-09-2470. [DOI] [PubMed] [Google Scholar]
- 56.Campbell ID, Humphries MJ. Integrin Structure, Activation, and Interactions. Cold Spring Harb Perspect Biol. 2011;3:a004994. doi: 10.1101/cshperspect.a004994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, Singh S, Williams C, Soplop N, Uryu K, Pharmer L, King T, Bojmar L, Davies AE, Ararso Y, Zhang T, Zhang H, Hernandez J, Weiss JM, Dumont-Cole VD, Kramer K, Wexler LH, Narendran A, Schwartz GK, Healey JH, Sandstrom P, Labori KJ, Kure EH, Grandgenett PM, Hollingsworth MA, de Sousa M, Kaur S, Jain M, Mallya K, Batra SK, Jarnagin WR, Brady MS, Fodstad O, Muller V, Pantel K, Minn AJ, Bissell MJ, Garcia BA, Kang Y, Rajasekhar VK, Ghajar CM, Matei I, Peinado H, Bromberg J, Lyden D. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sabatier F, Roux V, Anfosso F, Camoin L, Sampol J, Dignat-George F. Interaction of endothelial microparticles with monocytic cells in vitro induces tissue factor-dependent procoagulant activity. Blood. 2002;99(11):3962–3970. doi: 10.1182/blood.v99.11.3962. [DOI] [PubMed] [Google Scholar]
- 59.Segura E, Guérin C, Hogg N, Amigorena S, Théry C. CD8+ Dendritic Cells Use LFA-1 to Capture MHC-Peptide Complexes from Exosomes In Vivo. J Immunol. 2007;179(3):1489–1496. doi: 10.4049/jimmunol.179.3.1489. [DOI] [PubMed] [Google Scholar]
- 60.Gahmberg CG. Leukocyte adhesion: CD11/CD18 integrins and intercellular adhesion molecules. Curr Opin Cell Biol. 1997;9(5):643–650. doi: 10.1016/s0955-0674(97)80117-2. [DOI] [PubMed] [Google Scholar]
- 61.Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, Xiang J, Zhang T, Theilen TM, Garcia-Santos G, Williams C, Ararso Y, Huang Y, Rodrigues G, Shen TL, Labori KJ, Lothe IMB, Kure EH, Hernandez J, Doussot A, Ebbesen SH, Grandgenett PM, Hollingsworth MA, Jain M, Mallya K, Batra SK, Jarnagin WR, Schwartz RE, Matei I, Peinado H, Stanger BZ, Bromberg J, Lyden D. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. 2015;17:816–826. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Terrisse AD, Puech N, Allart S, Gourdy P, Xuereb JM, Payrastre B, SiÉ P. Internalization of microparticles by endothelial cells promotes platelet/endothelial cell interaction under flow. J Thromb Haemost. 2010;8(12):2810–2819. doi: 10.1111/j.1538-7836.2010.04088.x. [DOI] [PubMed] [Google Scholar]
- 63.Fonsato V, Collino F, Herrera MB, Cavallari C, Deregibus MC, Cisterna B, Bruno S, Romagnoli R, Salizzoni M, Tetta C, Camussi G. Human Liver Stem Cell-Derived Microvesicles Inhibit Hepatoma Growth in SCID Mice by Delivering Antitumor MicroRNAs. Stem Cells. 2012;30(9):1985–1998. doi: 10.1002/stem.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Paget S. The Distribution of Secondary Growths in Cancer of the Breast. Lancet. 1889;133(3421):571–573. [PubMed] [Google Scholar]
- 65.Ghazarian H, Idoni B, Oppenheimer SB. A glycobiology review: carbohydrates, lectins, and implications in cancer therapeutics. Acta Histochem. 2011;113(3):236–247. doi: 10.1016/j.acthis.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Echevarria J, Royo F, Pazos R, Salazar L, Falcon-Perez JM, Reichardt NC. Microarray-Based Identification of Lectins for the Purification of Human Urinary Extracellular Vesicles Directly from Urine Samples. Chembiochem. 2014;15(11):1621–1626. doi: 10.1002/cbic.201402058. [DOI] [PubMed] [Google Scholar]
- 67.Escrevente C, Keller S, Altevogt P, Costa J. Interaction and uptake of exosomes by ovarian cancer cells. BMC Cancer. 2011;11:108. doi: 10.1186/1471-2407-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Balaj L, Atai NA, Chen W, Mu D, Tannous BA, Breakefield XO, Skog J, Maguire CA. Heparin affinity purification of extracellular vesicles. Sci Rep. 2014;5 doi: 10.1038/srep10266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Christianson HC, Svensson KJ, van Kuppevelt TH, Li JP, Belting M. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc Natl Acad Sci USA. 2013;110(43):17380–17385. doi: 10.1073/pnas.1304266110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fei F, Joo EJ, Tarighat SS, Schiffer I, Paz H, Fabbri M, Abdel-Azim H, Groffen J, Heisterkamp N. B-cell precursor acute lymphoblastic leukemia and stromal cells communicate through Galectin-3. Oncotarget. 2015;6(13):11378–11394. doi: 10.18632/oncotarget.3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gomes J, Gomes-Alves P, Carvalho SB, Peixoto C, Alves PM, Altevogt P, Costa J. Extracellular Vesicles from Ovarian Carcinoma Cells Display Specific Glycosignatures. Biomolecules. 2015;5(3):1741–1761. doi: 10.3390/biom5031741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barrès C, Blanc L, Bette-Bobillo P, André S, Mamoun R, Gabius HJ, Vidal M. Galectin-5 is bound onto the surface of rat reticulocyte exosomes and modulates vesicle uptake by macrophages. Blood. 2010;115(3):696–705. doi: 10.1182/blood-2009-07-231449. [DOI] [PubMed] [Google Scholar]
- 73.Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, LeBleu VS, Mittendorf EA, Weitz J, Rahbari N, Reissfelder C, Pilarsky C, Fraga MF, Piwnica-Worms D, Kalluri R. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523:177–182. doi: 10.1038/nature14581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Atai N, Balaj L, van Veen H, Breakefield X, Jarzyna P, Van Noorden CF, Skog J, Maguire C. Heparin blocks transfer of extracellular vesicles between donor and recipient cells. J Neurooncol. 2013;115(3):343–351. doi: 10.1007/s11060-013-1235-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Franzen CA, Simms PE, Van Huis AF, Foreman KE, Kuo PC, Gupta GN. Characterization of Uptake and Internalization of Exosomes by Bladder Cancer Cells. Biomed Res Int. 2014;2014:619829. doi: 10.1155/2014/619829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Günthert U, Hofmann M, Rudy W, Reber S, Zöller M, Hauβmann I, Matzku S, Wenzel A, Ponta H, Herrlich P. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell. 1991;65(1):13–24. doi: 10.1016/0092-8674(91)90403-l. [DOI] [PubMed] [Google Scholar]
- 77.Saunderson SC, Dunn AC, Crocker PR, McLellan AD. CD169 mediates the capture of exosomes in spleen and lymph node. Blood. 2014;123(2):208–216. doi: 10.1182/blood-2013-03-489732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cossetti C, Iraci N, Mercer Tim R, Leonardi T, Alpi E, Drago D, Alfaro-Cervello C, Saini Harpreet K, Davis Matthew P, Schaeffer J, Vega B, Stefanini M, Zhao C, Muller W, Garcia-Verdugo Jose M, Mathivanan S, Bachi A, Enright Anton J, Mattick John S, Pluchino S. Extracellular Vesicles from Neural Stem Cells Transfer IFN-γ via Ifngr1 to Activate Stat1 Signaling in Target Cells. Mol Cell. 2014;56(2):193–204. doi: 10.1016/j.molcel.2014.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gabrielli M, Battista N, Riganti L, Prada I, Antonucci F, Cantone L, Matteoli M, Maccarrone M, Verderio C. Active endocannabinoids are secreted on extracellular membrane vesicles. EMBO Rep. 2015;16(2):213–220. doi: 10.15252/embr.201439668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Segura E, Amigorena S, Théry C. Mature dendritic cells secrete exosomes with strong ability to induce antigen-specific effector immune responses. Blood Cells Mol Dis. 2005;35(2):89–93. doi: 10.1016/j.bcmd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 81.Théry C, Duban L, Segura E, Veron P, Lantz O, Amigorena S. Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes. Nat Immunol. 2002;3(12):1156–1162. doi: 10.1038/ni854. [DOI] [PubMed] [Google Scholar]
- 82.Parolini I, Federici C, Raggi C, Lugini L, Palleschi S, De Milito A, Coscia C, Iessi E, Logozzi M, Molinari A, Colone M, Tatti M, Sargiacomo M, Fais S. Microenvironmental pH Is a Key Factor for Exosome Traffic in Tumor Cells. J Biol Chem. 2009;284(49):34211–34222. doi: 10.1074/jbc.M109.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Svensson KJ, Christianson HC, Wittrup A, Bourseau-Guilmain E, Lindqvist E, Svensson LM, Mörgelin M, Belting M. Exosome Uptake Depends on ERK1/2-Heat Shock Protein 27 Signaling and Lipid Raft-mediated Endocytosis Negatively Regulated by Caveolin-1. J Biol Chem. 2013;288(24):17713–17724. doi: 10.1074/jbc.M112.445403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tian T, Zhu YL, Hu FH, Wang YY, Huang NP, Xiao ZD. Dynamics of exosome internalization and trafficking. J Cell Physiol. 2013;228(7):1487–1495. doi: 10.1002/jcp.24304. [DOI] [PubMed] [Google Scholar]
- 85.Doherty GJ, McMahon HT. Mechanisms of Endocytosis. Annu Rev Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 86.Frühbeis C, Fröhlich D, Kuo WP, Amphornrat J, Thilemann S, Saab AS, Kirchhoff F, Möbius W, Goebbels S, Nave KA, Schneider A, Simons M, Klugmann M, Trotter J, Krämer-Albers EM. Neurotransmitter-Triggered Transfer of Exosomes Mediates Oligodendrocyte-Neuron Communication. PLoS Biol. 2013;11(7):e1001604. doi: 10.1371/journal.pbio.1001604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Heusermann W, Hean J, Trojer D, Steib E, von Bueren S, Graff-Meyer A, Genoud C, Martin K, Pizzato N, Voshol J, Morrissey DV, Andaloussi SEL, Wood MJ, Meisner-Kober NC. Exosomes surf on filopodia to enter cells at endocytic hot spots, traffic within endosomes, and are targeted to the ER. J Cell Biol. 2016;213(2):173–184. doi: 10.1083/jcb.201506084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Obregon C, Rothen-Rutishauser B, Gerber P, Gehr P, Nicod LP. Active Uptake of Dendritic Cell-Derived Exovesicles by Epithelial Cells Induces the Release of Inflammatory Mediators through a TNF-α-Mediated Pathway. Am J Pathol. 2009;175(2):696–705. doi: 10.2353/ajpath.2009.080716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mayor S, Pagano RE. Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol. 2007;8(8):603–612. doi: 10.1038/nrm2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2011;12(8):517–533. doi: 10.1038/nrm3151. [DOI] [PubMed] [Google Scholar]
- 91.Freeman SA, Grinstein S. Phagocytosis: receptors, signal integration, and the cytoskeleton. Immunol Rev. 2014;262(1):193–215. doi: 10.1111/imr.12212. [DOI] [PubMed] [Google Scholar]
- 92.Flannagan RS, Jaumouillé V, Grinstein S. The Cell Biology of Phagocytosis. Annu Rev Pathol: Mech Dis. 2012;7:61–98. doi: 10.1146/annurev-pathol-011811-132445. [DOI] [PubMed] [Google Scholar]
- 93.Wei X, Liu C, Wang H, Wang L, Xiao F, Guo Z, Zhang H. Surface Phosphatidylserine Is Responsible for the Internalization on Microvesicles Derived from Hypoxia-Induced Human Bone Marrow Mesenchymal Stem Cells into Human Endothelial Cells. PLoS ONE. 2016;11(1):e0147360. doi: 10.1371/journal.pone.0147360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nanbo A, Kawanishi E, Yoshida R, Yoshiyama H. Exosomes Derived from Epstein-Barr Virus-Infected Cells Are Internalized via Caveola-Dependent Endocytosis and Promote Phenotypic Modulation in Target Cells. J Virol. 2013;87(18):10334–10347. doi: 10.1128/JVI.01310-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lim JP, Gleeson PA. Macropinocytosis: an endocytic pathway for internalising large gulps. Immunol Cell Biol. 2011;89(8):836–843. doi: 10.1038/icb.2011.20. [DOI] [PubMed] [Google Scholar]
- 96.Nakase I, Kobayashi NB, Takatani-Nakase T, Yoshida T. Active macropinocytosis induction by stimulation of epidermal growth factor receptor and oncogenic Ras expression potentiates cellular uptake efficacy of exosomes. Sci Rep. 2015;5:10300. doi: 10.1038/srep10300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Koumangoye RB, Sakwe AM, Goodwin JS, Patel T, Ochieng J. Detachment of Breast Tumor Cells Induces Rapid Secretion of Exosomes Which Subsequently Mediate Cellular Adhesion and Spreading. PLoS ONE. 2011;6(9):e24234. doi: 10.1371/journal.pone.0024234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ohno Si, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N, Fujita K, Mizutani T, Ohgi T, Ochiya T, Gotoh N, Kuroda M. Systemically Injected Exosomes Targeted to EGFR Deliver Antitumor MicroRNA to Breast Cancer Cells. Mol Ther. 2013;21(1):185–191. doi: 10.1038/mt.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hall J, Prabhakar S, Balaj L, Lai CP, Cerione RA, Breakefield XO. Delivery of Therapeutic Proteins via Extracellular Vesicles: Review and Potential Treatments for Parkinson's Disease, Glioma, and Schwannoma. Cell Mol Neurobiol. 2016;36(3):417–427. doi: 10.1007/s10571-015-0309-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


