Abstract
Typhoid toxin is a unique A2B5 exotoxin and an important virulence factor for Salmonella Typhi, the cause of typhoid fever. In the decade since its initial discovery, great strides have been made in deciphering the unusual biological program of this toxin, which is fundamentally different from related toxins in many ways. Purified typhoid toxin administered to laboratory animals causes many of the symptoms of typhoid fever, suggesting that typhoid toxin is a central factor in this disease. Further advances in understanding the biology of this toxin will help guide the development of badly needed diagnostics and therapeutic interventions that target this toxin to detect, prevent or treat typhoid fever.
Introduction
Salmonella enterica serovar Typhi (S. Typhi), the cause of typhoid fever, is a human-adapted bacterial pathogen. The virulence of S. Typhi differs significantly from closely related, broad host range serovars of Salmonella enterica, which are a common source of food poisoning. These serovars typically cause short-term infections that remain confined to the gastrointestinal tract in healthy humans. By contrast, S. Typhi causes life-threatening, systemic infections and, for a subset of those who suffer the disease, it causes a life-long chronic infection [1]. Long-term carriers shed high levels of the bacterium asymptomatically and are thought to be crucial for the transmission of typhoid fever since humans are the only known reservoir of S. Typhi. The genetic factors and molecular mechanisms that dictate S. Typhi's host restriction and unique pathogenesis have been an enduring mystery and the subject of considerable investigation. While these issues are undoubtedly multifaceted, accumulating evidence suggests that a recently discovered toxin, dubbed typhoid toxin, is an important factor underlying S. Typhi's unique virulence traits. A decade of research into typhoid toxin has shown that it is distinct from related toxins in nearly every facet of its biology including its composition, the environment in which it is produced, its secretion and its intricate trafficking and delivery pathway. Collectively, these studies distinguish typhoid toxin as a remarkable evolutionary twist on the bacterial exotoxin and a central virulence factor that shapes S. Typhi's distinctive interaction with its human host. Here, we review studies that have shed light on typhoid toxin's unique biological program and its roles in S. Typhi virulence and host restriction.
The merging of two AB-type toxins and their adaptation to Salmonella's intracellular niche
The discovery of typhoid toxin arose from investigations into an ˜5 Kbp S. Typhi genomic islet that contains homologs of components of two distinct bacterial exotoxins: cytolethal distending toxin (CDT) and pertussis toxin [2*,3**]. Both CDT and pertussis toxin are AB-type toxins and are comprised of an active (A) subunit that exerts the toxin's effects and delivery (B) subunits that bind specific receptors on the surface of target host cells to mediate toxin uptake [4-6]. The S. Typhi locus contains homologs of the active subunits of both toxins as well of one of pertussis toxin's delivery subunits, but lacks homologs of the other three pertussis toxin delivery subunits as well as both CDT delivery subunits [2*,3**, 7]. CDT is a genotoxin; it's active subunit, CdtB, is a DNase that causes double stranded breaks in host cell chromosomal DNA, resulting in cell cycle arrest [8]. Initial studies into the S. Typhi cdtB homolog indicated that it is not expressed by S. Typhi grown under standard laboratory growth conditions, however its expression is very strongly induced following host cell invasion when S. Typhi resides within a specialized intracellular compartment known as a Salmonella containing vacuole (SCV) [2*]. Clear signs of CdtB intoxication, including cellular distension and cell cycle arrest, were observed in cultured cells infected with S. Typhi, which was shown to be dependent on encoding a functional cdtB and on S. Typhi internalization [2*]. A subsequent investigation into the neighboring pertussis toxin homologs, dubbed pltA and pltB (pertussis-like toxin A and B), indicated that they have a similar intracellular-specific expression pattern [3**]. Consistent with its homology to pertussis toxin's active subunit, pltA was shown to encode a functional ADP-ribosyltransferase [3**]. Collectively these findings suggested that S. Typhi might encode simpler, but functional, versions of both of these toxins. Surprisingly, however, S. Typhi's CdtB-mediated toxicity was found to strictly require both pltA and pltB [3**]. An assortment of genetic and biochemical evidence has since clearly established that CdtB, PltA and PltB assemble to form a single AB-type holotoxin that has been named typhoid toxin [3**,9**].
AB-type toxins are generally produced and secreted by extracellular bacteria and subsequently gain access to host cells via receptor-mediated uptake processes [10]. The observation that typhoid toxin is exclusively produced by intracellular S. Typhi implied that it could potentially directly intoxicate the cell in which it was produced and thus circumvent the need for cellular uptake. Upon S. Typhi infection of cultured mammalian cells, typhoid toxin can be visualized by fluorescence microscopy as discrete puncta that that radiate from SCVs [3**, 11*]. These puncta have been shown not to be destined for an alternate intracellular location, but instead to represent vesicle carrier intermediates exporting typhoid toxin out of the cell [3**, 11*]. Indeed, neutralizing antibody added exogenously to the culture medium completely prevents CdtB intoxication of S. Typhi infected cells, indicating that toxin produced within a cell cannot intoxicate that cell without first being exported to the extracellular space [3**]. This unique autocrine/paracrine delivery mechanism is a remarkable evolutionary adaptation that enables S. Typhi to deploy an AB-type exotoxin from within its quintessential intracellular niche.
The typhoid toxin locus is invariably present in the genomes of typhoidal strains of S. enterica (including both S. Typhi and a genetically similar serotype S. Paratyphi A, which causes an almost indistinguishable disease), but is absent from those associated with food poisoning such as S. Typhimurium and S. Enteritidis [12]. In addition to typhoidal Salmonellae, the typhoid toxin islet can also be found in other Salmonella lineages not commonly associated with human infection [12,13]. This includes a distinct clade of the enterica subspecies whose ecologies are poorly defined as well as the bongori species and the arizonae and diarizonae supspecies, which are associated with cold-blooded hosts such as reptiles [12,13]. Typhoid toxin's phylogenetic distribution suggests that it has been horizontally acquired in multiple independent events by different branches of the Salmonella genus. Based on the available genomic sequencing data, typhoid toxin is exclusively found in Salmonellae. Together with its unusual composition and delivery mechanism, this suggests that typhoid toxin is highly adapted to Salmonella's distinctive virulence program.
The structure of typhoid toxin reveals a unique A2B5 architecture
The amalgamation of two exotoxins into a single complex raised questions about the evolutionary path and the physical organization that would permit the delivery of two distinct toxin enzymes by a single B subunit. Important insights into these issues were provided by the crystal structure of typhoid toxin, which was solved to 2.4 Å resolution (Figure 1A) [9**]. The structure shows a unique A2B5 architecture for the toxin, in which 5 molecules of PltB assemble with a single molecule of both PltA and CdtB [9**]. The overall structure of the holotoxin forms a pyramid shape with a PltB homopentamer at the base, PltA in the center and CdtB at the apex [9**]. The C-terminal portion of PltA forms an α-helix that inserts into a channel at the center of the PltB pentamer which provides extensive hydrophobic interactions to support a stable PltB-PltA complex [9**]. CdtB does not interact with PltB and makes minimal direct contact with PltA. Instead, CdtB's attachment to the toxin is covalent, dictated by a single disulfide bond between Cys269 of CdtB and Cys214 of PltA [9**]. Mutations that disrupt this disulfide bond prevent the in vitro assembly of the holotoxin, preclude CdtB from trafficking out of the SCV during infection and abolish CdtB-induced toxicity, underscoring its essential role in fastening CdtB to the PltAB complex [9**]. While PltA and its homologs contain two cysteine residues that form an intramolecular disulfide bond, Cys214 of PltA, which enables the disulfide linkage with CdtB, provides PltA with a third cysteine residue not found in any of its close relatives [9**]. Likewise, despite strong sequence conservation within the CdtB family, Cys269 is unique to the typhoid toxin gene [9**]. The emergence of the critical residues that allow the formation of the intermolecular disulfide bond that joins CdtB to the PltAB complex must have been a pivotal step in the evolution of this remarkable toxin.
Figure 1. Atomic structures of typhoid toxin.
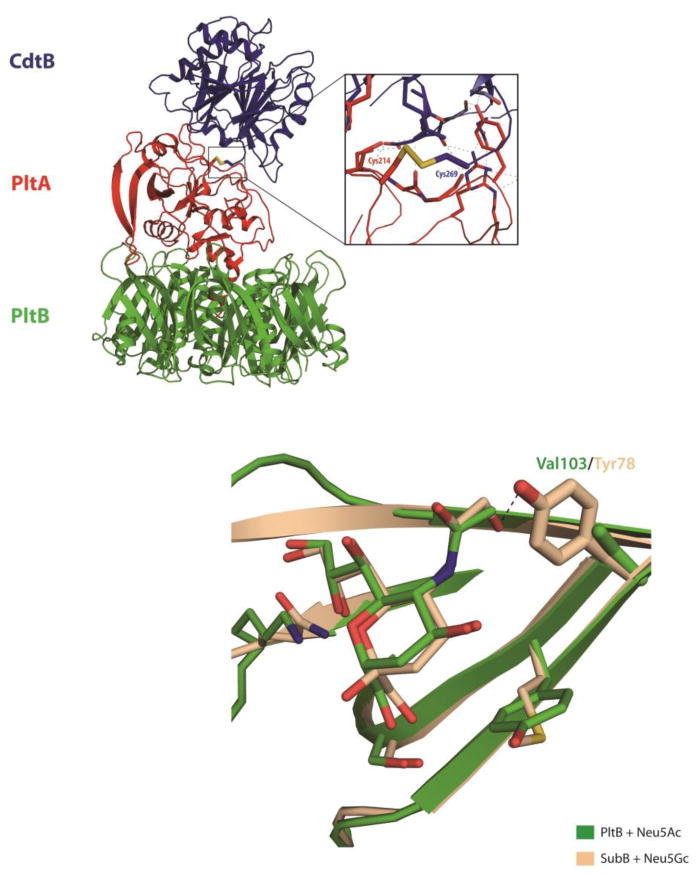
(A) The overall structure of the typhoid holotoxin complex is shown as a ribbon cartoon. CdtB, PltA and PltB are shown in blue, red and green, respectively. The inset shows a detailed view of a critical disulphide bond between PltA Cys214 and CdtB Cys269. (B) Comparison of the sugar binding sites of PltB and SubB bound to Neu5Ac and Neu5Gc, respectively. Critical residues that differ between SubB (Tyr78) and PltB (Val103) are highlighted as sticks. Other interacting amino acids and sugars are shown in lines. PltB and Neu5Ac are shown in green, SubB and Neu5Gc in light brown.
Recognition and intoxication of target cells
Typhoid toxin that has been secreted and exported to an extracellular environment is delivered to target cells in a process that is initiated by interactions between its delivery platform and specific host cell receptors. Using affinity purification coupled with mass spectrometry, podocalyxin 1 and CD45 were identified as key receptors targeted by typhoid toxin for epithelial cells and leukocytes, respectively [9**]. Like the B subunits of all known AB5-type toxins, PltB binds specific glycan moieties that decorate the surface of these molecules rather than the proteins themselves [9**]. The specificity of PltB has been well characterized by measuring the binding of purified typhoid toxin to large collections of glycan molecules arrayed on a solid surface. Consistent with typhoid toxin's broad target cell specificity, it was shown to bind a range of glycans with preferential binding to termini with the consensus sequences Neu5Ac2-3Galβ1-3/β1-4Glc/GlcNAc, found mostly on glycoproteins and Neu5Acα2-8Neu5Ac, a moiety associated with gangliosides [9**].
While typhoid toxin is able to bind a range of different glycans, it exhibits a clear preference for those featuring terminal sialic acids. Human sialoglycans differ from those produced by other mammals due to a loss-of-function mutation in the gene encoding CMP-N-acetylneuraminic acid hydroxylase (CMAH), which occurred in the human lineage sometime after its separation from its closest relatives, the great apes [14]. CMAH mediates the conversion of N-acetylneuramic acid (Neu5Ac) to N-glycolylneuramic acid (Neu5Gc). Therefore, while sialoglycans terminated in Neu5Gc are abundant in most mammals, human sialoglycans are primarily terminated in Neu5Ac. Remarkably, typhoid toxin exhibits a very strong binding preference for glycans terminated in Neu5Ac over otherwise identical glycans terminated in Neu5Gc, despite the fact that these molecules differ by a single oxygen atom [15**]. Cultured human cells fed high levels of Neu5Gc, which is metabolically incorporated into their surface glycans, are largely resistant to typhoid toxin compared to those grown in standard medium or those fed Neu5Ac [15**]. Furthermore, typhoid toxin exhibits much stronger binding to human red blood cells, lymphocytes and tissues, which contain Neu5Ac-decorated glycans, compared to those of chimpanzees, whose sialoglycans are terminated primarily in Neu5Gc [15**]. Typhoid toxin's inability to bind surface glycans that are prevalent in other mammals might be an important factor underlying S. Typhi's inability to cause typhoid fever in other species (see below). It is also noteworthy that the typhoid toxin locus appears to be prevalent in the genomes of Salmonellae that infect reptiles [12,13] and that the limited available data suggest that Neu5Gc-terminated glycans might be rare or absent in this lineage [16].
The crystal structure of PltB bound to Neu5Ac provides substantial insight into the structural bases for this specificity. The glycan binding motif of PltB is structurally very similar to that of SubB, the B subunit of subtilase toxin, which preferentially binds Neu5Gc terminated glycans [15**]. Structural comparison between Neu5Ac bound PltB and Neu5Gc bound SubB shows that these two glycans are located at the very similar positions within the binding pocket and several amino acids conserved between these two proteins are involved in the protein-glycan interaction (Figure 1B) [15**]. However, a crucial distinction in these binding pockets is that a tyrosine residue (tyr78) from SubB that forms direct hydrogen bond with the extra OH group from the Neu5Gc is missing in PltB [15**]. Instead, PltB has a valine residue at this position (val103), which cannot stabilize Neu5Gc's distinctive hydroxyl group, providing an explanation for typhoid toxin's inability to bind Neu5Gc-terminated glycans [15**].
The trafficking processes that follow typhoid toxin-receptor binding and culminate in host cell intoxication have not been studied in detail. However, it is likely that typhoid toxin follows an endocytic and retrograde trafficking pathway that is similar to other AB-type toxins including both CDT and pertussis toxin. This would result in typhoid toxin trafficking to the endoplasmic reticulum (ER), where it is expected that its inter- and intramolecular disulfide bonds would be reduced, the holotoxin would disassemble, and PltA and CdtB would be translocated to the cytoplasm. Like other CdtB homologs, the coding sequence of S. Typhi CdtB contains the required information for its translocation from the target cell cytoplasm to the nucleus [2*]. All phenotypes that have been attributed to typhoid toxin to date stem from the DNase activity of CdtB and the DNA damage and cell cycle arrest it elicits in intoxicated cells [2*,3**, 9**]. However, despite only ˜60% sequence identity to its closest homolog, PltA retains all of the crucial amino acid residues and structural features conserved amongst related ADP-ribosyltransferases and is capable of ADP-ribosylating one or more proteins in host cell lysates [3**]. This strongly suggests that PltA's enzymatic activity is an important aspect of typhoid toxin biology. Identifying the cellular target(s) of PltA and the consequences and biological importance of PltA intoxication will be important to attain a more complete picture of typhoid toxin's function.
Typhoid toxin secretion and export
Typhoid toxin is exclusively produced by intracellular S. Typhi and its autocrine/paracrine intoxication mechanism demands a far more intricate delivery process than is required for typical AB-type toxins (Figure 2). The first step in this process is the secretion of typhoid toxin from the bacterial cell into the lumen of the SCV. The three components of typhoid toxin all possess canonical secretion signals that imply that they are secreted across the inner membrane into the periplasmic space via the Sec machinery [17*]. It is within the oxidative environment of the periplasm that the three components are thought to assemble to form the holotoxin. The process by which typhoid toxin is transported out of the bacterium from periplasmic space is not fully understood, however it is known to strictly require ttsA (for typhoid toxin secretion protein A), a small gene encoded within the typhoid toxin genomic islet [17*]. ttsA encodes a putative N-acetyl-β-D-muramidase that is homologous to phage endolysins, which degrade the cell wall to facilitate phage release from bacterial cells [17-19]. In contrast to these enzymes, TtsA-dependent secretion of typhoid toxin does not coincide with bacterial lysis, indicating that the availability and activity of TtsA must by locally restricted [17*]. Consistent with this hypothesis, the specific determinants of TtsA's secretion function were mapped to its peptidoglycan-binding domain, which differs from that of its phage relatives in subtle but significant ways [17*].
Figure 2. Secretion and export pathways of typhoid toxin.
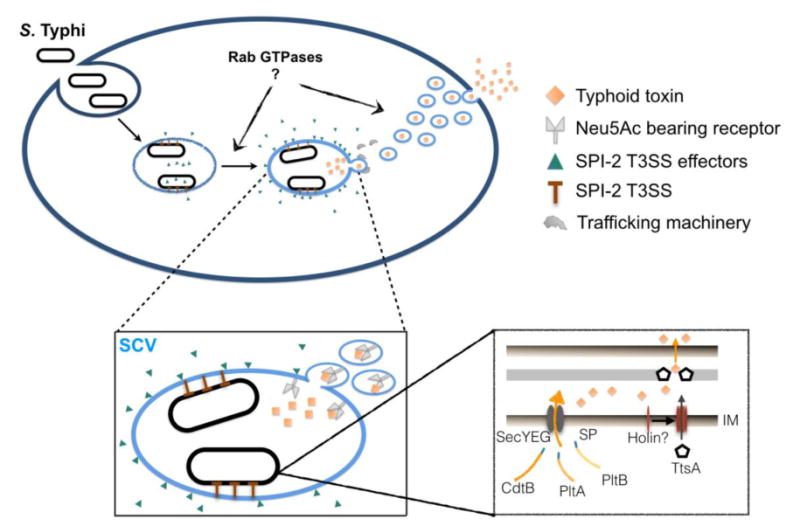
Expression of typhoid toxin is induced shortly after invasion S. Typhi invasion of host cells. Its subunits, CdtB, PltA and PltB, each possess a canonical N-terminal Sec signal peptide indicating that the Sec machinery mediates translocation of the subunits across the cytoplasmic membrane to the periplasm, where holotoxin assembly is thought to occur. Secretion of toxin from the periplasm requires TtsA, an endolysin homolog thought to facilitate toxin secretion through enzymatically modifying the peptidoglycan layer. TtsA lacks a Sec signal sequence and it has been hypothesized that its translocation to the periplasm might be exerted by a yet unidentified holin. Following secretion from the bacterium, the holotoxin is sorted from the lumen of SCV to packaging carrier intermediates, which subsequently export typhoid toxin from the cell. Typhoid toxin packaging requires the specific SCV environment generated through the actions of SPI-2 T3SS-secreted effector proteins, as well as interactions between PltB and specific lumenal Neu5Ac-sialylated glycan packaging receptors. Sec signal peptide (SP), inner membrane (IM), peptidoglycan (PG), outer membrane (OM), Salmonella-containing vacuole (SCV).
Accordingly, a phage endolysin homolog that was unable to complement a ttsA mutation to permit typhoid toxin secretion was converted to a functional replacement by introducing a single mutation to its peptidoglycan-binding domain [17*]. TtsA activity is also thought to be controlled through careful regulation of its access to the periplasm. TtsA does not contain a secretion signal and when highly expressed and provided with free access to the periplasm, it is capable of promoting bacterial lysis. The mechanism by which TtsA gains access to its periplasmic substrate is unknown, however one possibility is that, like its phage homologs, TtsA might traverse the inner membrane through pores formed by small membrane proteins known as holins. Endolysin homologs can also be found adjacent to other bacterial toxins or large extracellular enzymes, suggesting that this secretion mechanism might be widespread amongst Gram negative bacteria. Indeed, following its initial characterization for typhoid toxin, a similar secretion mechanism has been described for a chitinolytic enzyme from Serratia marcescens [20].
Once typhoid toxin reaches the lumen of the SCV, it is sorted into the vesicle carrier intermediates that export the toxin to the extracellular space [11*]. Packaging of typhoid toxin into vesicles carrier intermediates was recently shown to require the interaction of PltB with specific sialylated glycan packaging receptors [11*]. In agreement with PltB's glycan binding specificities, sorting into vesicle carrier intermediates was effectively blocked in cells fed Neu5Gc [11*]. Results using CRISPR/CAS9-generated cell lines deficient in N-glycosylation or ganglioside synthesis indicate that both glycolipids and glycoproteins are relevant to typhoid toxin export, however glycoproteins are likely to be the dominant receptors [11*]. The specific transport machinery responsible for the trafficking of typhoid toxin-containing vesicles to the extracellular environment has not been defined. The formation of typhoid toxin vesicle carriers requires S. Typhi to reach a specialized compartment sculpted through the action of effectors released by its SPI-2 type III secretion system (T3SS). Additionally, two host Rab GTPases, Rab29 and Rab40B, have been shown to be important for toxin export, although this connection is not understood [21]. Collectively, these observations indicate that the maturation of the SCV is crucial in order to engage highly specific receptors and vesicular trafficking machinery that mediate typhoid toxin export. This is particularly interesting in light of recent studies that show that disparities in S. Typhi's complement of T3SS secreted effectors leads to significant differences in the trafficking and composition of the S. Typhi SCV compared to that of broad host range serovars such as S. Typhimurium [21,22].
The role of typhoid toxin in S. Typhi virulence and host restriction
Accumulating evidence indicates that typhoid toxin is a major factor contributing to the unique pathogenesis of S. Typhi and the development of typhoid fever. The strongest evidence for this comes from studies that have examined the effects of administering purified toxin intravenously to C57BL/6 mice. While mice that received a mutant version of typhoid toxin featuring a catalytically inactive CdtB showed no adverse effects, those that received wild-type toxin or toxin featuring a catalytically inactive version of PltA displayed many of the pathognomonic symptoms of typhoid fever and ultimately died [9**]. While toxin administration did not lead to the development of fever, intoxicated mice lost weight, suffered a decrease in the number of circulating white blood cells with a near complete depletion of neutrophils and showed clear signs of lethargy, malaise and stupor - symptoms associated with the acute phase of typhoid fever [9**]. Mice were completely resistant to toxin featuring a mutation within the glycan binding site of PltB that renders it unable to bind and intoxicate target cells, indicating that B subunit mediated toxin targeting is essential for the observed effects [15**]. Mice encode a functional version of CMAH and are thus capable of producing Neu5Gc-terminated sialoglycans that would be resistant to typhoid toxin. However, for the specific mouse line used, most of its tissues contained high levels of Neu5Ac, likely due to low levels of CMAH expression, explaining its susceptibility to typhoid toxin [23]. Consistent with this, transgenic mice engineered to express high levels of CMAH in all tissues were completely resistant to typhoid toxin administration, even at doses 200-fold higher than those that are lethal to wild-type mice [15**].
Elucidating the role of typhoid toxin in S. Typhi virulence has been hampered by S. Typhi's host restriction and the resulting lack of a suitable animal model. The TLR11-/- mouse was also proposed as a model to study S. Typhi infection [24]; however, these observations have been proven to be irreproducible by other laboratories [25,26]. Utilization of humanized mice did not efficiently overcome the host specification of S. Typhi, however, this model did suggest that the typhoid toxin might play a role in the establishment of a persistent infection [27]. A role for typhoid toxin in persistence was also proposed by a recent study in which mice were infected by S. Typhimurium engineered to express the three genes encoding the components of typhoid toxin [28]. However, fundamental issues relating the design of these experiments make the relevance of these observations highly questionable. For example, S. Typhimurium is a poor surrogate for S. Typhi infections. Furthermore, the S. Typhimurium strain used in this study did not encode ttsA, which is essential for typhoid toxin secretion, indicating that typhoid toxin cannot have been secreted and exported in its normal fashion by this strain [17*]. Further development of animal models will be crucial to define typhoid toxin's role in S. Typhi pathogenesis.
S. Typhi is an exclusively human pathogen, which is most likely the net effect of a number of contributing factors. Like many other host-adapted bacterial pathogens, the S. Typhi genome has undergone significant degradation leading to an unusually high number of pseduogenes; this most likely contributes to its host restriction [29]. There is also evidence, however, that typhoid toxin could also be a significant factor in S. Typhi's inability to cause typhoid fever in non-human hosts. This is suggested by typhoid toxin's ability to cause many of the symptoms of typhoid fever in laboratory animals, coupled with its inability to elicit these effects in animals producing high levels of the Neu5Gc-terminated sialoglycans produced by most mammals. Indeed, while S. Typhi is unable to establish a productive infection in most animals, it can efficiently infect chimpanzees [30]. Consistent with typhoid toxin's inability to bind chimpanzee cells and tissues [15**], chimpanzees experimentally infected with S. Typhi exhibited a mild disease that lacked many of the same typhoid fever symptoms that typhoid toxin elicits in laboratory mice [30]. It is not known at what point in S. Typhi's evolutionary history it acquired the typhoid toxin islet, nor is it clear what specific role typhoid toxin plays in S. Typhi pathogenesis. It is tempting to speculate, however, that the acquisition of typhoid toxin might have been a relatively early event in S. Typhi evolution that was instrumental in shaping a fundamentally different virulence program. In this scenario, typhoid toxin's inability to effectively intoxicate other mammals could have been a major driving force in S. Typhi's host-adaptation.
Conclusions
Exotoxins play a major role in shaping the diseases caused by many important bacterial pathogens. Despite S. Typhi's considerable impact on global health and extensive scientific scrutiny, typhoid toxin eluded detection until very recently. Research to further unravel the biology of this unusual toxin will be crucial to define its role(s) in S. Typhi virulence and in the development typhoid fever. There are an estimated ˜20 million cases of typhoid fever worldwide each year, resulting in more than 200,000 deaths [31]. The high burden of this disease and the rise of antibiotic resistant S. Typhi underscore the need for novel interventions to combat typhoid fever. Typhoid toxin holds a great deal of promise as a target for such interventions, as highlighted by the fact that toxoid vaccines and anti-toxin therapeutics have proven to be been highly effective agents to combat other toxigenic bacterial pathogens [32-35]. Typhoid toxin's apparent role in the symptomology of typhoid fever suggests that agents that neutralize its activity could have therapeutic potential for typhoid fever patients. Furthermore, the currently available vaccines against S. Typhi offer incomplete protection and no vaccines are available that protect against S. Paratyphi. Typhoid toxin is highly immunogenic and a promising candidate as an antigen for the development of a vaccine that could potentially protect against typhoid fever caused by both S. Typhi and S. Paratyphi. Finally, the reliable diagnosis of typhoid fever is laborious and often beyond the capabilities of the health care facilities in the developing regions where the disease is endemic. This results in misdiagnosis of typhoid fever, inappropriate treatment plans and unreliable data on S. Typhi epidemiology. The strong immunogenicity of typhoid toxin suggests that it might be possible to develop straightforward and inexpensive serological tests that diagnose typhoid fever on the basis of the patient's immune response to the toxin.
Highlights.
Typhoid toxin is a unique A2B5 exotoxin and a key S. Typhi virulence factor
Its biological program differs from related toxins in many important ways
It is highly adapted to the intracellular niche S. Typhi adopts during infection
It binds Neu5Ac-terminated sialoglycans to enable its uptake into human cells
It appears to be a distinguishing factor in S. Typhi pathogenesis
Acknowledgments
Work discussed in this review was supported in part by National Institute of Allergy and Infectious Diseases grants AI055472 and AI079022 (to J.E.G.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.House D, Bishop A, Parry C, Dougan G, Wain J. Typhoid fever: pathogenesis and disease. Curr Opin Infect Dis. 2001;14:573–578. doi: 10.1097/00001432-200110000-00011. [DOI] [PubMed] [Google Scholar]
- 2*.Haghjoo E, Galan JE. Salmonella typhi encodes a functional cytolethal distending toxin that is delivered into host cells by a bacterial-internalization pathway. Proc Natl Acad Sci U S A. 2004;101:4614–4619. doi: 10.1073/pnas.0400932101. This study first demonstrated that S. Typhi is a toxigenic pathogen. It demonstates that S. Typhi-infected cells are intoxicated by CdtB and that this toxin is exclusively produced following infection by intracellular S. Typhi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3**.Spano S, Ugalde JE, Galan JE. Delivery of a Salmonella Typhi exotoxin from a host intracellular compartment. Cell Host Microbe. 2008;3:30–38. doi: 10.1016/j.chom.2007.11.001. This study represents the discovery of typhoid toxin. It identifies the three subunits of the toxin, demonstrates their assembly and, importantly, illustrates the unusual autocrine/paracrine delivery mechanism of typhoid toxin. [DOI] [PubMed] [Google Scholar]
- 4.Lara-Tejero M, Galan JE. Cytolethal distending toxin: limited damage as a strategy to modulate cellular functions. Trends Microbiol. 2002;10:147–152. doi: 10.1016/s0966-842x(02)02316-8. [DOI] [PubMed] [Google Scholar]
- 5.Guerra L, Cortes-Bratti X, Guidi R, Frisan T. The biology of the cytolethal distending toxins. Toxins (Basel) 2011;3:172–90. doi: 10.3390/toxins3030172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Locht C, Coutte L, Mielcarek N. The ins and outs of pertussis toxin. FEBS J. 2011;278:4668–82. doi: 10.1111/j.1742-4658.2011.08237.x. [DOI] [PubMed] [Google Scholar]
- 7.Parkhill J, Dougan G, James KD, Thomson NR, Pickard D, Wain J, Churcher C, Mungall KL, Bentley SD, Holden MGT, et al. Complete genome sequence of a multiple drug resistant Salmonella enterica serovar Typhi CT18. Nature. 2001;413:848–852. doi: 10.1038/35101607. [DOI] [PubMed] [Google Scholar]
- 8.Lara-Tejero M, Galan JE. A bacterial toxin that controls cell cycle progression as a deoxyribonuclease I-like protein. Science. 2000;290:354–357. doi: 10.1126/science.290.5490.354. [DOI] [PubMed] [Google Scholar]
- 9**.Song J, Gao X, Galan JE. Structure and function of the Salmonella Typhi chimaeric A(2)B(5) typhoid toxin. Nature. 2013;499:350–354. doi: 10.1038/nature12377. In this study, the structure of typhoid toxin is solved, demonstrating its unique A2B5 architechture. Furthermore, the glycan-decorated receptors that drive toxin uptake are first identified. Importantly, this study also demonstrates that purified typhoid toxin recapitulates most of the symptoms of the acute phase of typhoid fever when administered to laboratory animals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beddoe T, Paton AW, Le Nours J, Rossjohn J, Paton JC. Structure, biological functions and applications of the AB5 toxins. Trends Biochem Sci. 2010;35:411–418. doi: 10.1016/j.tibs.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11*.Chang SJ, Song J, Galan JE. Receptor-Mediated Sorting of Typhoid Toxin during Its Export from Salmonella Typhi-Infected Cells. Cell Host Microbe. 2016;20:682–689. doi: 10.1016/j.chom.2016.10.005. This study reveals that typhoid toxin export from its intravacuolar site of production requires PltB to bind Neu5Ac-decorated glycan receptors. It is also shown that packaging of typhoid toxin into vesicle carrier intermediates also requires a specific SCV environment that is disrupted in the absence of the SPI-2 T3SS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suez J, Porwollik S, Dagan A, Marzel A, Schorr YI, Desai PT, Agmon V, McClelland M, Rahav G, Gal-Mor O. Virulence gene profiling and pathogenicity characterization of non-typhoidal Salmonella accounted for invasive disease in humans. PLoS One. 2013;8:e58449. doi: 10.1371/journal.pone.0058449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desai PT, Porwollik S, Long F, Cheng P, Wollam A, Bhonagiri-Palsikar V, Hallsworth-Pepin K, Clifton SW, Weinstock GM, McClelland M. Eutionary Genomics of Salmonella enterica Subspecies. MBio. 2013;4 doi: 10.1128/mBio.00579-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chou HH, Hayakawa T, Diaz S, Krings M, Indriati E, Leakey M, Paabo S, Satta Y, Takahata N, Varki A. Inactivation of CMP-N-acetylneuraminic acid hydroxylase occurred prior to brain expansion during human evolution. Proc Natl Acad Sci U S A. 2002;99:11736–11741. doi: 10.1073/pnas.182257399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15**.Deng L, Song J, Gao X, Wang J, Yu H, Chen X, Varki N, Naito-Matsui Y, Galan JE, Varki A. Host adaptation of a bacterial toxin from the human pathogen Salmonella Typhi. Cell. 2014;159:1290–1299. doi: 10.1016/j.cell.2014.10.057. Unlike other mammals, humans lack the ability to produce the sialic acid Neu5Gc. This study reveals that typhoid toxin binds and is toxic toward cells producing Neu5Ac-decorated receptors, but not those expressing Neu5Gc-decorated receptors, which is explained at the molecular level by solving the stucture of typhoid toxin bound to Neu5Ac. This study demonstrates that Neu5Gc-rich cells of other mammals are resistant to typhoid toxin and provides strong evidence that this hinders the development of typhoid fever in these hosts, providing insights into a potential cause of S. Typhi's host restriction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schauer R, Srinivasan GV, Coddeville B, Zanetta JP, Guerardel Y. Low incidence of N-glycolylneuraminic acid in birds and reptiles and its absence in the platypus. Carbohydr Res. 2009;344:1494–1500. doi: 10.1016/j.carres.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 17*.Hodak H, Galan JE. A Salmonella Typhi homologue of acteriophage muramidases controls typhoid toxin secretion. EMBO Rep. 2013;14:95–102. doi: 10.1038/embor.2012.186. This study identifies TtsA as essential for typhoid toxin secretion. TtsA is a relative of bacteriophage endolysins and unique amino acids in TtsA's predicted peptidoglycan-binding domain are shown to enable its secretory functions. This study respesents the discovery of this novel secretion mechanism, which appears to have evolved from bacteriophage machinery that enables bacterial lysis and phage release. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stojkovic EA, Rothman-Denes LB. Coliphage N4 N-acetylmuramidase defines a new family of murein hydrolases. J Mol Biol. 2007;366:406–419. doi: 10.1016/j.jmb.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 19.Pei J, Grishin NV. COG3926 and COG5526: a tale of two new lysozyme-like protein families. Protein Sci. 2005;14:2574–2581. doi: 10.1110/ps.051656805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamilton JJ, Marlow VL, Owen RA, Costa Mde A, Guo M, Buchanan G, Chandra G, Trost M, Coulthurst SJ, Palmer T, et al. A holin and an endopeptidase are essential for chitinolytic protein secretion in Serratia marcescens. J Cell Biol. 2014;207:615–626. doi: 10.1083/jcb.201404127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spano S, Liu X, Galan JE. Proteolytic targeting of Rab29 by an effector protein distinguishes the intracellular compartments of human-adapted and broad-host Salmonella. Proc Natl Acad Sci U S A. 2011;108:18418–18423. doi: 10.1073/pnas.1111959108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spano S, Gao X, Hannemann S, Lara-Tejero M, Galan JE. A Bacterial Pathogen Targets a Host Rab-Family GTPase Defense Pathway with a GAP. Cell Host Microbe. 2016;19:216–226. doi: 10.1016/j.chom.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hedlund M, Tangvoranuntakul P, Takematsu H, Long JM, Housley GD, Kozutsumi Y, Suzuki A, Wynshaw-Boris A, Ryan AF, Gallo RL, et al. N-glycolylneuraminic acid deficiency in mice: implications for human biology and evolution. Mol Cell Biol. 2007;27:4340–4346. doi: 10.1128/MCB.00379-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mathur R, Oh H, Zhang D, Park SG, Seo J, Koblansky A, Hayden MS, Ghosh S. A mouse model of Salmonella typhi infection. Cell. 2012;151:590–602. doi: 10.1016/j.cell.2012.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mathur R, Zeng W, Hayden MS, Ghosh S. Mice Lacking TLR11 Exhibit Variable Salmonella typhi Susceptibility. Cell. 2016;164:829–830. doi: 10.1016/j.cell.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 26.Song J, Wilhelm CL, Wangdi T, Maira-Litran T, Lee SJ, Raetz M, Sturge CR, Mirpuri J, Pei J, Grishin NV, et al. Absence of TLR11 in Mice Does Not Confer Susceptibility to Salmonella Typhi. Cell. 2016;164:827–828. doi: 10.1016/j.cell.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song J, Willinger T, Rongvaux A, Eynon EE, Stevens S, Manz MG, Flavell RA, Galan JE. A mouse model for the human pathogen Salmonella typhi. Cell Host Microbe. 2010;8:369–376. doi: 10.1016/j.chom.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Del Bel Belluz L, Guidi R, Pateras IS, Levi L, Mihaljevic B, Rouf SF, Wrande M, Candela M, Turroni S, Nastasi C, et al. The Typhoid Toxin Promotes Host Survival and the Establishment of a Persistent Asymptomatic Infection. PLoS Pathog. 2016;12:e1005528. doi: 10.1371/journal.ppat.1005528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McClelland M, Sanderson KE, Clifton SW, Latreille P, Porwollik S, Sabo A, Meyer R, Bieri T, Ozersky P, McLellan M, et al. Comparison of genome degradation in Paratyphi A and Typhi, human-restricted serovars of Salmonella enterica that cause typhoid. Nat Genet. 2004;36:1268–1274. doi: 10.1038/ng1470. [DOI] [PubMed] [Google Scholar]
- 30.Edsall G, Gaines S, Landy M, Tigertt WD, Sprinz H, Trapani RJ, Mandel AD, Benenson AS. Studies on infection and immunity in experimental typhoid fever. I. Typhoid fever in chimpanzees orally infected with Salmonella typhosa. J Exp Med. 1960;112:143–166. doi: 10.1084/jem.112.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crump JA, Mintz ED. Global trends in typhoid and paratyphoid Fever. Clin Infect Dis. 2010;50:241–246. doi: 10.1086/649541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baxter R, Hansen J, Timbol J, Pool V, Greenberg DP, Johnson DR, Decker MD. Post-licensure safety surveillance study of routine use of tetanus toxoid, reduced diphtheria toxoid and 5-component acellular pertussis vaccine. Hum Vaccin Immunother. 2016;12:2742–2748. doi: 10.1080/21645515.2016.1201622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmitt CK, Meysick KC, O'Brien AD. Bacterial toxins: friends or foes? Emerg Infect Dis. 1999;5:224–234. doi: 10.3201/eid0502.990206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schneemann A, Manchester M. Anti-toxin antibodies in prophylaxis and treatment of inhalation anthrax. Future Microbiol. 2009;4:35–43. doi: 10.2217/17460913.4.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel K, Cai S, Singh BR. Current strategies for designing antidotes against botulinum neurotoxins. Expert Opin Drug Discov. 2014;9:319–333. doi: 10.1517/17460441.2014.884066. [DOI] [PubMed] [Google Scholar]


